Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.104015
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: June 15, 2025
Processing time: 188 Days and 15.3 Hours
Gastrectomy is the cornerstone of treatment for gastric cancer. Since the introduction of minimally invasive techniques, the main challenge for surgeons has been to achieve the same surgical radicality, adequate lymphadenectomy, and negative resection margins as with the open approach. Previous Eastern trials showed non-inferiority of laparoscopic gastrectomy, whereas Western trials reported a higher number of complications. This may depend on the different eli
Core Tip: Robotic gastrectomy is likely to become the way forward for treating gastric cancer. It offers technical advantages in terms of lower rate of intraoperative blood loss and postoperative complications, with comparable survival and lymph node retrieval to open surgery. Our systematic review of randomized control trials analyzes the potential benefits of robotic systems, sheds light on the remaining uncertainties, and outlines future lines of research. Further evidence is needed to confirm its advantages in postneoadjuvant cases.
- Citation: Marrelli D, Carbone L, Poto GE, Fusario D, Gjoka M, Andreucci E, Piccioni SA, Calomino N, Sandini M, Roviello F. Minimally invasive lymphadenectomy for gastric cancer: Could the robotic approach provide any benefits than laparoscopy? World J Gastrointest Oncol 2025; 17(6): 104015
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/104015.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.104015
The lymphatic route is the main manner by which gastric cancer (GC) spreads, and lymph node involvement strongly correlates with prognosis after potentially curative resection (R0)[1,2]. It is therefore unanimously acknowledged that lymphadenectomy is a critical step in gastric surgery. The extent of lymph node dissection has been a subject of considerable debate for some time. After years of intensive research, hundreds of studies and many clinical trials, the international scientific community has reached a consensus. Most guidelines recommend the D2 lymphadenectomy as the “gold standard” for non-early-stage disease in patients suitable for this procedure, with adequate physiological reserves and a life expectancy sufficient to justify an extensive surgical approach rather than a palliative intervention[3-5].
Indeed, D2 lymphadenectomy could provide at least a potential clinical benefit, if correctly performed: (1) By increasing the number of nodes removed, and achieving adequate disease staging (> 16 nodes) in most cases[6,7]; (2) Removing potentially metastatic nodes with consequent increase of surgical radicality[8-10]; (3) A reduction in GC recurrence during the follow-up; and (4) A possible improvement in long-term survival. It is noteworthy that the Dutch trial demonstrated the cancer-specific survival benefit of D2 in a 15-year follow-up analysis[11]. The D1 and D2 survival curves overlapped until 2.5 years, and then diverged significantly in favor of the D2 procedure in the later follow-up periods. This finding may be indicative of locoregional disease control achieved through adequate lymph node clearance. Similar to the Dutch trial, the recent results of the Italian trial showed the survival benefit of the D2 at 2 years after surgical treatment[12]. Its results confirmed the oncological role of lymph node dissection, as described and standardized by the Japanese authors, in Western patients and led to the inclusion of the D2 procedure in curative gastrectomy for non-metastatic GC[3,4].
Assuming that D2 dissection is the basic principle in the surgical approach to GC, the main issue is its reproducibility with minimally invasive surgery (MIS). We critically evaluate the advantages and limitations of MIS gastrectomy, particularly in oncological outcomes, surgical complexity, and postoperative complications. By reviewing existing randomized controlled trials (RCTs), this article determined whether robotic gastrectomy offers significant benefits or if further randomization in clinical trials is still necessary.
Several trials conducted in East Asia compared laparoscopic vs open gastrectomy; the main surgical and oncological selection criteria of these trials were clinical tumor stage (early/advanced forms), extent of gastrectomy (distal/total gastrectomy), and expertise of the centers involved. Table 1 shows a list of Eastern RCTs (last access in PubMed [MEDLINE] in October 2024). The medical subject headings and keywords “Randomized Controlled Trial,” “Clinical Trial,” “Gastric surgery,” “Gastrectomy,” “Gastric Cancer,” “Laparoscopic,” “Robotic,” “Lymphadenectomy,” and “Minimally Invasive” were investigated and combined to obtain the maximal number of articles. The articles were then screened for the presence of the following defined eligibility criteria according to the PICO format: P - population: Patients enrolled in an RCT; I and C - intervention and comparator: Minimally invasive gastrectomy for GC compared to laparoscopic or open approaches; O - outcomes of interest: Morbidity, lymphadenectomy, survival. Only published RCTs with full text in English language were included. The reference lists of all selected publications were hand searched for additional relevant articles. The following data were recorded and detailed in tables: Mono- or multi-centricity, country of origin, year of publication, study period, sample size, approach, surgical procedure, pre- or peri-operative treatment, pathological tumor-node-metastasis (TNM). Studies were excluded if the rate of D2 lymphadenectomies could not be determined. Additionally, trials analyzing hand-assisted laparoscopic gastrectomy or those enrolling patients undergoing exclusively or predominantly gastrectomy with Billroth I reconstruction were not considered.
| Trial | Ref. | Country | Study period | Patients | Patients/ | Study design | Approach (n) | Surgery (n) | Neo adjuvant (%) | Pathological T (n) | D2 (%)2 | Primary outcomes | Main results | |||||||
| Open | Laparoscopic | Robotic | Distal | Total1 | 1 | 2 | 3 | 4 | x | |||||||||||
| KLASS trial | Kim et al[77], 2010 | South Korea | 2006 to 2007 | 3424 | 24 | cT1N0-1, cT2N0 | 161 | 179 | 0 | 342 | 0 | 0 | / | / | / | / | / | 68.7 | 30-day morbidity and mortality | No significant difference in postoperative complication rates (10.5% in the laparoscopic vs 14.7% in the open group; P = 0.137) and in mortality (1.1% vs 0; P = 0.497) |
| JLSSG0901 | Inaki et al[78], 2015 | Japan | 2009 to 2013 | 1744 | 2 | cT2-4a N0-2 | 89 (not analyzed) | 86 | 0 | 174 | 0 | 0 | 32 | 18 | 19 | 17 | 0 | 86 | Anastomotic leakage or pancreatic fistula in laparoscopic group | Single-arm: 4.7% patients underwent laparoscopic gastrectomy had anastomotic leakage or pancreatic fistula (4/86; 95%CI: 1.3-11.5; 1-sided P = 0.00024) |
| Etoh et al[17], 2023 | 2009 to 2016 | 46 | 2 | cT2-4a N0-2 | 233 | 227 | 0 | 460 | 0 | 0 | 128 | 122 | 123 | 87 | 0 | 100 | 5-year relapse-free survival | Relapse-free survival did not differ between groups: 73.9% in the open and 75.7% in the laparoscopic group (HR = 0.96; 95%CI: 0.72-1.26; P = 0.03) | ||
| Cui | Cui et al[79], 2015 | Japan | 2010 to 2012 | 2704 | 141 | cT1-4a N0-3 | 142 | 128 | 0 | 148 | 122 | 0 | / | / | / | / | / | 100 | Short-term surgical outcome | Laparoscopic group had similar number of harvested lymph nodes (29.3 vs 30.1; P = 0.574), less blood loss, longer operation time and faster recovery (P < 0.05) than open |
| KLASS-01 | Kim et al[80], 2016 | South Korea | 2006 to 2010 | 13834 | 24 | cT1N0-1, cT2N0 | 657 | 726 | 0 | 1360 | 23 | 0 | 11027 | 224 | 41 | 1 | 10 | 60 | 30-day morbidity and mortality | The overall complication rate was significantly lower in the laparoscopic (13%) than open group (19.9%); P = 0.001. Mortality was similar (0.6% vs 0.3%; P = 0.687) |
| Kim et al[14], 2019 | 2006 to 2010 | 13584 | 24 | cT1N0-1, cT2N0 | 645 | 714 | 0 | 1345 | 13 | 0 | 1097 | / | / | / | / | 60 | 5-year overall survival | Overall survival rates were 94.2% in the laparoscopic and 93.3% in the open group; P = 0.64 | ||
| CLASS-01 | Hu et al[81], 2016 | China | 2012 to 2014 | 10394 | 33 | cT1-4a N0-3 | 520 | 519 | 0 | 1015 | 24 | 39.4 | 248 | / | / | / | / | 99.7 | 30-day morbidity and mortality | Postoperative morbidity was 15.2% vs 12.9% (95%CI: -1.9 to 6.6; P = 0.285) and mortality rate was 0.4% vs zero (95%CI: -0.4 to 1.4; P = 0.249) in the laparoscopic and open group, respectively |
| Yu et al[82], 2019 | 2012 to 2014 | 10394 | 33 | cT2-4a N0-3 | 520 | 519 | 0 | 1015 | 24 | 39.4 | 248 | / | / | / | / | 99.7 | 3-year disease-free survival | Disease-free survival did not differ between groups: 76.5% in the laparoscopic and 77.8% in the open group (log-rank P = 0.59; HR = 1.10; 95%CI: 0.84-1.43; P = 0.49) | ||
| Huang et al[16], 2022 | 2012 to 2014 | 10394 | 33 | cT2-4a N0-3 | 520 | 519 | 0 | 1015 | 24 | 39.4 | 248 | / | / | / | / | 99.7 | 5-year overall survival | Overall survival did not differ between groups with each tumor stage: 72.6% in the laparoscopic and 76.3% in the open group (log-rank P = 0.19; HR = 1.17; 95%CI: 0.93-1.48; P = 0.19) | ||
| JCOG 0912 | Katai et al[13], 2017 | Japan | 2010 to 2013 | 9124 | 7.5 | cT1N0-1, cT2N0 | 471 | 441 | 0 | 675 | 14 | 0 | 788 | 83 | 31 | 9 | 1 | 24.9 | Short-term surgical outcome | Operative time was longer in laparoscopic than in open group (278 vs 194 minutes; P < 0.001), while blood loss was smaller (38 vs 115 mL, P < 0.001) |
| Katai et al[18], 2019 | 2010 to 2013 | 9124 | 7.5 | cT1N0-1, cT2N0 | 471 | 441 | 0 | 675 | 1 | 0 | 823 | 69 | 19 | 1 | 0 | 24.9 | 5-year relapse-free survival | 5-year relapse-free survival was 94.0% in the open and 95.1% in the laparoscopic group (HR = 0.84; 90%CI: 0.56-1.27; P = 0.0075) | ||
| Hikage et al[83], 2023 | 2010 to 2013 | 8814 | 7 | cT1N0-1, cT2N0 | 447 | 434 | 0 | 653 | 05 | 0 | / | / | / | / | / | / | Associations between surgery-related factors and the development of late complications | The surgical approach was not a risk factor for any late complications | ||
| Akiyama et al[84], 2023 | 2010 to 2013 | 8154 | 7 | cT1N0-1, cT2N0 | / | / | 0 | 578 | 16 | 0 | 738 | 51 | 20 | 5 | 1 | 17.3 | Frequency and location of lymph node metastases | 10.9% had positive lymph node metastases. For cancer located in middle third of the stomach, metastases were widely located in each lymph node sites; for cancer located in lower third sites No. 4sb and 9 showed no metastasis | ||
| Shi | Shi et al[85], 2018 | China | 2010 to 2012 | 3223 | 133 | cT2-4a N0-3 | 156 | 166 | 0 | 196 | 126 | 0 | 0 | 65 | 257 | 0 | 0 | 100 | Short-term surgical outcomes | Laparoscopic group had longer operation time (P < 0.001), less estimated blood loss (P < 0.001), and less intraoperative transfusion (P = 0.048) than open. The average number of retrieved lymph nodes was 32 in both groups (P = 0.377) |
| Shi et al[86], 2019 | 2010 to 2012 | 3223 | 133 | cT2-4a N0-3 | 156 | 166 | 0 | 196 | 126 | 0 | 0 | 65 | 257 | 0 | 0 | 100 | 5-year overall survival | Overall survival rate was 49.0% in the laparoscopic and 50.7% in the open group; log-rank P = 0.59. No differences in each tumor stages | ||
| COACT 1001 | Park et al[87], 2018 | South Korea | 2010 to 2011 | 195 | 21 | cT2-4a N0-3 | 95 | 100 | 0 | 190 | 5 | 0 | / | / | / | / | / | 45.6 | Noncompliance rate of the lymph node dissection | No significant differences between laparoscopic (47%) and open groups (43.2%); P = 0.648. For clinical stage III disease, the noncompliance rate was 52% vs 25%; P = 0.043 |
| Wang | Wang et al[88], 2019 | China | 2014 to 2017 | 4424 | 26 | cT2-4a N0-3 | 234 | 208 | 0 | 418 | 24 | 0 | 110 | 80 | 136 | 116 | 0 | 99.6 | 30-day morbidity and mortality | No significant differences between laparoscopic (13.1%) and open groups (17.7%); P = 0.174. Independent risk factors were age (OR ≥ 60) and BMI (OR ≥ 25). No operation-related death occurred in both arms |
| Li | Li et al[89], 2019 | China | 2015 to 2017 | 954 | 37 | cT2-4a N0-3 | 47 | 48 | 0 | 92 | 3 | 100 | 187 | 16 | 36 | 18 | 0 | 84.2 | 3-year recurrence-free survival (ongoing); short-term clinical outcomes | Laparoscopic group had lower postoperative complication rate (20% vs 46%; P = 0.007) after neoadjuvant, and better adjuvant chemotherapy completion (OR = 4.39; 95%CI: 1.63-11.80; P = 0.003) |
| KLASS-02 | Lee et al[15], 2019 | South Korea | 2011 to 2015 | 10114 | 23 | cT2-4a N0-1 | 498 | 513 | 0 | 985 | 26 | 0 | 267 | 218 | 274 | 252 | 0 | 99.4 | 30-day morbidity and 90-day mortality | Morbidity was significantly lower after laparoscopic (16.6%) than after open gastrectomy (24.1%); P = 0.003. 90-day mortality was similar in both groups (laparoscopic 0.4% vs open 0.6%; P = 0.682) |
| Son et al[90], 2022 | 2011 to 2015 | 9744,5 | 22 | cT2-4a N0-1 | 482 | 492 | 0 | 947 | 27 | 0 | 262 | 217 | 267 | 228 | 0 | 99.7 | 5-year overall survival and relapse-free survival | No significant difference in the 5-year overall survival (88.9% vs 88.7%; P = 0.30) and relapse-free survival (79.5% vs 81.1%; P = 0.658) between laparoscopic and open groups. Most common types of recurrence were peritoneal carcinomatosis (42.1%) | ||
| Hyung et al[91], 2020 | 2011 to 2015 | 9744,5 | 22 | cT2-4a N0-1 | 482 | 492 | 0 | 947 | 27 | 0 | 262 | 217 | 267 | 228 | 0 | 99.7 | 3-year relapse-free survival | Relapse-free survival did not differ between groups: 81.3% in the laparoscopic and 80.3% in the open group (log-rank P = 0.827; HR = 1.035; 95%CI: 0.762-1.406; P = 0.039) | ||
| CLASS-02 | Liu et al[92], 2020 | China | 2017 to 2018 | 214 | 10 | cT1N0-1, cT2N0 | 109 | 105 | 0 | 0 | 214 | 0 | 1447 | 39 | 19 | 5 | 0 | 83.2 | 30-day morbidity and mortality | No significant difference in the overall postoperative complication rate (18.1% vs 17.4%) and in mortality (1% vs 0) |
| Lu | Lu et al[50], 2021 | China | 2017 to 2020 | 2953 | 121 | cT1-4a N0-3 | 0 | 142 | 141 | 283 | 12 | 0 | 92 | 42 | 104 | 45 | 0 | 67.5 | 30-day postoperative outcomes, quality of lymphadenectomy | Surgical morbidity was comparable in the robotic and laparoscopic groups (3.5% vs 6.3%; P = 0.279). Higher extraperigastric lymph nodes were retrieved in the robotic group (17.6 vs 15.8; P = 0.018) |
| Lu et al[93], 2024 | 2017 to 2020 | 2833 | 118 | cT1-4a N0-3 | 0 | 142 | 141 | 283 | 0 | 0 | 92 | 42 | 104 | 45 | 0 | 67.5 | 3-year disease-free survival | 3-year disease-free survival was 85.8% and 73.2% in the RDG and LDG groups (P = 0.011). Difference in local recurrence rate (2.1% vs 7.7%), while no difference in peritoneal and liver metastasis | ||
| Ojima | Ojima et al[54], 2021 | Japan | 2018 to 2020 | 2364 | 47 | cT1-4a N0-3 | 0 | 119 | 113 | 160 | 76 | 0 | 131 | 24 | 51 | 30 | 0 | 47 | Postoperative intra-abdominal infectious complications of C-D grade > 2 | No significant difference in the incidence of intra-abdominal infectious complications (8.4% in the laparoscopic vs 6% in the robotic group; P = 0.47) |
For early-stage disease treated with distal gastrectomy, two large RCT - MIS vs open - have been completed: The Japanese JCOG0912 [laparoscopic assisted distal gastrectomy (LADG) vs open distal gastrectomy (ODG)][13] and the Korean KLASS-01 (similar selection criteria)[14]. In JCOG0912, less than 30% of cases underwent D2 lymphadenectomy compared to approximately 60% of cases in KLASS-01. Both trials demonstrated non-inferiority of laparoscopic approach compared to open in both early and late outcomes. The surgical volume of involved centers was 7.5 and 24 patients/center per year, respectively.
For advanced forms (clinical stage II-III) treated with distal gastrectomy, three large trials from East have been concluded: The Korean trial KLASS-02 (distal gastrectomy with D2 lymphadenectomy and total omentectomy)[15], and the Chinese trial CLASS-01 (distal gastrectomy with D2 lymphadenectomy for GC without bulky nodes) demonstrated the non-inferiority of MIS vs D2 ODG for the established primary end-points (3-year recurrence-free survival and 3-year disease-free survival, respectively)[16]. The trial from the (JLSSG0901) randomized 502 patients from 37 institutions[17]. Five-year recurrence-free survival of LADG vs ODG were overlapping, with 73.9% (68.7%-79.5%) and 75.7% (70.5%-81.2%), respectively. There were also no significant differences in postoperative complications, overall survival and recurrence patterns between the two groups. The authors concluded that LADG with D2 lymph node dissection, when performed by qualified surgeons, could become a standard treatment for locally advanced GC.
Regarding total gastrectomy, some prospective studies are available for both early and advanced disease, but the quality of evidence is still inferior to that for LADG. JCOG1401 is a prospective, non-randomized, single-arm trial that confirmed the safety and efficacy of laparoscopy-assisted total gastrectomy (LATG) or laparoscopy-assisted proximal gastrectomy for clinical stage I proximal GC. The percentage of D2 dissection was 6.1%[18]. A similar trial has been conducted in 170 patients with clinical stage I GC of the upper third (19 institutions from South Korea, KLASS-03). Morbidity and mortality rates were “acceptable”: 20.6% and 0.6% respectively, with 9.4% of grade ≥ III complications, and 1.9% reoperation rate. However, a control group was lacking, and the rate of D2 dissection was 38.2%[19].
Some trials are still ongoing: The CLASS 02-01 and the KLASS-06. The CLASS02-01 is a multicenter randomized, controlled non-inferiority trial, with primary end-point morbidity/mortality rates. Two hundred patients with clinical stage I GC will be randomized to LATG (100) or open total gastrectomy (OTG) group (100)[20]. The KLASS-06 trial is a Phase 3 randomized trial comparing OTG and LATG for advanced GC located in the upper body of the stomach.
One issue with the Eastern RCT is that locally advanced GC was managed with upfront surgery. By contrast, the standard approach in the West for fit patients with locally advanced disease now involves surgery following neoadjuvant or perioperative chemotherapy (NAC)[21]. In this context, several RCTs have been performed and recently published in Europe. Table 2 shows a list of Western RCTs (last access in PubMed [MEDLINE] in October 2024).
| Trial | Ref. | Country | Study period | Patients | Patients/ | Study design | Approach (n) | Surgery (n) | Neo adjuvant (%) | Pathological T (n) | D2 (%)1 | Primary outcomes | Main results | |||||||
| Open | Laparoscopic | Robotic | Distal | Total | 1 | 2 | 3 | 4 | x | |||||||||||
| Huscher | Huscher et al[94], 2005 | Italy | 1998 to 2001 | 59 | 24.5 | cT1-4 N0-2 | 29 | 30 | 0 | 59 | 0 | 0 | 13 | 15 | 21 | 10 | 0 | 69.5 | Short-term surgical outcome, 5-year overall and disease-free survival | Laparoscopic group had similar mean number of harvested lymph nodes (33.4 vs 30; P > 0.05) and operative mortality (27.6% vs 26.7%; P > 0.05) than open. Five-year overall and disease-free survival rates were similar between groups; P > 0.05 |
| STOMACH | van der Wielen et al[23], 2021 | European Union | 2015 to 2018 | 96 | 2 | cT1-4a N0-3 | 49 | 47 | 0 | 0 | 96 | 100 | 142 | 9 | 36 | 27 | 0 | 42.7 | Oncological safety, measured as the number of resected lymph nodes and radicality | Mean number of resected lymph nodes was 43.4 ± 17.3 in open and 41.7 ± 16.1 in minimally invasive group (P = 0.612) after neoadjuvant. No significant differences in R0 resection between laparoscopic (98%) and open groups (93.6%); P = 0.617 |
| LOGICA | van der Veen et al[22], 2021 | Netherland | 2015 to 2018 | 215 | 6.5 | cT1-4a N0-3 | 105 | 110 | 0 | 123 | 92 | 72.2 | 292 | 26 | 89 | 55 | 0 | 99.5 | Hospital stay | Median hospital stay was 7 days (interquartile range, 5-9) in both groups; P = 5.34 |
| Ribeiro | Ribeiro et al[72], 2022 | Brazil | 2015 to 2020 | 60 | 12.5 | cT1-4a N0-1 | 31 | 0 | 29 | 51 | 9 | 0 | 26 | 12 | 11 | 11 | 0 | 100 | Short-term surgical outcome | Robotic group had similar mean number of harvested lymph nodes (41.3 vs 42.4; P = 0.805), longer surgical time (354 vs 215 minutes; P < 0.001), and less bleeding (124 vs 276 mL; P < 0.001) compared to open |
The LOGICA trial randomized a total of 227 patients with resectable GC to laparoscopic (115) or open gastrectomy (112). Of note, more than 70% of included patients were previously submitted to NAC, and a D2 procedure was declared in almost all patients[22]. The main oncological results (R0 resection rate > 95%, median number of removed nodes of 29) were comparable in the two groups. However, survival results are still limited to 1-year follow-up; although no significant differences in postoperative complications and mortality were observed, the 90-day mortality (10.4% and 9.1%, respectively) was not negligible, when considering that most cases were curatively resected. The European STOMACH trial compared OTG and LATG after NAC[23]. The number of randomized patients was rather small, 96 in total. The oncological and surgical results were not significantly different, with a very high number of lymph nodes removed (median > 40) and a leak rate of 10.2% vs 8.5% in OTG and LATG, respectively. Although this rate is not particularly low (given that gastroesophageal junction tumors were excluded), it is noteworthy that the anastomotic technique in open gastrectomy was circular in 49% of cases and linear in 51%. Overall, the results of the LOGICA and STOMACH trials may indicate that D2 gastrectomy after NAC may be a more complex operation than upfront surgery, even with an open approach, supporting previous studies[24-26].
In general, the current literature suggests that laparoscopic gastrectomy for GC is a feasible and safe procedure in experienced hands. However, several specific issues need to be addressed, and definitive and robust long-term data are required before it can become standard practice, particularly in the West. These include considerations of patient, gastric tumor, and surgeon characteristics.
First, the typology of patients should be considered. Even when considering RCTs for advanced forms, the median age was much lower in Eastern (KLASS-02: 59 years, CLASS01: 57 years) than European patients (LOGICA: 67 years), with a low comorbidity rate. Similarly, the median body mass index (BMI) was 22-23 in the former, and the JLSSG0901 included only patients with BMI < 30. The laparoscopic approach in overweight patients is considered a more complex procedure. In the JCO0912, the incidence of grade III/IV complications in BMI > 25 group was almost doubled in the LADG (8.4%) than ODG (4.4%)[18]. Pancreatic fistula also seems to be more common in obese patients after laparoscopic approach. Regarding lymphadenectomy, a BMI > 25 may also partly influence oncological radicality, as it has been reported as an independent factor for non-compliance after LATG[27].
Second, the different tumor characteristics may play a role when translating the results of trials in clinical practice. In Eastern RCTs, more locally advanced forms (bulky nodes, Bormann type IV) were excluded. In fact, in KLASS-02, the mean number of metastatic nodes was 3.5, 45% of cases were N0 and only 33% of cases were stage III, similar to the JLSSG0901 trial[15,17]. These data differ significantly from what would be considered “locally advanced” in the West[2,21]. The survival results of KLASS-02 (5-year overall survival > 80% in pathological stage III treated with upfront surgery) and JLSSG0901 are hardly achievable in Western patients at the same stage[1]. Despite the generally good prognosis of the RCTs, it should be noted that in clinically serosa exposed cases (approximately 15% of cases in JLSSG0901), the recurrence rate in the LADG was 46% compared to 35% in the ODG. Although not statistically significant, this trend merits further investigation in dedicated studies. Another important difference between the East and West is the use of NAC in locally advanced disease. As mentioned above, clinical stages II/III are treated with upfront surgery in the East and with NAC in the West. In the former, NAC is only indicated in patients with bulky lymph nodes[5]. Both LOGICA and STOMACH showed no oncological or surgical differences between open and laparoscopic approaches. However, long-term results are lacking and a trend towards a higher risk of postoperative complications after NAC has been suggested. Indeed, recent real-world experience suggests that conversion rates and morbidity are increased after laparoscopic gastrectomy in patients undergoing NAC[28]. Regardless of tumor characteristics in Western patients, a significant proportion of cases involve posterior (stations 8p, 12p/b, 13) and para-aortic lymph nodes[10,29]. These patients may benefit from a more extended (D2plus/D3) lymphadenectomy[30]. The latest JGCA guidelines also indicate that in cases with bulky lymph nodes, NAC and para-aortic dissection should be considered. These procedures generally require an open approach and are difficult to perform laparoscopically[31].
Third is the surgeon. In the Eastern trials, a rigorous selection was required before entering the trials. In KLASS-02, only surgeons who had performed 50 cases each of laparoscopic distal gastrectomy and ODG in high-volume centers (> 80 cases/year) were included in the study. Following validation in a separate clinical trial (KLASS-02-QC), a steering committee reviewed videos of the surgical fields for every 10 cases. The JLSSG0901 required the Endoscopic Surgical Skill Qualification System certification[32]. Indeed, data from literature indicate that the learning curve for a laparoscopic gastrectomy is 44 to 36 based on anastomotic leakage rate[33,34]. A rapid overview of published studies highlights that 79/83 studies on laparoscopic/robotic learning curve come from East. It is well known that the surgical volume of gastrectomy is much lower in the West than in the East. For example, more than 50% of patients with GC in Italy are operated on in centers with a volume of < 10 cases/year[35]. In addition, the changing epidemiology of GC, with a relative increase in diffuse, proximal tumors and synchronous peritoneal dissemination, has led to a further reduction in early-stage cases in the West, which now represent a minority of total GC cases (less than 30%)[2,36]. Therefore, an adequate learning curve for laparoscopic gastrectomy is hardly achievable for Western surgeons during their training program.
The use of robotic gastrectomy has increased worldwide, as has the amount of published case series[37]. These numbers are expected to grow exponentially in the future as patents expire and costs come down. With the entry of new competitors, increased market competition is expected to drive down prices for robotic platforms, consumables, and maintenance. Additionally, the impending expiration of key patents will likely allow for the adaptation and integration of third-party instruments and drapes, reducing dependency on proprietary robotic tools and further lowering costs. Moreover, a recent cost analysis reported that the overall cost increase for robotic surgery was primarily due to indirect costs, such as capital equipment, supplies, and maintenance costs. Direct costs, like medical and nursing costs, were lower for robotic surgery than for laparoscopy[38]. Robotic systems are claimed to facilitate complex reconstruction after gastrectomy, reduce the technical difficulties with their magnified vision and stable movements, and avoid early conversion even in patients with advanced GC[39-41]. Enhanced precision, particularly in complex procedures such as extended lymphadenectomies and anastomosis, may further help prevent costly complications. However, numerous large-scale studies have not demonstrated a clear superiority compared to laparoscopy[42,43]. This could be due to the large differences in the selected cohorts between the studies. Eastern trials enrolled patients with lower BMI (mean 23 kg/m2vs 28 kg/m2 in the West), clinical stage I-II, and tumors located in the lower third of the stomach. On the other hand, 55%-75% of cancers in the West were locally advanced and located in the upper or middle third of the stomach, resulting in a higher rate of total gastrectomy[44,45].
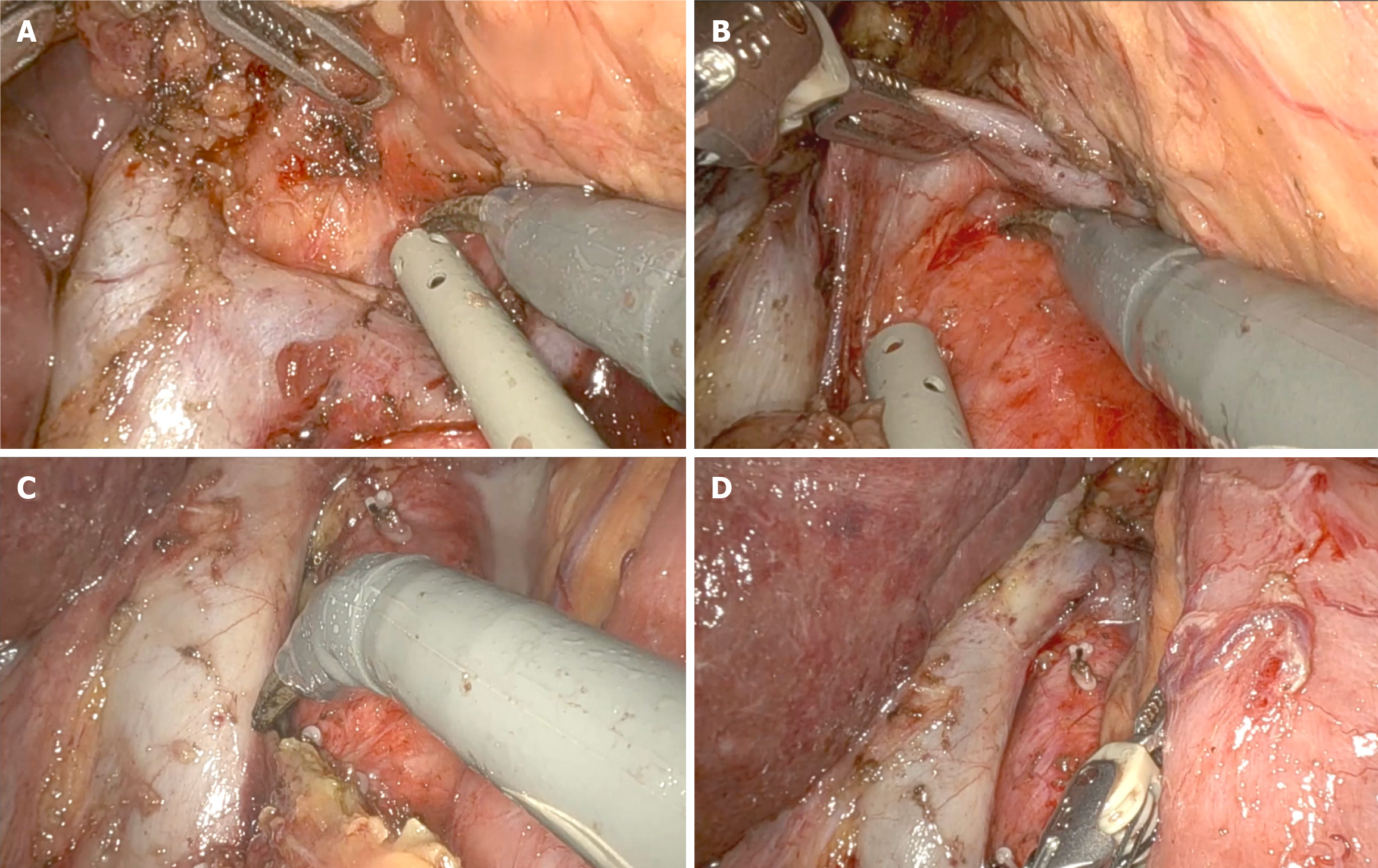
Previous reports have shown improved 3-year survival with robotic gastrectomy in patients with stage I/II GC[46]. It is generally accepted that the robotic approach can provide accurate lymphadenectomy, allowing better staging of the disease[47,48]. A recent update of the Italian Research Group for Gastric Cancer database shows a strong correlation between removed and positive lymph nodes, with this relationship reaching a plateau when 40-50 lymph nodes are examined. It suggests that survival for patients with the same number of metastatic nodes is significantly influenced by the total number of nodes removed: 12% for less than 10 nodes, 26% for 11 to 15 nodes, and 60% for more than 25 nodes[31]. As the number of lymph nodes harvested can be a predictor of survival, more extensive lymphadenectomy (i.e., removal of para-aortic and/or posterior nodes) may be indicated in GC with a high risk of extraperigastric metastases, such as located in the upper third of the stomach, with serosa involvement or exhibiting bulky nodes in the second level perigastric nodal stations[10]. No statistical differences in morbidity, mortality rates and mean hospital stay were observed compared to D2 lymphadenectomy[49]. The robotic system allows to mimic open surgical principles to perform more extended lymphadenectomy, including para-aortic nodes. In a Chinese RCT (283 patients in one high-volume hospital), more extraperigastric lymph nodes were retrieved in the robotic assisted distal gastrectomy group (RADG) (17.6 ± 5.8) than LADG (15.8 ± 6.6), particularly in the para-aortic stations (Figure 1). The noncompliance rate, defined as the absence of nodes from more than one station that should have been excised, was lower (7.7% vs 16.9%)[50]. Interestingly, the RADG group showed less intense inflammatory responses and fewer postoperative complications (9.2% vs 17.6%), resulting in faster postoperative recovery and earlier initiation of adjuvant chemotherapy.
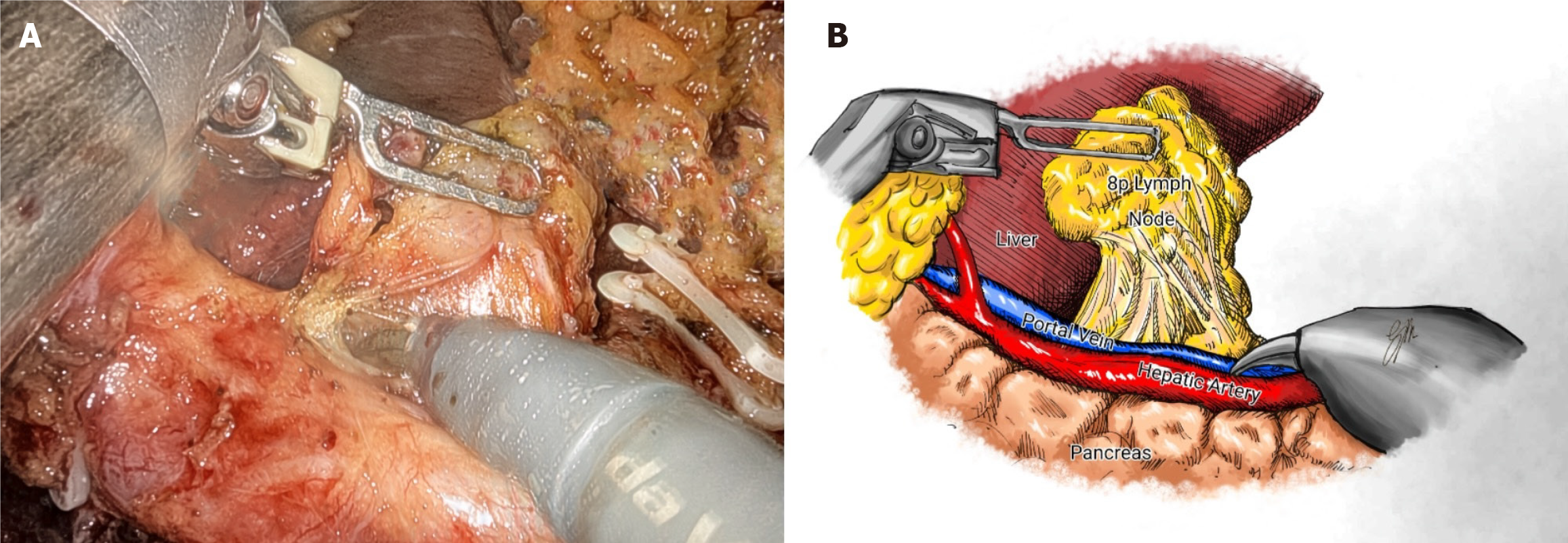
A known problem with MIS is pancreatic fistula caused by blunt trauma from compressing the pancreas and thermal injury from continuous use of energy devices[51]. A higher incidence of pancreatic fistula was described for laparoscopic approach when compared with robotic assisted (22.5% vs 10%)[52]. Assuming that this is probably related to the higher rate of pancreatic injury during lymph node dissection at stations 6, 8a, 9, 12, and 13, the highly articulated instruments and the possibility of retroversion facilitate the access to these stations better than the retraction of the human first assistant can guarantee (Figure 2)[53]. Similarly, a Japanese trial (236 patients in two institutions) reported fewer overall (8.8% vs 19.7%) and major (5.3% vs 16.2%) complications with the robotic approach, with no cases of pancreatic fistula and a similar rate of intra-abdominal infectious complications[54].
The number of patients with high BMI is increasing in Western countries[55]. As a result, less experienced surgeons tend to favor open over minimally invasive techniques to achieve adequate lymphadenectomy and negative resection margins. Recent series have shown an advantage of robotic gastrectomy in the incidence of grade > II postoperative complications in the obese cohort (11.5% vs 22.0%)[56]. Near-infrared fluorescence imaging (NIR) is an emerging technique. It may help surgeons locate tumors, follow lymph nodes and map critical anatomical structures also in the presence of elevated levels of visceral fat. Eastern series reported a higher number of lymph nodes retrieved with NIR in whole population (48.9 vs 35.2) and patients with high BMI (48.4 vs 39.8), without increasing the operative time[57,58]. However, some clinical situations, such as postchemotherapy interstitial fibrosis or cytotoxicity, may limit the use of NIR during surgery, especially in Western countries that emphasize the use of neoadjuvant therapy.
Several clinical trials have reported the efficacy of NAC in downstaging the primary tumor and increasing R0 resection rates, albeit with increased operative time and risk of intraoperative complications. Indeed, distortion of anatomical planes of dissection, interstitial fibrosis, and sclerotic tissue changes, may increase surgical difficulty[31]. It is intuitive that a greater number of lymph nodes to be excised, along with the side effects of chemotherapy, contribute to a steeper learning curve. Nonetheless, preoperative treatment does not eliminate the need for a complete radical resection when oncological resectability is to be pursued. RADG showed to be a potential therapeutic option for patients with advanced GC who underwent preoperative chemo/radio-chemotherapy[59]. In an Asian propensity score-matched analysis of 67 patients per group, RADG showed lower intraoperative blood loss and higher lymph node retrieval (50.7 vs 39.5, more extraperigastric and suprapancreatic), with similar operative time and postoperative complication rates to LADG. It suggests a clear technical advantage after NAC (SOX 2 cycles)[60]. Similar results have been confirmed in the Western context, where a low prevalence of disease favors the technical proficiency required for more advanced clinical stages. A series of 32 patients who underwent robotic surgery were less likely to have R+ (6.3% vs 20%) or inadequate lymphadenectomy (25% vs 43.6%) compared to open surgery[61]. These data suggest that robotic gastrectomy can be performed by lower-volume surgeons in Western centers serving older populations with higher BMI and comorbidities. However, these are analyses of retrospectively collected patients. So far, only four trials have investigated the outcomes after NAC, two from Eastern countries (NAC: 39.4% of 1039 cases and 100% of 95 cases) and two from Western countries (STOMACH: 100% of 96 cases, LOGICA: 72.2% of 215 cases). However, all of these studies included patients who underwent laparoscopic surgery. More RCT focused on robotic surgery are expected to be conducted soon.
Anastomotic leakage is the Achille’s heel of the surgeon. Many predictive factors have been identified: Patient-related (preoperative nutritional status, smoking, respiratory and cardiological pathologies), tumor-related (mixed-type histology), and operative variables, D2 or more lymphadenectomy, laparoscopic approach (15.1% vs 7.7% in open gastrectomy), multivisceral resection and reconstruction technique, with circular anastomosis at high risk of developing leakage[62,63]. The impact of surgeon experience has also been documented as an independent risk factor for leakage, ranging from 12% for less experienced surgeons (< 30 gastrectomies) to 2.2% for highly experienced surgeons[64]. Although the introduction of laparoscopy initially limited the use of hand sewing due to its technical requirements, the greater flexibility of robotic systems has made hand sewn esophagojejunostomy easier and more feasible (leakage in 1 out of 54 cases)[65]. The incidence of postoperative leakage tends to decrease after the first 15 cases, reflecting the robotic learning curve for gastrectomy[66]. Similar advantages have been described with the Albert-Lembert with knotless barbed sutures method for esophagojejunostomy during robotic assisted total gastrectomy (1 of 31 cases), with no occurrence of anastomotic stenosis, and the intracorporeal phi-shaped esophagojejunostomy (1 of 11 cases)[67,68]. The Upper Gastrointestinal International Robotic Association registry reported anastomotic leak rates of 10% (4.5% circular stapled, 3.4% hand sewn, 2.1% linear stapled) and 2% (all linear stapled) after robotic assisted total gastrectomy and RADG, respectively. Robotic linear staplers also appear to be associated with fewer anastomotic strictures than circular staplers due to easier calibration of the anastomotic angle and more precise reinforcement of the anastomosis[69]. With the aim of overcoming surgeon-related bias in demonstrating non-inferiority of robotic gastrectomy, completed trials have defined precise inclusion criteria for participants (Figure 3).
Longer operative time is usually associated with robotic approach. The increase in operating times is generally attributed to the docking time, so defined “junk time”, which can take up to half an hour[70]. An interesting analysis of the first 32 robotic cases showed a gain in operating time of 90 minutes per day of reduced hospital stay[61]. Indeed, the large heterogeneity in published meta-analyses makes it difficult to interpret the evidence. A 2021 umbrella review (a review of meta-analyses) concluded that robotic gastrectomy is associated with less intraoperative bleeding, faster oral intake and shorter hospital stay, with an acceptable level of evidence rather than laparoscopic gastrectomy[71]. Similar results were found when compared with the open approach. The Brazilian trial (60 patients in a single center) showed a halving of bleeding and no difference in postoperative complication and mortality rates[72]. The simplicity of technical gesture may reduce the number of surgeries required to achieve an acceptable rate of postoperative complications, making robotic approach an advantageous option for Western centers with lower case volumes. Cumulative sum analyses of operative time ranged from 65 cases in Eastern countries[73] to 25-27 cases in the West[74].
Robotic surgery has been considered a safe and effective option for the treatment of GC since its introduction in 2003[75]. Prospective studies from the late 2010s were primarily conducted in East Asia, where early-stage GC is more common, and laparoscopy has long been the standard surgical approach for treating the disease. On the other hand, emerging evidence comes from the West, where the low incidence of disease implies limited study cohorts. Significant changes in the market dynamics may lead to substantial cost reductions soon and a higher number of patients enrolled.
Given the heterogeneity of published studies, this comprehensive review highlights its potential key advantages: (1) Comparable amount of lymph nodes retrieved as guaranteed by highly experienced laparoscopic surgeons, and consequent accurate staging; (2) More acceptable rates of pancreatic fistula and other postoperative complications; (3) Easier dissection in patients with high BMI or in case of delayed surgery; and (4) Feasible anastomotic reconstruction after total gastrectomy. To date, published randomized trials comparing open/laparoscopic and robotic gastrectomy have not yet shown a clear oncological advantage in terms of survival or recurrence rates[76].
Is it time to stop randomization? Certainly, optimizing operating times and reducing operating costs as competition grows may expand robotic surgery’s accessibility, encourage larger trials, and potentially establish it as the standard of care for GC. To support what has been argued above, artificial intelligence-driven image interpretation and computer vision further support robotic surgery by enhancing decision-making and automation. It may have many practical clinical implications such as safe dissection and lymph node mapping. Furthermore, the real-time data transmission enables procedures to be performed from anywhere, overcoming geographical barriers, addressing medical resource imbalances, and facilitating remote collaboration.
| 1. | Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F; Italian Research Group for Gastric Cancer (IRGGC). Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Marrelli D, Polom K, de Manzoni G, Morgagni P, Baiocchi GL, Roviello F. Multimodal treatment of gastric cancer in the west: Where are we going? World J Gastroenterol. 2015;21:7954-7969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 3. | De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, Tomezzoli A, Berselli M, Catalano F, Di Leo A, Framarini M, Giacopuzzi S, Graziosi L, Marchet A, Marini M, Milandri C, Mura G, Orsenigo E, Quagliuolo V, Rausei S, Ricci R, Rosa F, Roviello G, Sansonetti A, Sgroi G, Tiberio GA, Verlato G, Vindigni C, Rosati R, Roviello F. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017;20:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 666] [Article Influence: 222.0] [Reference Citation Analysis (0)] |
| 5. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 593] [Article Influence: 296.5] [Reference Citation Analysis (2)] |
| 6. | Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH, Noh SH. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687-4693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Yang SY, Roh KH, Kim YN, Cho M, Lim SH, Son T, Hyung WJ, Kim HI. Surgical Outcomes After Open, Laparoscopic, and Robotic Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2017;24:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, Saragoni L, Tomezzoli A, Kurihara H; Italian Research Group for Gastric Cancer. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Siewert JR. Gastric cancer: the dispute between East and West. Gastric Cancer. 2005;8:59-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Marrelli D, Ferrara F, Giacopuzzi S, Morgagni P, Di Leo A, De Franco L, Pedrazzani C, Saragoni L, De Manzoni G, Roviello F. Incidence and Prognostic Value of Metastases to "Posterior" and Para-aortic Lymph Nodes in Resectable Gastric Cancer. Ann Surg Oncol. 2017;24:2273-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1306] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 12. | Degiuli M, Reddavid R, Tomatis M, Ponti A, Morino M, Sasako M; of the Italian Gastric Cancer Study Group (IGCSG). D2 dissection improves disease-specific survival in advanced gastric cancer patients: 15-year follow-up results of the Italian Gastric Cancer Study Group D1 versus D2 randomised controlled trial. Eur J Cancer. 2021;150:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 14. | Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019;5:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 377] [Article Influence: 75.4] [Reference Citation Analysis (1)] |
| 15. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 16. | Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Yu J, Zheng C, Liu F, Li Z, Zhao G, Zhang J, Chen P, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg. 2022;157:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 17. | Etoh T, Ohyama T, Sakuramoto S, Tsuji T, Lee SW, Yoshida K, Koeda K, Hiki N, Kunisaki C, Tokunaga M, Otsubo D, Takagane A, Misawa K, Kinoshita T, Cho H, Doki Y, Nunobe S, Shiraishi N, Kitano S; Japanese Laparoscopic Surgery Study Group (JLSSG). Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial. JAMA Surg. 2023;158:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 18. | Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M, Okitsu H, Yoshikawa T, Terashima M; Stomach Cancer Study Group of Japan Clinical Oncology Group. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 19. | Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 20. | He H, Li H, Su X, Li Z, Yu P, Huang H, Huang C, Ye J, Li Y, Suo J, Yu J, Li G, Xu Z, Zhao G, Cao H, Hu J, Du X, Liu F, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Study on safety of laparoscopic total gastrectomy for clinical stage I gastric cancer: the protocol of the CLASS02-01 multicenter randomized controlled clinical trial. BMC Cancer. 2018;18:944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1634] [Article Influence: 272.3] [Reference Citation Analysis (0)] |
| 22. | van der Veen A, Brenkman HJF, Seesing MFJ, Haverkamp L, Luyer MDP, Nieuwenhuijzen GAP, Stoot JHMB, Tegels JJW, Wijnhoven BPL, Lagarde SM, de Steur WO, Hartgrink HH, Kouwenhoven EA, Wassenaar EB, Draaisma WA, Gisbertz SS, van der Peet DL, May AM, Ruurda JP, van Hillegersberg R; LOGICA Study Group. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J Clin Oncol. 2021;39:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 23. | van der Wielen N, Straatman J, Daams F, Rosati R, Parise P, Weitz J, Reissfelder C, Diez Del Val I, Loureiro C, Parada-González P, Pintos-Martínez E, Mateo Vallejo F, Medina Achirica C, Sánchez-Pernaute A, Ruano Campos A, Bonavina L, Asti ELG, Alonso Poza A, Gilsanz C, Nilsson M, Lindblad M, Gisbertz SS, van Berge Henegouwen MI, Fumagalli Romario U, De Pascale S, Akhtar K, Jaap Bonjer H, Cuesta MA, van der Peet DL. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24:258-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 24. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1499] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 26. | Claassen YHM, Hartgrink HH, Dikken JL, de Steur WO, van Sandick JW, van Grieken NCT, Cats A, Trip AK, Jansen EPM, Meershoek-Klein Kranenbarg WM, Braak JPBM, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH. Surgical morbidity and mortality after neoadjuvant chemotherapy in the CRITICS gastric cancer trial. Eur J Surg Oncol. 2018;44:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Lin GT, Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Huang CM. Lymph Node Noncompliance Affects the Long-Term Prognosis of Patients with Gastric Cancer after Laparoscopic Total Gastrectomy. J Gastrointest Surg. 2020;24:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Bracale U, Corcione F, Pignata G, Andreuccetti J, Dolce P, Boni L, Cassinotti E, Olmi S, Uccelli M, Gualtierotti M, Ferrari G, De Martini P, Bjelović M, Gunjić D, Cuccurullo D, Sciuto A, Pirozzi F, Peltrini R. Impact of neoadjuvant therapy followed by laparoscopic radical gastrectomy with D2 lymph node dissection in Western population: A multi-institutional propensity score-matched study. J Surg Oncol. 2021;124:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Bencivenga M, Verlato G, Mengardo V, Scorsone L, Sacco M, Torroni L, Giacopuzzi S, de Manzoni G. Is There Any Role for Super-Extended Limphadenectomy in Advanced Gastric Cancer? Results of an Observational Study from a Western High Volume Center. J Clin Med. 2019;8:1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Marrelli D, Piccioni SA, Carbone L, Petrioli R, Costantini M, Malagnino V, Bagnacci G, Rizzoli G, Calomino N, Piagnerelli R, Mazzei MA, Roviello F. Posterior and Para-Aortic (D2plus) Lymphadenectomy after Neoadjuvant/Conversion Therapy for Locally Advanced/Oligometastatic Gastric Cancer. Cancers (Basel). 2024;16:1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Marano L, Carbone L, Poto GE, Restaino V, Piccioni SA, Verre L, Roviello F, Marrelli D. Extended Lymphadenectomy for Gastric Cancer in the Neoadjuvant Era: Current Status, Clinical Implications and Contentious Issues. Curr Oncol. 2023;30:875-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Tanigawa N, Lee SW, Kimura T, Mori T, Uyama I, Nomura E, Okuda J, Konishi F. The Endoscopic Surgical Skill Qualification System for gastric surgery in Japan. Asian J Endosc Surg. 2011;4:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Chan KS, Oo AM. Learning curve of laparoscopic and robotic total gastrectomy: A systematic review and meta-analysis. Surg Today. 2024;54:509-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Brenkman HJF, Claassen L, Hannink G, van der Werf LR, Ruurda JP, Nieuwenhuizen GAP, Luyer MDP, Kouwenhoven EA, van Det MJ, van Berge Henegouwen MI, Gisbertz SS, Stoot JHMB, Hulsewé KWE, van Workum F, van Hillegersberg R, Rosman C. Learning Curve of Laparoscopic Gastrectomy: A Multicenter Study. Ann Surg. 2023;277:e808-e816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Lorenzon L, Biondi A, Agnes A, Scrima O, Persiani R, D'Ugo D. Quality Over Volume: Modeling Centralization of Gastric Cancer Resections in Italy. J Gastric Cancer. 2022;22:35-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Koemans WJ, Lurvink RJ, Grootscholten C, Verhoeven RHA, de Hingh IH, van Sandick JW. Synchronous peritoneal metastases of gastric cancer origin: incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer. 2021;24:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Shibasaki S, Suda K, Hisamori S, Obama K, Terashima M, Uyama I. Robotic gastrectomy for gastric cancer: systematic review and future directions. Gastric Cancer. 2023;26:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 39. | Lim SH, Lee HM, Son T, Hyung WJ, Kim HI. Robotic surgery for gastric tumor: current status and new approaches. Transl Gastroenterol Hepatol. 2016;1:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 41. | Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Yang C, Shi Y, Xie S, Chen J, Zhao Y, Qian F, Hao Y, Tang B, Yu P. Short-term outcomes of robotic- versus laparoscopic-assisted Total Gastrectomy for advanced gastric Cancer: a propensity score matching study. BMC Cancer. 2020;20:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Ye SP, Shi J, Liu DN, Jiang QG, Lei X, Qiu H, Li TY. Robotic-assisted versus conventional laparoscopic-assisted total gastrectomy with D2 lymphadenectomy for advanced gastric cancer: short-term outcomes at a mono-institution. BMC Surg. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | van Boxel GI, Ruurda JP, van Hillegersberg R. Robotic-assisted gastrectomy for gastric cancer: a European perspective. Gastric Cancer. 2019;22:909-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer. 2010;13:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Ryan S, Tameron A, Murphy A, Hussain L, Dunki-Jacobs E, Lee DY. Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma: Propensity-Matched Analysis. Surg Innov. 2020;27:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Cianchi F, Indennitate G, Trallori G, Ortolani M, Paoli B, Macrì G, Lami G, Mallardi B, Badii B, Staderini F, Qirici E, Taddei A, Ringressi MN, Messerini L, Novelli L, Bagnoli S, Bonanomi A, Foppa C, Skalamera I, Fiorenza G, Perigli G. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg. 2016;16:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Marrelli D, Pedrazzani C, Neri A, Corso G, DeStefano A, Pinto E, Roviello F. Complications after extended (D2) and superextended (D3) lymphadenectomy for gastric cancer: analysis of potential risk factors. Ann Surg Oncol. 2007;14:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Lu J, Zheng CH, Xu BB, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Huang CM, Li P. Assessment of Robotic Versus Laparoscopic Distal Gastrectomy for Gastric Cancer: A Randomized Controlled Trial. Ann Surg. 2021;273:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 51. | Fujita T, Ohta M, Ozaki Y, Takahashi Y, Miyazaki S, Harada T, Iino I, Kikuchi H, Hiramatsu Y, Kamiya K, Konno H. Collateral thermal damage to the pancreas by ultrasonic instruments during lymph node dissection in laparoscopic gastrectomy. Asian J Endosc Surg. 2015;8:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Seo HS, Shim JH, Jeon HM, Park CH, Song KY. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res. 2015;194:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Li ZY, Zhou YB, Li TY, Li JP, Zhou ZW, She JJ, Hu JK, Qian F, Shi Y, Tian YL, Gao GM, Gao RZ, Liang CC, Shi FY, Yang K, Wen Y, Zhao YL, Yu PW; Robotic, Laparoscopic Surgery Committee of Chinese Research Hospital Association. Robotic Gastrectomy Versus Laparoscopic Gastrectomy for Gastric Cancer: A Multicenter Cohort Study of 5402 Patients in China. Ann Surg. 2023;277:e87-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 54. | Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, Nakai T, Nakamori M, Ohi M, Kusunoki M, Yamaue H. Short-term Outcomes of Robotic Gastrectomy vs Laparoscopic Gastrectomy for Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2021;156:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 55. | Tsekrekos A, Lovece A, Chrysikos D, Ndegwa N, Schizas D, Kumagai K, Rouvelas I. Impact of obesity on the outcomes after gastrectomy for gastric cancer: A meta-analysis. Asian J Surg. 2022;45:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Komatsu M, Kinoshita T, Akimoto E, Yoshida M, Nagata H, Habu T, Okayama T, Yura M. Advantages of robotic gastrectomy for overweight patients with gastric cancer: a comparison study of robotic gastrectomy and conventional laparoscopic gastrectomy. Surg Today. 2023;53:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Pang HY, Liang XW, Chen XL, Zhou Q, Zhao LY, Liu K, Zhang WH, Yang K, Chen XZ, Hu JK. Assessment of indocyanine green fluorescence lymphography on lymphadenectomy during minimally invasive gastric cancer surgery: a systematic review and meta-analysis. Surg Endosc. 2022;36:1726-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Kim KY, Hwang J, Park SH, Cho M, Kim YM, Kim HI, Hyung WJ. Superior lymph node harvest by fluorescent lymphography during minimally invasive gastrectomy for gastric cancer patients with high body mass index. Gastric Cancer. 2024;27:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Tanaka T, Suda K, Shibasaki S, Serizawa A, Akimoto S, Nakauchi M, Matsuoka H, Inaba K, Uyama I. Safety and feasibility of minimally invasive gastrectomy following preoperative chemotherapy for highly advanced gastric cancer. BMC Gastroenterol. 2024;24:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Tian Y, Guo H, Hu Y, Yang P, Liu Y, Zhang Z, Ding P, Zheng T, Fan L, Zhang Z, Li Y, Zhao Q. Safety and efficacy of robotic-assisted versus laparoscopic distal gastrectomy after neoadjuvant chemotherapy for advanced gastric cancer. Surg Endosc. 2023;37:6761-6770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 61. | Rodriguez MJ, Ore AS, Schawkat K, Kennedy K, Bullock A, Pleskow DK, Critchlow J, Moser AJ. Treatment burden of robotic gastrectomy for locally advanced gastric cancer (LAGC): a single western experience. Ann Transl Med. 2021;9:1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (2)] |
| 62. | Trapani R, Rausei S, Reddavid R, Degiuli M; ITALIAN RESEARCH GROUP FOR GASTRIC CANCER (GIRCG) Clinical Investigators. Risk factors for esophago-jejunal anastomosis leakage after total gastrectomy for cancer. A multicenter retrospective study of the Italian research group for gastric cancer. Eur J Surg Oncol. 2020;46:2243-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Kodera Y, Yoshida K, Kumamaru H, Kakeji Y, Hiki N, Etoh T, Honda M, Miyata H, Yamashita Y, Seto Y, Kitano S, Konno H. Introducing laparoscopic total gastrectomy for gastric cancer in general practice: a retrospective cohort study based on a nationwide registry database in Japan. Gastric Cancer. 2019;22:202-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 64. | Kanaji S, Ohyama M, Yasuda T, Sendo H, Suzuki S, Kawasaki K, Tanaka K, Fujino Y, Tominaga M, Kakeji Y. Can the intraoperative leak test prevent postoperative leakage of esophagojejunal anastomosis after total gastrectomy? Surg Today. 2016;46:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427-6437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Salvador-Rosés H, Escartín A, Muriel P, Santamaría M, González M, Jara J, Vela F, Olsina JJ. Robotic versus open approach in total gastrectomy for gastric cancer: a comparative single-center study of perioperative outcomes. J Robot Surg. 2023;17:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Wang Z, Ren S, Wang B. The ALBS (Albert-Lembert with Knotless Barbed Sutures) Method for Esophagojejunostomy After Totally Robotic Radical Total Gastrectomy. J Gastrointest Surg. 2023;27:2272-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Zhang S, Khaliq J, Li D, Jiang X, Sun R, Li Y. Robotic total gastrectomy with π-shaped esophagojejunostomy using a linear stapler as a novel technique. World J Surg Oncol. 2018;16:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | de Jongh C, Cianchi F, Kinoshita T, Kingma F, Piccoli M, Dubecz A, Kouwenhoven E, van Det M, Mala T, Coratti A, Ubiali P, Turner P, Kish P, Borghi F, Immanuel A, Nilsson M, Rouvelas I, Hӧlzen JP, Rouanet P, Saint-Marc O, Dussart D, Patriti A, Bazzocchi F, van Etten B, Haveman JW, DePrizio M, Sabino F, Viola M, Berlth F, Grimminger PP, Roviello F, van Hillegersberg R, Ruurda J; UGIRA Collaborative Group. Surgical Techniques and Related Perioperative Outcomes After Robot-assisted Minimally Invasive Gastrectomy (RAMIG): Results From the Prospective Multicenter International Ugira Gastric Registry. Ann Surg. 2024;280:98-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 70. | Liu H, Kinoshita T, Tonouchi A, Kaito A, Tokunaga M. What are the reasons for a longer operation time in robotic gastrectomy than in laparoscopic gastrectomy for stomach cancer? Surg Endosc. 2019;33:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 71. | Marano L, Fusario D, Savelli V, Marrelli D, Roviello F. Robotic versus laparoscopic gastrectomy for gastric cancer: an umbrella review of systematic reviews and meta-analyses. Updates Surg. 2021;73:1673-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Ribeiro U Jr, Dias AR, Ramos MFKP, Yagi OK, Oliveira RJ, Pereira MA, Abdalla RZ, Zilberstein B, Nahas SC, Cecconello I. Short-Term Surgical Outcomes of Robotic Gastrectomy Compared to Open Gastrectomy for Patients with Gastric Cancer: a Randomized Trial. J Gastrointest Surg. 2022;26:2477-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Kim MS, Kim WJ, Hyung WJ, Kim HI, Han SU, Kim YW, Ryu KW, Park S. Comprehensive Learning Curve of Robotic Surgery: Discovery From a Multicenter Prospective Trial of Robotic Gastrectomy. Ann Surg. 2021;273:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 74. | Staderini F, Barbato G, Bottari A, Russo E, Fortuna L, Giudici F, Coratti F, Stacchini L, Indennitate G, Cianchi F. Effects of the learning curve on operative time and lymph node harvesting during robotic gastrectomy. Int J Med Robot. 2023;19:e2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 770] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 76. | Solaini L, Avanzolini A, Pacilio CA, Cucchetti A, Cavaliere D, Ercolani G. Robotic surgery for gastric cancer in the west: A systematic review and meta-analyses of short-and long-term outcomes. Int J Surg. 2020;83:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 621] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 78. | Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39:2734-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 79. | Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, Zhang C, Yang H, Zhang N, Tan F, Jiang B, Di J, Wang Z, Ji J, Su X. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32:241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (1)] |
| 81. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 82. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 524] [Article Influence: 87.3] [Reference Citation Analysis (1)] |
| 83. | Hikage M, Hato S, Uemura K, Yura M, Sato Y, Matsushita H, Cho H, Hiki N, Kunisaki C, Inoue K, Choda Y, Boku N, Yoshikawa T, Katai H, Terashima M. Late complication after gastrectomy for clinical stage I cancer: supplementary analysis of JCOG0912. Surg Endosc. 2023;37:2958-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 84. | Akiyama Y, Katai H, Kitabayashi R, Nunobe S, Koeda K, Yura M, Sato Y, Yoshikawa T, Terashima M. Frequency of lymph node metastasis according to tumor location in clinical T1 early gastric cancer: supplementary analysis of the Japan Clinical Oncology Group study (JCOG0912). J Gastroenterol. 2023;58:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 85. | Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, Luo H, Chen J, Yu P. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2018;32:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 86. | Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, Luo H, Chen J, Yu P. Long-term oncologic outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surgery. 2019;165:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, Jeong O, Yoon KY, Lee JH, Lee SE, Yu W, Jeong SH, Kim T, Kim S, Nam BH; COACT group. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg. 2018;267:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 88. | Wang Z, Xing J, Cai J, Zhang Z, Li F, Zhang N, Wu J, Cui M, Liu Y, Chen L, Yang H, Zheng Z, Wang X, Gao C, Wang Z, Fan Q, Zhu Y, Ren S, Zhang C, Liu M, Ji J, Su X. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg Endosc. 2019;33:33-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 89. | Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2019;154:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 90. | Son SY, Hur H, Hyung WJ, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Yang HK, Han SU; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: 5-Year Outcomes of the KLASS-02 Randomized Clinical Trial. JAMA Surg. 2022;157:879-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 91. | Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU; Korean Laparoendoscopic Gastrointestinal Surgery Study Group. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. 2020;38:3304-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 92. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 93. | Lu J, Xu BB, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Yao ZH, Zheng CH, Huang CM. Robotic versus laparoscopic distal gastrectomy for resectable gastric cancer: a randomized phase 2 trial. Nat Commun. 2024;15:4668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Reference Citation Analysis (0)] |
| 94. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |