Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107382
Revised: April 14, 2025
Accepted: May 7, 2025
Published online: June 15, 2025
Processing time: 83 Days and 23.6 Hours
The rapid advancement of single-cell sequencing (SCS) technology has provided new insights into the relationship between inflammatory bowel disease (IBD) and colorectal cancer (CRC). This technique allows for detailed cellular analysis, enabling researchers to uncover the infiltration patterns of immune cells within the gut microenvironment and their roles in disease progression. This review summarizes significant research findings on the interplay between IBD and CRC, the characteristics of immune cell infiltration, and potential therapeutic targets identified through SCS. The aim is to offer references for future clinical studies and treatment strategies in this field.
Core Tip: Single-cell sequencing technologies are revolutionizing the understanding of immune cell infiltration in colorectal cancer and inflammatory bowel disease. By providing a granular, cell-level view of immune dynamics in these diseases, single-cell RNA sequencing helps identify specific immune cell subpopulations, their roles in disease progression, and their interactions within the tumor or inflammatory microenvironment. This technology enhances the precision of immune profiling, offering new insights for early detection, prognostic markers, and personalized immunotherapies. However, challenges such as data complexity and spatial resolution limitations remain, and ongoing technological innovations are expected to further refine these capabilities.
- Citation: Zhang Z, Wang HM, Xu ZX, Luan WY, Lin SX, Miao YD. Application of single-cell sequencing in the study of immune cell infiltration in inflammatory bowel disease and colorectal cancer. World J Gastrointest Oncol 2025; 17(6): 107382
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107382.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107382
Colorectal cancer (CRC), as a major global public health problem, has shown a continuous upward trend in its disease burden. The latest epidemiological data showed that in 2022, the number of new cases of CRC reached 1.92 million (9.6% of malignant tumors) and the number of related deaths exceeded 900000 (9.3%). CRC ranks third in the global spectrum of cancer incidence and is the second most common cause of cancer-related death[1]. According to statistical projections, the projected number of cases and deaths from CRC will increase to 3.2 million and 1.6 million, respectively, by 2040[2]. Despite the continuous innovation of screening methods and comprehensive treatment programs, the 5-year survival rate of CRC still shows significant clinical stage dependence, plummeting from 91% in stage I to 12% in stage IV[3]. Behind this precipitous decline in survival rate lies a complex multistage carcinogenesis mechanism: From adenoma formation triggered by mutations in driver genes such as adenomatous polyposis coli (APC) and Kirsten-ras (KRAS), to malignant transformation caused by abnormal epigenetic modifications, and ultimately metastatic progression through angiogenesis and immune escape[4], especially immune cell infiltration in the intestinal microenvironment is considered one of the important factors in tumorigenesis and progression[5].
In addition, the dysfunction of the immune system in a long-term chronic inflammatory state is prone to provide a favorable environment for the growth and proliferation of cancer cells, which leads to the development of CRC. Notably, inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis (UC), which exhibits chronic intestinal inflammation, is one of the important risk factors for CRC, and its incidence is increasing globally year by year[6]. Studies have shown that IBD is closely pathogenetically linked to CRC, especially in the long-term chronic inflammatory state, where the intestinal mucosal damage-repair cycle leads to the dysregulation of key immune-mediated pathways through reactive oxygen species (ROS) accumulation and pro-inflammatory factor storms, significantly accelerating epithelial cell genomic instability. Meanwhile, long-term intestinal immune dysregulation, especially the imbalance of immune cell subpopulations, not only exacerbates the intestinal inflammatory response, but may also directly promote carcinogenesis[7]. In addition, chronic intestinal inflammation induces DNA damage and uncontrolled proliferation of epithelial cells through sustained activation of nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) pathways. Macrophages are polarized to secrete interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), which abnormally cross-link with the Wnt/β-catenin pathway of intestinal stem cells, promoting adenoma formation and playing a central role in the inflammation-carcinogenesis axis[8]. Another study demonstrated that the risk of CRC development in patients with extensive UC is 5-7 times higher than that in the general population, and the cancerous process is shortened to 8-10 years[9]. Therefore, it is important to explore the pathological relationship between IBD and CRC and the role played by immune cell infiltration for early detection and prevention of CRC.
Immune cells play a crucial role in the pathogenesis of CRC and IBD. In CRC, immune cell infiltration in the tumor microenvironment is closely associated with disease progression, especially changes in the ratio of cluster of differentiation 8-positive (CD8+) T cells and regulatory T cells (Tregs), which is an important indicator of prognosis and response to therapy[10,11]. Studies have shown that the type and number of immune cells infiltrated by a tumor have a significant impact on the tumor's ability to grow and metastasize[12]. In IBD, dysfunction of immune cells is considered as one of the pathological features involving both innate and adaptive immunity[13], and in particular, imbalance in the ratio of T helper 17 cells (Th17) cells to Tregs is associated with the severity of the disease[14,15]. In addition, abnormal activation of immune cells in chronic inflammatory states not only exacerbates the intestinal inflammatory response, but also promotes intestinal tumorigenesis, e.g., the abnormal activation of intestinal immune cells, which is common in patients with IBD, is closely associated with tumor formation[16]. Therefore, an in-depth study of the role of immune cells in CRC and IBD and revealing their relationship with disease progression is crucial for the development of novel immunotherapeutic strategies.
In recent years, the emergence of single-cell sequencing (SCS) technologies has provided powerful tools for the study of tumor and inflammatory microenvironments. While traditional bulk sequencing techniques are often unable to reveal the heterogeneity and dynamics between cells, SC RNA sequencing is able to analyze these complex biological features in depth at the SC level. By analyzing SCS in patients with CRC and IBD, researchers are not only able to identify specific immune cell subpopulations but also gain insights into the roles of these subpopulations in disease progression, thus providing new perspectives for understanding the biological mechanisms of the diseases[6,11]. Moreover, SCS combined with pseudo-temporal trajectory analysis can track the dynamic changes of cells during the disease process, which in turn helps us understand the response of the tumor microenvironment to drug therapy and provides an important basis for clinical prognosis and treatment planning[17]. SCS, on the other hand, can reveal the expression profiles of different cell types and states, providing researchers with more detailed biological information and advancing our profound understanding of the disease microenvironment[10,18]. More importantly, with the continuous development of SCS technology, the introduction of new techniques such as spatial analysis has enabled us to further explain the heterogeneity of gene expression in the tumor microenvironment, opening up new research directions[19]. In addition, SCS is able to combine multiomics data to provide more comprehensive biological information, which can help develop personalized treatment strategies[20]. Therefore, SCS technology shows great potential and value for application in the study of CRC and IBD, contributing to the development of precision medicine.
The aim of this review is to review the application of SCS in CRC and IBD, focusing on the research progress of immune cell infiltration. Concentrating on its central role in the identification of immune cell subpopulations, monitoring dynamic changes, as well as the tumor microenvironment and inflammatory response, this review highlights how this technology reveals the characteristics of immune cell infiltration in the disease microenvironment and its dynamic changes and elucidates the association between immunosuppressive mechanisms and tumor escape. It also comprehensively demonstrates how this technology can deepen our understanding of the pathogenesis of CRC and IBD and provide personalized immune therapies with new ideas for personalized immunotherapy. Meanwhile, we will explore the potential of this technology in guiding precise treatment strategies and optimizing the efficacy prediction of immune checkpoint inhibitors (ICIs). We will also analyze the technical bottlenecks, such as the limitation of spatial resolution and the complexity of data analysis, and look forward to the development of this technology in the direction of SC multiomics integration and spatial-temporal dynamic tracking. By systematically analyzing the research results in this field, we aim to build a bridge between basic research and clinical practice, and promote the innovation of therapeutic strategies targeting the immune microenvironment.
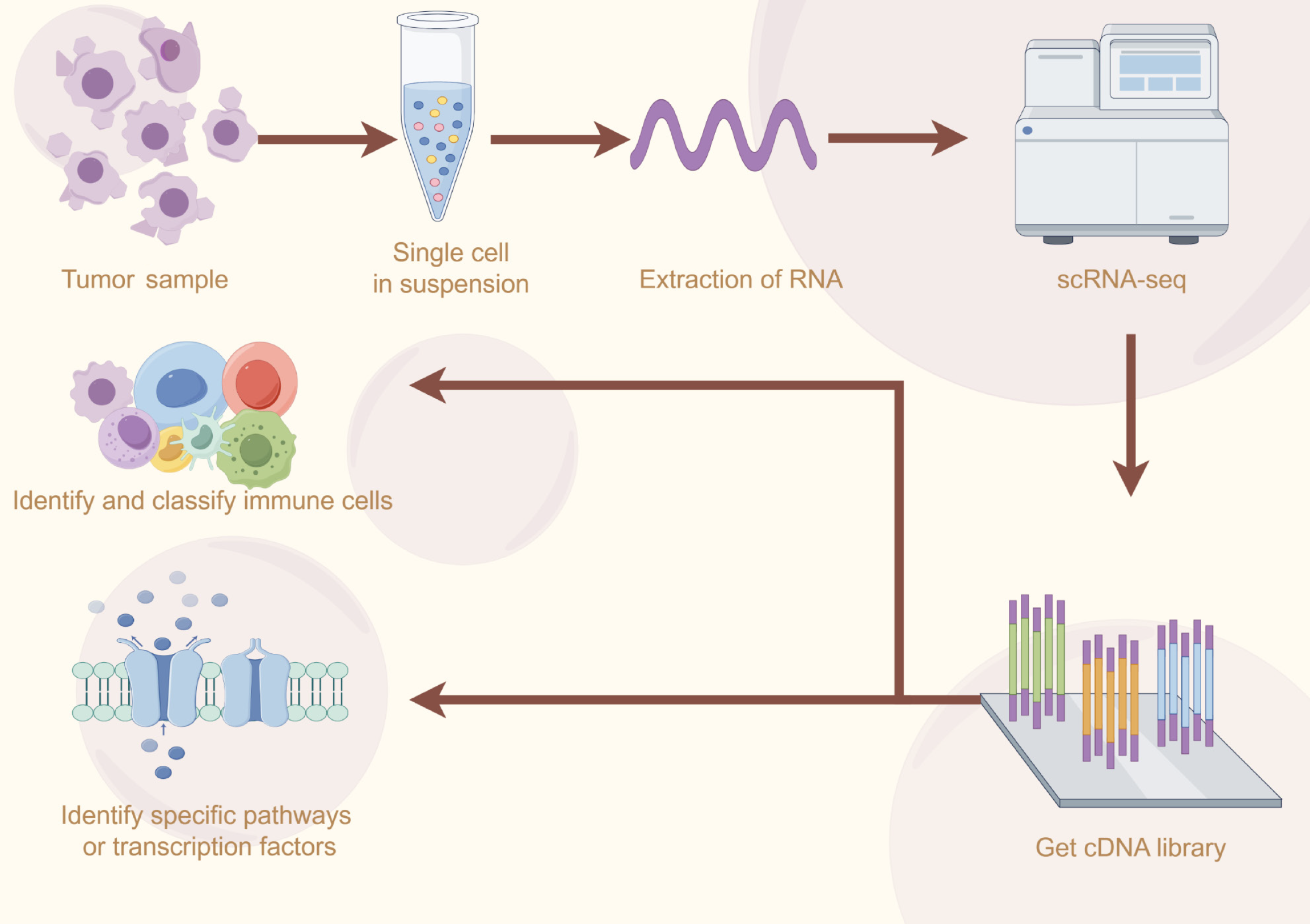
SC RNA sequencing is an emerging and powerful bioinformatics technology that is capable of capturing transcriptomic information of cells at the SC level, revealing regulatory relationships among complex genes, and tracking developmental trajectories of different populations[21] (Figure 1). The basic principle is to extract and reverse transcribe RNA from a single cell into complementary DNA (cDNA) and amplify it, then perform high-throughput sequencing, and finally prepare a complete cDNA library[22]. Currently, the mainstream SC RNA sequencing platforms include 10× Genomics, BD Rhapsody, and Fluidigm etc. Among them, 10× Genomics utilizes microfluidic technology to achieve high-throughput SC capture and sequencing by with high sensitivity and accuracy, which is suitable for the analysis of large-scale samples and is one of the most widely used SC RNA sequencing platforms[23]. In addition, the ICELL8 platform, DNBelab C4 high-throughput SC platform, SeekOne dual-platform system, and other platforms also have different advantages as well as application scenarios, which provide researchers with more diverse choices (Table 1).
| Platform | Technical features | Data quality | Application scenarios | Comparative advantage |
| 10× genomics | A system based on liquid droplets | High | High-throughput sequencing of cells | High-throughput, easy to operate, and large-scale cell atlas construction |
| BD rhapsody | A technology based on micropores | Medium | High unique molecular identifier detection sensitivity | Using imaging systems, populations with lower cell viability can be detected |
| Fluidigm C1 | Isolation of single cells in a size and shape-dependent manner based on an automated microfluidic platform | High | High gene detection rate (> 10000) | Single-cell full-length transcripts were analyzed and complete cDNA libraries were prepared |
| ICELL8 | Nanopore-based cell trapping and relying on cell and transcript specific barcodes to obtain individual cell transcript counts | High | High-throughput single-cell RNA sequencing | Ease of use and fast turnaround time allow experiments on a variety of biological samples |
| Illumina | Sequencing was performed based on the captured mtDNA | Medium | High-throughput sequencing of single cells | High throughput and high precision |
Transcriptome sequencing methods such as smart-sequencing and drop-sequencing also have their own unique advantages. Smart-sequencing is more sensitive to gene detection and has better performance in detecting genes with low expression levels and cellular interactions[24]. Drop-sequencing is favored for its lower cost and simple operation process[25]. In addition, sequencing methods with combined multiomics applications (e.g., SC assay for transposase-accessible chromatin using sequencing, cellular indexing of transcriptomes and epitopes by sequencing [CITE-seq], RNA end-associated purification by sequencing) also have unique application areas and advantages. The continuous development of these platforms and sequencing methods has advanced SC technology, leading to its increasing application in fields such as cancer research, developmental biology, and immunology[26]. Data analysis methods, on the other hand, include normalization, dimensionality reduction (e.g., principal component analysis[27], T-distributed stochastic neighbor embedding[28], or uniform manifold approximation and projection[29]) and cluster analysis (e.g., Seurat and Scanpy)[30], and the combination of multiple analytical methods has enabled researchers to gain a deeper understanding of cellular functional status and interactions.
With the development of SC RNA sequencing technology, the ability to more precisely identify and classify new subtypes of immune cells that exhibit unique transcriptomic profiles in different physiological and pathological states can provide us with a new perspective on immune processes in disease and potentially offer new targets for precision therapy[31,32]. In addition, SCS can also help us classify tumor-infiltrating T cells in detail and reveal the clinical significance of different heterogeneous populations of Tregs. It can also reveal the gene expression differences and antigen presentation relationship between B cell and plasma cell subpopulations in the tumor microenvironment, providing new ideas for understanding tumor immunotherapy. The continuous development of SCS has made it possible to discriminate between different tumor-associated macrophage (TAM) subpopulations, thus revealing the relationship between different subpopulations and gene expression, and tracking the developmental profiles of each subpopulation, thus searching for potential prognostic biomarker therapeutic targets[33]. Through high-throughput SCS technology, researchers are able to efficiently identify rare cell populations in tumors or other pathological states, which can help us understand their potential role in tumor immunity or immunosuppression, and thus develop targeted therapies aimed at modulating their activity[34].
Disease-specific transcription factors play a key role in immune cell function and fate determination. Through SCS, researchers have been able to identify transcription factors that are altered in specific disease states. These transcription factors not only regulate immune cell development and function but are also involved in the remodeling of signaling pathways. For example, in CRC, specific transcription factors are closely associated with immune cell infiltration in the tumor microenvironment, which provides potential targets for the development of new therapeutic strategies[35,36]. In addition, through SCS, researchers are able to identify cell types and their interactions that play a key role in disease progression. For example, in chronic inflammatory diseases, specific immune cell populations have been found to correlate with disease severity, and the functional state and metabolic profiles of these cells provide new clues for understanding disease progression[37,38].
The spatial and temporal dynamics of the immune microenvironment are key to understanding tumor development and therapeutic responses. SC technology enables researchers to monitor the distribution, heterogeneity, and dynamic changes of immune cells in the tumor microenvironment in real time[39]. The research has shown that immune cells in the tumor microenvironment are not only unevenly distributed spatially but also exhibit dynamic changes in time. For example, the proportions of specific immune cell subtypes change with tumor progression during tumor growth, and such changes may affect immune escape and therapeutic responses to tumors[3,40]. In addition, by comparing SC data from primary and metastatic tumors, researchers have revealed differences in the immune microenvironment of tumors at different stages of progression, which may be associated with altered tumor aggressiveness and therapeutic response[41]. Other studies have shown that immune cells in the tumor microenvironment not only differ in number but also exhibit significant heterogeneity in their functional status[36]. SCS, by analyzing T cells in the tumor microenvironment, has revealed that T cells in the tumor microenvironment exhibit heterogeneity in gene expression related to tumor progression, and these different expression patterns may be associated with immune escape and therapeutic responses to tumors[42].
The immune microenvironment of CRC is usually characterized by immune escape and immunosuppression. Tumor cells suppress the immune response through several mechanisms, such as upregulation of immune checkpoint molecules and recruitment of suppressor immune cells to evade host immune surveillance[36,43]. Another mechanism may be that CRC achieves immune evasion by downregulating the expression of major histocompatibility complex molecules and reducing the presentation of tumor antigens, thereby evading recognition by immune cells[44,45]. Furthermore, the basis of early immune escape in CRC may be due to low neoantigen expression and poor T-cell initiation due to insufficient antigenic stimulation[46]. Another study found that alterations in the immune-metabolic microenvironment of CRC are also involved in the process of tumor immune escape. CRC usually exhibits aberrant glycolysis leading to a state of cellular acidosis, which hinders immune cell activity, and the resulting nutritional deficiencies in turn affect the development of Tregs and M2-type macrophages. In addition, activation of G protein-coupled receptor 81 signaling leads to overexpression of programmed death ligand 1 (PD-L1) and reduces the antigen-presenting capacity of dendritic cells (DCs). Together, these two pathways contribute to the process of CRC immune escape[47]. These immune escape mechanisms lead to a state of immunosuppression in the tumor microenvironment, resulting in an impaired antitumor immune response, which in turn promotes tumor growth and metastasis. There are also complex interactions between the tumor microenvironment and tumor cells in CRC. These interactions not only affect the proliferation and metastasis of tumor cells but also regulate the function and infiltration of immune cells. For example, tumor cells can attract immune cell infiltration by secreting cytokines and chemokines, as well as affecting immune cell activity by altering the metabolic state in the microenvironment[36,48]. At the same time, cancer cells create a unique tumor microenvironment for their growth by modulating the function of immune cells. CRC can promote the secretion of IL-4 secretion by exosomes, thus promoting the polarization of M2 macrophages[49]. As another example, tumor cells can attract monocytes into the tumor microenvironment by secreting C-C motif chemokine ligand 2, and these monocytes are subsequently polarized to M2-type macrophages, which then promote tumor growth and metastasis[50]. The dynamic balance of such interactions is crucial for understanding the immune escape mechanisms of CRC and developing new immunotherapeutic strategies.
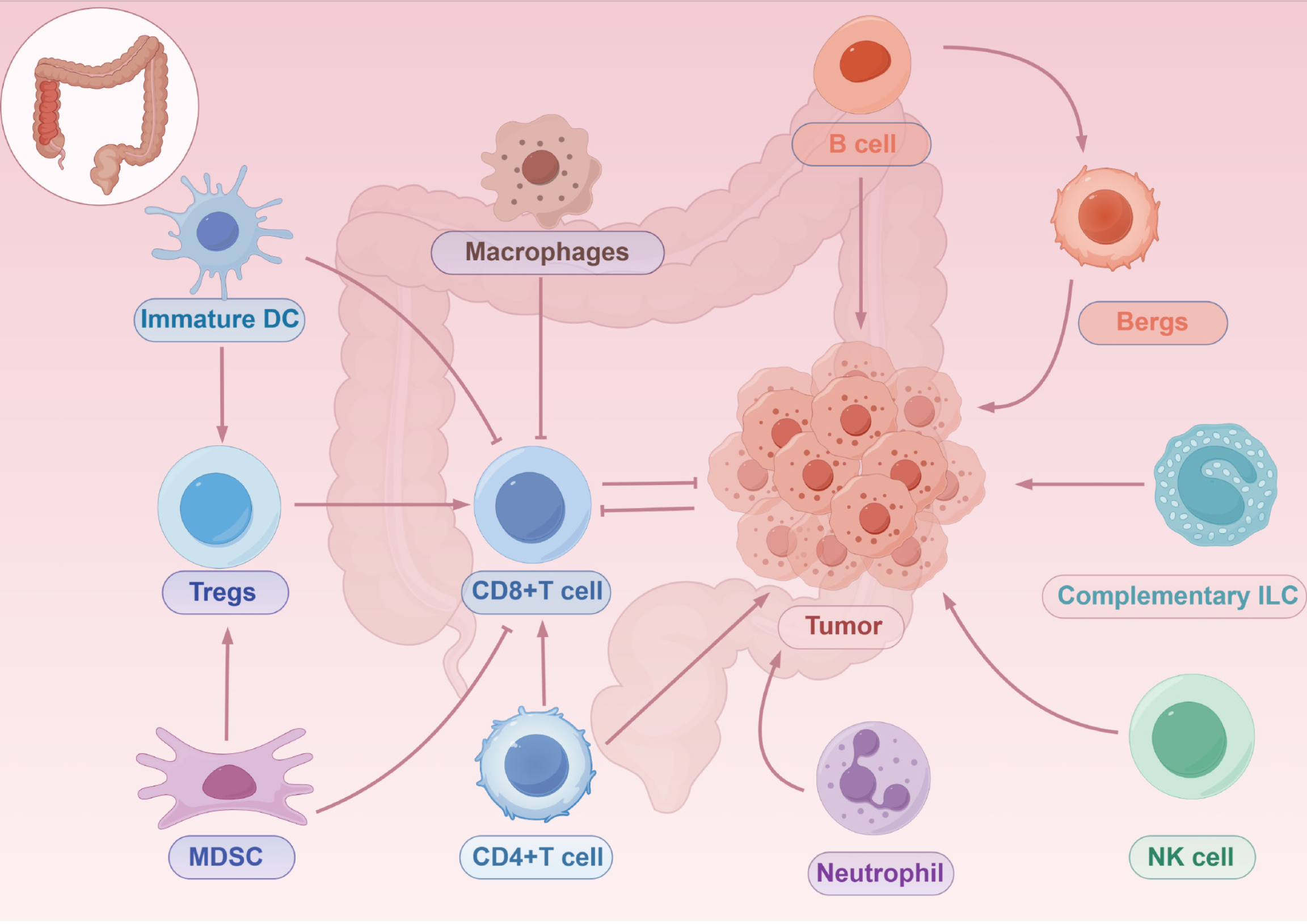
In the immune microenvironment of CRC, immune cells can be categorized into two main groups, myeloid lineage cells (including macrophages, DCs, and neutrophils) and lymphoid lineage cells (including natural and adaptive lymphocytes), based on different sources and functions[51] (Figure 2). Myeloid-derived suppressor cells (MDSCs) are derived from immature myeloid lineage cells and play a major role in CRC by suppressing T cells and promoting the development of Tregs, thus participating in tumor cell genesis as well as helping the cells evade the surveillance of the host immune system[52]. Macrophages, also known as TAMs, can be classified into M1 and M2 types depending on their response to stimuli, with the M1 type having antitumor effects while the M2 type promotes tumor growth and metastasis[53]. Studies have shown that TAMs can undergo dynamic switching in response to changes in tumor microenvironment. The polarization of TAMs is regulated by a variety of cytokines, growth factors, or multiple signaling molecules produced by tumors and other stromal cells, and plays a critical role in CRC progression[54]. DCs are highly differentiated antigen-presenting cells that efficiently promote T-cell activation to trigger an antitumor immune response, while also inhibiting tumor-associated factors that promote CRC immune tolerance and cancer progression, limiting the initiation of an antitumor immune response and enhancing immune escape in CRC, which has a dual effect on CRC progression[55,56]. Although considered to have antitumor defense functions, there is still evidence that some neutrophil populations, known as tumor-associated neutrophils (TANs), have tumor-supportive functions. Similar to TAMs, TANs are categorized into two phenotypes: N1 and N2. Similarly, N1 produces chemokines and cytokines with antitumor effects, while N2 exhibits anti-inflammatory effects that promote tumor growth[57]. In addition, neutrophil extracellular traps (NETs) function in assisting tumor cell growth, invasion, and metastasis in CRC, but the exact mechanism still needs to be explored in depth[51,58].
Natural lymphocytes include natural killer (NK) cells, helper lymphocytes, and lymphoid-induced histiocytes, a group of cells that lack adaptive antigen receptors and are capable of responding to a wide range of intracellular and extracellular pathogens[51]. As the earliest natural lymphocytes to be identified, their role in immune surveillance is well documented. NK cells also have a role in inhibiting CRC tumor metastasis and killing tumor cells. In addition to cytotoxic effects similar to those of CD8+ T cells, NK cells produce cytokines and chemokines to regulate other immune cells[59]. Moreover, NK cells also play an important role in CRC metastasis as well as in prognosis[60,61]. Helper natural lymphocytes play a critical role in early antigenic defense and inflammation, influencing the inflammatory microenvironment within the tumor. Using SCS, some studies have found that innate lymphoid cells (ILCs) are dynamically regulated in CRC, and ILC1 undergoes a dynamic switch in function during CRC progression and plays a role in advanced tumors. ILC2, which has three subpopulations and is present only in CRC tissues and is not detected in healthy tissues, has a role in promoting tumor growth. ILC3 can be stimulated by transforming growth factor beta (TGF-β) and converted to ILCregs, which act at the stage of CRC progression to promote tumor development[62,63].
B cells are the main component of immune cells infiltrated in CRC, but the significance of B cells in CRC still needs to be further explored. The degree of prognosis and cancer progression in CRC correlates with the infiltration of B cells. The distribution of B cell subtypes in the tumor microenvironment changes dynamically, with some exhibiting pro-tumorigenic properties, and others influencing the prognosis of CRC through different gene expression. In addition, there is a subpopulation of B cells called regulatory B cells (Bregs), which regulate physiological functions such as tumor cell growth, invasion, or metastasis through the secretion of certain cytokines or regulatory enzymes, thus providing a potential mechanism for immunotherapy of CRC to improve the survival and cure rates of patients with CRC[64,65].
T cells are the most abundant immune cells in CRC. Over several decades, a large number of investigators has studied and demonstrated the functions of various subtypes of T cells and their roles in CRC. Th1 cells, as well as their derived cytokines, are generally considered to be prognostically relevant in CRC, and these cells secrete signaling molecules such as interferon gamma (IFN-γ), TNF-α, and IL-2 to inhibit the proliferation of cancer cells, promote apoptosis, reduce angiogenesis, and recruit CD8+ T cells[66,67]. Th2 cells express the transcription factor GATA-binding protein 3 and secrete IL-4 and IL-5, which may be relevantly associated with chronic inflammation and cancer progression[68], whereas Th2 cells also polarize macrophages into the M2 phenotype, which is involved in tumorigenesis[69]. However, another study showed that Th2 cells can recruit eosinophils, thus exerting antitumor effects[70]. Th9 cells are characterized by the secretion of IL-9 and expression of the transcription factor PU.1. A study reported that Th9 cells are activated in patients with CRC, which suggests a pro-tumorigenic role for Th9 and IL-9[71]. In addition, IL-9 promotes the proliferation of CD8+ T cells in a dependent manner, thus exerting antitumor effects[72]. The most important feature of Th17 cells is the secretion of the IL-17 family containing six cytokines[73,74]. In CRC, IL-17A is involved in tumor angiogenesis as well as inducing tumor-associated stroma to secrete pro-tumorigenic factors through the STAT3 pathway. On the other hand, Th17 also tends to recruit neutrophils and CD8+ T cells into tumor tissues to exert antitumor effects[75,76]. Another study showed that its secretion of IF-17F has antitumor effects[77]. Th22 is transformed from naive T cells by the transcription factor aryl hydrocarbon receptor, which is characterized by the secretion of IL-22. IL-22 promotes the proliferation of cancer cells and is associated with the development of CRC[78,79]. However, another study showed that Th22 is associated with a better prognosis in patients with CRC and that IL-22 recruits neutrophils with antitumor capacity[80]. Activated cytotoxic T lymphocytes (CTLs) also release cytokines to enhance NK cell responses and recruit macrophages, resulting in a coordinated immune defense response with multicellular involvement[51]. CTLs not only kill cancer cells, but also help to convert the tumor microenvironment into a more tumor-suppressive microenvironment, thus making CTLs highly relevant to the prognosis and survival of patients with CRC[81,82]. In CRC, there are two main subpopulations of gamma delta (γδ) T cells, one of which produces IFN-γ and exhibits tumor progression suppression, whereas the other produces IL17-A and supports tumor growth[83]. However, in human CRC, the role of such cells seems to be uncertain and subtype-dependent. IL17A-expressing γδ T cells promote tumor cell growth, whereas IFN-γ-expressing γδ T cells inhibit CRC cells[84,85].
In CRC, one of the key mechanisms of immune escape is aberrant activation of the immune checkpoint pathway. PD-L1 is a glycoprotein that plays a role in decreasing T-cell activity and inducing apoptosis through activation of the programmed cell death protein 1 (PD-1) receptor. High expression of PD-L1/PD-1 is a common phenomenon in the CRC tumor microenvironment[86]. The expression of PD-L1 is significantly increased in tumor metastases, leading to functional inhibition of T cells and thus promoting immune escape from the tumor[87]. In addition, overexpression of PD-L1/PD-1 mediates cellular functions such as adhesion, invasion, and the proliferation of CRC cells[88]. Meanwhile, PD-L1/PD-1 promotes tumor development by regulating signaling pathways such as phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin, which further promotes CRC metastasis and serves as a marker of poor CRC prognosis[89]. CTL-associated antigen-4 (CTLA-4), another important immune checkpoint, and its activation in Tregs is also an important mechanism of immune escape. By inhibiting the expression of CD80/CD86 on the surface of antigen-presenting cells, CTLA-4 further impairs the activation ability of T cells, creating a favorable tumor-growth immunosuppressive environment[90]. The interaction of these mechanisms allows CRC cells to effectively evade host immune surveillance. In the tumor microenvironment of CRC, TAMs and MDSCs form a complex immunosuppressive network. TAMs support tumor development by secreting immunosuppressive factors and promoting tumor cell growth, while MDSCs enhance tumor immune escape by inhibiting the function of CD8+ T cells[32]. In addition, TAMs play a role in directly inhibiting the proliferation and activity of T cells to promote immune escape from tumors as well as inducing the expansion of Tregs, which further suppresses the body’s antitumor immune response[91]. Some research has shown that TAMs and MDSCs have synergistic effects on promoting tumor growth and metastasis and enhancing tumor invasiveness, and this mutually reinforcing relationship further exacerbates the immunosuppressive state of the tumor microenvironment, thus enabling tumor cells to evade host immune attacks[92].
CD8+ T-cell infiltration density and IFN-γ signaling score are considered important prognostic markers for patients with CRC. It has been shown that increased CD8+ T-cell infiltration is significantly associated with patient survival, especially in patients with high microsatellite instability (MSI-H) and tumor mutational load[10,93]. Additionally, activation of the IFN-γ signaling pathway has been strongly associated with an improved immune microenvironment in tumors and patient prognosis[94]. Although CD8+ T-cell infiltration and IFN-γ activity scores have been established as important prognostic indicators in CRC, their clinical translation is still limited by the lack of standardization of the assay process. Spatial heterogeneity is a central challenge: Studies have shown that CD8+ T-cell density at the invasive margins of CRC significantly outperforms the predictive efficacy of overall survival in the tumor core, and that randomized single-field-of-view testing alone may result in a 30% increased risk of prognostic misclassification[95]. ICIs are rapidly becoming the mainstay of therapy for many solid tumors due to their superior efficacy. ICIs block immunosuppressive tumor signaling by targeting receptor or ligand checkpoint proteins, such as PD-L1 and CTLA-4[96]. The use of ICIs has been demonstrated in the treatment of CRC showing exciting results. Patients with MSI-H CRC are more immunogenic due to the presence of mismatch repair defects in their genes and thus code-shifting mutations, and subsequently typically exhibit stronger immune responses and possess better immunotherapeutic responses[51,93]. In conclusion, another ICI targeting NK group 2 member A, has been developed to promote NK cell function and has shown potential to enhance the efficacy of anti-PD-1 therapy in microsatellite stable metastatic CRC[97]. With the intensive study of the immune microenvironment in CRC, the development of new targets against immune cells is also advancing. For example, colony-stimulating factor 1 receptor inhibitors of TAMs show good promise, which are able to remodel the tumor microenvironment and enhance the anti-tumor activity of immune cells. In addition, indoleamine 2,3-dioxygenase inhibitors that inhibit tryptophan metabolism have been recognized as promising new targets to enhance immune responses by altering the metabolic microenvironment[10,94].
The immune microenvironment of IBD consists of a variety of cells and their interactions, including intestinal epithelial cells, immune cells, and intestinal microorganisms. An imbalance of intestinal flora and abnormal activation of the immune system are important mechanisms leading to dysregulation of immune tolerance in IBD. Changes in intestinal flora not only affect the infiltration of immune cells but may also modulate the immune response in the gut through the production of metabolites such as short-chain fatty acids[98,99]. Dysregulation of intestinal flora can lead to abnormal activation of the immune system, which in turn triggers chronic inflammation and tumorigenesis[100]. For example, in patients with CD, an increase in specific bacterial groups such as adherent invasive Escherichia coli is associated with immunosuppression, which may promote tumor progression by suppressing anti-tumor immune responses. In addition, the synergistic effect of mucosal barrier disruption and antigen exposure plays an important role in the pathogenesis of IBD. The integrity of the intestinal epithelium is critical for maintaining immune tolerance. When the intestinal barrier is damaged, microorganisms and their metabolites in the gut can come into direct contact with immune cells, leading to abnormal activation and overreaction of the immune system[101]. This aberrant activation not only causes a localized inflammatory response but also triggers a systemic immune response and may lead to a dysregulation of the balance between beneficial and harmful intestinal bacteria, thus affecting the course of IBD as well as promoting the malignant process of IBD[102]. During the pathology of IBD, infiltration of immune cells not only leads to inflammation and injury in the intestine but may also promote intestinal fibrosis and tumorigenesis. In chronic inflammatory states, immune cells in the intestine further exacerbate the functional damage and inflammatory response of the intestine by secreting pro-inflammatory factors and cytokines, creating a vicious cycle of inflammation[103,104]. Chemokines and cell adhesion molecules (CAMs) play a key role in immune cell infiltration. For example, C-X-C motif chemokine receptor 3 (CXCR3)-CXC motif chemokine ligand 10 (CXCL10) is highly expressed in tissue infiltration as well as activated T cells, thereby promoting recruitment of effector cells to the inflammatory tissues of the intestine, and these cells play an important role in antitumor immunity in IBD[105]. In addition, CAMs such as intercellular cell adhesion molecule-1 significantly increase expression in active IBD, promoting the migration and infiltration of immune cells by enhancing their adhesion to endothelial cells[18,106].
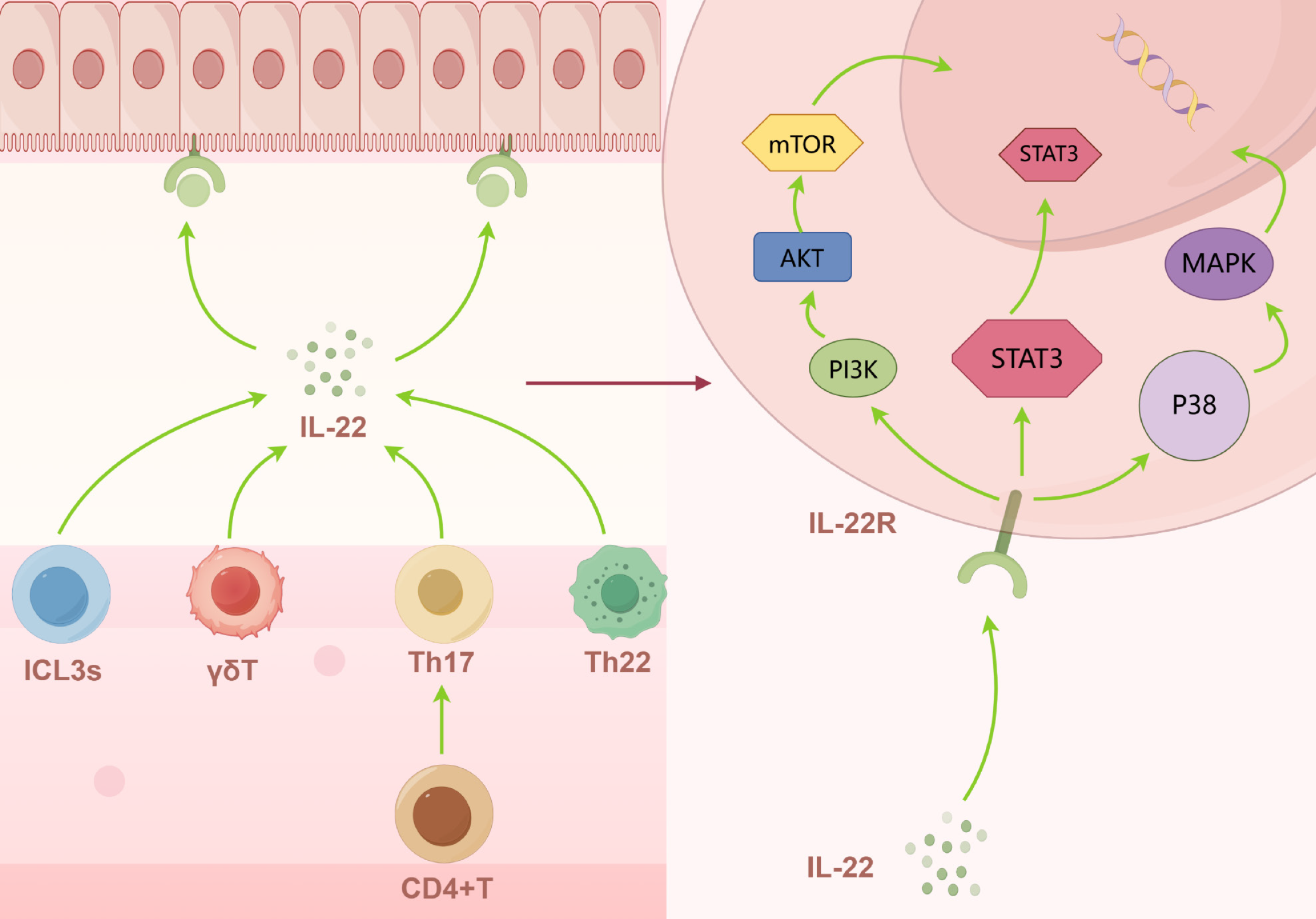
In the immune microenvironment of IBD, the dynamic balance of T-cell subsets plays a crucial role in the development and progression of the disease. Overactivation of Th1 and Th17 cells, and elevated levels of cytokines such as IL-12 and IL-23, are usually considered to be responsible for the production of CD. By contrast, the development and progression of UC is associated with the involvement of Th2 and Th9 and their major cytokines IL-13 and IL-5[107,108]. In addition, dysregulation of Th22 can also enhance the production of inflammatory mediators such as IL-6 by human colonic myofibroblasts through the production of excess IL-22, which subsequently induces inflammation to produce IBD[109]. Furthermore, by activating STAT3, IL-22 promotes the expression of different genes involved in mucus production, epithelial cell proliferation, epithelial regeneration, and wound healing, which subsequently reduces intestinal inflammatory responses[110]. However, the role of IL-22 in IBD remains controversial. Most studies suggest that IL-22 promotes epithelial cell proliferation and the production of antimicrobial agents, thereby exerting protective effects. Whereas IL-22 can also induce inflammation, animal experiments have shown that the severe epithelial tissue damage exhibited during colitis in mesenchymal stem cell-deficient mice is due to IL-22 overproduction, and that blocking IL-22 reverses this severe damage and reduces the severity of IBD as well as decreases tumor load (Figure 3)[111,112]. Meanwhile, functional inhibition and reduced number of Tregs lead to loss of tolerogenic immunity, which further exacerbates the state of immune imbalance and promotes chronic inflammation in the gut. In addition, the development of IBD is associated with an imbalance in the ratio of Th17/Tregs, which is regulated by a variety of factors including cytokines and co-stimulatory signals[113,114].
Macrophages exhibit significant polarization and functional heterogeneity in the immune response to IBD. In the microenvironment of IBD, a significant decrease in M2 was observed in addition to a significant increase in M1 type[115]. The M1 type promotes the inflammatory response by participating in tissue destruction through the release of pro-inflammatory factors, whereas the M2 type counteracts inflammation by promoting repair functions and fibrotic processes[12]. In another study, it was also found that the M2 type does not only inhibit inflammation but also produces adverse effects such as fibrosis and granulomas[116]. The inflammatory infiltration of lymphoplasmacytic cells in IBD is a well-established fact[117], and B-cells and the antibodies they produce play an important role in the pathology; however, exactly how B cells play a role in IBD still needs to be investigated. However, the inflammatory profile of B cells is different in CD and UC, where patients with CD usually show systemic anti-IgG and IgA responses and constitutive secretion of IL-8 by B cells, which shows a positive correlation with the degree of disease activity. Patients with UC, on the other hand, are more likely to show increased IgG expression alone and increased Toll-like receptor 2 (TLR2) expression by circulating B cells, suggesting that these cells play an active role in a given situation[118,119]. In addition, studies of Bregs could lead to a better understanding of the pro-inflammatory mechanisms and interactions of the B-cell family in IBD, leading to the development of more effective therapeutic regimens[120].
The pathological process of IBD is closely related to the cascade response of pro-inflammatory cytokines. Innate immune cells recognize pathogen-associated molecular patterns through pattern recognition receptors, which activate the NF-κB and mitogen-activated protein kinase signaling pathways, leading to the release of early inflammatory factors such as TNF-α, IL-1β, and IL-6[121]. During the adaptive immunity phase, IL-12 and IL-23 drive the differentiation of Th1 and Th17 cells, respectively, and secrete effectors such as IFN-γ and IL-17A/F, forming a positive feedback loop to amplify the inflammatory response. In addition, the IL-23/Th17 axis promotes the production of antimicrobial peptides and chemokines by intestinal epithelial cells through the activation of STAT3 signaling, while suppressing the immunoregulatory function of Tregs[122]. In the chronic phase inflammatory microenvironment, factors such as TGF-β and IL-13 drive the fibrosis process by inducing fibroblast activation and cupping apoptosis[123]. Functional phenotypic switching of immune cells is a central event in the pathologic process of IBD that determines inflammatory homeostasis. In macrophages, for example, the M1 type activates the TLR4/NF-κB pathway to secrete TNF-α and IL-6 to drive mucosal injury, whereas the M2 type secretes IL-10 and TGF-β through a STAT6-dependent mechanism to promote tissue repair[124]. SCS studies have demonstrated that intestinal macrophages from patients with IBD exhibit a “mixed M1-M2 phenotype” with a metabolic signature that maintains a pro-inflammatory state by inhibiting hypoxia inducible factor 1 alpha (HIF-1α) degradation. T-cell phenotypic switching is also critical in adaptive immunity, where IL-6/STAT3 signaling promotes Th17 differentiation and suppresses Treg function, while IL-2/STAT5 reverses this imbalance[125].
The interaction between immune cells and the intestinal barrier is a central mechanism for maintaining mucosal homeostasis, and its dynamic imbalance directly drives the chronic inflammatory process in IBD[126]. Intraepithelial lymphocytes (IELs) defend against pathogen invasion by activating the STAT3 signaling pathway through the secretion of IL-22, inducing goblet cell differentiation and enhancing mucin 2 secretion[127]. IL-22 expression in IELs is significantly reduced in patients with IBD, leading to thinning of the mucus layer and a decrease in antimicrobial peptides, which promote a systemic inflammatory response. However, IL-22 also inhibits systemic inflammatory responses by inducing lipopolysaccharide-binding proteins. Therefore, the role of IL-22 in IBD remains to be determined[128,129]. Macrophages in the lamina propria promote the proliferation of leucine rich repeat containing G protein-coupled receptor 5-positive intestinal stem cells through the Wnt/β-catenin pathway, but they are transformed into a pro-inflammatory phenotype in the inflammatory microenvironment, releasing TNF-α and ROS, and disrupting epithelial intercellular tight junctions[130]. DCs extend transepithelial synapses through CX3CR1-dependent mechanisms, ingest commensal bacteria and induce Treg differentiation to maintain immune tolerance. By contrast, TLR4-hyperactivated DCs promote Th17 polarization and degrade basement membrane collagen via matrix metalloproteinase 9 (MMP9), exacerbating barrier leakage[131]. Neutrophil infiltration is the hallmark event of the acute phase of IBD, and its release of NETs containing myeloperoxidase (MPO) and histone H3 forms microulcers by activating the epithelial apoptotic pathway. In addition, neutrophils produce high levels of ROS leading to epithelial barrier damage and can activate redox-sensitive inflammatory pathways, which in turn release proteases, pro-inflammatory factors and mediators, further damaging the epithelial barrier and recruiting neutrophils in a vicious cycle[132,133].
The assessment of disease activity in IBD relies on the integrated analysis of multidimensional immune markers that not only reflect the intensity of mucosal inflammation but also predict treatment response and risk of recurrence. Among serum markers, IL-6 and soluble IL-2 receptor (IL-2R) are positively correlated with endoscopic ulcer severity, and their elevated levels are suggestive of overactivation of the Th1/Th17 pathway[134]. C-reactive protein is considered to be one of the important proteins in acute inflammation, and a correlation between its elevated levels and IBD has been demonstrated. Anti-neutrophil cytoplasmic antibodies and anti-glycoprotein 2 immunoglobulin G (GP2-IgG) antibodies are disease-specific in UC and CD, respectively, and high titers of GP2-IgG have been associated with the risk of intestinal stenosis in CD. In addition, the role of other markers such as nitric oxide (NO), TNF-α, tumorigenicity suppressor 2, and TNF-α-inducible protein 6 biomarkers still needs to be further explored, but should be considered as potential biomarkers[135,136]. Regarding fecal markers, calreticulin released by neutrophils is the current gold standard for sensitive identification of endoscopically active lesions[137]. By contrast, lactoferrin and calreticulin C reflect intestinal bleeding and macrophage activation status, respectively. Notably, neutrophil gelatinase-associated lipid transport protein, intestinal alkaline phosphatase, MMPs, and MPO each have advantages and disadvantages in terms of diagnostic sensitivity and specificity as potential biomarkers for the detection of IBD[138,139]. Among the mucosal immunomarkers, intraepithelial CD8+ T-cell density and TNF-like ligand 1A expression levels significantly correlate with the depth of histologic remission by SCS[140]. Emerging markers such as circulating free DNA methylation profiles and exosome microRNA-223 enable noninvasive activity grading by liquid biopsy techniques, and their combination with intestinal ultrasound elastography may enhance predictive accuracy[138].
Precision intervention with biologics has significantly improved the response rate of IBD by targeting specific immune pathways and individualized dosing strategies. Currently, biologics focus on three major classes of targets: TNF, leukocyte migration-related molecules, and the IL-12/23 pathway. Anti-TNF monoclonal antibodies exert their efficacy by neutralizing soluble/transmembrane TNF-α and inducing apoptosis in membrane-bound TNF-expressing cells, but their response rates are affected by genetic polymorphisms and anti-drug antibodies[141]. Gut-selective anti-integrin therapies offer symptom-specific benefits in patients with concomitant pregnancy, elderly patients, and patients with a history of prior malignancy by blocking lymphocyte homing to the gut[142]. IL-23 inhibitors such as ustekinumab, a class of monoclonal antibodies that inhibit Th17 differentiation by blocking the p40 subunit, are used in patients with moderate-to-severe disease who have responded poorly to conventional therapies and are intolerant of conventional agents patients[143,144]. In addition, emerging therapies such as SMAD7 antisense oligonucleotides and IL-6 trans-signaling inhibitors achieve intestinal mucosal repair by modulating the TGF-β and IL-6 pathways. Although it still takes a long time to prove their safety and efficacy, the available clinical data have shown promising results as a potential therapeutic tool[145,146].
The treatment of IBD is shifting from broad-spectrum immunosuppression to precision interventions targeting pathogenic cell clones and microbial-immune interactions, with breakthrough potential demonstrated by intestinal flora transplantation (FMT) and CAR-T cell therapy. FMT modulates the immune microenvironment by reestablishing host-microbial homeostasis, which improves clinical symptoms and enhances the response of the patient to immunotherapy. FMT may be a more powerful manipulation of the gut microbiota than the use of probiotics and antibiotics, and has been used in patients with Clostridium difficile-infected IBD[147,148]. CAR-T cell therapy, a therapeutic approach using anti-inflammatory autologous or allogeneic cells, is currently focusing on CD19+ B-cell and IL-23R targeted CAR-T cells, which may have potential for treating difficult-to-treat conditions. This approach has the potential to have a major impact on the treatment of refractory IBD. However, it will take a long time for such therapies to reduce their cost and to discover more efficient therapeutic targets[149]. In addition, although side effects of CAR-T therapy such as cytokine release syndrome and immune effect-associated neurotoxicity syndrome are rare in the treatment of chronic inflammatory diseases, further controlled studies and investigations are needed to assess the safety of CAR-T cell therapy. Therefore, these important adverse events should be carefully considered in the risk-benefit consideration of CAR-T therapy for IBD[149].
There is significant heterogeneity in the distribution of cellular subpopulations in the immune microenvironment of CRC and IBD but also some commonalities. It has been shown that patients with CRC have a significantly higher proportion of monocyte-macrophages and a decreasing proportion of B-cell CD4+ and CD8+ T cells as the disease continues to progress. In IBD, however, the proportions of B-cell and T-cell subsets showed a richer distribution, suggesting that the two diseases have different biases in immunosuppressive and pro-inflammatory responses[16,150]. The molecular characterization networks of CRC and IBD show significant commonality and specificity in the inflammatory-carcinogenic transformation. Genome-wide analyses have shown that the Wnt/β-catenin pathway is persistently activated in CRC due to mutations in the APC gene, whereas IBD-associated hyperproliferative tissues are more reliant on IL-6/STAT3 and NF-κB signaling to drive proliferation, and IBD-associated CRC exhibits a higher level of genome-wide DNA methylation aberrations compared to sporadic CRC[151,152]. In addition, in IBD macrophages and DCs secrete IL-6, which in turn activates STAT3 phosphorylation in CD4 + T cells and neutrophils to produce a storm of pro-inflammatory factors. In turn, concomitant STAT3 induces differentiation of Th17 cells that secrete IL-17 and IL-22, driving intestinal inflammation[153]. In CRC, IL-22 activates and promotes the binding of STAT3 to the polycomb repressive complex 2 promoter and stimulates the proliferative metastasis and anti-apoptotic capacity of colon cancer cells by targeting cell cycle protein deterrents[78]. It has also been shown that CRC malignant cells and IBD epithelial cells share more than 50% genomic overlap, suggesting common molecular features between inflammation and cancer. The intersection and divergence of these molecular networks provide a theoretical basis for the development of CRC prevention strategies based on inflammatory regulation and therapies targeting the tumor immune microenvironment[154].
The immune microenvironment of CRC and IBD shows significant temporal evolutionary features with disease progression. Long-term cohort studies have shown that p53 and KRAS are frequently mutated early in the transition from chronic inflammation to allopatric hyperplasia in patients with IBD, whereas APC mutations occur later in the disease progression. In addition, the interplay of gut microbes and impaired intestinal mucosal barriers also play a role in the development of CRC, and the temporal changes in the above mechanisms ultimately promote carcinogenesis[155]. The SC transcriptome further revealed that nicotinamide-phosphate ribosyltransferase affects the TAM phenotype during CRC development by promoting HIF-1α expression and STAT3 phosphorylation, decreasing stimulator of interferon genes signaling and induction of type I IFN-responsive genes, and regulating fatty acid metabolism through increasing nicotinamide adenine dinucleotide-positive pools and decreasing nicotinamide levels, thereby promoting sirtuin 1 activity. TAM phenotype, thereby contributing to immune-heterogeneous tumor microenvironment remodeling for CRC progression[156]. Notably, blocking IL-6 trans-signaling during the “time window” of IBD to CRC transformation significantly reversed the Th17/Treg imbalance and inhibited tumorigenesis, whereas interventions at later stages had limited effect, suggesting the necessity of time-sequence-specific therapy[157]. The immunotherapy response characteristics of CRC and IBD show significant differences due to disease heterogeneity and dynamic remodeling of the microenvironment. Clinical studies have shown that patients with MSI-H CRC can respond up to 40%-60% to anti-PD-1 therapy by a mechanism associated with high CXCL13 expression by tumor-infiltrating CD8+ T cells, whereas the efficacy of anti-TNF therapies in patients with IBD has been associated with the ratio of Bregs to Tregs in the intestinal mucosa[158,159]. The SCS further revealed that anti-PD-1 treatment-resistant CRCs had clusters of lymphocyte activation gene 3-positive/CD4+ T-cell depletion induced by fibroblast activation protein-positive fibroblasts via TGF-β signaling, whereas patients with IBD ineffective on anti-TNF-α therapy exhibited an expansion of the IL-23R+ intrinsic lymphoid cell subset and sustained activation of STAT3 phosphorylation in epithelial cells[160].
There are multiple shared molecular switches in the pathologic processes of CRC and IBD that drive disease progression by regulating the inflammation-cancer axis. It has been shown that the STAT3 signaling pathway plays a central role in both, with the IL-6/STAT3 axis promoting Th17 cell differentiation and epithelial barrier damage in IBD[151], whereas STAT3 is phosphorylated by activation of cytokines, such as IL-22 and IL-17, in CRC, thereby sustaining tumor cell growth and damaging the intestinal epithelial mucosa[161]. The epigenetic regulator DNA demethylase 2 (ten-eleven translocation 2 [TET2]) has also been shown to act as a shared switch, and aberrant expression of TET2 in the intestinal mucosa of patients with IBD leads to aberrant epigenetic modification of cellular DNA, which further promotes carcinogenesis in IBD[162]. In contrast, overexpression of TET2 in CRC significantly reduces the methylation level of the hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta (HADHB) promoter, which inhibits HADHB gene expression, activates the focal adhesion kinase signaling pathway, and ultimately promotes CRC metastasis[163]. In short, the Wnt/β-catenin pathway exhibits a “dose effect” in IBD-associated allopatric hyperplasia and CRC, where low levels of β-catenin activation promote epithelial reparative hyperplasia, whereas β-catenin accumulation due to inactivation of the APC gene drives the self-renewal of CRC stem cells. This dynamic balance is regulated by the Ring finger protein 43/zinc Ring finger 3 complex[164].
In response to the pathological molecular features of CRC and IBD, several types of novel therapeutic strategies based on molecular typing have emerged in recent years. monoclonal antibodies targeting IL-23 and IL-12 in IBD play a therapeutic role by improving the expression of immune cells and maintaining the integrity of the epithelial barrier[165]. In addition, clustered regularly interspaced short palindromic repeats-mediated gene editing and targeted drug delivery technologies have been successfully applied to IBD, providing therapeutic genome editing with site-specificity and more precise drug delivery for inflammatory diseases[166]. Ovo like zinc finger 2, a Wnt/β-catenin pathway-specific antagonist for CRC, ameliorates CRC by blocking Wnt signaling by promoting the recruitment of protein deacetylase 1 to the transcription factor 4 beta catenin complex[167].
The precision diagnostic system for CRC and IBD is gradually changing from traditional pathology to a multiomics integration model. Liquid biopsy technology based on circulating tumor DNA has higher sensitivity and specificity compared with traditional biomarker testing, but this technology still requires long-term research[168]. Combined fecal microbiome-metabolome analysis further improved the diagnostic specificity, as the abundance of Clostridium perfringens was positively correlated with the concentration of succinate metabolites in patients with CRC, whereas the reduction of Mycobacterium avium spp. in the feces of patients with active stages of IBD was significantly correlated with the elevated levels of secondary bile acids, and the combined markers of the two could differentiate inflammatory carcinoma in IBD from sporadic CRC[169]. In addition, the combination of spatial transcriptomics and SCS has enabled an integrated exploration of primary CRC and CRC as well as secondary CRC from a molecular point of view, and deciphered the associated cellular composition, providing a molecular basis for discriminating between different types of CRC[170].
Clinical decision support systems for CRC and IBD are being upgraded with precision by integrating multi-omics data with artificial intelligence (AI) technology. AI-assisted computer-aided detection system for colorectal tumors integrated with colonoscopy improves adenoma detection and reduces adenoma underdiagnosis[171]. The AI-assisted endoscopic imaging system provides an endoscopic evaluation system for IBD that objectifies inflammation, underlying lesion detection, and prognostic testing for disease recurrence, thus providing a rapid and accurate platform for clinicians to make interim decisions[172]. A prospective cohort study demonstrated that the AI-based mayo endoscopic score predicted clinical recurrence at 12 months with individual receiver operating characteristic curve area under the curve values of 0.61 and 0.60, respectively, and had a sensitivity and accuracy of 96.9% and 93.4%, respectively, which markedly improved the diagnostic accuracy and internal reproducibility of IBD[173]. In addition, a multicenter randomized controlled trial reported a 7.5% improvement in overall adenoma detection rate (ADR) and 1.7% improvement in late ADR in ADR during AI-assisted colonoscopy relative to conventional colonoscopy, demonstrating the benefit of AI-assisted colonoscopy in asymptomatic subjects undergoing CRC screening[174]. Although the application of these tools has substantially improved the accuracy of early diagnosis of CRC and provided an immediate, highly standardized, and highly reliable tool for assessing the staging or grading of IBD, their clinical dissemination still needs to address the challenges of data heterogeneity and ethical compliance[175,176].
The current application of SC and spatial multiomics technologies in CRC and IBD research still has significant experimental limitations: The low efficiency of cell capture in SCS leads to the leakage of rare cell subpopulations. In the commonly used platforms, the cell capture rate of 10× Genomics Chromium is about 65%, while that of BD Rhapsody is only about 30%, and a large number of cells are missed, resulting in incomplete cell capture and resulting in data bias[177]. The loss of cell activity during dissociation further exacerbates the data bias, which still needs to be solved by further development of cell capture technology[178]. In terms of technology preference, droplet-based microfluidic platforms have significant differences in the capture efficiency of epithelial cells and immune cells, which can easily lead to distortion of the resolution of the mucosal immune microenvironment, whereas spatial transcriptomic technologies are limited in resolution and are difficult to accurately locate synaptic signaling between tumor-immune cells[179]. Although novel in situ sequencing technologies have improved detection sensitivity to the single-molecule level through multiplexed probe hybridization, their high cost and long experimental cycles have limited clinical scale application[180]. In the future, a low-cost, high-fidelity SC multimodal capture platform needs to be developed and combined with AI-driven experimental condition optimization algorithms to systematically break through the bottleneck of existing technologies[181]. In addition, clinical translation of SCS faces three key bottlenecks: Lack of standardization in sample preprocessing, crisis of reproducibility in bioinformatics analysis, and lagging technology adaptation in regulatory frameworks. Tissue dissociation methods and preservation conditions significantly affect cell capture rates and transcriptome integrity, and stress gene expression in frozen samples can be up to three times higher than in fresh samples, making it difficult to compare data across studies[182]. Secondly, fragmentation of the analytical process and manual parameter dependency fluctuate false-negative rates for key biomarkers by more than 30%, threatening the reliability of clinical decision-making[183]. Finally, the existing United States Food and Drug Administration approval framework lacks compatibility with SC, high-dimensional data, and traditional validation criteria fail to address technical noise and dynamic threshold definition challenges[184]. Therefore, to break through the above bottlenecks, it is necessary to collaboratively promote standardized protocols for clinical-grade operations, AI-driven analysis tools for the whole process, and innovative regulatory evidence systems, so as to realize the leap from scientific research exploration to clinical implementation.
In recent years, SC and spatial multiomics technologies have made breakthroughs in multi-dimensional analysis of the disease microenvironment: Microfluidics-based ultra-high-throughput SC multiomics sequencing platforms have increased the cell capture throughput to the millions, and realized the simultaneous detection of full-length transcripts and surface proteins, which reveals the cellular interactions network[185]. Fourth-generation sequencing methods such as in situ sequencing combine traditional imaging and analysis techniques with state-of-the-art sequencing technology to provide a new approach for studying tissue heterogeneity. Although in situ sequencing still has shortcomings in providing whole transcriptome profiles despite its current shortcomings, it is undisputed that this technology still has a great potential[186]. In addition, multimodal genomics integration technologies such as TEA-seq and CITE-seq combined with clustered regularly interspaced short palindromic repeats screening can simultaneously capture the transcriptome, epitome, and hundreds of surface proteins of single cells, and successfully analyze the epitope-transcription co-regulation mechanism of Wnt pathway enhancers in CRC stem cells, which can provide a new target for targeted therapy[187]. Future novel AI-based automated analysis platforms such as DeepCell[188] and CellXGene[189] enable SCS data sharing and repurposing through standardized architecture and management, and allow access by multi-disciplinary researchers, providing a concise and rapid platform for the development of various fields.
The rapid development of SCS technology has provided unprecedented depth and precision for the study of the immune microenvironment in CRC and IBD. Through the integrated analysis of SC transcriptome, epigenome and spatial genomics, researchers are able to reveal the heterogeneity of immune cells in the tumor and inflammatory microenvironments, the dynamic evolutionary patterns and their functional states, thus deeply resolving the core mechanisms of the diseases. In CRC, SC sequencing technology clarified the key mechanisms of immune escape (e.g., activation of the PD-1/PD-L1 axis, M2 polarization of TAM) and identified prognostic markers such as CD8+ T-cell infiltration and the IFN-γ signaling pathway. In IBD, on the other hand, the technique revealed the role of Th17/Treg imbalance, aberrant macrophage polarization, and gut flora-immune interaction network in chronic inflammation. In addition, the common pathogenic mechanisms of the two provide a theoretical basis for the development of cross-disease therapeutic targets. However, current research still faces technical bottlenecks and clinical translational challenges. The cell capture efficiency, technology preference and data analysis complexity of SCS limit the resolution of rare cell subpopulations and spatial interactions, while the reproducibility of results and lack of standardized processes also need to be addressed. In the future, there is still a need to break through the existing limitations through technological innovation and AI-driven analysis platforms, as well as to strengthen the synergy between bioinformatics and basic research to promote the development of precision medicine. Looking ahead, further optimization of SC technologies will help personalized design of immunotherapy strategies, such as biomarker screening based on patient-specific immune profiles, development of combination therapies targeting immunosuppressive microenvironments, and precise regulation of gut flora-immunity interactions. Through interdisciplinary collaboration and deep integration of multiomics data, SCS is expected to serve as a bridge connecting basic research and clinical applications, and ultimately provide more efficient and precise therapeutic solutions for patients with CRC and IBD.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 7795] [Article Influence: 7795.0] [Reference Citation Analysis (2)] |
| 2. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 888] [Article Influence: 444.0] [Reference Citation Analysis (1)] |
| 3. | Bazyari MJ, Saadat Z, Firouzjaei AA, Aghaee-Bakhtiari SH. Deciphering colorectal cancer progression features and prognostic signature by single-cell RNA sequencing pseudotime trajectory analysis. Biochem Biophys Rep. 2023;35:101491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 4. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 902] [Article Influence: 112.8] [Reference Citation Analysis (2)] |
| 5. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 808] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 6. | Keever-Keigher MR, Harvey L, Williams V, Vyhlidal CA, Ahmed AA, Johnston JJ, Louiselle DA, Grundberg E, Pastinen T, Friesen CA, Chevalier R, Smail C, Shakhnovich V. Genomic insights into pediatric intestinal inflammatory and eosinophilic disorders using single-cell RNA-sequencing. Front Immunol. 2024;15:1420208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Porter RJ, Arends MJ, Churchhouse AMD, Din S. Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines. J Crohns Colitis. 2021;15:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8161] [Article Influence: 544.1] [Reference Citation Analysis (0)] |
| 9. | Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389-16397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Huang X, Sun T, Wang J, Hong X, Chen H, Yan T, Zhou C, Sun D, Yang C, Yu T, Su W, Du W, Xiong H. Metformin Reprograms Tryptophan Metabolism to Stimulate CD8+ T-cell Function in Colorectal Cancer. Cancer Res. 2023;83:2358-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 11. | Lv X, Ma W, Miao X, Hu S, Xie H. Navigating colorectal cancer prognosis: A Treg-related signature discovered through single-cell and bulk transcriptomic approaches. Environ Toxicol. 2024;39:3512-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Wen R, Zhou L, Peng Z, Fan H, Zhang T, Jia H, Gao X, Hao L, Lou Z, Cao F, Yu G, Zhang W. Single-cell sequencing technology in colorectal cancer: a new technology to disclose the tumor heterogeneity and target precise treatment. Front Immunol. 2023;14:1175343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 225] [Reference Citation Analysis (0)] |
| 14. | Padoan A, Musso G, Contran N, Basso D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr Issues Mol Biol. 2023;45:5534-5557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 15. | Song Y, Yuan M, Xu Y, Xu H. Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 16. | Lv H, Mu Y, Zhang C, Zhao M, Jiang P, Xiao S, Sun H, Wu N, Sun D, Jin Y. Comparative analysis of single-cell transcriptome reveals heterogeneity and commonality in the immune microenvironment of colorectal cancer and inflammatory bowel disease. Front Immunol. 2024;15:1356075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Zhao Y, Li ZX, Zhu YJ, Fu J, Zhao XF, Zhang YN, Wang S, Wu JM, Wang KT, Wu R, Sui CJ, Shen SY, Wu X, Wang HY, Gao D, Chen L. Single-Cell Transcriptome Analysis Uncovers Intratumoral Heterogeneity and Underlying Mechanisms for Drug Resistance in Hepatobiliary Tumor Organoids. Adv Sci (Weinh). 2021;8:e2003897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Yang S, Zhang D, Sun Q, Nie H, Zhang Y, Wang X, Huang Y, Sun Y. Single-Cell and Spatial Transcriptome Profiling Identifies the Transcription Factor BHLHE40 as a Driver of EMT in Metastatic Colorectal Cancer. Cancer Res. 2024;84:2202-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Lee Y, Bogdanoff D, Wang Y, Hartoularos GC, Woo JM, Mowery CT, Nisonoff HM, Lee DS, Sun Y, Lee J, Mehdizadeh S, Cantlon J, Shifrut E, Ngyuen DN, Roth TL, Song YS, Marson A, Chow ED, Ye CJ. XYZeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Yan Q, Zhao Z, Liu D, Li J, Pan S, Duan J, Liu Z. Novel immune cross-talk between inflammatory bowel disease and IgA nephropathy. Ren Fail. 2024;46:2337288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 1052] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 22. | Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 902] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 23. | An Z, Liu W, Li W, Wei M, An C. Application of single-cell RNA sequencing in head and neck squamous cell carcinoma. Chin J Cancer Res. 2023;35:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Wang X, He Y, Zhang Q, Ren X, Zhang Z. Direct Comparative Analyses of 10X Genomics Chromium and Smart-seq2. Genomics Proteomics Bioinformatics. 2021;19:253-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 25. | Hashimshony T, Senderovich N, Avital G, Klochendler A, de Leeuw Y, Anavy L, Gennert D, Li S, Livak KJ, Rozenblatt-Rosen O, Dor Y, Regev A, Yanai I. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 2016;17:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 26. | Han Y, Huang C, Pan Y, Gu X. Single Cell Sequencing Technology and Its Application in Alzheimer's Disease. J Alzheimers Dis. 2024;97:1033-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Ringnér M. What is principal component analysis? Nat Biotechnol. 2008;26:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1189] [Article Influence: 69.9] [Reference Citation Analysis (2)] |
| 28. | Kobak D, Berens P. The art of using t-SNE for single-cell transcriptomics. Nat Commun. 2019;10:5416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 29. | Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 2848] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 30. | Qu L, Li S, Qiu HJ. Applications of single-cell RNA sequencing in virology. Yi Chuan. 2020;42:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Yang X, Qi Q, Pan Y, Zhou Q, Wu Y, Zhuang J, Xu J, Pan M, Han S. Single-Cell Analysis Reveals Characterization of Infiltrating T Cells in Moderately Differentiated Colorectal Cancer. Front Immunol. 2020;11:620196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Zhang Q, Liu Y, Wang X, Zhang C, Hou M, Liu Y. Integration of single-cell RNA sequencing and bulk RNA transcriptome sequencing reveals a heterogeneous immune landscape and pivotal cell subpopulations associated with colorectal cancer prognosis. Front Immunol. 2023;14:1184167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 33. | Wang J, Zhu N, Su X, Gao Y, Yang R. Novel tumor-associated macrophage populations and subpopulations by single cell RNA sequencing. Front Immunol. 2023;14:1264774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 34. | Zhang X, Yang L, Deng Y, Huang Z, Huang H, Wu Y, He B, Hu F. Single-cell RNA-Seq and bulk RNA-Seq reveal reliable diagnostic and prognostic biomarkers for CRC. J Cancer Res Clin Oncol. 2023;149:9805-9821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Willems A, Panchy N, Hong T. Using Single-Cell RNA Sequencing and MicroRNA Targeting Data to Improve Colorectal Cancer Survival Prediction. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Lin K, Chowdhury S, Zeineddine MA, Zeineddine FA, Hornstein NJ, Villarreal OE, Maru DM, Haymaker CL, Vauthey JN, Chang GJ, Bogatenkova E, Menter D, Kopetz S, Shen JP. Identification of Colorectal Cancer Cell Stemness from Single-Cell RNA Sequencing. Mol Cancer Res. 2024;22:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Haskins IN, Wang BD, Bernot JP, Cauley E, Horvath A, Marks JH, Lee NH, Agarwal S. Genomics of Black American colon cancer disparities: An RNA sequencing (RNA-Seq) study from an academic, tertiary referral center. Surgery. 2021;170:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Kasashima H, Fukui Y, Kitayama K, Miki Y, Yoshii M, Fukuoka T, Tamura T, Shibutani M, Toyokawa T, Ree S, Tanaka H, Yashiro M, Maeda K. [The Role of Cancer-Associated Fibroblasts in Modulating Tumor Immunity in Colorectal Cancer]. Gan To Kagaku Ryoho. 2023;50:958-959. [PubMed] |
| 39. | Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, Cheng Y, Huang S, Liu Y, Jiang S, Liu J, Huang X, Wang X, Qiu S, Xu J, Xi R, Bai F, Zhou J, Fan J, Zhang X, Gao Q. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022;12:134-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 550] [Article Influence: 137.5] [Reference Citation Analysis (0)] |
| 40. | Nie H, Lin P, Zhang Y, Wan Y, Li J, Yin C, Zhang L. Single-cell meta-analysis of inflammatory bowel disease with scIBD. Nat Comput Sci. 2023;3:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Mo CK, Liu J, Chen S, Storrs E, Targino da Costa ALN, Houston A, Wendl MC, Jayasinghe RG, Iglesia MD, Ma C, Herndon JM, Southard-Smith AN, Liu X, Mudd J, Karpova A, Shinkle A, Goedegebuure SP, Abdelzaher ATMA, Bo P, Fulghum L, Livingston S, Balaban M, Hill A, Ippolito JE, Thorsson V, Held JM, Hagemann IS, Kim EH, Bayguinov PO, Kim AH, Mullen MM, Shoghi KI, Ju T, Reimers MA, Weimholt C, Kang LI, Puram SV, Veis DJ, Pachynski R, Fuh KC, Chheda MG, Gillanders WE, Fields RC, Raphael BJ, Chen F, Ding L. Tumour evolution and microenvironment interactions in 2D and 3D space. Nature. 2024;634:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 42. | Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3043] [Cited by in RCA: 2435] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 43. | Parcesepe P, Giordano G, Laudanna C, Febbraro A, Pancione M. Cancer-Associated Immune Resistance and Evasion of Immune Surveillance in Colorectal Cancer. Gastroenterol Res Pract. 2016;2016:6261721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Zhang B, Li J, Hua Q, Wang H, Xu G, Chen J, Zhu Y, Li R, Liang Q, Wang L, Jin M, Tang J, Lin Z, Zhao L, Zhang D, Yu D, Ren J, Zhang T. Tumor CEMIP drives immune evasion of colorectal cancer via MHC-I internalization and degradation. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 45. | Kong J, Xu S, Zhang P, Zhao Y. CXCL1 promotes immune escape in colorectal cancer by autophagy-mediated MHC-I degradation. Hum Immunol. 2023;84:110716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Westcott PMK, Sacks NJ, Schenkel JM, Ely ZA, Smith O, Hauck H, Jaeger AM, Zhang D, Backlund CM, Beytagh MC, Patten JJ, Elbashir R, Eng G, Irvine DJ, Yilmaz OH, Jacks T. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat Cancer. 2021;2:1071-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 47. | Nicolini A, Ferrari P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Front Immunol. 2024;15:1353787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 48. | Sell T, Klotz C, Fischer MM, Astaburuaga-García R, Krug S, Drost J, Clevers H, Sers C, Morkel M, Blüthgen N. Oncogenic signaling is coupled to colorectal cancer cell differentiation state. J Cell Biol. 2023;222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Lin X, Wang S, Sun M, Zhang C, Wei C, Yang C, Dou R, Liu Q, Xiong B. miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J Hematol Oncol. 2019;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 50. | Wang S, Pang L, Liu Z, Meng X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: a novel therapeutic target. BMC Cancer. 2021;21:767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Ferkel SAM, Holman EA, Sojwal RS, Rubin SJS, Rogalla S. Tumor-Infiltrating Immune Cells in Colorectal Cancer. Neoplasia. 2025;59:101091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Sieminska I, Baran J. Myeloid-Derived Suppressor Cells in Colorectal Cancer. Front Immunol. 2020;11:1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 53. | Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 54. | Zhong X, Chen B, Yang Z. The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression. Cell Physiol Biochem. 2018;45:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Chistiakov DA, Orekhov AN, Bobryshev YV. Dendritic Cells in Colorectal Cancer and a Potential for their Use in Therapeutic Approaches. Curr Pharm Des. 2016;22:2431-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Ji S, Miao G, Du S, Wang H, Yang X, Li A, Lu Y, Wang X, Zhao X. The current role of dendritic cells in the progression and treatment of colorectal cancer. Cancer Biol Med. 2024;21:769-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Masui H, Kawada K, Obama K. Neutrophil and Colorectal Cancer. Int J Mol Sci. 2024;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Khan U, Chowdhury S, Billah MM, Islam KMD, Thorlacius H, Rahman M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Marchalot A, Mjösberg J. Innate lymphoid cells in colorectal cancer. Scand J Immunol. 2022;95:e13156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Tang YP, Xie MZ, Li KZ, Li JL, Cai ZM, Hu BL. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020;20:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 61. | Fionda C, Scarno G, Stabile H, Molfetta R, Di Censo C, Gismondi A, Paolini R, Sozzani S, Santoni A, Sciumè G. NK Cells and Other Cytotoxic Innate Lymphocytes in Colorectal Cancer Progression and Metastasis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020;30:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 63. | Qi J, Crinier A, Escalière B, Ye Y, Wang Z, Zhang T, Batista L, Liu H, Hong L, Wu N, Zhang M, Chen L, Liu Y, Shen L, Narni-Mancinelli E, Vivier E, Su B. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep Med. 2021;2:100353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 64. | Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. 2022;19:441-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 358] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 65. | Liu H, Li Z, Han X, Li Z, Zhao Y, Liu F, Zhu Z, Lv Y, Liu Z, Zhang N. The prognostic impact of tumor-infiltrating B lymphocytes in patients with solid malignancies: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2023;181:103893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20:662-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 1043] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 67. | Rentschler M, Braumüller H, Briquez PS, Wieder T. Cytokine-Induced Senescence in the Tumor Microenvironment and Its Effects on Anti-Tumor Immune Responses. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 69. | Braumüller H, Mauerer B, Andris J, Berlin C, Wieder T, Kesselring R. The Cytokine Network in Colorectal Cancer: Implications for New Treatment Strategies. Cells. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 70. | Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 71. | Gerlach K, Popp V, Wirtz S, Al-Saifi R, Gonzalez Acera M, Atreya R, Dregelies T, Vieth M, Fichtner-Feigl S, McKenzie ANJ, Rosenbauer F, Weigmann B, Neurath MF. PU.1-driven Th9 Cells Promote Colorectal Cancer in Experimental Colitis Models Through Il-6 Effects in Intestinal Epithelial Cells. J Crohns Colitis. 2022;16:1893-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Wang C, Lu Y, Chen L, Gao T, Yang Q, Zhu C, Chen Y. Th9 cells are subjected to PD-1/PD-L1-mediated inhibition and are capable of promoting CD8 T cell expansion through IL-9R in colorectal cancer. Int Immunopharmacol. 2020;78:106019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity. 2019;50:892-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 970] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 74. | Razi S, Baradaran Noveiry B, Keshavarz-Fathi M, Rezaei N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine. 2019;116:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 75. | Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, Governa V, Han J, Huber X, Droeser RA, Zuber M, Adamina M, Bolli M, Rosso R, Lugli A, Zlobec I, Terracciano L, Tornillo L, Zajac P, Eppenberger-Castori S, Trapani F, Oertli D, Iezzi G. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66:692-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 76. | Song M, Liang J, Wang L, Li W, Jiang S, Xu S, Tang L, Du Q, Liu G, Meng H, Zhai D, Shi S, Yang Y, Zhang L, Zhang B. IL-17A functions and the therapeutic use of IL-17A and IL-17RA targeted antibodies for cancer treatment. Int Immunopharmacol. 2023;123:110757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 77. | Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, Fang W, Xiong B, Wan Y, Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS One. 2012;7:e34959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Sun D, Lin Y, Hong J, Chen H, Nagarsheth N, Peng D, Wei S, Huang E, Fang J, Kryczek I, Zou W. Th22 cells control colon tumorigenesis through STAT3 and Polycomb Repression complex 2 signaling. Oncoimmunology. 2016;5:e1082704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Huang YH, Cao YF, Jiang ZY, Zhang S, Gao F. Th22 cell accumulation is associated with colorectal cancer development. World J Gastroenterol. 2015;21:4216-4224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Tosti N, Cremonesi E, Governa V, Basso C, Kancherla V, Coto-Llerena M, Amicarella F, Weixler B, Däster S, Sconocchia G, Majno PE, Christoforidis D, Tornillo L, Terracciano L, Ng CKY, Piscuoglio S, von Flüe M, Spagnoli G, Eppenberger-Castori S, Iezzi G, Droeser RA. Infiltration by IL22-Producing T Cells Promotes Neutrophil Recruitment and Predicts Favorable Clinical Outcome in Human Colorectal Cancer. Cancer Immunol Res. 2020;8:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 81. | Zheng Z, Wieder T, Mauerer B, Schäfer L, Kesselring R, Braumüller H. T Cells in Colorectal Cancer: Unravelling the Function of Different T Cell Subsets in the Tumor Microenvironment. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 82. | Zou Q, Hu B, Yu HC, Ren DL. [Characteristics of CD8+ T cell infiltration in colorectal cancer and their correlation with prognosis]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 83. | Mikulak J, Oriolo F, Bruni E, Roberto A, Colombo FS, Villa A, Bosticardo M, Bortolomai I, Lo Presti E, Meraviglia S, Dieli F, Vetrano S, Danese S, Della Bella S, Carvello MM, Sacchi M, Cugini G, Colombo G, Klinger M, Spaggiari P, Roncalli M, Prinz I, Ravens S, di Lorenzo B, Marcenaro E, Silva-Santos B, Spinelli A, Mavilio D. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 84. | Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287-7296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 85. | Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, Chen Z, Wang K, Zhang T, Xu J, Han Y, Zhang T, Wu X, Wang J, Gong W, Zheng S, Qiu F, Yan J, Huang J. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 86. | Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G, Qin Y, Chen Y, Cui K, Zhou L, Bian Z, Fei B, Huang S, Huang Z. Colorectal Cancer-Derived Small Extracellular Vesicles Promote Tumor Immune Evasion by Upregulating PD-L1 Expression in Tumor-Associated Macrophages. Adv Sci (Weinh). 2022;9:2102620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 87. | Zhang Y, Xie R, Zhang H, Zheng Y, Lin C, Yang L, Huang M, Li M, Song F, Lu L, Yang M, Liu Y, Wei Q, Li J, Chen J. Integrin β7 Inhibits Colorectal Cancer Pathogenesis via Maintaining Antitumor Immunity. Cancer Immunol Res. 2021;9:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Fang L, Feng R, Liang W, Liu FF, Bian GL, Yu C, Guo H, Cao Y, Liu M, Zuo J, Peng Y, Zhao J, Sun RX, Shan J, Wang J. Overexpression of PD-L1 causes germ cells to slough from mouse seminiferous tubules via the PD-L1/PD-L1 interaction. J Cell Mol Med. 2022;26:2908-2920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Cao Y, Liang W, Fang L, Liu MK, Zuo J, Peng YL, Shan JJ, Sun RX, Zhao J, Wang J. PD-L1/PD-L1 signalling promotes colorectal cancer cell migration ability through RAS/MEK/ERK. Clin Exp Pharmacol Physiol. 2022;49:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 90. | Wang D, Peng L, Hua L, Li J, Liu Y, Zhou Y. Mapk14 is a Prognostic Biomarker and Correlates with the Clinicopathological Features and Immune Infiltration of Colorectal Cancer. Front Cell Dev Biol. 2022;10:817800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Huang R, Kang T, Chen S. The role of tumor-associated macrophages in tumor immune evasion. J Cancer Res Clin Oncol. 2024;150:238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 92. | van Baarle L, De Simone V, Schneider L, Santhosh S, Abdurahiman S, Biscu F, Schneider R, Zanoletti L, Siqueira de Mello R, Verbandt S, Hu Z, Stakenborg M, Ke BJ, Stakenborg N, Salvador Laureano R, García-Reyes B, Henn J, Toma M, Vanmechelen M, Boeckxstaens G, De Smet F, Garg AD, Ibiza S, Tejpar S, Wehner S, Matteoli G. IL-1R signaling drives enteric glia-macrophage interactions in colorectal cancer. Nat Commun. 2024;15:6079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 93. | Xue J, Yu X, Xue L, Ge X, Zhao W, Peng W. Intrinsic β-catenin signaling suppresses CD8(+) T-cell infiltration in colorectal cancer. Biomed Pharmacother. 2019;115:108921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 94. | Zheng X, Song J, Yu C, Zhou Z, Liu X, Yu J, Xu G, Yang J, He X, Bai X, Luo Y, Bao Y, Li H, Yang L, Xu M, Song N, Su X, Xu J, Ma X, Shi H. Single-cell transcriptomic profiling unravels the adenoma-initiation role of protein tyrosine kinases during colorectal tumorigenesis. Signal Transduct Target Ther. 2022;7:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 95. | Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1476] [Article Influence: 210.9] [Reference Citation Analysis (0)] |
| 96. | Zhao W, Jin L, Chen P, Li D, Gao W, Dong G. Colorectal cancer immunotherapy-Recent progress and future directions. Cancer Lett. 2022;545:215816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 97. | Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front Immunol. 2021;12:808964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 98. | Yang X, Yabe-Wada T, Han J, Saito F, Ogasawara C, Yamada S, Onai N. PCBP1 acts as a regulator of CCL2 expression in macrophages to induce recruitment of monocyte-derived macrophages into the inflamed colon. Int Immunol. 2023;35:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 99. | Xiong M, Sun W. Research progress of probiotics and their protective strategy in the field of inflammatory bowel disease treatment: A review. Medicine (Baltimore). 2024;103:e40401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | George S, Johdi NA. Single Cell RNA Sequencing in Colorectal Cancer Immunology: Recent Updates, Application, and Emerging Challenges. Curr Mol Med. 2025;. [PubMed] [DOI] [Full Text] |
| 101. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 102. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1274] [Article Influence: 67.1] [Reference Citation Analysis (2)] |
| 103. | Wang Z, Chang Y, Sun H, Li Y, Tang T. Advances in molecular mechanisms of inflammatory bowel disease-associated colorectal cancer (Review). Oncol Lett. 2024;27:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 104. | De Cecco F, Franceschelli S, Panella V, Maggi MA, Bisti S, Bravo Nuevo A, D'Ardes D, Cipollone F, Speranza L. Biological Response of Treatment with Saffron Petal Extract on Cytokine-Induced Oxidative Stress and Inflammation in the Caco-2/Human Leukemia Monocytic Co-Culture Model. Antioxidants (Basel). 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | Trivedi PJ, Adams DH. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. J Crohns Colitis. 2018;12:S641-S652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 106. | Danese S, Semeraro S, Marini M, Roberto I, Armuzzi A, Papa A, Gasbarrini A. Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation. Dig Liver Dis. 2005;37:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 107. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1114] [Article Influence: 123.8] [Reference Citation Analysis (1)] |
| 108. | Mahapatro M, Erkert L, Becker C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 109. | Gomez-Bris R, Saez A, Herrero-Fernandez B, Rius C, Sanchez-Martinez H, Gonzalez-Granado JM. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 110. | Arshad T, Mansur F, Palek R, Manzoor S, Liska V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front Immunol. 2020;11:2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 111. | Yan J, Yu J, Yuan S, Tang W, Ma W, Yang X, Liu Y, Liang H, Zhong X, Shao J, Cao Y, Mao C, Near R, Gao W. Musculin is highly enriched in Th17 and IL-22-producing ILC3s and restrains pro-inflammatory cytokines in murine colitis. Eur J Immunol. 2021;51:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Zhao N, Liu C, Li N, Zhou S, Guo Y, Yang S, Liu H. Role of Interleukin-22 in ulcerative colitis. Biomed Pharmacother. 2023;159:114273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 113. | Geem D, Harusato A, Flannigan K, Denning TL. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Yan JB, Luo MM, Chen ZY, He BH. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J Immunol Res. 2020;2020:8813558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 115. | Liu X, Ren X, Zhou L, Liu K, Deng L, Qing Q, Li J, Zhi F, Li M. Tollip Orchestrates Macrophage Polarization to Alleviate Intestinal Mucosal Inflammation. J Crohns Colitis. 2022;16:1151-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 116. | Zhang K, Guo J, Yan W, Xu L. Macrophage polarization in inflammatory bowel disease. Cell Commun Signal. 2023;21:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 117. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1532] [Article Influence: 117.8] [Reference Citation Analysis (5)] |
| 118. | Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Castro-Dopico T, Colombel JF, Mehandru S. Targeting B cells for inflammatory bowel disease treatment: back to the future. Curr Opin Pharmacol. 2020;55:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 120. | Zogorean R, Wirtz S. The yin and yang of B cells in a constant state of battle: intestinal inflammation and inflammatory bowel disease. Front Immunol. 2023;14:1260266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 121. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1965] [Article Influence: 178.6] [Reference Citation Analysis (1)] |
| 122. | Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 545] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 123. | Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015;50:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 124. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2061] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 125. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1538] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 126. | Hou Q, Huang J, Ayansola H, Masatoshi H, Zhang B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front Immunol. 2020;11:623691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 127. | Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O'Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 128. | Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973-5981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 129. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 564] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 130. | Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 603] [Cited by in RCA: 706] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 131. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1870] [Cited by in RCA: 1821] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 132. | Drury B, Hardisty G, Gray RD, Ho GT. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol Gastroenterol Hepatol. 2021;12:321-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 133. | Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, Figliuzzi MM, Caprioli F, Stolfi C, Monteleone I, Monteleone G. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J Crohns Colitis. 2019;13:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 134. | Mavropoulou E, Mechie NC, Knoop R, Petzold G, Ellenrieder V, Kunsch S, Pilavakis Y, Amanzada A. Association of serum interleukin-6 and soluble interleukin-2-receptor levels with disease activity status in patients with inflammatory bowel disease: A prospective observational study. PLoS One. 2020;15:e0233811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Liu D, Saikam V, Skrada KA, Merlin D, Iyer SS. Inflammatory bowel disease biomarkers. Med Res Rev. 2022;42:1856-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 136. | Pavlidis P, Komorowski L, Teegen B, Liaskos C, Koutsoumpas AL, Smyk DS, Perricone C, Mytilinaiou MG, Stocker W, Forbes A, Bogdanos DP. Diagnostic and clinical significance of Crohn's disease-specific pancreatic anti-GP2 and anti-CUZD1 antibodies. Clin Chem Lab Med. 2016;54:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 137. | Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE. Calprotectin: from biomarker to biological function. Gut. 2021;70:1978-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 138. | Zhang J, Guo Z, Wang Z, Zhu W, Li Q. Fecal miR-223 is a noninvasive biomarker for estimating Crohn's disease activity. Immun Inflamm Dis. 2023;11:e1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 139. | D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, Van Olmen G, Rutgeerts P. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 140. | Garcia P, Hartman D, Choudry H, Pai RK. CD8 + T-cell Density Is an Independent Predictor of Survival and Response to Adjuvant Chemotherapy in Stage III Colon Cancer. Appl Immunohistochem Mol Morphol. 2023;31:69-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 141. | Vande Casteele N, Feagan BG, Gils A, Vermeire S, Khanna R, Sandborn WJ, Levesque BG. Therapeutic drug monitoring in inflammatory bowel disease: current state and future perspectives. Curr Gastroenterol Rep. 2014;16:378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 142. | Triantafillidis JK, Zografos CG, Konstadoulakis MM, Papalois AE. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J Gastroenterol. 2024;30:2068-2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (3)] |
| 143. | Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 330] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 144. | Mocci G, Tursi A, Scaldaferri F, Napolitano D, Pugliese D, Capobianco I, Bartocci B, Blasi V, Savarino EV, Maniero D, Redavid C, Lorenzon G, Cuomo A, Donnarumma L, Gravina AG, Pellegrino R, Bodini G, Pasta A, Marzo M, Serio M, Scarcelli A, Rodinò S, Sebkova L, Maconi G, Cataletti G, Luppino I, Checchin D, Ferronato A, Gaiani F, Kayali S, Felice C, Pranzo G, Catarella D, D'Agostino D, Di Bartolo E, Lombardi G, Patturelli M, Bendia E, Bolognini L, Balducci D, Quatraccioni C, Martini F, Mucherino C, D'Antonio E, Montesano L, Vespere G, Sedda S, D'Onofrio V, De Luca L, Spagnuolo R, Luzza F, Fanigliulo L, Rocco G, Sacchi C, Zampaletta C, Grossi L, Lorenzetti R, Aragona G, Perazzo P, Forti G, Allegretta L, Cazzato AI, Scorza S, Cortellini F, Capone P, Villani GD, Di Fonzo M, Iacopini F, Tonti P, Neve V, Colucci R, Elisei W, Monterubbianesi R, Faggiani R, Pica R, Pagnini C, Graziani MG, Di Paolo MC, Onidi FM, Saba F, Dore MP, Usai-Satta P, Picchio M, Papa A. Long-Term Effectiveness and Safety of Ustekinumab in Crohn's Disease: Results from a Large Real-Life Cohort Study. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 145. | Naganuma M, Takeno M, Çelik AF, Moots R, Pinton P, Hisamatsu T. Assessment of IL-6 Pathway Inhibition in Gastrointestinal Behçet's Disease from Immunological and Clinical Perspectives. Biomedicines. 2025;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 146. | Sands BE, Feagan BG, Sandborn WJ, Schreiber S, Peyrin-Biroulet L, Frédéric Colombel J, Rossiter G, Usiskin K, Ather S, Zhan X, D'Haens G. Mongersen (GED-0301) for Active Crohn's Disease: Results of a Phase 3 Study. Am J Gastroenterol. 2020;115:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 147. | Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 148. | Lötstedt B, Stražar M, Xavier R, Regev A, Vickovic S. Spatial host-microbiome sequencing reveals niches in the mouse gut. Nat Biotechnol. 2024;42:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 149. | Neurath MF, Sands BE, Rieder F. Cellular immunotherapies and immune cell depleting therapies in inflammatory bowel diseases: the next magic bullet? Gut. 2024;74:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 150. | Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao R, Kang B, Zhang Q, Huang JY, Konno H, Guo X, Ye Y, Gao S, Wang S, Hu X, Ren X, Shen Z, Ouyang W, Zhang Z. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018;564:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 881] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 151. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1767] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 152. | Rajamäki K, Taira A, Katainen R, Välimäki N, Kuosmanen A, Plaketti RM, Seppälä TT, Ahtiainen M, Wirta EV, Vartiainen E, Sulo P, Ravantti J, Lehtipuro S, Granberg KJ, Nykter M, Tanskanen T, Ristimäki A, Koskensalo S, Renkonen-Sinisalo L, Lepistö A, Böhm J, Taipale J, Mecklin JP, Aavikko M, Palin K, Aaltonen LA. Genetic and Epigenetic Characteristics of Inflammatory Bowel Disease-Associated Colorectal Cancer. Gastroenterology. 2021;161:592-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 153. | Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 154. | Pavelescu LA, Enache RM, Roşu OA, Profir M, Creţoiu SM, Gaspar BS. Predictive Biomarkers and Resistance Mechanisms of Checkpoint Inhibitors in Malignant Solid Tumors. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 155. | Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 156. | Hong SM, Lee AY, Kim BJ, Lee JE, Seon SY, Ha YJ, Ng JT, Yoon G, Lim SB, Morgan MJ, Cha JH, Lee D, Kim YS. NAMPT-Driven M2 Polarization of Tumor-Associated Macrophages Leads to an Immunosuppressive Microenvironment in Colorectal Cancer. Adv Sci (Weinh). 2024;11:e2303177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 157. | West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Görtz D, This S, Stockenhuber K, Pott J, Friedrich M, Ryzhakov G, Baribaud F, Brodmerkel C, Cieluch C, Rahman N, Müller-Newen G, Owens RJ, Kühl AA, Maloy KJ, Plevy SE; Oxford IBD Cohort Investigators, Keshav S, Travis SPL, Powrie F. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 560] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 158. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4925] [Article Influence: 615.6] [Reference Citation Analysis (0)] |
| 159. | Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-α agents - Comparison among therapeutic TNF-α antagonists. Cytokine. 2018;101:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 160. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1216] [Article Influence: 202.7] [Reference Citation Analysis (0)] |
| 161. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 162. | Dziaman T, Gackowski D, Guz J, Linowiecka K, Bodnar M, Starczak M, Zarakowska E, Modrzejewska M, Szpila A, Szpotan J, Gawronski M, Labejszo A, Liebert A, Banaszkiewicz Z, Klopocka M, Foksinski M, Marszalek A, Olinski R. Characteristic profiles of DNA epigenetic modifications in colon cancer and its predisposing conditions-benign adenomas and inflammatory bowel disease. Clin Epigenetics. 2018;10:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 163. | Li L, Jiang M, Wang W, Cao X, Ma Q, Han J, Liu Z, Huang Y, Chen Y. DNA demethylase TET2-mediated reduction of HADHB expression contributes to cadmium-induced malignant progression of colorectal cancer. Ecotoxicol Environ Saf. 2024;280:116579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 164. | Schatoff EM, Goswami S, Zafra MP, Foronda M, Shusterman M, Leach BI, Katti A, Diaz BJ, Dow LE. Distinct Colorectal Cancer-Associated APC Mutations Dictate Response to Tankyrase Inhibition. Cancer Discov. 2019;9:1358-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 165. | Verstockt B, Salas A, Sands BE, Abraham C, Leibovitzh H, Neurath MF, Vande Casteele N; Alimentiv Translational Research Consortium (ATRC). IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 98] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 166. | Yan X, Pan Q, Xin H, Chen Y, Ping Y. Genome-editing prodrug: Targeted delivery and conditional stabilization of CRISPR-Cas9 for precision therapy of inflammatory disease. Sci Adv. 2021;7:eabj0624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 167. | Ye GD, Sun GB, Jiao P, Chen C, Liu QF, Huang XL, Zhang R, Cai WY, Li SN, Wu JF, Liu YJ, Wu RS, Xie YY, Chan EC, Liou YC, Li BA. OVOL2, an Inhibitor of WNT Signaling, Reduces Invasive Activities of Human and Mouse Cancer Cells and Is Down-regulated in Human Colorectal Tumors. Gastroenterology. 2016;150:659-671.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 168. | Zhou H, Zhu L, Song J, Wang G, Li P, Li W, Luo P, Sun X, Wu J, Liu Y, Zhu S, Zhang Y. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol Cancer. 2022;21:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (1)] |
| 169. | Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, Casero D, Courtney H, Gonzalez A, Graeber TG, Hall AB, Lake K, Landers CJ, Mallick H, Plichta DR, Prasad M, Rahnavard G, Sauk J, Shungin D, Vázquez-Baeza Y, White RA 3rd; IBDMDB Investigators, Braun J, Denson LA, Jansson JK, Knight R, Kugathasan S, McGovern DPB, Petrosino JF, Stappenbeck TS, Winter HS, Clish CB, Franzosa EA, Vlamakis H, Xavier RJ, Huttenhower C. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1844] [Reference Citation Analysis (0)] |
| 170. | Li R, Liu X, Huang X, Zhang D, Chen Z, Zhang J, Bai R, Zhang S, Zhao H, Xu Z, Zeng L, Zhuang L, Wen S, Wu S, Li M, Zuo Z, Lin J, Lin D, Zheng J. Single-cell transcriptomic analysis deciphers heterogenous cancer stem-like cells in colorectal cancer and their organ-specific metastasis. Gut. 2024;73:470-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 171. | Hassan C, Spadaccini M, Mori Y, Foroutan F, Facciorusso A, Gkolfakis P, Tziatzios G, Triantafyllou K, Antonelli G, Khalaf K, Rizkala T, Vandvik PO, Fugazza A, Rondonotti E, Glissen-Brown JR, Kamba S, Maida M, Correale L, Bhandari P, Jover R, Sharma P, Rex DK, Repici A. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy : A Systematic Review and Meta-analysis. Ann Intern Med. 2023;176:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 172. | Sundaram S, Choden T, Mattar MC, Desai S, Desai M. Artificial intelligence in inflammatory bowel disease endoscopy: current landscape and the road ahead. Ther Adv Gastrointest Endosc. 2021;14:26317745211017809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 173. | Ogata N, Maeda Y, Misawa M, Takenaka K, Takabayashi K, Iacucci M, Kuroki T, Takishima K, Sasabe K, Niimura Y, Kawashima J, Ogawa Y, Ichimasa K, Nakamura H, Matsudaira S, Sasanuma S, Hayashi T, Wakamura K, Miyachi H, Baba T, Mori Y, Ohtsuka K, Ogata H, Kudo SE. Artificial Intelligence-assisted Video Colonoscopy for Disease Monitoring of Ulcerative Colitis: A Prospective Study. J Crohns Colitis. 2025;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 174. | Xu H, Tang RSY, Lam TYT, Zhao G, Lau JYW, Liu Y, Wu Q, Rong L, Xu W, Li X, Wong SH, Cai S, Wang J, Liu G, Ma T, Liang X, Mak JWY, Xu H, Yuan P, Cao T, Li F, Ye Z, Shutian Z, Sung JJY. Artificial Intelligence-Assisted Colonoscopy for Colorectal Cancer Screening: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023;21:337-346.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 175. | Stidham RW, Takenaka K. Artificial Intelligence for Disease Assessment in Inflammatory Bowel Disease: How Will it Change Our Practice? Gastroenterology. 2022;162:1493-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 176. | Yao L, Lu Z, Yang G, Zhou W, Xu Y, Guo M, Huang X, He C, Zhou R, Deng Y, Wu H, Chen B, Gong R, Zhang L, Zhang M, Gong W, Yu H. Development and validation of an artificial intelligence-based system for predicting colorectal cancer invasion depth using multi-modal data. Dig Endosc. 2023;35:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 177. | Salcher S, Heidegger I, Untergasser G, Fotakis G, Scheiber A, Martowicz A, Noureen A, Krogsdam A, Schatz C, Schäfer G, Trajanoski Z, Wolf D, Sopper S, Pircher A. Comparative analysis of 10X Chromium vs. BD Rhapsody whole transcriptome single-cell sequencing technologies in complex human tissues. Heliyon. 2024;10:e28358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 178. | Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1429] [Cited by in RCA: 1178] [Article Influence: 147.3] [Reference Citation Analysis (1)] |
| 179. | Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2637] [Cited by in RCA: 4104] [Article Influence: 410.4] [Reference Citation Analysis (0)] |
| 180. | Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348:aaa6090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1718] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 181. | He X, Liu X, Zuo F, Shi H, Jing J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin Cancer Biol. 2023;88:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 122] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 182. | Denisenko E, Guo BB, Jones M, Hou R, de Kock L, Lassmann T, Poppe D, Clément O, Simmons RK, Lister R, Forrest ARR. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol. 2020;21:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 183. | Luecken MD, Büttner M, Chaichoompu K, Danese A, Interlandi M, Mueller MF, Strobl DC, Zappia L, Dugas M, Colomé-Tatché M, Theis FJ. Benchmarking atlas-level data integration in single-cell genomics. Nat Methods. 2022;19:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 530] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 184. | Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker Qualification: Toward a Multiple Stakeholder Framework for Biomarker Development, Regulatory Acceptance, and Utilization. Clin Pharmacol Ther. 2015;98:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 185. | Yin Y, Jiang Y, Lam KG, Berletch JB, Disteche CM, Noble WS, Steemers FJ, Camerini-Otero RD, Adey AC, Shendure J. High-Throughput Single-Cell Sequencing with Linear Amplification. Mol Cell. 2019;76:676-690.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 186. | Ke R, Mignardi M, Hauling T, Nilsson M. Fourth Generation of Next-Generation Sequencing Technologies: Promise and Consequences. Hum Mutat. 2016;37:1363-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 187. | Yi H, Li G, Long Y, Liang W, Cui H, Zhang B, Tan Y, Li Y, Shen L, Deng D, Tang Y, Mao C, Tian S, Cai Y, Zhu Q, Hu Y, Chen W, Fang L. Integrative multi-omics analysis of a colon cancer cell line with heterogeneous Wnt activity revealed RUNX2 as an epigenetic regulator of EMT. Oncogene. 2020;39:5152-5164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 188. | Bannon D, Moen E, Schwartz M, Borba E, Kudo T, Greenwald N, Vijayakumar V, Chang B, Pao E, Osterman E, Graf W, Van Valen D. DeepCell Kiosk: scaling deep learning-enabled cellular image analysis with Kubernetes. Nat Methods. 2021;18:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 189. | CZI Cell Science Program, Abdulla S, Aevermann B, Assis P, Badajoz S, Bell SM, Bezzi E, Cakir B, Chaffer J, Chambers S, Cherry JM, Chi T, Chien J, Dorman L, Garcia-Nieto P, Gloria N, Hastie M, Hegeman D, Hilton J, Huang T, Infeld A, Istrate AM, Jelic I, Katsuya K, Kim YJ, Liang K, Lin M, Lombardo M, Marshall B, Martin B, McDade F, Megill C, Patel N, Predeus A, Raymor B, Robatmili B, Rogers D, Rutherford E, Sadgat D, Shin A, Small C, Smith T, Sridharan P, Tarashansky A, Tavares N, Thomas H, Tolopko A, Urisko M, Yan J, Yeretssian G, Zamanian J, Mani A, Cool J, Carr A. CZ CELLxGENE Discover: a single-cell data platform for scalable exploration, analysis and modeling of aggregated data. Nucleic Acids Res. 2025;53:D886-D900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |