Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107364
Revised: April 3, 2025
Accepted: April 17, 2025
Published online: June 15, 2025
Processing time: 84 Days and 6.7 Hours
The association between adiposity parameters and incident hepatocellular carcinoma (HCC) in individuals with nonalcoholic fatty liver disease (NAFLD) is yet to be evaluated.
To investigate the risk of HCC according to body mass index (BMI) and waist circumference (WC) in people with NAFLD.
This population-based cohort study included Korean National Health Insurance Service examination participants with NAFLD (n = 1110773). NAFLD was defined as a fatty liver index of ≥ 30. The risk of HCC was determined by Cox proportional hazards regression according to BMI and WC after adjusting for age, sex, health behaviors, income, comorbidities, and WC or BMI.
HCC was diagnosed in 4773 (0.43%) participants during a median follow-up of 10.3 years. A U-shaped association between BMI or WC and HCC was observed, with the highest risk observed in the lowest BMI and WC groups. Compared to normal BMI, the adjusted hazard ratio (aHR) of the underweight BMI group was 2.02 [95% confidence interval (CI): 1.25-3.28]. The lowest risk was found in groups with overweight BMI (aHR = 0.67, 95%CI: 0.60-0.73; reference: normal BMI) and WC: 85-89.9/80-84.9 cm for men/women (aHR = 0.55, 95%CI: 0.49-0.63; reference: < 80/< 75 cm). Subgroup analyses of age, sex, health behaviors, and fatty liver index showed consistent results.
The development of HCC shows a U-shaped relationship with BMI and WC in people with NAFLD, with the highest risk in underweight individuals.
Core Tip: While the risk of hepatocellular carcinoma (HCC) increases with body mass index and waist circumference (WC) in the general population, the risk in nonalcoholic fatty liver disease (NAFLD) is unknown. This population-based study of people with NAFLD demonstrated a U-shaped association of body mass index and WC with HCC, with the highest risk in the underweight and lowest WC groups. These findings suggest that surveillance for incident HCC is necessary in populations with NAFLD, irrespective of obesity, with particular attention to individuals with low adiposity.
- Citation: Jung HN, Heo JH, Roh E, Kim BJ, Lee M, Kim JK, Kim JH, Han B, Han KD, Kang JG, Lee SJ, Ihm SH. Risk of hepatocellular carcinoma according to body mass index and waist circumference in nonalcoholic fatty liver disease. World J Gastrointest Oncol 2025; 17(6): 107364
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107364.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107364
Nonalcoholic fatty liver disease (NAFLD) has a global prevalence of 38% and represents a broad spectrum of disease that can progress from simple steatosis to hepatocellular carcinoma (HCC)[1]. As the most common primary cancer of the liver, HCC ranks sixth and third in overall cancer incidence and mortality, respectively, in 2022[2]. Despite the relatively low incidence of HCC in NAFLD (0.44 per 1000 person-years), it rises rapidly to 5.29 per 1000 person-years after progression to steatohepatitis, and nearly half of NAFLD-driven HCCs develop before the transition to cirrhosis[3].
Previous studies in the general population have shown an increased risk of developing HCC with higher body mass index (BMI)[4,5]. A meta-analysis of 11 cohort studies reported a significant 17% and 89% higher risk of HCC in individuals who are underweight and obese, respectively, compared to those with normal weight[4]. Similar to BMI, the risk of HCC increased dose-dependently with greater waist circumference (WC) in a pooled analysis of more than 2.5 million participants, with a 1.59-fold higher risk in the highest WC group than in the lowest WC group[6].
In contrast, the opposite association between BMI and HCC was suggested by a recent systematic review on the NAFLD population[7]. Individuals who are not overweight or obese had a 77% increased risk of HCC compared to those who are overweight or obese[7]. However, the studies included in this pooled analysis had a limited number of participants; thus, only 0-9 events of incident HCC were recorded in a group who was not overweight or obese[7]. Additionally, there is a lack of investigation on the development of HCC according to BMI categories and WC, which is a surrogate marker of central adiposity, in the NAFLD population. Therefore, we aimed to evaluate the association of BMI or WC with the risk of incident HCC using a population-based cohort consisting only of individuals with NAFLD.
We used health insurance claims and health examination data from the Korean National Health Insurance Service (NHIS), which covers 97% of the total population of South Korea with minimal attrition. Health examination data included self-reported questionnaires, anthropometric measurements, and laboratory results. Height, weight, and WC were measured while the participants were clothed in light attire. WC was measured at the midline between the bottom of the lower rib and the iliac crest while the person was standing. Blood pressure was measured in a sitting position after a rest period of at least 5 minutes. Blood samples for glucose, lipid profiles, creatinine, and γ-glutamyl transferase (GGT) levels were collected after an overnight fast.
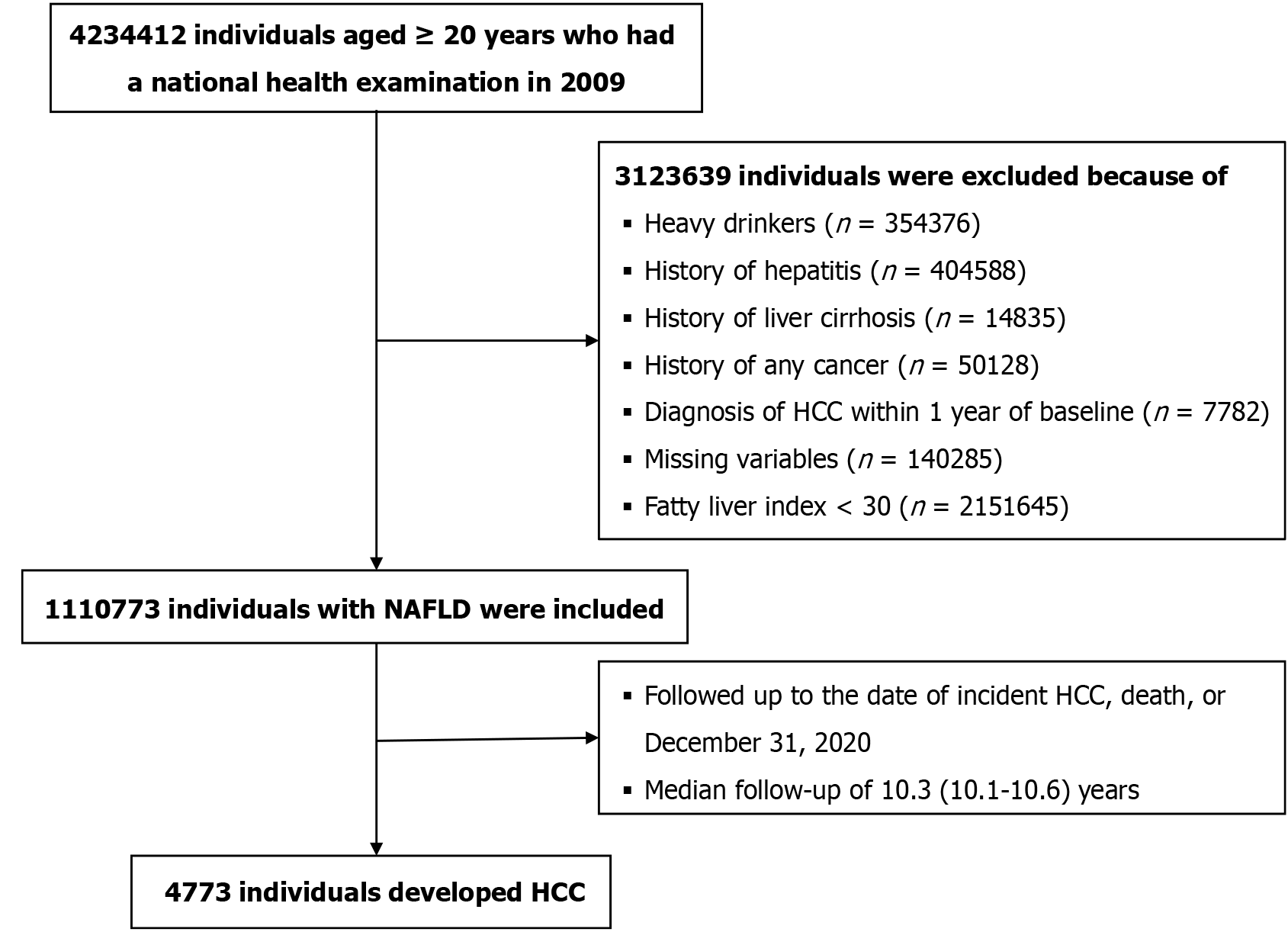
A representative sample cohort (n = 4234412) was created by randomly selecting 40% of the total population aged ≥ 20 years who underwent a health examination in 2009, as the NHIS restricted access to the full dataset (Figure 1). Heavy alcohol drinkers and those with a history of viral hepatitis were excluded based on the definition of NAFLD (Supplementary Table 1)[8]. We excluded individuals diagnosed with HCC within 1 year of baseline to reduce the potential for reverse causation. Following the exclusion of individuals with missing variables, the final cohort comprised 1110773 participants with NAFLD. Participants were followed up from the date of the health examination until the date of HCC occurrence, death, or December 31, 2020, whichever came first.
This study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital, approval No. HALLYM 2024-04-026. Owing to strict confidentiality measures that ensure anonymity in the NHIS database, the need for written informed consent was waived.
The European Association for the Study of the Liver prioritizes serum biomarkers for diagnosing NAFLD in epidemiological studies because of their high availability and cost-effectiveness[9]. The fatty liver index (FLI) is one of the most established steatosis scoring systems[9]. FLI was used to diagnose NAFLD in this study because it was not feasible to perform liver imaging or biopsy in all participants during routine health examinations. The FLI is calculated using the following formula, which yields values ranging from 0 to 100: (e0.953 × Ln (triglycerides) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × WC ± 15.745)/(1 + e0.953 × Ln (triglycerides) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × WC ± 15.745) × 100[10]. The cut-off value of the FLI for the discrimination of NAFLD was defined as ≥ 30, which is optimal threshold in the general population of Korea, with an area under the receiver operating characteristic curve of 0.82 [95% confidence interval (CI): 0.81-0.84][11].
The primary outcome was newly diagnosed HCC, defined by the International Classification of Diseases 10th Revision code (C22.0) and the National Cancer Registry reimbursement code (V193)[12]. All Korean individuals diagnosed with cancer were enlisted in a cancer registry administered by the NHIS to qualify for special medical assistance for cancer-related expenses through rigorous verification by oncology experts and health insurance personnel. Obesity was defined as a BMI ≥ 25 kg/m2 according to the World Health Organization Asia-Pacific criteria[13]. Abdominal obesity was determined by WC ≥ 90 cm and ≥ 85 cm for men and women, according to the guidelines of the Korean Society for the Study of Obesity[14]. Definitions of other comorbidities are described in Supplementary Table 1.
Baseline characteristics were compared between groups according to HCC development during follow-up using independent t-tests for continuous variables and χ2d tests for categorical variables. Participants were divided into five BMI categories: BMI < 18.5 (underweight), 18.5-22.9 (normal weight), 23-24.9 (overweight), 25-29.9 (class I obesity), and ≥ 30 kg/m2 (class II or III obesity)[13]. Additionally, participants were divided into WC categories defined as follows: < 80/< 75, 80-84.9/75-79.9, 85-89.9/80-84.9, 90-94.9/85-89.9, 95-99.9/90-94.9, and ≥ 100/≥ 95 cm for men/women. The incidence rates of HCC for each BMI and WC category were calculated by dividing the number of new cases by 10000 person-years. Cox proportional hazard regression models were used to determine hazard ratios (HRs) and corresponding 95%CIs for developing HCC. The regression models included the unadjusted model (Model 1); Model 2 adjusted for age and sex; Model 3 adjusted for the covariates of Model 2 and for smoking, alcohol consumption, physical activity, income status, the presence of hypertension, dyslipidemia, and diabetes mellitus and WC or BMI for the analysis of BMI and WC groups, respectively. The risk of HCC in each BMI and WC category was calculated with reference to the normal weight and < 80/< 75 cm groups. The risk of HCC development in each BMI category was evaluated based on the presence of abdominal obesity. Subgroup analyses for the development of HCC by BMI and WC were conducted according to age groups and the FLI with likelihood ratio tests for interaction. Furthermore, the potential confounding effects of sex, smoking, and drinking status on the association between BMI and HCC were evaluated using subgroup analysis. Statistical significance was set at a two-sided P < 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, United States). The statistical methods of this study were reviewed by Han KD from Soongsil University, Republic of Korea.
The mean age and percentage of men were 49.2 years (SD = 13.4) and 73.5%, respectively. During 11.2 million person-years of follow-up (median: 10.3 years, interquartile range: 10.1-10.6), 0.43% of the total population (n = 4773) were newly diagnosed with HCC. Compared with individuals who did not develop HCC, those who developed HCC were older, more likely to be men, exercised regularly, and had low income (Table 1). Individuals who developed HCC had a higher proportion of hypertension and diabetes mellitus but less dyslipidemia. They had a lower prevalence of obesity but a higher prevalence of abdominal obesity than those without HCC.
| Characteristics | Hepatocellular carcinoma | P value | |
| No (n = 1106000) | Yes (n = 4773) | ||
| Age (year), mean ± SD | 49.1 ± 13.4 | 59.6 ± 11.1 | < 0.001 |
| Men | 813054 (73.5) | 3791 (79.4) | < 0.001 |
| Smoking | < 0.001 | ||
| Never | 517549 (46.8) | 2080 (43.6) | |
| Former | 211304 (19.1) | 1064 (22.3) | |
| Current | 377147 (34.1) | 1629 (34.1) | |
| Alcohol consumption | 0.224 | ||
| None | 509324 (46.1) | 2240 (46.9) | - |
| Light-to-moderate | 596676 (54.0) | 2533 (53.1) | - |
| Regular exercise | 200883 (18.2) | 977 (20.5) | < 0.001 |
| Low income (< 25%) | 213577 (19.3) | 1126 (23.6) | < 0.001 |
| Anthropometrics | |||
| Body mass index (kg/m2) | 26.4 ± 2.8 | 26.0 ± 3.1 | < 0.001 |
| Waist circumference (cm) | 88.0 ± 6.6 | 88.9 ± 7.2 | < 0.001 |
| Systolic blood pressure (mmHg) | 127.6 ± 14.6 | 130.7 ± 15.7 | < 0.001 |
| Diastolic blood pressure (mmHg) | 79.7 ± 9.8 | 80.3 ± 10.3 | < 0.001 |
| Laboratory findings, mean ± SD | |||
| Fasting glucose (mmol/L) | 5.7 ± 1.6 | 6.3 ± 2.2 | < 0.001 |
| Total cholesterol (mmol/L) | 5.4 ± 1.0 | 5.0 ± 1.0 | < 0.001 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.8 | 1.4 ± 0.7 | 0.046 |
| LDL cholesterol (mmol/L) | 3.1 ± 1.1 | 2.8 ± 1.1 | < 0.001 |
| Triglycerides (mmol/L), median (interquartile range) | 2.0 (1.99-2.00) | 1.7 (1.65-1.70) | < 0.001 |
| eGFR (mL/minute/1.73 m2) | 85.4 ± 46.9 | 83.3 ± 27.9 | 0.002 |
| Comorbidities | |||
| Hypertension | 416881 (37.7) | 2600 (54.5) | < 0.001 |
| Dyslipidemia | 309920 (28.0) | 1108 (23.2) | < 0.001 |
| Diabetes mellitus | 155120 (14.0) | 1481 (31.0) | < 0.001 |
| Obesity | 753485 (68.1) | 2968 (62.2) | < 0.001 |
| Abdominal obesity | 505999 (45.8) | 2423 (50.8) | < 0.001 |
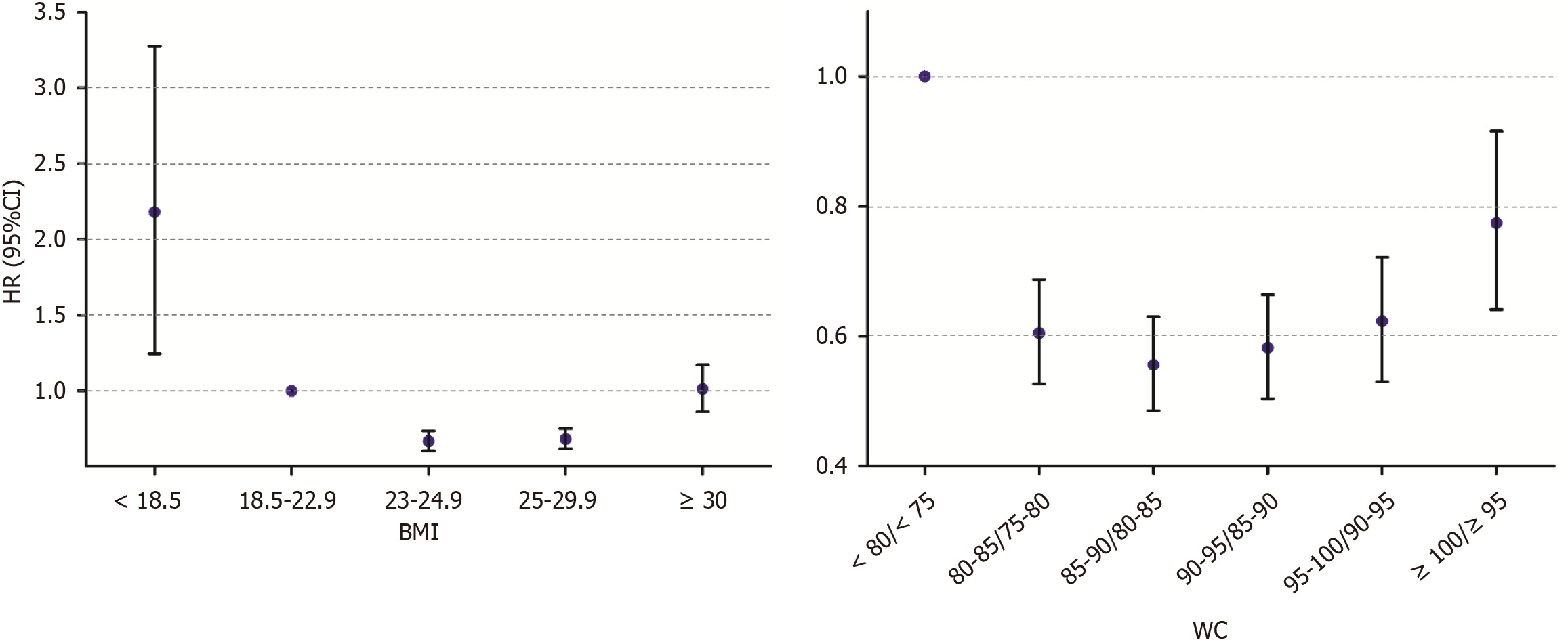
A U-shaped relationship was observed between BMI and HCC development (Figure 2, Supplementary Table 2). In the fully adjusted model, the underweight group had the highest risk of HCC, with a 2-fold higher risk than the normal weight group [Model 3: adjusted HR (aHR) = 2.02, 95%CI: 1.25-3.28]. The lowest risk was observed in the overweight group (Model 3: aHR = 0.67, 95%CI: 0.60-0.73). The risk in individuals with class II obesity or higher was not different from that in individuals with normal weight (Model 3: AHR = 1.01, 95%CI: 0.86-1.17).
Abdominal obesity was significantly associated with the relationship between BMI and HCC (Table 2; P for interaction = 0.012). The association between BMI and HCC maintained a U-shaped pattern regardless of abdominal obesity, with the highest risk of HCC in the underweight group. Among people with normal abdominal circumference, the lowest risk of HCC was found in the class I obesity group compared to the normal weight group (Model 3: AHR = 0.62, 95%CI: 0.56-0.69). In contrast, people with abdominal obesity in combination with overweight or class I obesity had a similar risk of incident HCC as people with normal weight.
| Characteristics | BMI (kg/m2) | n | Event (n) | Person-years | Incidence rate | Hazard ratio (95%CI) | P for interaction |
| No abdominal obesity | < 18.5 | 828 | 15 | 7139 | 21.0 | 1.77 (1.06-2.96) | 0.012 |
| 18.5-22.9 | 86305 | 616 | 854725 | 7.2 | 1 (reference) | - | |
| 23-24.9 | 212179 | 825 | 2149559 | 3.8 | 0.65 (0.59-0.73) | - | |
| 25-29.9 | 295403 | 867 | 3012025 | 2.9 | 0.62 (0.56-0.69) | - | |
| ≥ 30 | 7636 | 27 | 77780 | 3.5 | 1.15 (0.78-1.69) | - | |
| Abdominal obesity | < 18.5 | 53 | 2 | 457 | 43.8 | 5.89 (1.43-24.2) | - |
| 18.5-22.9 | 6575 | 50 | 61235 | 8.2 | 1 (reference) | - | |
| 23-24.9 | 48380 | 297 | 474737 | 6.3 | 0.92 (0.68-1.24) | - | |
| 25-29.9 | 353051 | 1651 | 3555405 | 4.6 | 0.99 (0.75-1.32) | - | |
| ≥ 30 | 100363 | 423 | 1018134 | 4.2 | 1.43 (1.06-1.94) | - |
The association between WC and HCC development showed a U-shaped relationship, with the highest risk of HCC in the lowest WC group (Figure 2, Supplementary Table 2). The group with a pre-stage of abdominal obesity (WC of 85-89.9/80-84.9 cm) had the lowest risk of HCC in the adjusted models compared to the group with the lowest WC (Model 3: aHR = 0.55, 95%CI: 0.49-0.63). The highest WC group had a 23% lower risk of HCC compared to the lowest WC group (Model 3: aHR = 0.77, 95%CI: 0.64-0.92).
Subgroup analysis by age showed that a U-shaped association between BMI and HCC generally persisted in all subgroups (P for interaction = 0.632) (Supplementary Table 3). For all participants aged ≥ 40 years, the overweight and class I obesity groups had a significantly lower risk of HCC than the normal weight group. A U-shaped relationship between BMI and HCC remained consistent across the subgroups of sex (P for interaction = 0.339), smoking status (P for interaction = 0.536), alcohol consumption (P for interaction = 0.753), and FLI (P for interaction = 0.211) (Supplementary Table 4, Supplementary Figure 1).
All subgroups stratified by age showed a U-shaped relationship between WC and HCC risk, although the association differed according to age (P for interaction = 0.048) (Supplementary Table 5). People with WC between 80-99.9 cm for men and 75-94.9 cm for women in all age groups had a significantly lower risk of developing HCC than the lowest WC group, except for the 95-99.9/90-94.9 cm group aged < 40 years. People with FLI: 30-60 and ≥ 60 consistently had the highest risk of HCC in the lowest WC group (P for interaction = 0.093) (Supplementary Figure 1). A decreasing risk of HCC with increasing WC was observed in the subgroup with FLI: 30-60, whereas individuals with a FLI ≥ 60 showed a U-shaped pattern.
This nationwide population-based cohort study with 11.2 million person-years of follow-up demonstrated a U-shaped relationship between BMI or WC and the risk of incident HCC in individuals with NAFLD. The highest risk of developing HCC was observed in the lowest BMI (underweight) and WC (< 80/< 75 cm) groups. The lowest risk of HCC was found in the overweight group and in the group with WC of 85-89.9/80-84.9 cm, which immediately precedes the criteria for abdominal obesity. The risk in individuals with class II or III obesity did not differ from that in individuals with normal weight. The group with the highest WC had a significantly lower risk than the group with the lowest WC. Subgroup analyses according to age, sex, health-related behaviors, and FLI showed consistent results. The current findings suggest an association between NAFLD and HCC in both the presence and absence of obesity, and an even higher risk of HCC in apparently lean individuals.
In contrast to a positive association between BMI or WC and HCC in the general population[4-6], the exceptional association in individuals with NAFLD may be attributed to the heterogeneous characteristics of NAFLD between lean and obese individuals. Although excessive fat accumulation is considered a hallmark of NAFLD, the global prevalence of NAFLD without being overweight or obese, commonly referred to as “lean NAFLD,” is estimated to be 5.1% overall and 19.2% of the NAFLD population[15]. A higher incidence of liver-related adverse outcomes has been reported in people with lean NAFLD compared to those with obese NAFLD, including severe liver disease and all-cause and liver-specific mortality[15,16]. Extending these results by dividing the participants into BMI and WC categories, we showed that underweight individuals and those with low WC may be more susceptible to HCC among those with NAFLD grouped as a lean phenotype.
Several lines of evidence suggest that the severity of obesity does not reflect the degree of liver damage once NAFLD has developed[17,18]. The rates of nonalcoholic steatohepatitis in Italian individuals with NAFLD were not statistically different among the normal weight, overweight, and obese groups, although the rates of metabolic syndrome were higher with greater body weight[17]. Similarly, in 431 individuals with biopsy-confirmed NAFLD, higher WC was significantly associated with more metabolic risk factors but not severe fibrosis[18]. Taken together, individuals with NAFLD and weight or WC in the low-to-normal range may have an independent mechanism from those with obesity for developing severe liver diseases, including HCC.
One potential explanation for the vulnerability to HCC in individuals with non-obese NAFLD may be the higher prevalence of genetic variants associated with liver disease progression. Recent data from a Korean health screening center reported that the GG allele of patatin-like phospholipase domain-containing protein 3 (PNPLA3) and the CT or TT allele of transmembrane 6 superfamily 2 (TM6SF2) are two genetic variants significantly associated with lean NAFLD[19]. In the Japanese population, the odds ratio for NAFLD in carriers of the G allele of PNPLA3 relative to non-carriers was 3.5 times higher in individuals who have normal weight, whereas no significant difference was found in those with obesity[20]. Guanine substitution in PNPLA3 exacerbates hepatic triglyceride accumulation, fibrosis, and, ultimately, HCC development in NAFLD[21]. A meta-analysis also revealed a significant association between the TM6SF2 polymorphism and risk of HCC[22]. Overall, the genetic variants represented by PNPLA3 and TM6SF2 may be putative drivers of HCC in individuals with non-obese NAFLD.
Moreover, a distinct gut microbiome has been observed in lean NAFLD compared to obese NAFLD in both Korean and Caucasian populations, including reduced microbial diversity in lean NAFLD[23,24]. Given that gut dysbiosis influences immune surveillance, hepatic inflammation, and progression to HCC[25], an imbalanced microbiome in lean individuals with NAFLD may precipitate HCC in the absence of obesity. Additionally, unmeasured coexisting characteristics in individuals with lean NAFLD may predispose them to HCC development. For example, high-fructose intake, typically from soft drinks, is strongly implicated in NAFLD and HCC progression[26]. As another cause, chronic malnutrition in underweight individuals may impair the immune response to tumorigenesis[27]. Although the dietary and nutritional statuses of the participants were not determined in our study, the consumption of sweetened beverages or undernutrition may be associated with incident HCC in populations with lean NAFLD.
A new nomenclature of metabolic dysfunction-associated steatotic liver disease (MASLD) was introduced to replace NAFLD by a consensus of multinational liver societies in 2023[28]. The discrepancy between MASLD and NAFLD appears to be minimal, as only 2.3% and 0.2% of NAFLD diagnoses in community- and hospital-based data, respectively, did not meet the criteria for MASLD[29]. Furthermore, the lower BMI groups in our study had higher glucose and triglyceride levels and similar blood pressures compared to the higher BMI groups (Supplementary Table 6), possibly meeting the cardiometabolic criteria for MASLD. Therefore, the current findings on the association between BMI or WC and the risk of HCC may be valid under the new term of MASLD but need future confirmation.
This study presents the strength of nationwide data involving more than 1.1 million participants with NAFLD. In addition to the previous binary classification of lean and non-lean NAFLD, our study is novel in that it demonstrates the association of each BMI or WC category with incident HCC. However, this study has a few limitations. First, owing to the observational study design, only association and not causality could be evaluated. Second, the diagnosis of liver steatosis was based on the FLI without radiological or histological evaluations, which can accurately assess the severity of steatosis. However, the FLI is a preferred surrogate marker for NAFLD in epidemiological studies because of its accessibility to the general population and cost-effectiveness[9]. Third, the operational definition of HCC raises concerns regarding inaccurate diagnoses. However, HCC was defined using the National Cancer Registry, which is expertly enrolled and directly managed by the NHIS. Finally, our findings must be validated to determine whether they apply to populations beyond Korea.
This nationwide population-based study of cohorts with NAFLD found a U-shaped association between BMI or WC and the development of HCC, with the highest risk observed in individuals who were underweight or WC < 80/< 75 cm for men/women. People who were overweight or in the pre-stage of abdominal obesity had the lowest risk of HCC. Surveillance of incident HCC in populations with NAFLD is needed, regardless of the presence of obesity, with particular vigilance in people with low adiposity.
We thank the staff of the Big Data Steering Department of the National Health Insurance Service and the participants in this study.
| 1. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1334] [Article Influence: 667.0] [Reference Citation Analysis (2)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 7345] [Article Influence: 7345.0] [Reference Citation Analysis (2)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7477] [Article Influence: 830.8] [Reference Citation Analysis (0)] |
| 4. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Jun BG, Kim M, Shin HS, Yi JJ, Yi SW. Impact of overweight and obesity on the risk of hepatocellular carcinoma: a prospective cohort study in 14.3 million Koreans. Br J Cancer. 2022;127:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Rahmani J, Kord Varkaneh H, Kontogiannis V, Ryan PM, Bawadi H, Fatahi S, Zhang Y. Waist Circumference and Risk of Liver Cancer: A Systematic Review and Meta-Analysis of over 2 Million Cohort Study Participants. Liver Cancer. 2020;9:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Souza M, Diaz I, Barchetta I, Mantovani A. Gastrointestinal cancers in lean individuals with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2024;44:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3716] [Article Influence: 161.6] [Reference Citation Analysis (2)] |
| 9. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3165] [Article Influence: 351.7] [Reference Citation Analysis (4)] |
| 10. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 1990] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 11. | Cho EJ, Jung GC, Kwak MS, Yang JI, Yim JY, Yu SJ, Chung GE. Fatty Liver Index for Predicting Nonalcoholic Fatty Liver Disease in an Asymptomatic Korean Population. Diagnostics (Basel). 2021;11:2233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, Kim HJ, Kong HJ, Im JS, Seo HG; Community of Population-Based Regional Cancer Registries*. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res Treat. 2023;55:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective : redefining obesity and its treatment. Sydney: Health Communications Australia, 2000: 15-21. |
| 14. | Kim KK, Haam JH, Kim BT, Kim EM, Park JH, Rhee SY, Jeon E, Kang E, Nam GE, Koo HY, Lim JH, Jeong JE, Kim JH, Kim JW, Park JH, Hong JH, Lee SE, Min SH, Kim SJ, Kim S, Kim YH, Lee YJ, Cho YJ, Rhie YJ, Kim YH, Kang JH, Lee CB; Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO). Evaluation and Treatment of Obesity and Its Comorbidities: 2022 Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity. J Obes Metab Syndr. 2023;32:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 15. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 16. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun. 2018;2:48-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 17. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1914] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 18. | Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, Consonni D, Fatta E, Lombardi R, Marchesini G, Fargion S. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Park H, Yoon EL, Chung GE, Choe EK, Bae JH, Choi SH, Kim M, Hwang W, Kim HL, Yang SY, Jun DW. Genetic and Metabolic Characteristics of Lean Nonalcoholic Fatty Liver Disease in a Korean Health Examinee Cohort. Gut Liver. 2024;18:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Nishioji K, Mochizuki N, Kobayashi M, Kamaguchi M, Sumida Y, Nishimura T, Yamaguchi K, Kadotani H, Itoh Y. The Impact of PNPLA3 rs738409 Genetic Polymorphism and Weight Gain ≥10 kg after Age 20 on Non-Alcoholic Fatty Liver Disease in Non-Obese Japanese Individuals. PLoS One. 2015;10:e0140427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Trépo E, Romeo S, Zucman-Rossi J, Nahon P. PNPLA3 gene in liver diseases. J Hepatol. 2016;65:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Tang S, Zhang J, Mei TT, Guo HQ, Wei XH, Zhang WY, Liu YL, Liang S, Fan ZP, Ma LX, Lin W, Liu YR, Qiu LX, Yu HB. Association of TM6SF2 rs58542926 T/C gene polymorphism with hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2019;19:1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Yun Y, Kim HN, Lee EJ, Ryu S, Chang Y, Shin H, Kim HL, Kim TH, Yoo K, Kim HY. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS One. 2019;14:e0213692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, Bayoumi A, Metwally M, Azardaryany MK, Coulter S, Choo JM, Younes R, Rosso C, Liddle C, Adams LA, Craxì A, George J, Eslam M. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology. 2020;71:1213-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 25. | Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C, Kilic K, Schneider CV, Schierwagen R, Strnad P, Wirtz TH, Marschall HU, Latz E, Lelouvier B, Saez-Rodriguez J, de Vos W, Strowig T, Trebicka J, Trautwein C. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. 2022;13:3964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 26. | Zhou P, Chang WY, Gong DA, Xia J, Chen W, Huang LY, Liu R, Liu Y, Chen C, Wang K, Tang N, Huang AL. High dietary fructose promotes hepatocellular carcinoma progression by enhancing O-GlcNAcylation via microbiota-derived acetate. Cell Metab. 2023;35:1961-1975.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 27. | Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119-28; quiz 1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 318] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1250] [Article Influence: 625.0] [Reference Citation Analysis (0)] |
| 29. | Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80:e54-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 205.0] [Reference Citation Analysis (0)] |