Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107272
Revised: April 7, 2025
Accepted: April 22, 2025
Published online: June 15, 2025
Processing time: 86 Days and 11.7 Hours
Squamous cell carcinoma (SCC) of the colon is a rare malignant tumor with an unclear pathogenesis. Its clinical presentation is similar to that of adenocarcinoma, and there are no standard treatment guidelines. Treatment for SCC of the colon is mainly based on the protocols for colon adenocarcinoma. In advanced stages, colon SCC is highly invasive, prone to distant metastasis, and has a worse prog
The patient presented with abdominal pain and was diagnosed with SCC of the descending colon following colonoscopy. Preoperative examinations did not reveal any obvious metastasis to other organs, and the patient underwent laparoscopic radical resection of the descending colon cancer. During surgery, suspi
This case highlights the aggressive nature of colorectal SCC with atypical meta
Core Tip: This case represents the first documented primary squamous cell carcinoma of the descending colon with pancreatic metastasis, highlighting the dual rarity of both the primary site and metastatic behavior. It underscores the challenges in managing this aggressive malignancy, including rare metastatic patterns and severe postoperative complications, while providing critical insights for optimizing multidisciplinary therapeutic strategies in colorectal squamous cell carcinoma.
- Citation: Hu RR, Liu M, Li HY. Primary squamous cell carcinoma of the descending colon with pancreatic metastasis: A case report. World J Gastrointest Oncol 2025; 17(6): 107272
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107272.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107272
Primary squamous cell carcinoma (SCC) of the colon is extremely rare, as it accounts for less than 0.5% of all colorectal malignancies. Furthermore, its pathogenesis remains unclear, and is potentially associated with chronic inflammation, human papillomavirus infection, or abnormal differentiation of stem cells[1]. Colorectal SCC shares clinical symptoms with adenocarcinoma, but primary colorectal SCC requires histopathological confirmation. It is highly invasive and prone to distant metastasis. Currently, there are no standard treatment guidelines for colorectal SCC[1]. Treatment methods for colorectal SCC include surgery, chemotherapy, and immunotherapy, but the mortality rate is higher than that of colo
A 67-year-old male presented with epigastric pain for over 2 months and weight loss of 15 kg.
The patient reported no hematochezia, nausea, vomiting, or changes in bowel habits.
There was no specific past medical history.
The patient had a 50-year history of smoking and alcohol consumption. His family history was unremarkable.
A firm, mildly tender mass in the left lower abdomen was identified on physical examination.
Tumor markers (carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen 19-9, and carbohydrate antigen 72-4) were negative. Routine blood tests, C-reactive protein (CRP), biochemistry, and thyroid function tests showed no signi
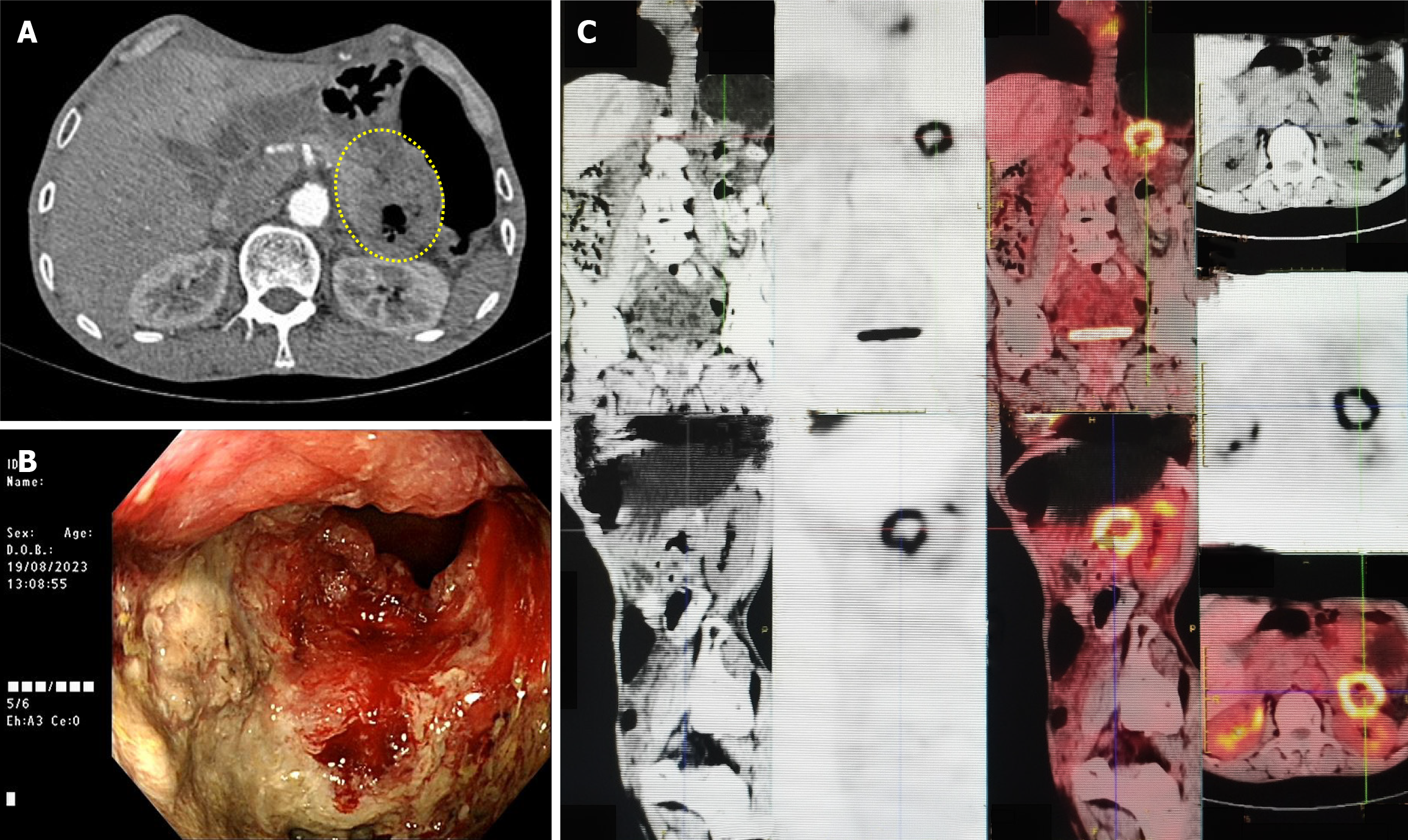
On contrast-enhanced computed tomography (August 19, 2023), descending colon wall thickening was observed (Figure 1A). On colonoscopy (August 19, 2023), an ulcerative, circumferential mass near the splenic flexure was identified (Figure 1B). On positron emission tomography-computed tomography (August 23, 2023), a hypermetabolic lesion with a maximum standardized uptake value of 10.0 was observed (Figure 1C).
Biopsy pathology suggested SCC of the colon.
On August 30, 2023, laparoscopic radical colectomy was attempted, but intraoperative findings revealed tumor infiltration into the pancreatic body/tail, splenic vessels, and splenic hilum, necessitating combined resection of the pancreatic body/tail and spleen.
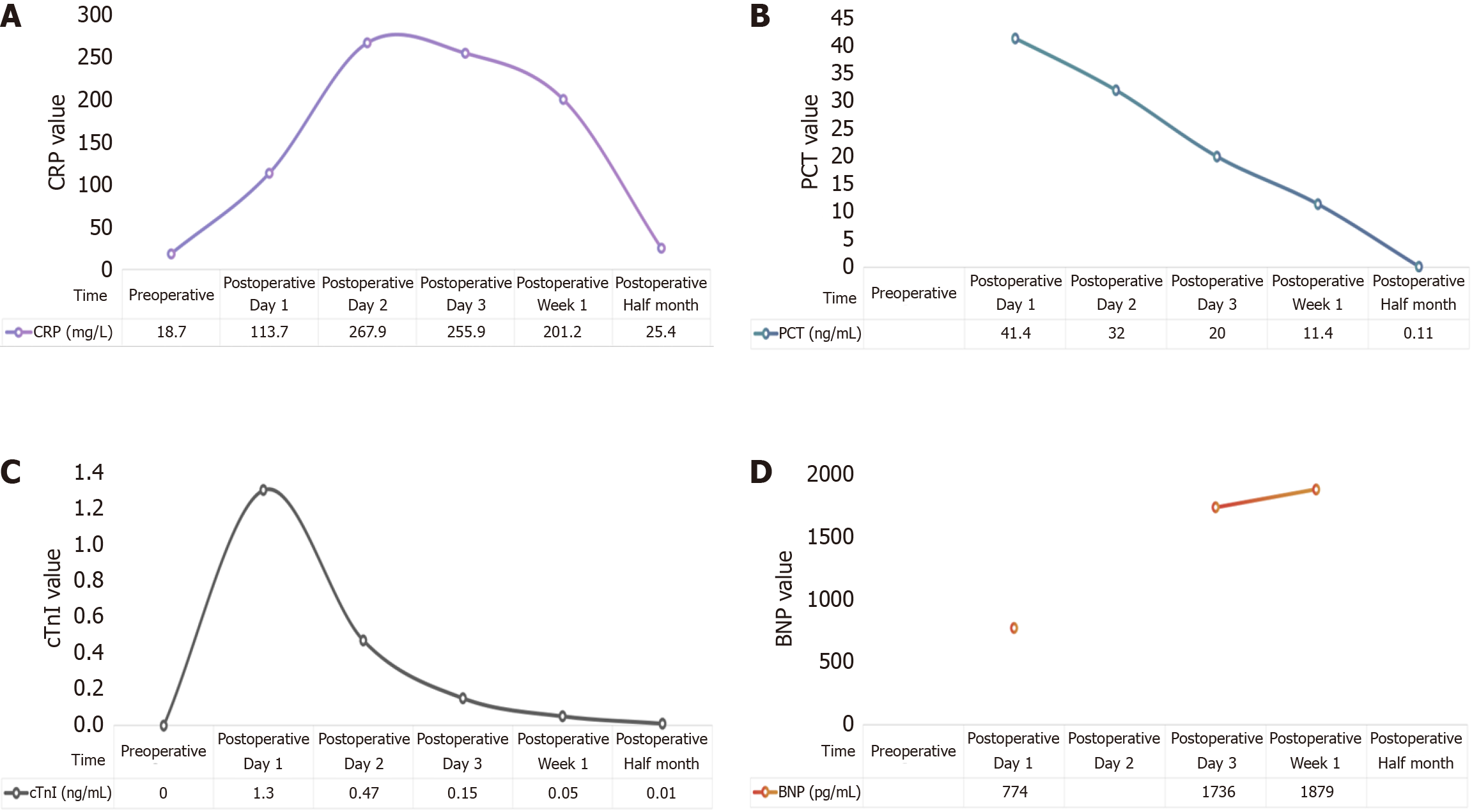
Postoperative pathology (Figure 2) confirmed tumor invasion through the entire intestinal wall and into the pancreas, with neural invasion (+). Immunohistochemical analysis demonstrated positive staining for squamous differentiation markers [P40, cytokeratin (CK) 5/6, P63] with strong pan-CK expression, along with CK7 positivity, while showing negativity for intestinal differentiation markers (CK20, caudal-type homeobox protein 2, villin) and somatostatin receptor 2. This immunoprofile supports the diagnosis of SCC and argues against a colorectal adenocarcinoma origin or neuroendocrine differentiation, with additional findings including a moderate proliferative index (Ki67 approximately 20%) and preserved vascular (CD34+) and lymphatic (D2-40+) architecture. Within 24 hours postoperatively, the patient developed agitation with elevated CRP and cardiac troponin I levels (Figure 3). Electrocardiography demonstrated acute non-ST-segment elevation myocardial infarction, attributed to perioperative ischemic hypoperfusion exacerbated by concurrent intra-abdominal infection. Empirical antimicrobial therapy with ceftriaxone was initiated alongside continuous negative-pressure peritoneal irrigation. Adjunctive therapies included antiplatelet therapy (aspirin), lipid-lowering therapy (atorvastatin calcium), and coronary vasodilation (tanshinone). Nutritional Risk Screening (2002) revealed a score of 6 points, prompting intravenous administration of glucose, amino acids, vitamins, and fat emulsion. On postoperative day 5, purulent drainage fluid cultured multidrug-resistant Escherichia coli, while Doppler ultrasonography of the lower extremities revealed bilateral calf dorsal muscular venous thrombosis. Antimicrobial therapy was escalated to ertapenem based on antimicrobial susceptibility testing, with concurrent initiation of low-molecular-weight heparin anticoagulation. Amylase-negative abdominal fluid analysis excluded pancreatic leakage. Following comprehensive therapeutic inter
Colon SCC was first reported by Schmidtmann and other scientists in 1919[1], and to date, approximately 150 cases have been documented in the literature[4]. The average age of patients with colorectal SCC is between 50 and 60 years, with a slightly greater incidence in women than in men. It is more commonly found in the rectum and right-sided colon. The clinical features of colorectal SCC often overlap with those of adenocarcinoma, including abdominal pain, hematochezia, changes in bowel habits, and weight loss[1,2]. However, colon SCC is more invasive and prone to metastasis, with the most common metastatic sites being the liver, peritoneum, and lungs[5]. Moreover, Williams et al[6] indicated that four criteria must be met before diagnosing primary SCC of the colon: (1) Exclusion of proximal extension from the anal canal; (2) Exclusion of a fistula between the squamous mucosa and the affected colon; (3) Exclusion of metastasis from another primary SCC; and (4) Histopathological evidence of colorectal SCC. The pathogenesis of colon SCC is still unclear, and several hypotheses have been proposed: (1) Abnormal transformation of pluripotent stem cells in the colon mucosa into squamous epithelium; (2) Chronic inflammatory damage to the intestinal mucosa, leading to basal cell proliferation into squamous cells, especially in relation to ulcerative colitis[6]; (3) Associations with infections such as human papillomavirus, schistosomiasis, and amebiasis[7]; (4) Synchronous or metachronous occurrence with other adenocarcinomas, including ovarian cancer, prostate cancer, endometrial cancer, and breast cancer[8]; and (5) Genetic mutations, such as those observed in Lynch syndrome[4].
The clinical management of colorectal SCC remains challenging due to the absence of standardized guidelines, with current strategies largely extrapolated from adenocarcinoma protocols. However, emerging evidence highlights distinct biological behaviors and therapeutic responses between these two entities. While a multidisciplinary team approach combining surgery with adjuvant chemotherapy or immunotherapy may improve outcomes in select patients[9-11], the rapid progression observed in this case (intraoperative discovery of pancreatic and splenic hilar infiltration) and severe postoperative complications (abdominal infection, myocardial infarction, deep vein thrombosis) leading to therapeutic failure underscore the aggressive nature of colorectal SCC. This aligns with the imaging findings by Yoon et al[12], demonstrating SCC’s propensity for local invasion into adjacent organs (e.g., small bowel, ureters) and poorer prognosis compared to adenocarcinoma at equivalent tumor-node-metastasis stages[12,13]. Notably, the atypical pancreatic metastasis pattern in this case deviates from the previously reported hepatic/peritoneal/pulmonary metastatic dominance[5], necessitating a reevaluation of SCC’s metastatic mechanisms.
Although large-scale studies by Larkins et al[9] confirmed significantly prolonged overall survival with surgery plus adjuvant chemotherapy (hazard ratio = 2.66), our patient’s 3-month survival - attributable to postoperative complications precluding adjuvant therapy - exposes limitations in current therapeutic frameworks. Conventional chemotherapy regimens (e.g., 5-fluorouracil-based XELOX) exhibit limited efficacy in certain colorectal SCC cases, while emerging evidence supports the potential superiority of taxane-carboplatin regimens or programmed cell death protein-1 inhibitor-chemotherapy combinations[14-17]. The therapeutic role of radiotherapy in colorectal SCC remains inadequately defined, with limited high-quality evidence supporting its routine use. While definitive chemoradiation has demonstrated efficacy in rectal SCC (5-year local control rate: 72%, Sturgeon et al[18]), extrapolation to colonic SCC lacks robust validation. Furthermore, the immunohistochemical profile of this case (CK7+) correlates with findings by Singhal et al[19], where CK7 positivity, as demonstrated in this case (Figure 2J), correlates with epithelial-mesenchymal transition markers, suggesting a mechanistic link to metastatic potential[19], signifying its utility as a prognostic biomarker. Future directions should focus on the following: (1) Developing SCC-specific staging systems integrating molecular features (e.g., programmed death-ligand 1 expression, tumor mutational burden, CK7 status) and radiological assessments of invasiveness[12,17,19]; (2) Exploring neoadjuvant chemoradiation for locally advanced disease, informed by definitive chemoradiation strategies for rectal SCC[17]; and (3) Optimizing perioperative management to mitigate severe complications. Despite the unfavo
This case report describes a rare instance of descending colon SCC with pancreatic metastasis. The disease progressed rapidly, with multiple postoperative complications, and the patient ultimately died due to multiple organ failure. This case further confirms the high invasiveness and poor prognosis of colorectal SCC, emphasizing the importance of early diagnosis, accurate staging, and comprehensive treatment. Currently, the pathogenesis of this disease remains unclear, and there is a lack of standardized treatment protocols. Personalized treatment and multidisciplinary decision-making are critical for improving patient prognosis. In the future, further exploration of the role of imaging and molecular biomarkers in early diagnosis is needed, as is an evaluation of the clinical value of emerging therapies such as immunotherapy and targeted therapy to improve survival rates and quality of life. This case report provides valuable insights and references for clinicians in the diagnosis and treatment of colorectal SCC.
We gratefully acknowledge the clinical team for their dedicated patient care and the pathology department for their expert diagnostic support. Special thanks to the patient’s family for providing informed consent and contributing to medical knowledge.
| 1. | Schizas D, Katsaros I, Mastoraki A, Karela NR, Zampetaki D, Lazaridis II, Tsapralis D, Theodoropoulos GE. Primary Squamous Cell Carcinoma of Colon and Rectum: A Systematic Review of the Literature. J Invest Surg. 2022;35:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Sahoo BS, Prasad Das SA, Badwal S, Nundy S, Mehta NN. Obstructing primary squamous cell carcinoma of caecum: A case report. Ann Med Surg (Lond). 2022;78:103907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Tani R, Hori T, Yamada M, Yamamoto H, Harada H, Yamamoto M, Yazawa T, Tani M, Kamada Y, Aoyama R, Sasaki Y, Zaima M. Metachronous Pancreatic Metastasis from Rectal Cancer that Masqueraded as a Primary Pancreatic Cancer: A Rare and Difficult-to-Diagnose Metastatic Tumor in the Pancreas. Am J Case Rep. 2019;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Sidhu AS, Grewal H. An Unusual Tumor: Squamous Cell Carcinoma of the Colon in Lynch Syndrome. Cureus. 2024;16:e64477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Frizelle FA, Hobday KS, Batts KP, Nelson H. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum. 2001;44:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol. 1979;129:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Sotlar K, Köveker G, Aepinus C, Selinka HC, Kandolf R, Bültmann B. Human papillomavirus type 16-associated primary squamous cell carcinoma of the rectum. Gastroenterology. 2001;120:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Vezeridis MP, Herrera LO, Lopez GE, Ledesma EJ, Mittleman A. Squamous-cell carcinoma of the colon and rectum. Dis Colon Rectum. 1983;26:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Larkins MC, Bhatt A, Irish W, Kennedy KN, Burke A, Armel K, Honaker MD. Squamous cell carcinoma of the colon: evaluation of treatment modalities and survival. J Gastrointest Surg. 2024;28:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Ozuner G, Aytac E, Gorgun E, Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis. 2015;30:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol. 2009;15:4380-4386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Yoon EJ, Song SG, Kim JW, Kim HC, Kim HJ, Hur YH, Hong JH. Comprehensive CT Imaging Analysis of Primary Colorectal Squamous Cell Carcinoma: A Retrospective Study. Tomography. 2024;10:674-685. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (4)] |
| 13. | Zhao S, Guo J, Sun L, Lv J, Qiu W. Gemcitabine-based chemotherapy in colon squamous cell carcinoma: A case report and literature review. Mol Clin Oncol. 2017;6:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Wu X, Su S, Wei Y, Hong D, Wang Z. Case Report: A management strategy and clinical analysis of primary squamous cell carcinoma of the colon. Front Oncol. 2023;13:1265421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Copur S, Ledakis P, Novinski D, Mleczko KL, Frankforter S, Bolton M, Fruehling RM, VanWie E, Norvell M, Muhvic J. Squamous cell carcinoma of the colon with an elevated serum squamous cell carcinoma antigen responding to combination chemotherapy. Clin Colorectal Cancer. 2001;1:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Dahlbeck S, Kagan AR. Spinal cord compression, infection, and unknown primary cancers. Am J Clin Oncol. 2001;24:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Du J, Zhang P, Meng W, Xiao H. Squamous cell carcinoma of ascending colon with pMMR/MSS showed a partial response to PD-1 blockade combined with chemotherapy: A case report. Front Oncol. 2023;13:1051786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Sturgeon JD, Crane CH, Krishnan S, Minsky BD, Skibber JM, Rodriguez-Bigas MA, Chang GJ, You YN, Eng C, Das P. Definitive Chemoradiation for Squamous Cell Carcinoma of the Rectum. Am J Clin Oncol. 2017;40:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Singhal S, Singh RB, Potdar R. Metastatic squamous cell carcinoma of the colon: a conundrum in colorectal malignancies. BMJ Case Rep. 2021;14:e240573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |