Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105664
Revised: March 25, 2025
Accepted: May 12, 2025
Published online: June 15, 2025
Processing time: 125 Days and 5.3 Hours
RPF2 is a crucial factor in ribosome synthesis, which has been linked to the deve
To explore the role and mechanism of RPF2 in the pathogenesis of Helicobacter pylori (H. pylori) infection.
GES-1 was co-cultured with H. pylori in vitro to detect changes in the expression of RPF2. Overexpression and silencing of RPF2 were performed. Quantitative real-time polymerase chain reaction (q-PCR) and Western blot (WB) were used to determine mRNA and protein expression of RPF2, protein kinase B (AKT)/mam
H. pylori facilitated RPF2 expression and triggered AKT/mTOR signaling path
RPF2 plays a significant role in the pathogenic mechanism of H. pylori infection and may be useful in the detection and management of gastric cancer caused by H. pylori infection.
Core Tip: Helicobacter pylori (H. pylori) has a profound impact on gastric cancer development. RPF2 is overexpressed in cancer and can enhance cancer cell proliferation. However, the mechanism of RPF2 in the pathogenesis of H. pylori infection remains largely unknown. We found that H. pylori infection increased RPF2 expression, affecting the malignant behavior of gastric epithelial cells by regulating the protein kinase B/mammalian target of rapamycin pathway. This study is the first to explore the mechanism of RPF2 in the pathogenesis of H. pylori infection.
- Citation: Hua YQ, Guo KX, Ni P, Wang D, An TY, Gao YY, Zhang RG. RPF2 regulates the protein kinase B/mammalian target of rapamycin pathway in the pathogenesis of Helicobacter pylori. World J Gastrointest Oncol 2025; 17(6): 105664
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105664.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105664
Gastric cancer is one of the most common tumors in human beings with the fifth highest incidence and mortality rate among the causes of death worldwide and the highest prevalence in eight countries[1,2]. Among them, Helicobacter pylori (H. pylori) infection can be effectively prevented and treated as one of the main causes of gastric cancer development[3,4].
H. pylori is a microaerobic bacterium that stimulates leukocytes to secrete large amounts of cytokines to participate in immune and inflammatory responses. When an immune or inflammatory response occurs, the expression and function of the relevant inhibitory factors may become dysregulated and cause the persistence of H. pylori-induced gastric inflammation, which can lead to the development of gastric cancer[5]. The colonization of H. pylori in human stomach tissue is affected by many factors. Interactions between virulence factors, phase, variable genes, and host genetics collectively determine the outcome of infection, with specific bacterial virulence factors more likely to cause severe gastric disease when co-occurring with the genetic background of susceptible hosts, and this complex interplay makes the outcome of H. pylori infection significantly different among individuals[6].
Epithelial-mesenchymal transition (EMT) is the process by which epithelial cells acquire mesenchymal characteristics[7,8]. The occurrence of EMT contributes to embryogenesis and is essential for physiological processes such as wound healing; its pathologic reactivation plays a vital role in diseases such as organ fibrosis and cancer progression to meta
The AKT/mammalian target of rapamycin (mTOR) pathway is intricately involved in EMT progression[14,15]. In the pathogenic process of H. pylori infection, it can induce Dickkopf-related protein (DKK) 1 activation, promote the inte
RPF2, a protein-coding gene belonging to the BRIX family, can participate in the assembly of ribosomal large subunits and is required for the maturation of the 60S ribosomal subunit[18-20]. Ribosome biosynthesis is closely related to the development of many cancers, including gastric cancer[21-23]. Studies have shown that increased ribosome content in epithelial cells can promote cell migration after cancerous transformation[24]. RRS1, which forms a ribosomal subunit together with RPF2, is positively correlated with the development of various cancers[25-31]. In addition, studies have shown that RPF2 is highly expressed in patients with hepatocellular carcinoma, and its expression level correlates with hepatocellular carcinoma tumor node metastasis stage, tumor size, tumor differentiation, and vascular invasion[32]. In colorectal cancer, RPF2 triggers EMT by activating the AKT/GSK-3β signaling pathway in colon cancer cells, thereby enhancing the migratory and invasive capabilities of these cells[33]. However, the role and mechanism of RPF2 in H. pylori pathogenesis are still unclear.
Therefore, we investigated the role and mechanism of H. pylori infection on RPF2, the AKT/mTOR pathway, and EMT in gastric epithelial cells. This paper establishes the foundation for further defining the pathogenic mechanism of H. pylori infection and the identification of biological targets for early diagnosis and treatment.
H. pylori strain (MEL-HP27, Hp27) was isolated and cultured from the gastric tissue specimens of patients with chronic atrophic gastritis in Zhengzhou, identified for preservation, and cultured with Columbia blood agar medium in a constant temperature incubator at 37 °C, 50 mL/L carbon dioxide. The gastric mucosal epithelial cell line GES-1 was cultured in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin in a constant temperature incubator at 37 °C, 50 mL/L carbon dioxide.
H. pylori Hp27 was co-cultured with GES-1 for 24 hours at a multiplicity of infection of 100:1.
Total RNA was extracted from cells using Trizol reagent according to the manufacturer’s instructions. SYBR quantitative real-time polymerase chain reaction (qPCR) master mix was used to conduct q-PCR following reverse transcription per the manufacturer’s guidelines.
Proteins were isolated from cell lines using radio immunoprecipitation assay buffer with protease and phosphatase inhibitors. Protein samples were characterized using a bicinchoninic acid assay kit and then heated at 98 °C for 15 minutes. Gel electrophoresis was performed using 30 μg protein and then transferred to a polyvinylidene difluoride membrane. The membranes were labeled with primary antibody and incubated in a blocking buffer containing secondary antibody for 1 hour.
The RPF2 overexpression plasmid was constructed (by Shenzhen BGI Gene Technology Co., LTD, China) and transfected into GES-1 cells. The overexpression cell model was divided into four groups: PcDNA3.1 (+), pcDNA3.1 (+) + Hp27, pcDNA-RPF2 and pcDNA-RPF2 + Hp27. RPF2 small interfering (si) RNA was designed (by Heyuan Biotechnology Co., LTD, China) and transfected. The silencing cell model was divided into four groups: Si-negative control (NC), si-NC + Hp27, si-RPF2 and si-RPF2 + Hp27. Plasmids and siRNAs were transfected into GES-1 cells using Lipofectamine 3000 Reagent (Invitrogen) and opti-MEM (Gibco).
A 96-well plate was inoculated with 1500 cells/well. Cell viability was assayed by mixing cell counting kit 8 (CCK8) reagent with 100 μL of 10% DMEM.
According to the experimental grouping, a 200 μL sterile lance tip was used in the middle of each well to make a hard scratch along the vertical direction of the straightedge, and 2 mL of 2% antiserum-free cell culture medium was added to each well and incubated.
Gastric cancer tissues and the paracancerous tissues were sent to Aifang Biological Company for detection.
ImageJ was used to process imaging data. Differences between treatments were analyzed by t-test or analysis of variance. All statistical analyses were performed using GraphPad Prism 9.0, and the data were expressed as the mean ± SE. Differences were considered statistically significant when P < 0.05.
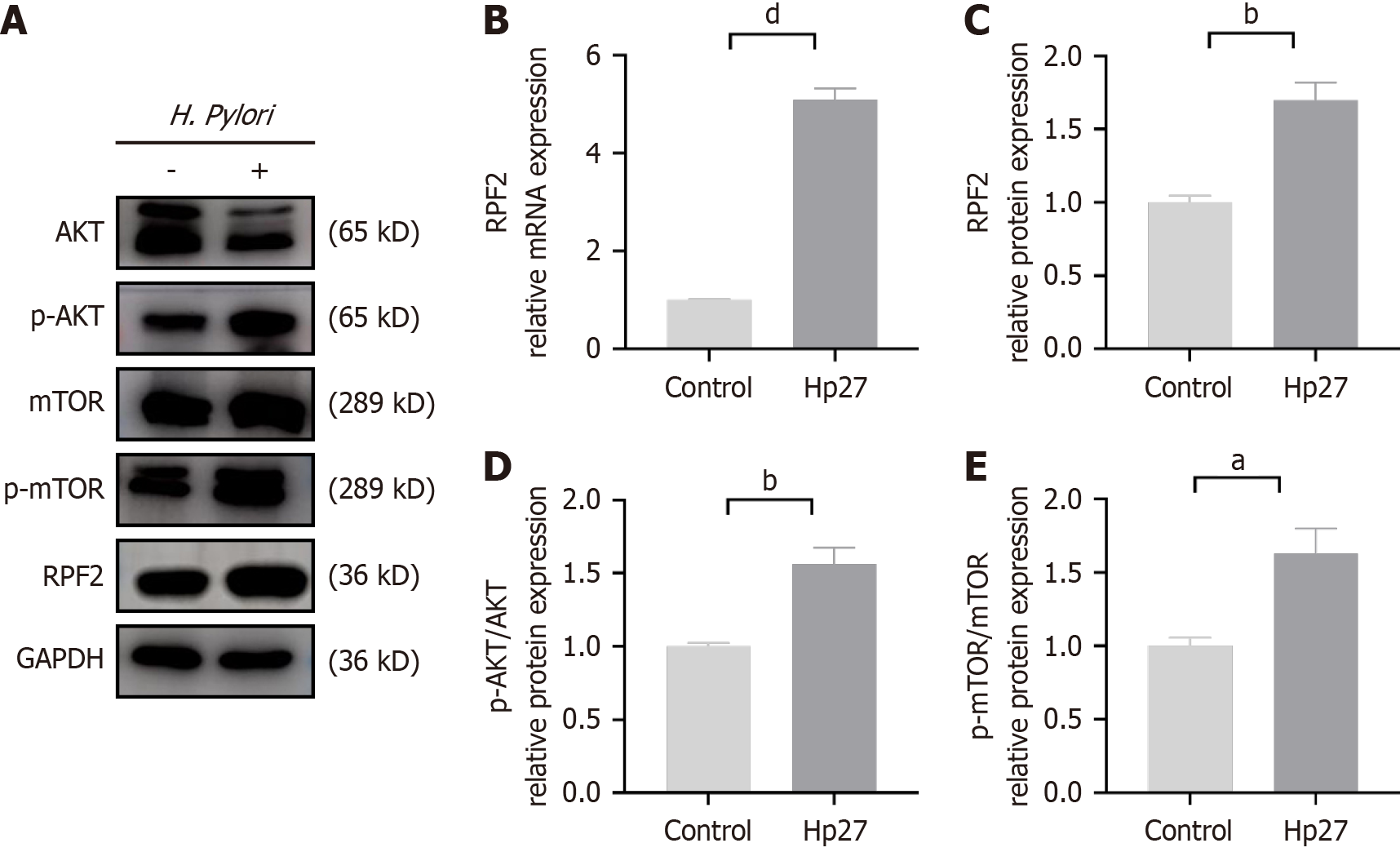
To verify the difference in RPF2 expression after H. pylori infection, we co-cultured H. pylori with GES-1 cells and per
To test the effect of H. pylori infection on the AKT/mTOR pathway, we performed immunoblotting. H. pylori infection resulted in increased phospho-AKT (p-AKT)/p-mTOR expression compared to the uninfected group (Figure 1A, D and E).
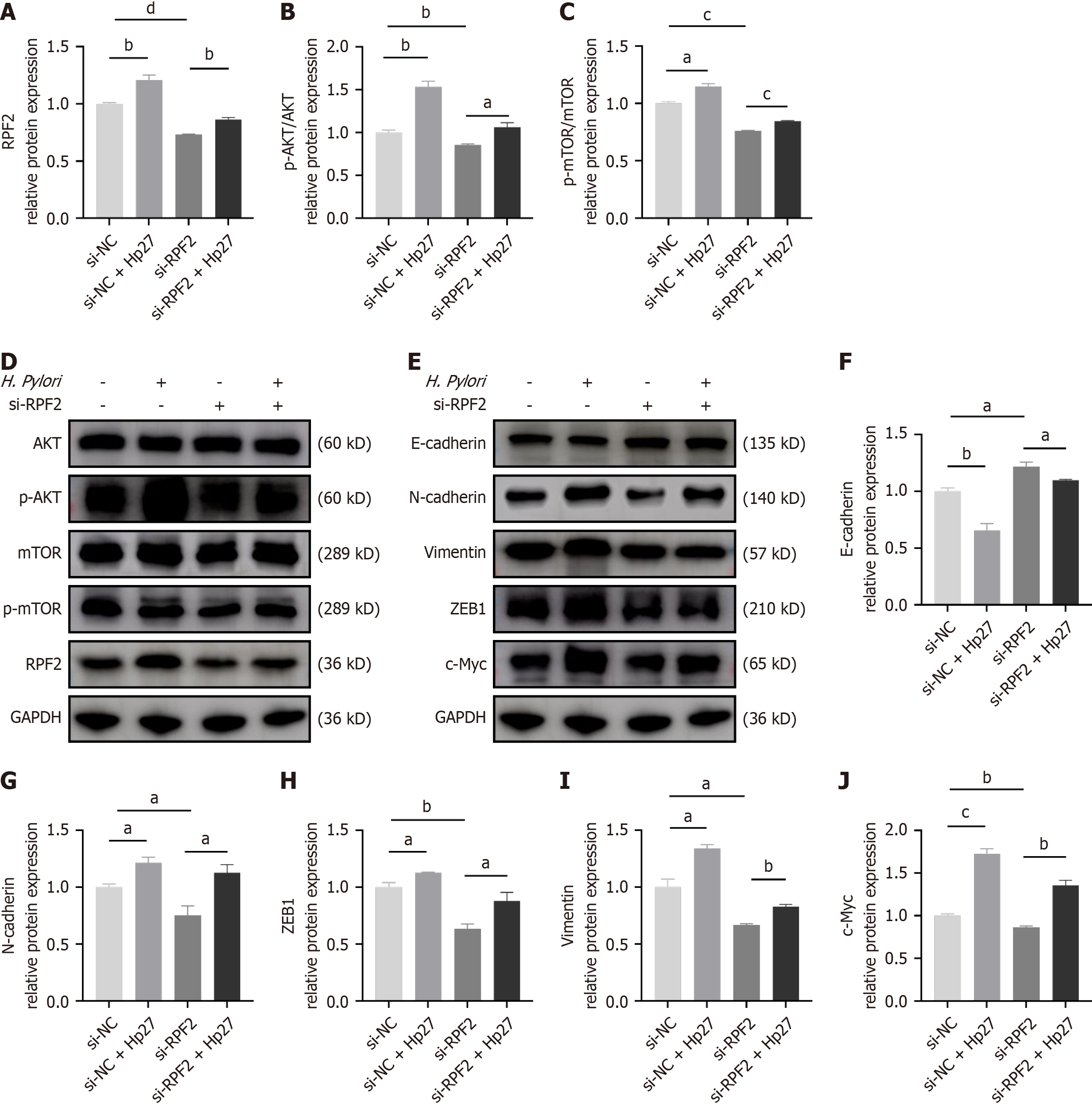
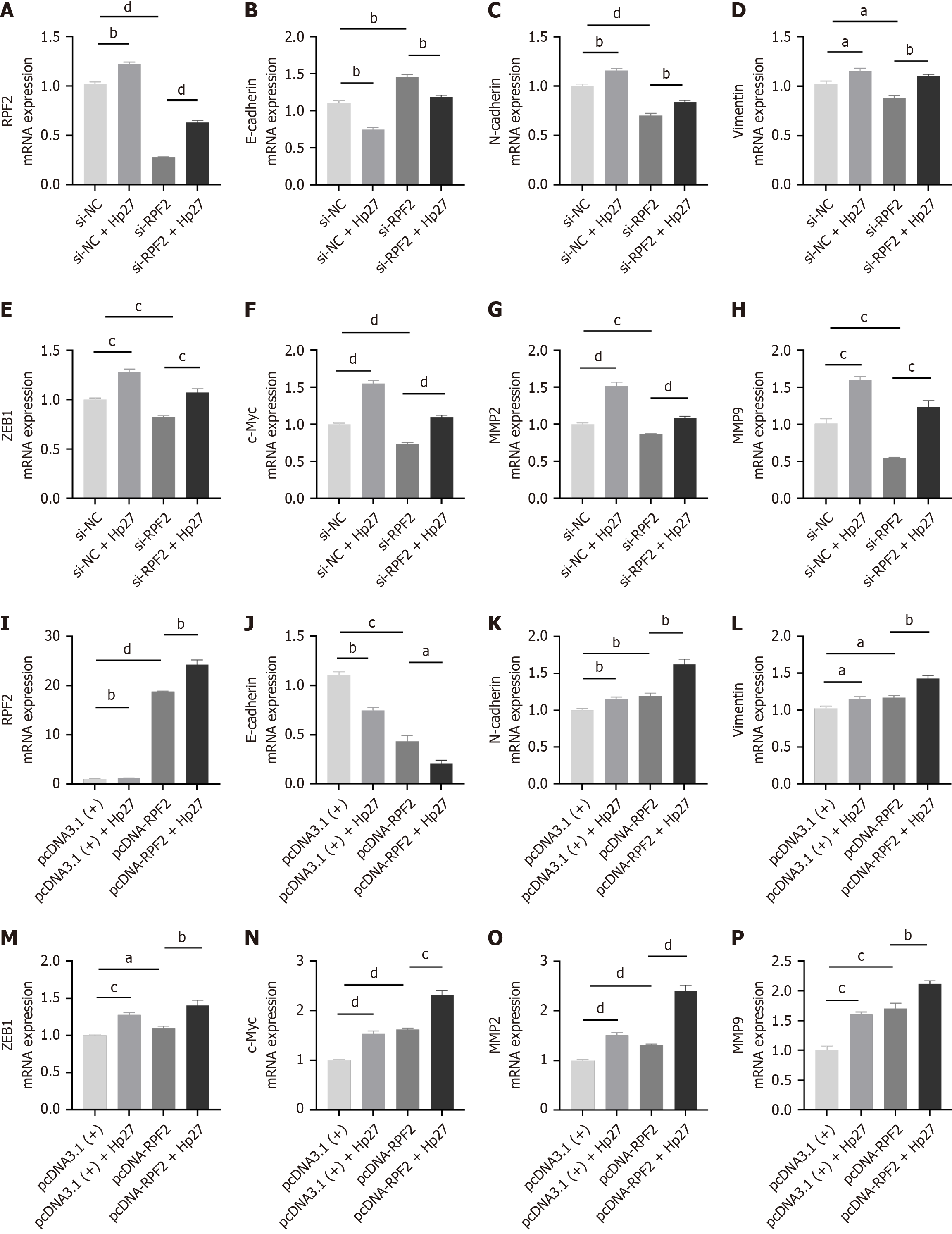
To verify the role of RPF2 in the AKT/mTOR pathway, we generated a cell model for silencing RPF2. We confirmed knock down at the protein level by Western blotting. We divided the cells into four groups: si-NC, si-NC + Hp27, si-RPF2, and si-RPF2 + Hp27. We performed Western blotting to detect protein expression of E-cadherin, N-cadherin, and vimentin and q-PCR to measure ZEB1, and c-Myc mRNA expression. H. pylori infection activated the AKT/mTOR pathway, promoted EMT, down-regulated E-cadherin, and up-regulated N-cadherin, vimentin, ZEB1, and c-Myc com
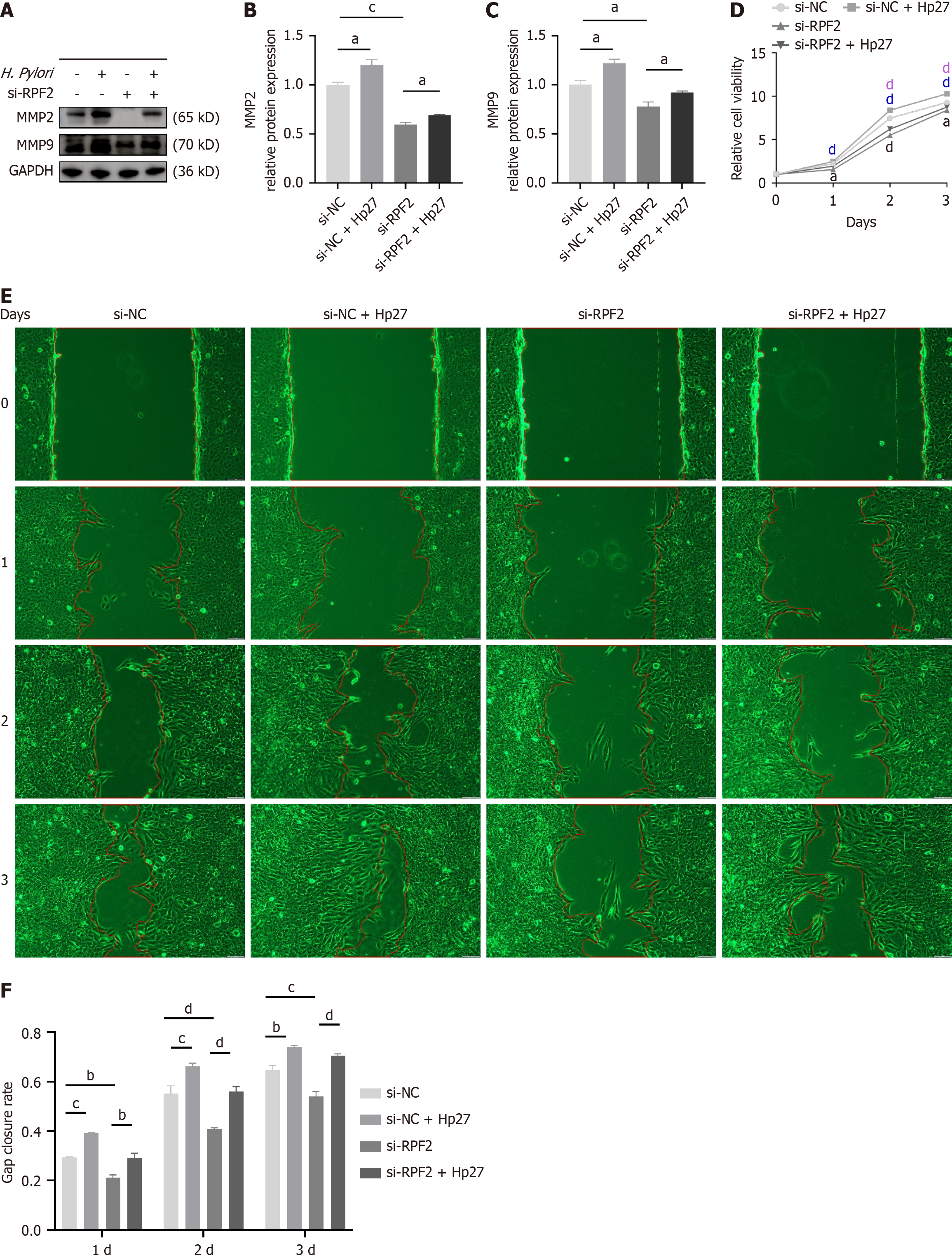
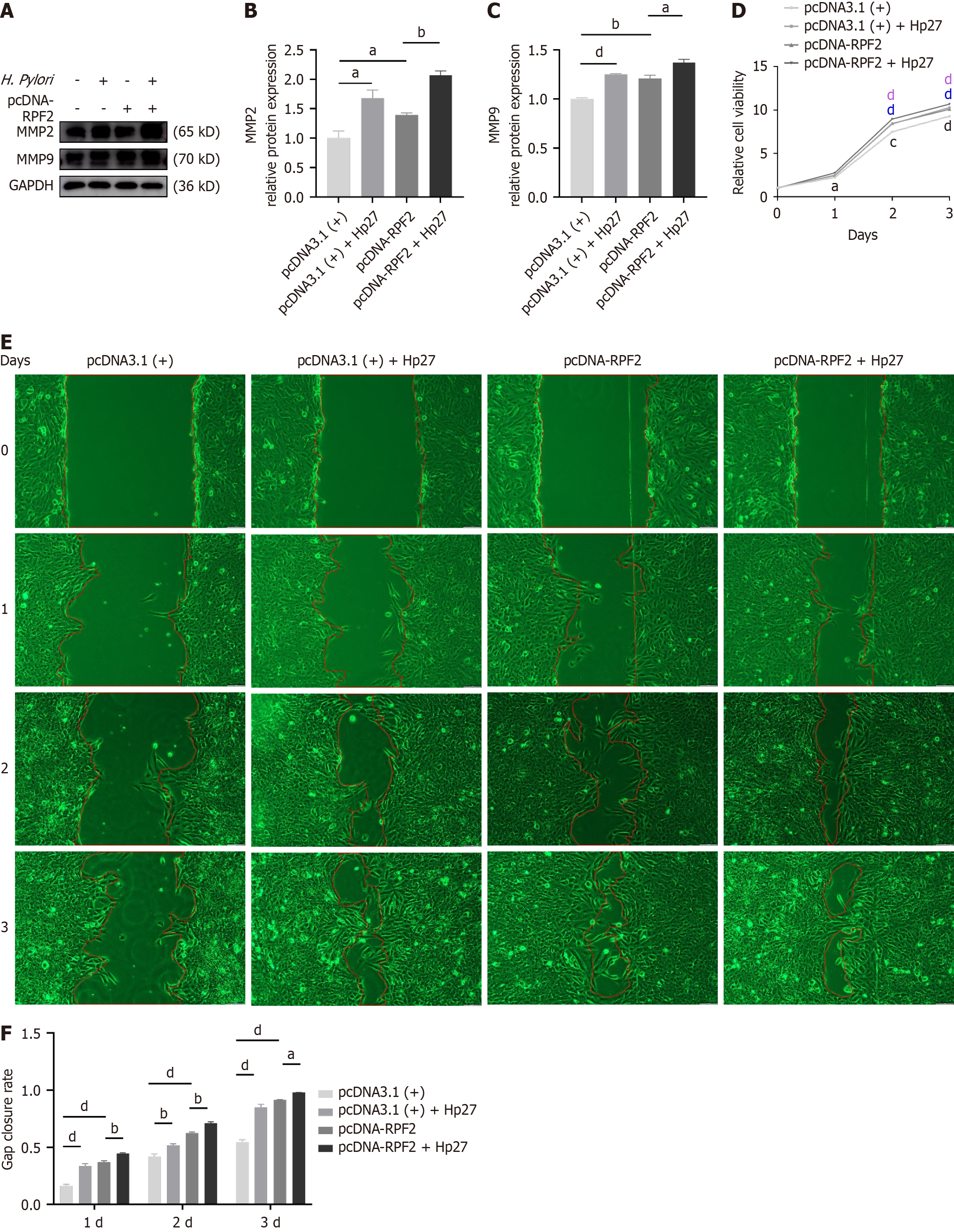
Using the same treatment groupings as above, we detected differences in the expression of RPF2, MMP2, and MMP9 at the mRNA and protein levels. Relative to the control group, the H. pylori-infected group exhibited upregulated mRNA and protein expressions of RPF2, MMP2, and MMP9, whereas knockdown of RPF2 resulted in decreased expression of RPF2, MMP2, and MMP9 (Figure 3G and H and Figure 4A-C); treatment of si-RPF2 cells with H. pylori resulted in increa
CCK8 assays demonstrated that cell viability was elevated in the H. pylori group compared to the control group and decreased in the si-RPF2 group (Figure 4D); cell viability was significantly elevated in the si-RPF2 + Hp27 group com
The results of scratch experiments showed that the migration ability of cells after H. pylori infection was significantly elevated compared to the control group, and the migration ability of cells in the si-RPF2 group was reduced (Figure 4E and F); the migration ability of the si-RPF2 + H. pylori group showed an upward trend compared to the si-RPF2 group (Figure 4E and F).
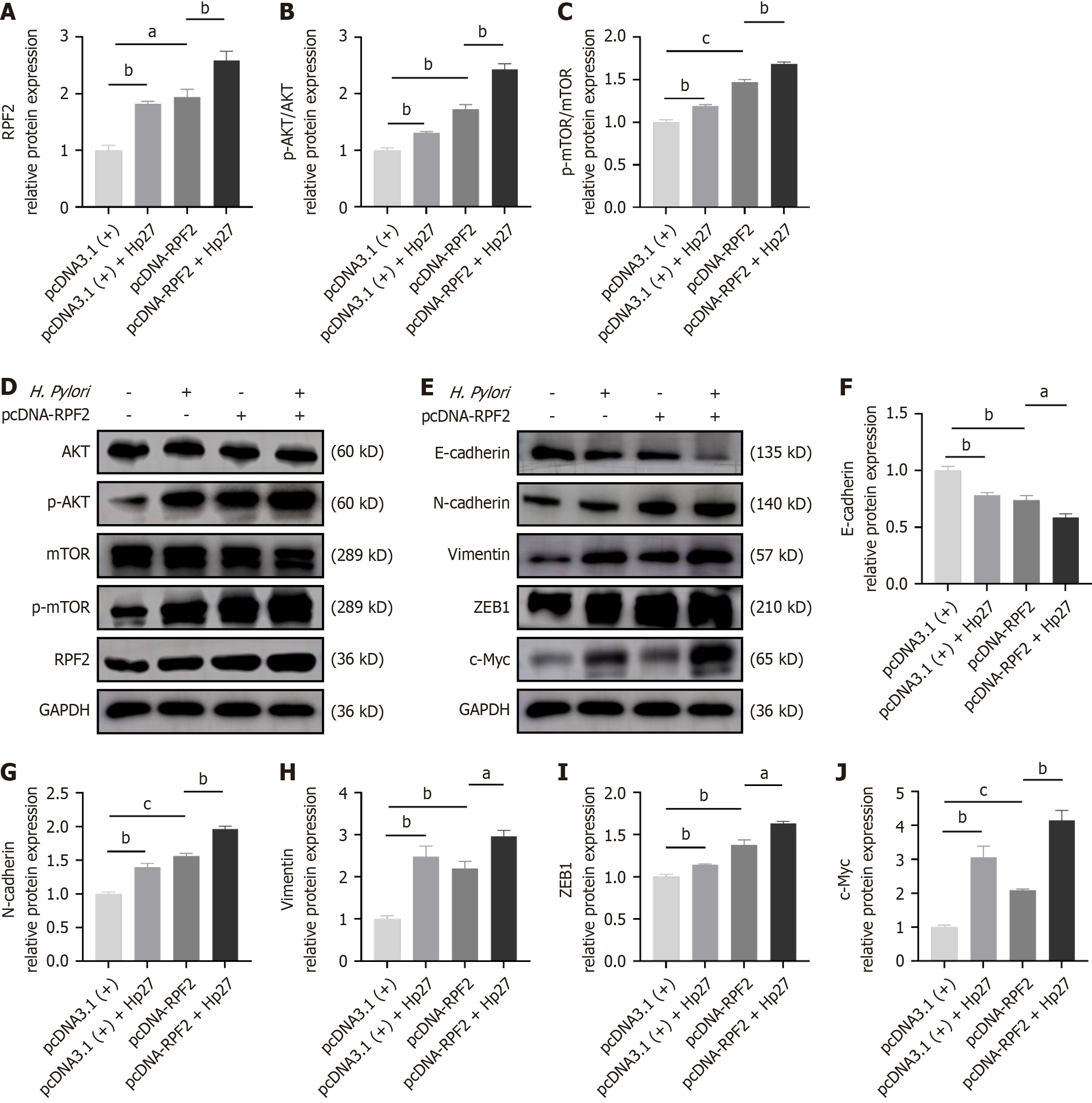
We generated cell models overexpressing RPF2 and divided cells into four groups: pcDNA3.1 (+), pcDNA3.1 (+) + Hp27, pcDNA-RPF2, and pcDNA-RPF2 + Hp27. We detected the expression of E-cadherin, N-cadherin, and vimentin by Western blotting and ZEB1 and c-Myc by q-PCR. RPF2 overexpression promoted the expression of p-AKT/p-mTOR proteins and EMT, and resulted in decreased E-cadherin expression and increased N-cadherin, vimentin, ZEB1, and c-Myc expression compared to the control group (Figure 5 and Figure 3I-N).
We examined the differences in MMP2 and MMP9 the mRNA and protein expression levels between each group. MMP2 and MMP9 mRNA and protein levels increased in the RPF2 group relative to the control group (Figure 3O and P and Figure 6A-C). The results of CCK8 and wound healing assays showed that cell viability was increased and migration was significantly increased after RPF2 overexpression compared to the control group (Figure 6D-F).
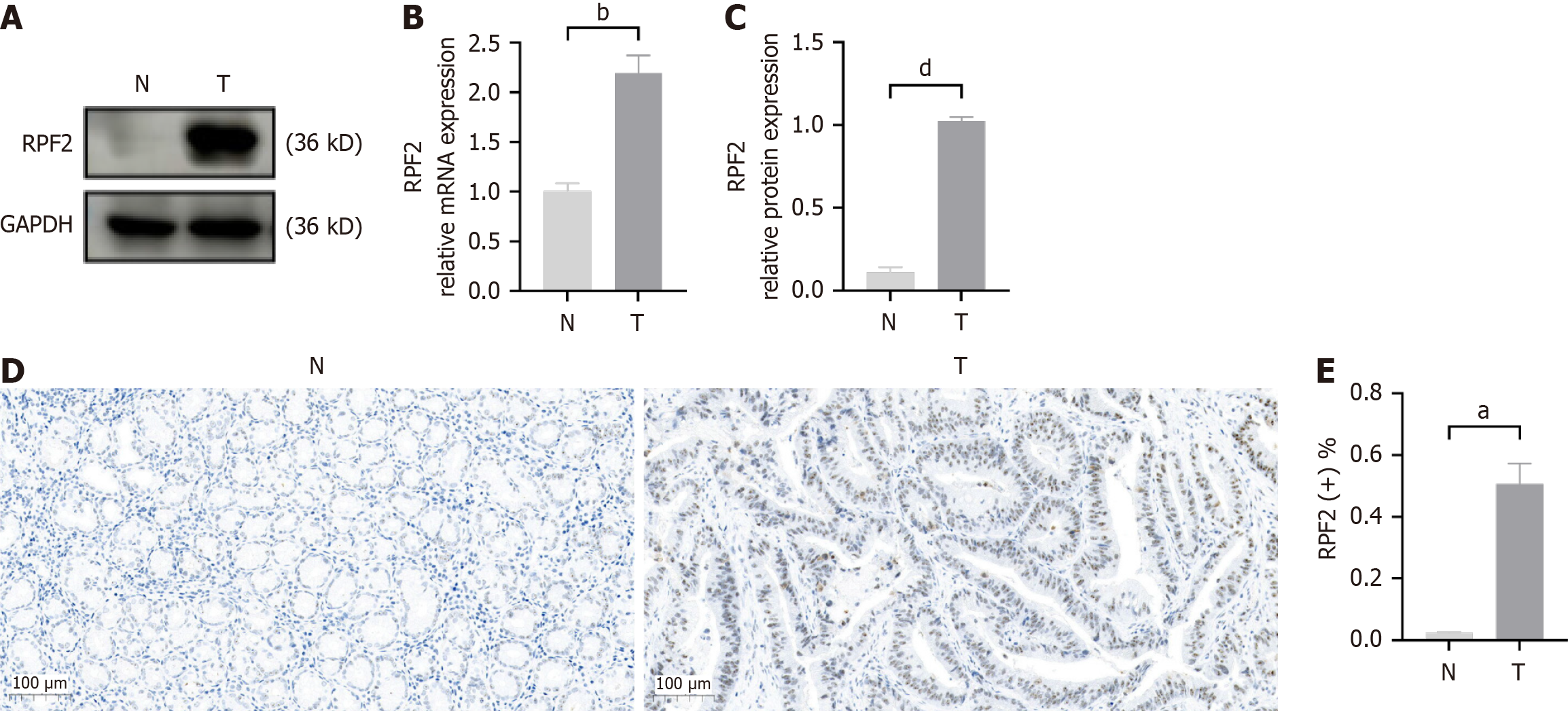
To verify the higher expression of RPF2 in the pathogenic process of H. pylori, we isolated mRNA and protein from cancer tissues and paracancerous tissues and found that RPF2 expression was significantly elevated in cancer tissues (Figure 7A-C). Immunohistochemistry results indicated that RPF2 was overexpressed in gastric cancer tissues, and RPF2 protein was primarily located in the nucleus, with a small cytoplasmic pool (Figure 7D and E).
H. pylori is one of the major causes of gastric carcinogenesis, and the increase of its drug resistance urges the need for more sensitive assays and markers to H. pylori infectious gastric cancer. This research represents the initial exploration into how H. pylori infection affects RPF2 expression and influences the proliferative and metastatic behaviors of gastric epithelial cells. Our data suggest that RPF2 may activate the AKT/mTOR pathway in the context of H. pylori infection, thereby facilitating EMT progression and increased tumor marker expression. These data might offer valuable insights for investigations into the early diagnosis and intervention of H. pylori-related gastric cancer.
Co-culturing H. pylori with gastric epithelial cells revealed that the RPF2 mRNA expression level significantly increased after H. pylori intervention. Immunoblotting results demonstrated that RPF2 protein levels increased after H. pylori infec
Numerous studies have shown that the AKT/mTOR pathway is a common oncogenic pathway indispensable for many biological processes such as cell growth, metastasis, survival, and metabolism[34-36]. mTORC2 can regulate cellular actin skeleton remodeling and EMT by regulating the phosphorylation status of protein kinase C and activating AKT. In addition, AKT activation leads to the accumulation of Snail in the nucleus, which triggers Twist expression, decreasing E-cadherin expression, which further contributes to actin cytoskeleton remodeling[14]. Studies have shown that CCNB2 is highly expressed in gastric cancer patients, and RNA sequencing results show that CCNB2 is related to PI3K/AKT signaling pathway activation[37]. The polyamidoamine (PAMAM)-wrapped miR-144 (PAMAM/miR-144) nanoparticle system significantly reduced EMT by targeting the mTOR signaling pathway in HGC-27 cells and significantly inhibited the growth of subcutaneous gastric cancer xenografts in nude mice[38]. These findings align with the results of our study on the AKT/mTOR pathway. This paper, for the first time, establishes the role of RPF2 in the AKT/mTOR pathway activation by H. pylori. Constructing a silencing/overexpression model of RPF2 found that RPF2 activated the AKT/mTOR pathway to induce gastric mucosal lesions in H. pylori; caused differential expression of EMT-related proteins, including E-cadherin, N-cadherin, vimentin, ZEB1, and c-Myc; and facilitated the development of EMT. The development of EMT is widely regarded as a fundamental mechanism for the development of malignant tumors including gastric, lung, and breast cancers. In this context, E-cadherin, a critical intercellular adhesion molecule, helps preserve the epithelial cell phenotype and promotes cell-cell adhesion. Additionally, N-cadherin, vimentin, and ZEB1, which are key regulators of the EMT process in tumor cells, can facilitate EMT by downregulating E-cadherin expression[7].
Ribosomes function as translators, and their biogenesis is a multistep process that begins in the nucleolus and ends in the cytoplasm. Any alteration in this process may lead to overactivation of ribosome biogenesis[32,39,40]. RPF2 promotes ribosome synthesis and enhances glycolysis through activation of the AKT/GSK-3β signaling pathway in colorectal cancer tissues and cell lines, promotes EMT, and enhances cell migration and invasion in vitro and in vivo, which leads to colon carcinogenesis[33,41]. In addition, RPF2 may promote drug resistance in colorectal cancer through the CARM1-MYCN pathway[42]. For intracellular RPF2 silencing/overexpression, q-PCR and immunoblotting showed that overexpression of RPF2 increased MMP2 and MMP9 expression, and CCK8 and wound healing assay showed that RPF2 overexpression \ significantly enhanced the proliferation ability and migration ability of gastric epithelial cells, which was enhanced after H. pylori infection, whereas RPF2 downregulation had the opposite effect. In clinical gastric cancer tissues, RPF2 expression was increased, and this finding is consistent with our in vitro studies suggesting that RPF2 may be a pro-cancer factor.
In summary, the present study demonstrated that RPF2 was highly expressed after H. pylori infection and promoted EMT through activation of the AKT/mTOR pathway, which enhanced cell proliferation and migration. RPF2 could serve as a potential biomarker for evaluating gastric cancer cell viability and migration capacity after H. pylori infection. Additionally, it might represent a novel therapeutic target for curbing the local invasion and distant metastasis of H. pylori-infected gastric cancer via small molecule inhibitors or siRNA.
RPF2 exhibited elevated expression in gastric cancer tissues, which activated the AKT/mTOR pathway in gastric epithelial cells, facilitated EMT in H. pylori-infected cells, and improved cell viability and migration. This study suggests for the first time that RPF2 has a vital role in the pathogenic mechanism of H. pylori infection, which may be of reference value for early diagnosis and therapeutic research of H. pylori-infected gastric cancer.
We would like to thank Fan XS, Liang TY and Cheng C (All from the Department of Epidemiology, School of Public Health, Zhengzhou University) for their assistance in experimental manipulation and valuable comments in this study.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8084] [Article Influence: 8084.0] [Reference Citation Analysis (2)] |
| 2. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1227] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 3. | Normile D. Eliminating a gut microbe could slash gastric cancers. Science. 2024;385:585-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Senchukova MA. Helicobacter Pylori and Gastric Cancer Progression. Curr Microbiol. 2022;79:383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Jafarzadeh A, Jafarzadeh Z, Nemati M, Yoshimura A. The Interplay Between Helicobacter pylori and Suppressors of Cytokine Signaling (SOCS) Molecules in the Development of Gastric Cancer and Induction of Immune Response. Helicobacter. 2024;29:e13105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Bhattacharjee A, Sahoo OS, Sarkar A, Bhattacharya S, Chowdhury R, Kar S, Mukherjee O. Infiltration to infection: key virulence players of Helicobacter pylori pathogenicity. Infection. 2024;52:345-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1891] [Article Influence: 270.1] [Reference Citation Analysis (0)] |
| 8. | Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 669] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 9. | Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 2021;40:e108647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 445] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 10. | Jamal Eddin TM, Nasr SMO, Gupta I, Zayed H, Al Moustafa AE. Helicobacter pylori and epithelial mesenchymal transition in human gastric cancers: An update of the literature. Heliyon. 2023;9:e18945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Fang Z, Zhang W, Wang H, Zhang C, Li J, Chen W, Xu X, Wang L, Ma M, Zhang S, Li Y. Helicobacter pylori promotes gastric cancer progression by activating the TGF-β/Smad2/EMT pathway through HKDC1. Cell Mol Life Sci. 2024;81:453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | He B, Hu Y, Wu Y, Wang C, Gao L, Gong C, Li Z, Gao N, Yang H, Xiao Y, Yang S. Helicobacter pylori CagA elevates FTO to induce gastric cancer progression via a "hit-and-run" paradigm. Cancer Commun (Lond). 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Chen ZW, Dong ZB, Xiang HT, Chen SS, Yu WM, Liang C. Helicobacter pylori CagA protein induces gastric cancer stem cell-like properties through the Akt/FOXO3a axis. J Cell Biochem. 2024;125:e30527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 333] [Article Influence: 111.0] [Reference Citation Analysis (1)] |
| 15. | An TY, Hu QM, Ni P, Hua YQ, Wang D, Duan GC, Chen SY, Jia B. N6-methyladenosine modification of hypoxia-inducible factor-1α regulates Helicobacter pylori-associated gastric cancer via the PI3K/AKT pathway. World J Gastrointest Oncol. 2024;16:3270-3283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (4)] |
| 16. | Luo M, Chen YJ, Xie Y, Wang QR, Xiang YN, Long NY, Yang WX, Zhao Y, Zhou JJ. Dickkopf-related protein 1/cytoskeleton-associated protein 4 signaling activation by Helicobacter pylori-induced activator protein-1 promotes gastric tumorigenesis via the PI3K/AKT/mTOR pathway. World J Gastroenterol. 2022;28:6769-6787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (3)] |
| 17. | Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020;262:118513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 18. | Castillo Duque de Estrada NM, Thoms M, Flemming D, Hammaren HM, Buschauer R, Ameismeier M, Baßler J, Beck M, Beckmann R, Hurt E. Structure of nascent 5S RNPs at the crossroad between ribosome assembly and MDM2-p53 pathways. Nat Struct Mol Biol. 2023;30:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Thoms M, Mitterer V, Kater L, Falquet L, Beckmann R, Kressler D, Hurt E. Suppressor mutations in Rpf2-Rrs1 or Rpl5 bypass the Cgr1 function for pre-ribosomal 5S RNP-rotation. Nat Commun. 2018;9:4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Asano N, Kato K, Nakamura A, Komoda K, Tanaka I, Yao M. Structural and functional analysis of the Rpf2-Rrs1 complex in ribosome biogenesis. Nucleic Acids Res. 2015;43:4746-4757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Zhang Y. LncRNA-encoded peptides in cancer. J Hematol Oncol. 2024;17:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 22. | Susanto TT, Hung V, Levine AG, Kerr CH, Yoo Y, Chen Y, Oses-Prieto JA, Fromm L, Fujii K, Wernig M, Burlingame AL, Ruggero D, Barna M. RAPIDASH: A tag-free enrichment of ribosome-associated proteins reveals compositional dynamics in embryonic tissues and stimulated macrophages. bioRxiv. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Park J, Wu J, Szkop KJ, Jeong J, Jovanovic P, Husmann D, Flores NM, Francis JW, Chen YC, Benitez AM, Zahn E, Song S, Ajani JA, Wang L, Singh K, Larsson O, Garcia BA, Topisirovic I, Gozani O, Mazur PK. SMYD5 methylation of rpL40 links ribosomal output to gastric cancer. Nature. 2024;632:656-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Ebright RY, Lee S, Wittner BS, Niederhoffer KL, Nicholson BT, Bardia A, Truesdell S, Wiley DF, Wesley B, Li S, Mai A, Aceto N, Vincent-Jordan N, Szabolcs A, Chirn B, Kreuzer J, Comaills V, Kalinich M, Haas W, Ting DT, Toner M, Vasudevan S, Haber DA, Maheswaran S, Micalizzi DS. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science. 2020;367:1468-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 25. | Hua Y, Song J, Peng C, Wang R, Ma Z, Zhang J, Zhang Z, Li N, Hou L. Advances in the Relationship Between Regulator of Ribosome Synthesis 1 (RRS1) and Diseases. Front Cell Dev Biol. 2021;9:620925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Wang R, Peng C, Song J, Hua Y, Wu Q, Deng L, Cao Y, Zhang J, Zhang L, Wu L, Hou L. Downregulated RRS1 inhibits invasion and metastasis of BT549 through RPL11-c-Myc-SNAIL axis. Int J Oncol. 2022;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Cao P, Yang A, Li P, Xia X, Han Y, Zhou G, Wang R, Yang F, Li Y, Zhang Y, Cui Y, Ji H, Lu L, He F, Zhou G. Genomic gain of RRS1 promotes hepatocellular carcinoma through reducing the RPL11-MDM2-p53 signaling. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Cong P, Tian L, Zheng Y, Zhang H, Liu Q, Wu T, Zhang Q, Wu H, Huang X, Xiong L. Genomic gain/methylation modification/hsa-miR-132-3p increases RRS1 overexpression in liver hepatocellular carcinoma. Cancer Sci. 2023;114:4329-4342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Chen F, Jin Y, Feng L, Zhang J, Tai J, Shi J, Yu Y, Lu J, Wang S, Li X, Chu P, Han S, Cheng S, Guo Y, Ni X. RRS1 gene expression involved in the progression of papillary thyroid carcinoma. Cancer Cell Int. 2018;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Liu C, Cao Y, Liu L, Sun F, Hou L. RRS1 knockdown inhibits the proliferation of neuroblastoma cell via PI3K/Akt/NF-κB pathway. Pediatr Res. 2025;97:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Yan X, Wu S, Liu Q, Zhang J. RRS1 Promotes Retinoblastoma Cell Proliferation and Invasion via Activating the AKT/mTOR Signaling Pathway. Biomed Res Int. 2020;2020:2420437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | An Y, Xia Y, Wang Z, Jin GZ, Shang M. Clinical significance of ribosome production factor 2 homolog in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2024;48:102289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Li H, Hu X, Cheng C, Lu M, Huang L, Dou H, Zhang Y, Wang T. Ribosome production factor 2 homolog promotes migration and invasion of colorectal cancer cells by inducing epithelial-mesenchymal transition via AKT/Gsk-3β signaling pathway. Biochem Biophys Res Commun. 2022;597:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol. 2022;85:69-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 362] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 35. | Shao Q, Zhang Z, Cao R, Zang H, Pei W, Sun T. CPA4 Promotes EMT in Pancreatic Cancer via Stimulating PI3K-AKT-mTOR Signaling. Onco Targets Ther. 2020;13:8567-8580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Wang J, Jiang C, Li N, Wang F, Xu Y, Shen Z, Yang L, Li Z, He C. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11:682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 37. | Hu M, Tao P, Wang Y, Zhu C, Ma Y, Liu X, Cai H. Knockdown of CCNB2 inhibits the tumorigenesis of gastric cancer by regulation of the PI3K/Akt pathway. Sci Rep. 2025;15:5703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Qian Y, Zhu D, Xu Q, Wang Y, Chen X, Hua W, Xi J, Lu F. PAMAM/miR-144 nanocarrier system inhibits the migration of gastric cancer by targeting mTOR signal transduction pathway. Colloids Surf B Biointerfaces. 2025;249:114492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Elhamamsy AR, Metge BJ, Alsheikh HA, Shevde LA, Samant RS. Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance. Cancer Res. 2022;82:2344-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 166] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 40. | Chen J, Zhang J, Zhang Z. Upregulation of GTPBP4 Promotes the Proliferation of Liver Cancer Cells. J Oncol. 2021;2021:1049104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Jiang C, Sun L, Wen S, Tian Y, Xu C, Xu Q, Xue H. BRIX1 promotes ribosome synthesis and enhances glycolysis by selected translation of GLUT1 in colorectal cancer. J Gene Med. 2024;26:e3632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Lu M, Hu X, Cheng C, Zhang Y, Huang L, Kong X, Li Z, Zhang Q, Zhang Y. RPF2 mediates the CARM1-MYCN axis to promote chemotherapy resistance in colorectal cancer cells. Oncol Rep. 2024;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |