Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106316
Revised: March 27, 2025
Accepted: April 22, 2025
Published online: June 15, 2025
Processing time: 112 Days and 0.6 Hours
Lynch syndrome (LS), an autosomal dominant genetic disorder, is distinguished by germline mutations in the DNA mismatch repair genes, including MLH1. These mutations confer an elevated risk for the development of colorectal cancer (CRC) and an array of other malignancies. Timely detection, facilitated by genetic profiling and stringent molecular surveillance, is crucial. It enables the im
This case presentation focuses on a 54-year-old male patient with a strong familial predisposition to colon cancer, who was identified to have LS-associated multiple colorectal neoplasms. Utilizing a comprehensive, multidisciplinary therapeutic strategy that encompassed precision medicine, immunotherapy with pembrolizumab, and stringent molecular residual disease monitoring, we effectively managed his advanced CRC. This tailored approach led to the achievement of sustained clinical remission exceeding 30 months, illustrating the promise of personalized treatment protocols in optimizing outcomes for individuals with LS and associated colorectal malignancies.
A synergistic, multidisciplinary approach is essential for managing LS-associated CRC, advocating for personalized care pathways in precision medicine.
Core Tip: This case report spotlights the critical value of a personalized, multidisciplinary approach in managing Lynch syndrome-associated colorectal cancer. It underscores how precision medicine, particularly immunotherapy with programmed death protein 1 inhibitors, and rigorous molecular surveillance can lead to markedly improved patient outcomes, including sustained clinical remission for over 30 months. The integration of these strategies sets a new precedent for treating this complex genetic disorder.
- Citation: Xu XX, Gao YH, Du CZ, Gao XX, Chen P, Fan RF, Li HT, Qiao Z. Molecular surveillance-informed personalized multidisciplinary therapy achieves prolonged survival in a patient with Lynch syndrome-associated colorectal cancer: A case report. World J Gastrointest Oncol 2025; 17(6): 106316
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106316.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106316
Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer (CRC), is a genetic disorder that significantly increases the risk of developing various types of cancer, with CRC being the most prevalent[1]. This syndrome is caused by mutations in the DNA mismatch repair (MMR) genes, which are crucial for maintaining the stability of the genome. The inheritance pattern of LS is autosomal dominant, meaning that an individual only needs one copy of the mutated gene to be at an increased risk for cancer. The impact of LS on public health is substantial, yet it remains underdiagnosed and often misunderstood[2]. The syndrome not only affects the individual but also has profound implications for family members who may carry the mutated gene unknowingly. Early detection and intervention are critical in managing LS, as they can significantly improve outcomes and reduce mortality rates. In the landscape of precision medicine, LS stands out as a paradigm for targeted therapeutics. The genetic aberrations characteristic of LS, particularly high microsatellite instability, render tumors more susceptible to immune checkpoint inhibitors, offering a beacon of hope for patients with this hereditary cancer syndrome[3]. Against this backdrop, the case of a 54-year-old male patient with advanced CRC associated with LS presents a compelling narrative. His medical journey, marked by a constellation of symptoms and a family history deeply intertwined with the specter of colon cancer, exemplifies the challenges and opportunities in the contemporary management of LS-associated neoplasms. This case study delves into the intricacies of a multidisciplinary therapeutic approach, precision medicine, and the pivotal role of molecular surveillance in achieving prolonged survival and remission, setting the stage for a deeper exploration of the interplay between genetics, personalized treatment, and clinical outcomes in LS.
A 54-year-old male patient presented to the hospital with a two-month history of rectal bleeding and occasional tenesmus in December 2021.
The patient had experienced rectal bleeding and occasional tenesmus for two months prior to his presentation. He had no significant weight loss. His symptoms did not improve with over-the-counter treatments, prompting medical evaluation.
The patient had no significant past medical history.
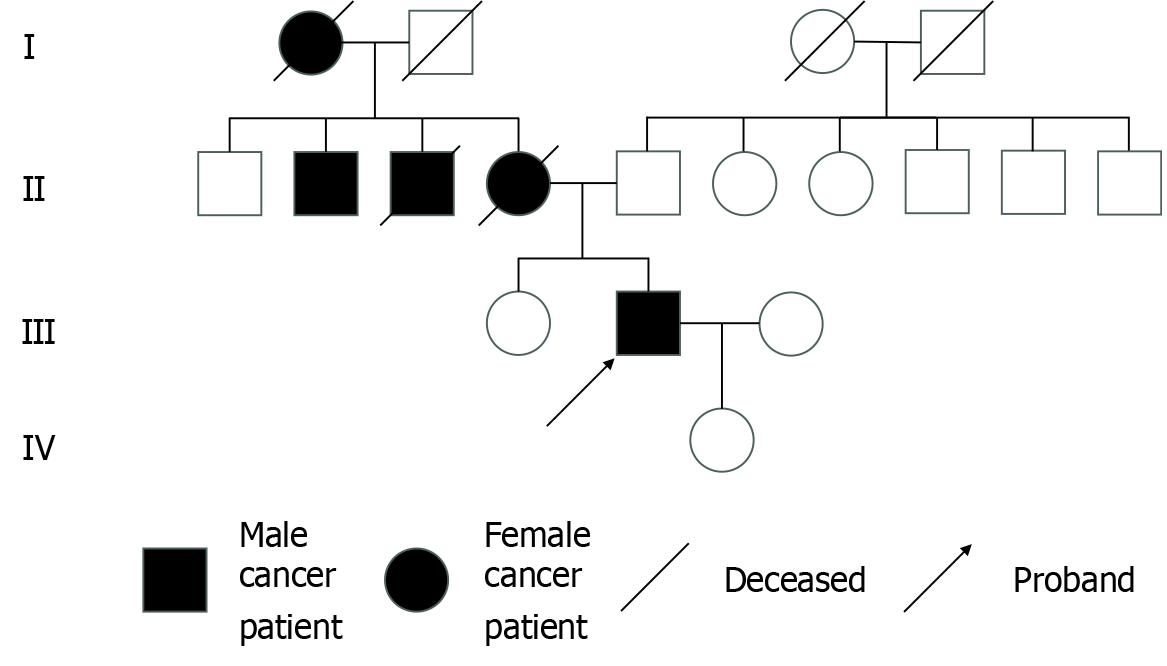
The patient’s family history was significant for colon cancer, with his mother and maternal uncle both having been diagnosed and deceased due to the disease (Figure 1).
On physical examination, the patient’s body mass index was 28.1 kg/m2, classifying him as overweight. A painful nodular mass, with a diameter of 1.5 cm, was found on the left penis. There was no obvious redness or swelling. The glans, testis, and epididymis were normal. No secretion was found at the urethral orifice.
Initial carcinoembryonic antigen level was 2.58 ng/mL, with subsequent levels rising to a peak of 12.15 ng/mL before gradually declining to 6.06 ng/mL. The genetic testing method used was next-generation sequencing technology based on the Illumina platform and hybridization capture method, conducted on the Illumina, Berry Genomics Sequencing Platform, and ADx-SEQ200 Plus Sequencer. The test covered 116 genes, including exons, splicing regions, fusion-related introns, and some chemotherapeutic single-nucleotide polymorphism sites, detecting point mutations, small fragment insertions/deletions, gene fusions, and polymorphic sites of enzymes related to chemotherapeutic drug metabolism within the target region. The test analyzed and interpreted tumor-driving gene mutations, providing molecular marker information for solid tumor-targeted therapy, immune checkpoint inhibitor therapy, and chemotherapy within the framework of evidence-based medicine to help clinicians formulate treatment plans.
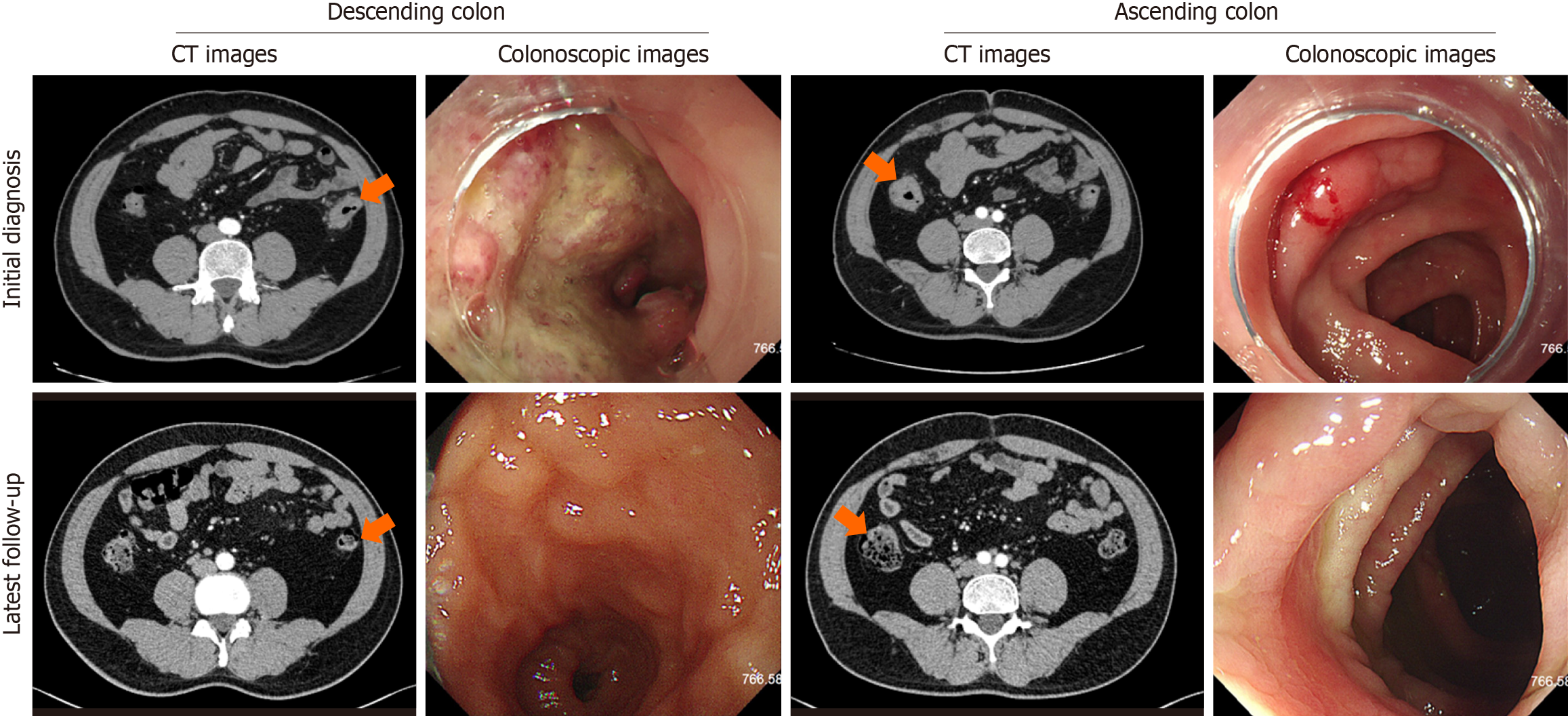
A colonoscopy identified multiple polyps in the colon and suspected malignant tumors in both the descending and ascending colon. Subsequent biopsies confirmed high-grade intraepithelial neoplasias in the ascending colon and poorly differentiated adenocarcinomas in the descending colon. The abdominal and pelvic computed tomography scans revealed mural thickening and enhancement in the colon, along with lymphadenopathy in the right iliac and bilateral inguinal regions, initially suggesting a stage IV disease (cT4N2-3M1). However, subsequent inguinal lymph node biopsy showed no evident neoplastic diseases, leading to a revised diagnosis of stage III (cT4N2-3M0). The patient underwent treatment, and follow-up computed tomography scans post-treatment demonstrated resolution of the mural thickening and enhancement in the colon, with significant reduction in the size and number of lymph nodes in the right iliac and bilateral inguinal regions, indicating a good response to therapy (Figure 2).
Genetic testing by next-generation sequencing identified a germline heterozygous MLH1 mutation, specifically the c.677G>A variant, in accordance with the Bethesda guidelines[4], which confirmed the diagnosis of LS.
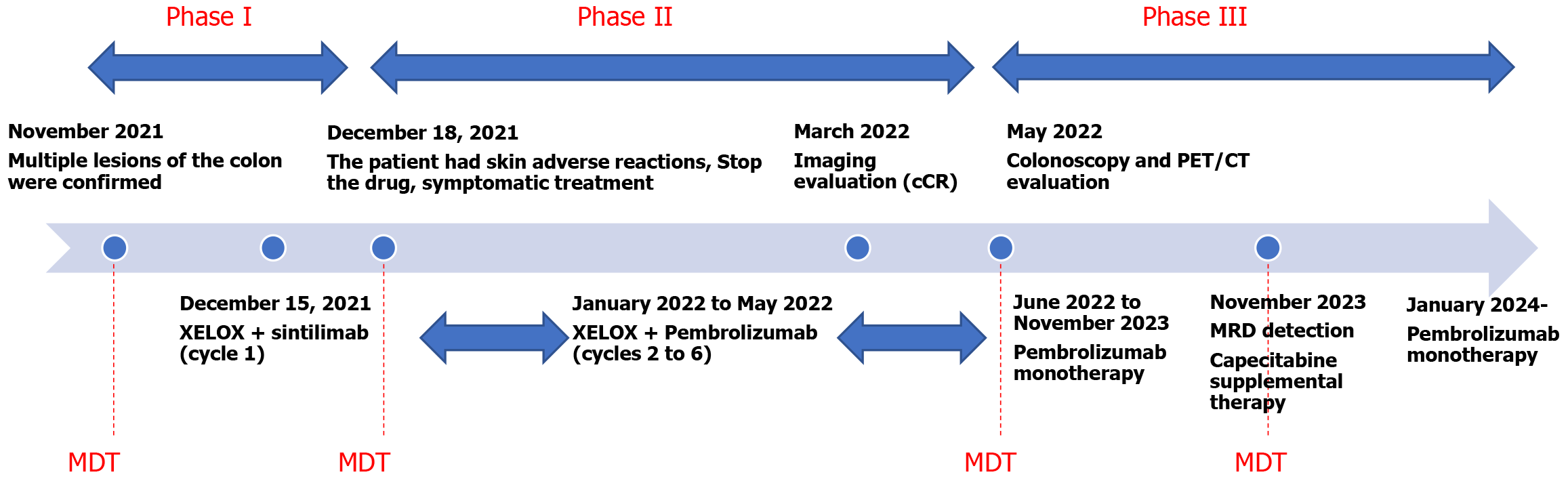
The treatment strategy was anchored in precision medicine, encompassing a tailored combination of chemotherapy, immunotherapy with programmed death protein 1 (PD-1) inhibitors, and rigorous molecular surveillance. The chemotherapy regimen consisted of oxaliplatin (130 mg/m2 intravenously on day 1) and capecitabine (1000 mg/m2 orally twice daily from day 1 to day 14), repeated every 3 weeks. For immunotherapy, the initial PD-1 inhibitor sintilimab was administered every 3 weeks, starting on December 15, 2021. However, due to a drug-induced rash, sintilimab was discontinued after the first cycle, and pembrolizumab was used for subsequent cycles from January 2022. Starting in 2024, the dosing interval of pembrolizumab was changed to every 2 months for maintenance therapy (Figure 3). Additionally, the patient took anti-emetics (metoclopramide tablets or ondansetron tablets as needed) during follow-up. No other medications were used.
The patient achieved sustained clinical complete remission for over 30 months.
In this case, LS was genetically confirmed through the detection of a germline mutation in the MLH1 gene. The clinical presentation of colorectal neoplasms with multiple polyps and poorly differentiated adenocarcinomas, along with a strong family history of colon cancer, supported this diagnosis. The presence of mural thickening and lymphadenopathy on imaging studies further confirmed the aggressive nature of the disease.
LS is a hereditary condition that significantly increases the risk of CRC and a spectrum of other malignancies[2,5,6]. This genetic predisposition is primarily driven by mutations in the DNA MMR genes, with MLH1, MSH2, MSH6, and PMS2 being the most commonly implicated, and is further influenced by EpCAM alterations which can lead to epigenetic silencing of the MSH2 gene, resulting in a LS phenotype. The presence of these mutations not only heightens the risk of cancer development but also confers a unique therapeutic opportunity: An increased responsiveness to immune checkpoint inhibitors. Particularly, the blockade of the PD-1 pathway has emerged as a promising therapeutic strategy for individuals with LS[7]. PD-1 checkpoint inhibitors are monoclonal antibodies that block the interaction between PD-1 on T cells and its ligands on tumor or stromal cells[8]. Normally, this interaction suppresses T cell activation, enabling tumors to evade immune detection. By inhibiting PD-1/programmed death-ligand 1 binding, these drugs restore T cell-mediated antitumor responses, enhancing immune clearance of cancer cells. The rationale for using PD-1 checkpoint inhibitors in this study is grounded in the molecular mechanisms involving MSH2 epigenetic silencing. In our case, MSH2 epigenetic silencing leads to MMR deficiency and increased microsatellite instability, resulting in a higher tumor mutation burden. This creates more antigenic mutations in the tumor microenvironment and increases T cell infiltration, thereby enhancing the sensitivity and efficacy of PD-1 inhibitors[9].
The era of precision medicine has brought about a paradigm shift in the management of LS-associated CRC. This personalized approach involves the careful tailoring of treatment strategies based on the intricate details of a patient’s genetic profile. By harnessing the power of genetic information, clinicians can now design treatment plans that are not only more likely to be effective but also more tolerable, minimizing the risk of adverse effects. Central to this precision approach is the concept of molecular surveillance, with a focus on monitoring molecular residual disease (MRD). This surveillance allows for the early detection of treatment response and the emergence of resistance, enabling clinicians to make informed, timely adjustments to treatment protocols. This is crucial in the management of LS, where the genetic landscape can be complex and dynamic.
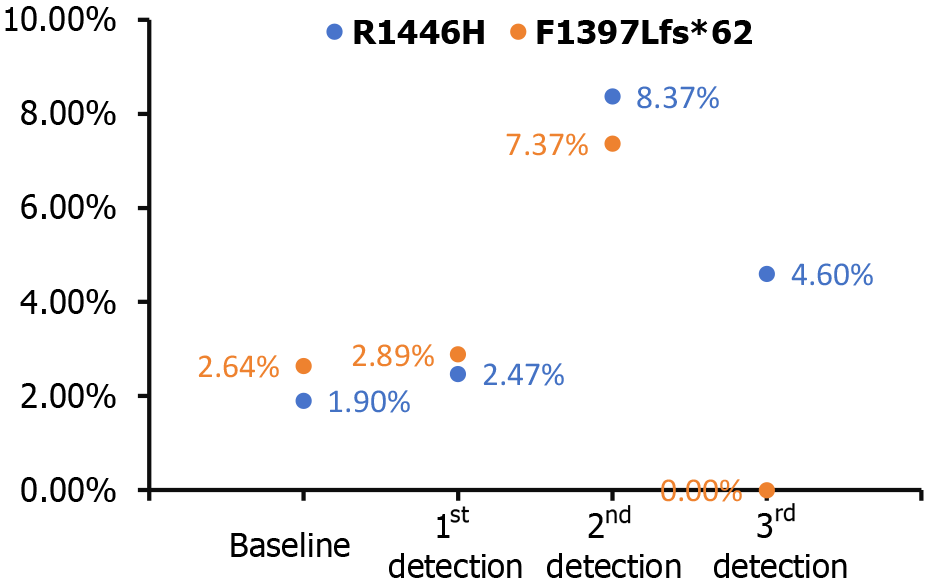
In this case of a patient with LS and multiple colorectal neoplasms, the optimal treatment strategy is one that is both personalized and multidisciplinary. It integrates precision medicine with immunotherapy and molecular surveillance to achieve the best possible outcomes. The initial treatment plan might include a combination of chemotherapy drugs, such as oxaliplatin and capecitabine in the XELOX regimen, alongside PD-1 monotherapy with sintilimab. However, due to drug-induced complications, such as a rash, the treatment may be refined to include pembrolizumab, another PD-1 inhibitor[10]. Moreover, the immunotherapy-related skin reaction is now considered a positive prognostic sign, suggesting a favorable response to the therapeutic intervention[11]. This individualized treatment approach, guided by the patient’s genetic profile and clinical features, has resulted in marked tumor regression and alleviation of symptoms, including hematochezia. Additionally, the tumor has achieved a complete clinical response, indicating that traditional imaging diagnostics and their predictive value may be diminished in this context. The heightened sensitivity to PD-1 blockade in LS patients has proven to be highly effective, and this is further enhanced by the vigilant monitoring of MRD. The detection of CREBBP mutations through MRD surveillance has revealed two specific alterations: R1446H and F1397 Lfs*62. R1446H is a missense mutation in exon 26, where the 1446th amino acid changes from arginine to histidine. F1397 Lfs*62 is a frameshift mutation caused by a deletion at position 4188 of exon 25, resulting in a shifted reading frame and protein truncation. This finding indicates potential molecular progression of the tumor, prompting a strategic adjustment to the treatment protocol by incorporating capecitabine alongside pembrolizumab. CREBBP, a gene associated with oncogenic activity, signifies the presence of a molecular signature that may portend a propensity for tumorigenesis, underscoring the importance of personalized therapeutic interventions. This intervention has successfully reduced circulating CREBBP levels, allowing for the continuation of effective monotherapy (Figure 4).
The patient’s sustained complete clinical response for over 30 months is a testament to the efficacy of this synergistic treatment approach. This case illustrates the importance of a comprehensive, personalized strategy that combines chemotherapy, immunotherapy, targeted therapy, genetic counseling, and rigorous monitoring. It sets a new standard for the care of patients with CRC induced by LS, demonstrating the potential of a synergistic approach to achieve superior outcomes in the management of this complex condition (Figure 3).
Our case underscores the critical role of a multidisciplinary therapeutic strategy in the management of LS-associated CRC. The incorporation of precision medicine, including personalized chemotherapy and immunotherapy, was instrumental in our patient’s management. Notably, MRD surveillance was crucial, enabling the detection of a CREBBP mutation that prompted a strategic shift to pembrolizumab, leading to sustained clinical complete remission. This underscores the importance of MRD surveillance in guiding real-time treatment adjustments and underscores the potential of precision medicine to enhance outcomes for patients with hereditary cancer syndromes. As the field of precision medicine advances, the integration of MRD surveillance is likely to become increasingly vital, setting a precedent for future management of LS and related conditions.
The authors sincerely thank the patient for providing informed consent to publish this case report and accompanying medical images in an anonymized manner.
| 1. | Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer. 2015;15:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 581] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Sinicrope FA. Lynch Syndrome-Associated Colorectal Cancer. N Engl J Med. 2018;379:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 4. | Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2219] [Article Influence: 105.7] [Reference Citation Analysis (1)] |
| 5. | Peltomäki P, Nyström M, Mecklin JP, Seppälä TT. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology. 2023;164:783-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 6. | Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin. 2018;68:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul Razak AR, Dotti K, Santoro A, Cohen RB, Gould M, Saraf S, Stein K, Han SW. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | He M, Yang T, Wang Y, Wang M, Chen X, Ding D, Zheng Y, Chen H. Immune Checkpoint Inhibitor-Based Strategies for Synergistic Cancer Therapy. Adv Healthc Mater. 2021;10:e2002104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 671] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 10. | Yu JH, Xiao BY, Tang JH, Li DD, Wang F, Ding Y, Han K, Kong LH, Ling YH, Mei WJ, Hong ZG, Liao LE, Yang WJ, Pan ZZ, Zhang XS, Jiang W, Ding PR. Efficacy of PD-1 inhibitors for colorectal cancer and polyps in Lynch syndrome patients. Eur J Cancer. 2023;192:113253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Wan G, Chen W, Khattab S, Roster K, Nguyen N, Yan B, Rajeh A, Seo J, Rashdan H, Zubiri L, Hadfield MJ, Demehri S, Yu KH, Lotter W, Gusev A, LeBoeuf NR, Reynolds KL, Kwatra SG, Semenov YR. Multi-organ immune-related adverse events from immune checkpoint inhibitors and their downstream implications: a retrospective multicohort study. Lancet Oncol. 2024;25:1053-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Reference Citation Analysis (0)] |