Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.103198
Revised: March 21, 2025
Accepted: April 21, 2025
Published online: June 15, 2025
Processing time: 115 Days and 6 Hours
Primary liver cancer, predominantly hepatocellular carcinoma (HCC), is a major cause of cancer-related mortality. Transarterial chemoembolization (TACE) is a key palliative option for unresectable HCC. However, prognostic outcomes after TACE vary significantly. This study evaluated the prognostic value of the fibrinogen and neutrophil-to-lymphocyte ratio (F-NLR) score, serum alpha-fetoprotein (AFP), and prealbumin (PA) in patients undergoing TACE.
To investigate the prognostic significance of F-NLR score, AFP, and PA in patients undergoing TACE.
Variables such as F-NLR score, AFP, PA, and other clinical indicators were assessed. Follow-ups determined prognosis as good or poor. Statistical asse
A retrospective analysis of 162 patients with primary liver cancer undergoing TACE was conducted. Low F-NLR scores and AFP levels and high PA were significantly associated with a good prognosis. The combined model, which integrated F-NLR, AFP, and PA, demonstrated a favorable prognostic predictive capability, with an area under the curve of 0.933.
Preoperative F-NLR, AFP, and PA are valuable prognostic predictors in patients with HCC undergoing TACE.
Core Tip: This study investigated the prognostic value of combining the fibrinogen and neutrophil-to-lymphocyte ratio (F-NLR) score, alpha-fetoprotein (AFP), and prealbumin (PA) in patients with primary liver cancer undergoing transarterial chemoembolization. Our findings indicate that integrating F-NLR, which reflects systemic inflammation and coagulation status, AFP as a marker of tumor burden, and PA as an indicator of nutritional status and liver function, significantly enhances prognostic accuracy. This combined approach provides a basis for personalized treatment strategies, enabling precise identification of high-risk patients and optimizing therapeutic plans to improve outcomes.
- Citation: Liu QQ, Li YD, Chen JX, Zhang LL, Guan RC, Zhao W, Meng LY. Prognostic value of preoperative fibrinogen, neutrophil-to-lymphocyte ratio, serum alpha-fetoprotein, and prealbumin for patients with primary liver cancer undergoing transarterial chemoembolization. World J Gastrointest Oncol 2025; 17(6): 103198
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/103198.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.103198
Primary liver cancer, which is primarily represented by hepatocellular carcinoma (HCC), is a leading cause of cancer-related mortality worldwide and characterized by increasing incidence and complex patterns of dissemination that challenge current therapeutic paradigms[1]. Trans-arterial chemoembolization (TACE) is a cornerstone palliative treatment for unresectable HCC and provides survival benefits by delivering chemotherapy directly to the tumor while obstructing its blood supply[2]. Despite the widespread use of TACE, there is a lack of reliable preoperative biomarkers to predict treatment outcomes, necessitating the development of integrated prognostic models. The variability in outcomes following TACE underscores the need for refined prognostic tools to tailor patient management effectively[3].
The application of biomarkers in oncological practice offers a pathway towards precision medicine by facilitating prognostic stratification and therapy optimization[4]. Fibrinogen (FIB), FIB and neutrophil-to-lymphocyte ratio (F-NLR), serum alpha-fetoprotein (AFP), and prealbumin (PA) are potential indicators of clinical outcomes in patients with liver malignancies[5]. Each marker provides insights into different aspects of tumor biology and host response, underpinning their combined prognostic utility[6].
FIB, an acute-phase reactant, is elevated in response to systemic inflammation, a state often accompanying malignancy[7]. FIB also contributes to tumorigenesis by modulating the tumor microenvironment dynamics, enhancing angiogenesis, and facilitating immune evasion[8]. Elevated FIB levels have been linked to poor outcomes in various cancers, underscoring its value as part of the F-NLR score[9]. The NLR, which reflects systemic inflammation, was derived from the balance between neutrophils and lymphocytes in circulation[10]. An elevated NLR, which indicates increased neutrophil-driven inflammation and relative lymphopenia, has been associated with adverse prognoses in cancer possibly because of an impaired antitumor immune response[11].
AFP, a traditional biomarker for HCC, correlates with tumor burden and aggressiveness[12]. It plays intricate roles in hepatocarcinogenesis and influences pathways related to cell proliferation, survival, and immune modulation[13]. Preoperative AFP levels serve as an independent prognostic factor that guides clinical decision-making and offers a window into tumor biology[14]. Post-TACE dynamics of AFP further indicate tumor responsiveness to treatment, adding layers of prognostic depth[15].
Serum PA, which primarily reflects nutritional status and liver function, is gaining attention as an additional prognostic tool. In patients with HCC, nutritional compromise is common and is driven by cancer-related cachexia, hepatic dysfunction, and treatment side effects. Low PA levels may indicate poor nutritional status and impaired hepatic synthetic function, which are crucial determinants of a patient’s ability to withstand treatment and disease progression. These components make PA a valuable addition to prognostic assessment, especially in liver-related oncological contexts[16,17].
Therefore, understanding the interplay of inflammation, coagulation, and nutrition in cancer progression and treatment response is critical. While individual biomarkers such as AFP and NLR have shown prognostic value, their combined use with PA, a marker of nutritional and hepatic function, has not been extensively studied in the context of TACE. This study aims to elucidate the role of these combined markers (F-NLR, AFP, and PA) in predicting clinical outcomes post-TACE Therefore, understanding the interplay of inflammation, coagulation, and nutrition in cancer progression and treatment response is critical. This study aims to clarify the role of combined markers in predicting clinical outcomes post-TACE and offer clinicians a nuanced tool to navigate the intricacies of liver cancer care.
A retrospective analysis was performed on 162 patients with primary liver cancer who underwent TACE at the Third Affiliated Hospital of Qiqihar Medical University (Qiqihar, China) from January 2019 to December 2023. After three cycles of treatment, short-term efficacy was assessed using solid tumor assessment criteria. The evaluation categories are as follows: Complete response (CR) is defined as the complete disappearance of the lesion on imaging with no new lesions; partial response (PR) is indicated by a reduction of more than 30% in the total diameter of the lesion; stable disease (SD) is characterized by a decrease of less than 30% in the total diameter; and progressive disease (PD) is identified by an increase of more than 20% in the sum of the maximum diameters of the lesions or the appearance of new lesions[18]. Patients demonstrating CR, PR, or SD were categorized as having a good prognosis (n = 88), whereas those exhibiting PD were grouped under poor prognosis (n = 74). Patient data were collected via the medical record system and included demographic characteristics, baseline disease-related features, blood test results, coagulation index, F-NLR score, AFP levels, and liver function indicators.
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Qiqihar Medical University. Informed consent was waived due to the retrospective nature of the study and the use of de-identified patient data, which presented no risk to patient care.
The inclusion criteria: (1) All patients had a confirmed diagnosis of primary liver cancer in accordance with the diagnostic criteria specified in the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition)[19]; (2) Age of 18 years or older; (3) Underwent TACE; and (4) Had complete medical records.
The exclusion criteria: (1) Patients with other uncontrolled malignant tumors; (2) Those who were not newly diagnosed and had previously received treatments, such as chemotherapy or radiotherapy for cancer, as these conditions could introduce variability in NLR measurements; (3) Patients with severe immune system disorders or infectious diseases, due to potential confounding effects on FIB levels; (4) Individuals with coagulation disorders; and (5) Pregnant or lactating women.
All patients underwent TACE. Under local anesthesia, the right femoral artery was accessed using the Seldinger technique. A 5F catheter was inserted, and selective angiography was performed to identify the tumor-feeding arteries. Each target artery was embolized with an emulsion of pirarubicin, lobaplatin, and ethiodized oil (Lipiodol), followed by gelatin sponge particles to occlude blood flow. Post-procedure cone-beam computed tomography confirmed adequate drug deposition in the tumor sites. Patients experienced minimal discomfort during and after the procedure, and no complications were reported. This standardized protocol was repeated every 5 days for a total of three cycles.
Both groups of patients provided 10 mL of fasting venous blood prior to treatment. A fully automated blood cell analyzer (model Mindray BC6800; Shenzhen Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) was employed to measure the levels of hemoglobin, white blood cells (WBCs), neutrophils, lymphocytes, platelets (PLTs), monocytes, red cell distribution width (RDW), and mean PLT volume (MPV). The blood was centrifuged at 4000 rpm for 10 minutes by using a low-temperature high-speed centrifuge (Mini1524; Zhuhai Hema Medical Instrument Co., Ltd., Zhuhai, China). Then the samples were stored at low temperatures for subsequent analysis. A fully automated coagulation analyzer (Sysmex CS-5100; Sysmex Corporation, Kobe, Hyogo, Japan) was employed to measure the levels of prothrombin time (PT), thrombin time (TT), and international normalized ratio (INR).
The plasma obtained post-centrifugation was analyzed for FIB levels using a fully automated coagulation analyzer (Sysmex CS-5100; Sysmex Corporation). NLR was then calculated based on the measured values. The normal NLR range for adults was 0.88-4.00. An F-NLR score model was developed by integrating FIB and NLR indicators. A receiver operating characteristic (ROC) curve was plotted to establish the thresholds for FIB and NLR and used to calculate the F-NLR score. The F-NLR scoring system ranges from 0 to 2 points, where 0 indicates that FIB and NLR levels are below their respective thresholds; 1 signifies that either FIB or NLR is at or above the threshold; and 2 indicates that both FIB and NLR levels meet or exceed the thresholds.
After centrifugation, the serum was analyzed to determine serum AFP levels by using chemiluminescence method (DXI800; Beckman Coulter, Inc., Brea, CA, United States). The Beckman Coulter fully automated biochemical analyzer (model AU5800) was employed to measure the levels of total bilirubin (TB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), PA, and gamma-glutamyl transferase (GGT).
Prior to data analysis, this study adhered to a rigorous data cleaning protocol aimed at identifying and rectifying any inconsistencies, errors, or missing values within the dataset. The comprehensive process included meticulous inspection of the dataset, removal of duplicate entries, correction of data entry inaccuracies, and systematic handling of missing values.
To address missing data, we used R 4.3.2 with the mouse package for implementing Multiple Imputation by Chained Equations, a robust and flexible approach for handling missing data across various types of variables.
Imputation was only applied when the proportion of missing data did not exceed 5% to maintain data integrity, thereby minimizing the risk of selection bias. Furthermore, sensitivity analyses were conducted by assuming the outcomes of lost-to-follow-up cases as worst-case and best-case scenarios. If no significant differences were observed between these extreme scenarios, then attrition had minimal effect on the final results, thus validating the robustness of the conclusions drawn. The final results presented were based on the dataset after missing value imputation.
Data analysis was performed using SPSS 29.0 statistical software (SPSS Inc., Chicago, IL, United States). Categorical data are presented as the (n [%]), and χ2 test was used. Continuous variables were first assessed for normal distribution using Shapiro-Wilk test. Normally distributed continuous data were presented as mean ± SD. Non-normally distributed data were analyzed using Wilcoxon rank-sum test, and the (median [25% quantile, 75% quantile]) was used for presentation. P < 0.05 was considered statistically significant. Correlation analysis was conducted using Spearman’s correlation for categorical variables. Further analyses, including correlation and ROC, were conducted. Predictive value was analyzed using xgbTree method, which is a well-established gradient boosted trees technique. This approach employs Classification and Regression Tree method to assess the prognostic implications of the F-NLR score in combination with serum AFP and PA for patients undergoing TACE.
The demographic characteristics of the participants were analyzed to assess their prognostic value when undergoing TACE for primary liver cancer (Table 1). No statistical differences were observed for sex, age, body mass index, diabetes, hypertension, smoking history, drinking history, family history of liver cancer, or cirrhosis (P > 0.05). However, significant differences were found for hepatitis C infection rates (good prognosis: 8 [9.09%], poor prognosis: 18 [24.32%]; χ2 = 6.923, P = 0.009), indicating a higher prevalence in the poor prognosis group. These findings suggest that while most demographic characteristics are similar between the two groups, the presence of hepatitis C infection may play a crucial role in influencing prognosis outcomes among patients.
| Parameters | Good prognosis group (n = 88) | Poor prognosis group (n = 74) | t/χ2 | P value |
| Sex (male) | 72 (81.82) | 58 (78.38) | 0.300 | 0.584 |
| Age (years) | 61.64 ± 8.25 | 61.78 ± 9.99 | 0.103 | 0.918 |
| Body mass index (kg/m²) | 24.46 ± 2.40 | 24.44 ± 2.41 | 0.036 | 0.971 |
| Diabetes | 13 (14.77) | 10 (13.51) | 0.052 | 0.819 |
| Hypertension | 23 (26.14) | 20 (27.03) | 0.016 | 0.898 |
| Smoking history | 24 (27.27) | 25 (33.78) | 0.808 | 0.369 |
| Drinking history | 26 (29.55) | 22 (29.73) | 0.001 | 0.980 |
| Family history of liver cancer | 14 (15.91) | 9 (12.16) | 0.463 | 0.496 |
| Hepatitis B | 67 (76.14) | 49 (66.22) | 1.946 | 0.163 |
| Hepatitis C | 8 (9.09) | 18 (24.32) | 6.923 | 0.009 |
| Cirrhosis | 66 (75) | 48 (64.86) | 1.980 | 0.159 |
No significant differences were observed for tumor thrombus, vascular invasion, Child-Pugh grade, Barcelona Clinic Liver Cancer grade, ascites, portal hypertension, or length of hospital stay (P > 0.05; Table 2). However, significant differences were found for tumor diameter (good prognosis: 4.2 cm [interquartile range, IQR: 3, 7], poor prognosis: 5.2 cm [IQR: 3.12, 10]; W = 2629.500, P = 0.035), number of tumors (good prognosis: 19 [21.59%], poor prognosis: 27 [36.49%]; χ2 = 4.387, P = 0.036), portal vein thrombosis (good prognosis: 45 [51.14%], poor prognosis: 52 [70.27%]; χ2 = 6.126, P = 0.013), and duration of surgery (good prognosis: 85 minutes [IQR: 80, 110], poor prognosis: 80 minutes [IQR: 70, 88]; W = 3948.000, P = 0.019). These results suggest that large tumor size, increased number of tumors, high incidence of portal vein thrombosis, and short surgical duration are associated with poor prognosis outcomes among patients.
| Parameters | Good prognosis group (n = 88) | Poor prognosis group (n = 74) | W/χ2 | P value |
| Tumor diameter (cm) | 4.2 (3, 7) | 5.2 (3.12, 10) | 2629.500 | 0.035 |
| Number of tumors | 19 (21.59) | 27 (36.49) | 4.387 | 0.036 |
| Tumor thrombus | 58 (65.91) | 44 (59.46) | 0.717 | 0.397 |
| Vascular invasion | 49 (55.68) | 47 (63.51) | 1.021 | 0.312 |
| Child-Pugh grade (A/BC) | 60 (68.18)/28 (31.82) | 52 (70.27)/22 (29.73) | 0.082 | 0.774 |
| BCLC grade (A/≥ B) | 42 (47.73)/46 (52.27) | 37 (50)/37 (50) | 0.083 | 0.773 |
| Ascites | 41 (46.59) | 42 (56.76) | 1.663 | 0.197 |
| Portal hypertension | 52 (59.09) | 46 (62.16) | 0.159 | 0.690 |
| Portal vein thrombosis | 45 (51.14) | 52 (70.27) | 6.126 | 0.013 |
| Length of hospital stay (days) | 7 (5, 14) | 7.5 (2, 15) | 3779.500 | 0.078 |
| Duration of surgery (minutes) | 85 (80, 110) | 80 (70, 88) | 3948.000 | 0.019 |
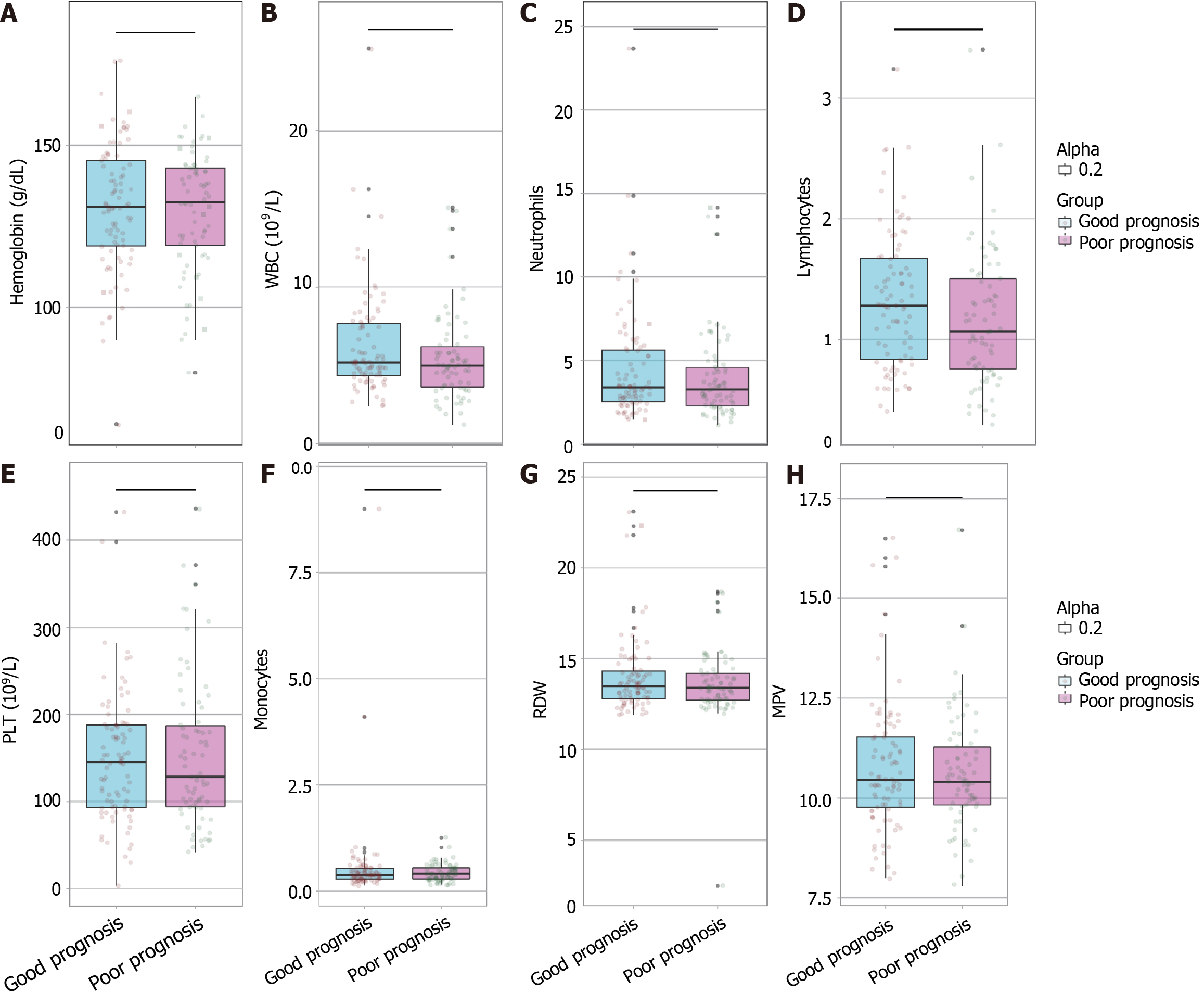
In comparing hematological parameters between the good prognosis group and the poor prognosis group, no significant differences were observed for hemoglobin levels, WBC count, neutrophils, lymphocytes, PLT count, monocytes, RDW, or MPV (all P > 0.05; Figure 1). The P values were 0.873, 0.108, 0.392, 0.059, 0.950, 0.968, 0.488, and 0.745 respectively, indicating that none of the evaluated blood cell-related parameters significantly differed between patients with good and poor prognosis outcomes.
No significant differences were observed for PT (P = 0.483), TT (P = 0.479), APTT (P = 0.813), or INR (P = 0.399; Table 3). These results indicate that coagulation parameters are similar between the two groups.
| Parameters | Good prognosis group (n = 88) | Poor prognosis group (n = 74) | W/t | P value |
| PT (second) | 12 (11.07, 13.12) | 12.2 (11.43, 12.9) | 3047.000 | 0.483 |
| TT | 17.20 ± 1.56 | 17.02 ± 1.77 | 0.709 | 0.479 |
| APTT | 28.4 (27.2, 29.95) | 28.6 (26.88, 30.2) | 3185.000 | 0.813 |
| INR | 1.04 (0.95, 1.15) | 1.06 (0.99, 1.14) | 3005.000 | 0.399 |
No significant differences were observed in FIB levels, but the good prognosis group had lower levels (2.66 [2.13, 3.2] g/L] than the poor prognosis group (2.67 [2.12, 3.71] g/L, P = 0.508; Table 4). NLR was significantly lower in the good prognosis group (3.18 ± 0.97) than in the poor prognosis group (3.87 ± 1.86; P = 0.005). Furthermore, the distribution of the F-NLR scores showed significant variation between the two groups. The good prognosis group had more participants with an F-NLR score of 0 or 1 (44.32% and 36.36%, respectively) compared with the poor prognosis group (20.27% and 43.24%, respectively). Meanwhile, the poor prognosis group had a higher proportion of participants with an F-NLR score of 2 (36.49%) compared with the good prognosis group (19.32%, P = 0.003). These findings indicate that the F-NLR score and its individual components may serve as significant prognostic indicators for patients undergoing TACE.
| Parameters | Good prognosis group (n = 88) | Poor prognosis group (n = 74) | W/t/χ2 | P value |
| FIB (g/L) | 2.66 (2.13, 3.2) | 2.67 (2.12, 3.71) | 3058.500 | 0.508 |
| NLR | 3.18 ± 0.97 | 3.87 ± 1.86 | 2.872 | 0.005 |
| F-NLR (0/1/2) | 39 (44.32)/32 (36.36)/17 (19.32) | 15 (20.27)/32 (43.24)/27 (36.49) | 11.818 | 0.003 |
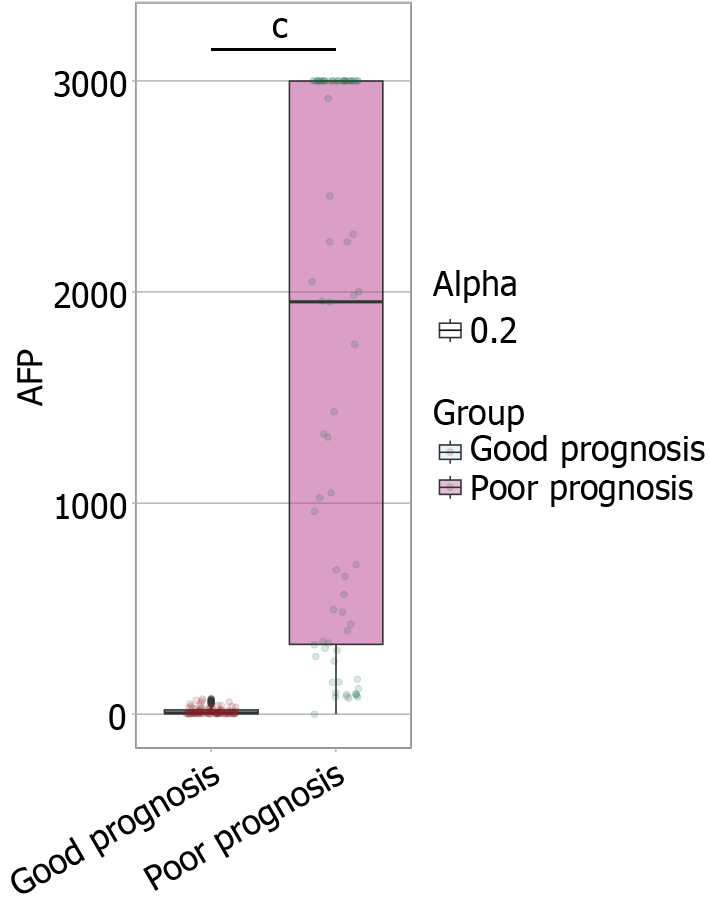
The AFP levels were significantly lower in the good prognosis group (6.27 [3.16, 20.38] ng/mL) than in the poor prognosis group (1954.46 [330.66, 3000] ng/mL, W = 88.000, P < 0.001; Figure 2).
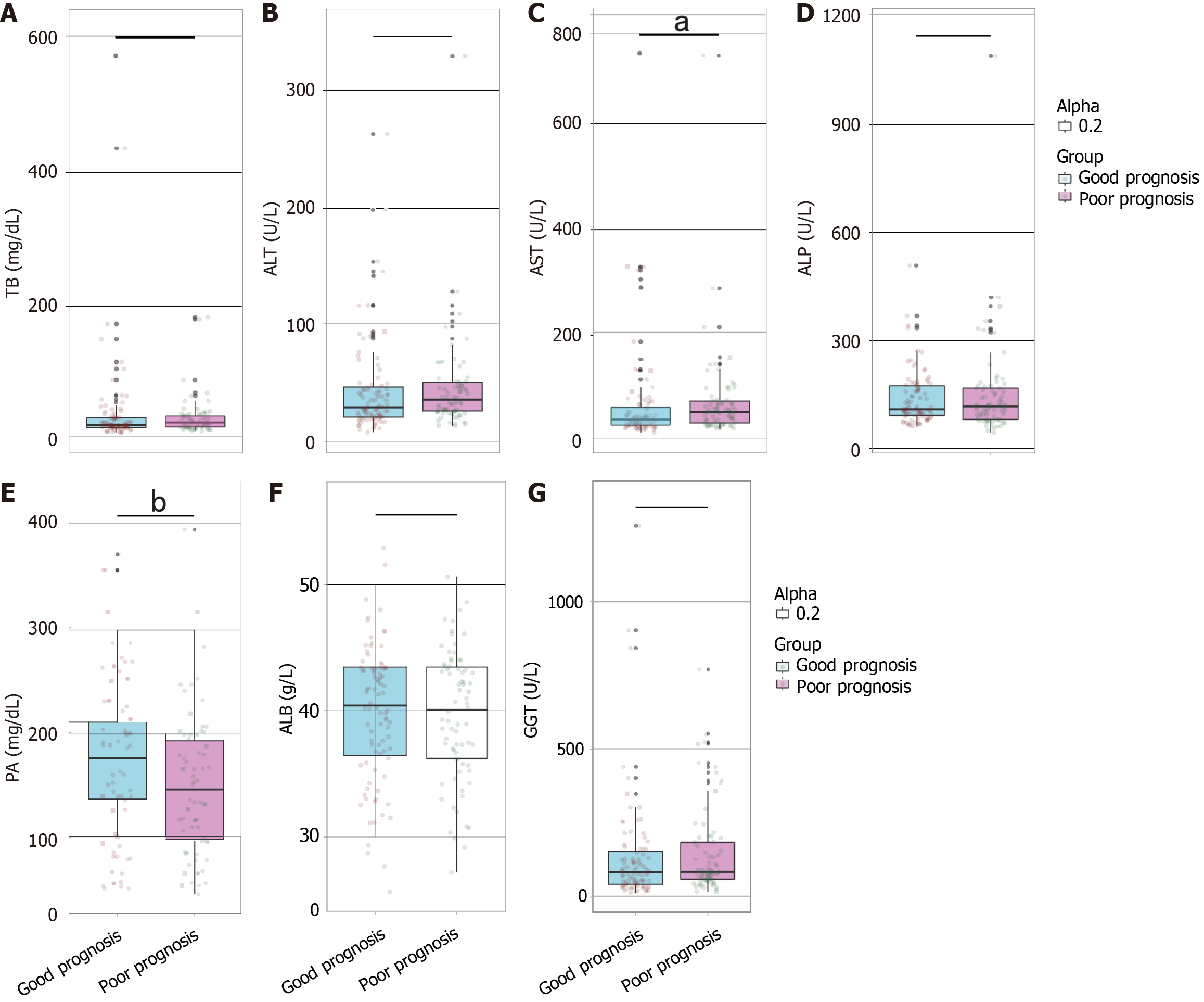
Biochemical parameters were compared between the good prognosis group and the poor prognosis group. Significant differences were observed for AST (W = 2574.500, P = 0.022) and PA (W = 4071.500, P = 0.006; Figure 3). No significant differences were found for TB (W = 2881.500, P = 0.209), ALT (W = 2811.500, P = 0.135), ALP (W = 3342.500, P = 0.772), ALB (t = 0.226, P = 0.821), or GGT (W = 2936.500, P = 0.283). These results suggest that AST and PA may serve as relevant indicators in distinguishing prognosis outcomes among patients, with AST and PA showing statistically significant differences that could reflect their potential roles in prognosis assessment.
The correlation between various parameters and poor prognosis was assessed. Significant correlations were identified for hepatitis C (r = 0.207, P = 0.008), tumor diameter (r = 0.166, P = 0.035), number of tumors (r = 0.165, P = 0.036), portal vein thrombosis (r = 0.194, P = 0.013), duration of surgery (r = -0.185, P = 0.018), AST (r = 0.181, P = 0.021), PA (r = -0.216, P = 0.006), FIB (r = 0.201, P = 0.010), and NLR (r = 0.191, P = 0.015). Additionally, AFP (r = 0.842, P < 0.001) and F-NLR (r = 0.266, P < 0.001) showed particularly strong correlations with poor prognosis. These findings indicate that several factors, including hepatitis C infection, large tumor size, high number of tumors, presence of portal vein thrombosis, long duration of surgery, high AST levels, low PA levels, high FIB levels, and high NLR are associated with a poor prognosis (Table 5).
| Parameters | r | P value |
| Hepatitis C (no/yes: 0/1) | 0.207 | 0.008 |
| Tumor diameter (cm) | 0.166 | 0.035 |
| Number of tumors (no/yes: 0/1) | 0.165 | 0.036 |
| Portal vein thrombosis (no/yes: 0/1) | 0.194 | 0.013 |
| Duration of surgery (minutes) | -0.185 | 0.018 |
| AFP (ng/mL) | 0.842 | p < 0.001 |
| AST (U/L) | 0.181 | 0.021 |
| PA (mg/L) | -0.216 | 0.006 |
| FIB (g/L) | 0.201 | 0.010 |
| NLR | 0.191 | 0.015 |
| F-NLR (0/1/2) | 0.266 | p < 0.001 |
The evaluated predictive factors for the prognosis of patients with primary liver cancer undergoing TACE demonstrated varying levels of diagnostic performance (Table 6). AFP demonstrated the highest area under the curve (AUC) (0.986) and Youden index (0.986), indicating its strong predictive capability with high sensitivity (0.986) and specificity (1.000). Other factors such as duration of surgery (AUC = 0.606, Youden index = 0.284), AST (AUC = 0.605, Youden index = 0.286), FIB (AUC = 0.617, Youden index = 0.268), NLR (AUC = 0.61, Youden index = 0.271), and F-NLR (AUC = 0.645, Youden index = 0.24) showed moderate predictive power. Hepatitis C (AUC = 0.576, Youden index = 0.152), tumor diameter (AUC = 0.596, Youden index = 0.21), number of tumors (AUC = 0.574, Youden index = 0.149), portal vein thrombosis (AUC = 0.596, Youden index = 0.192), and PA (AUC = 0.625, Youden index = 0.264) had lower but still notable predictive capability. These findings suggest that while multiple factors can contribute to prognosis prediction, AFP stands out as a particularly robust predictor among patients undergoing TACE.
| Parameters | Best_threshold | Sensitivities | Specificities | AUC | Youden_index | F1_score |
| Hepatitis C (no/yes: 0/1) | 0.500 | 0.243 | 0.909 | 0.576 | 0.152 | 0.360 |
| Tumor diameter (cm) | 9.100 | 0.324 | 0.886 | 0.596 | 0.210 | 0.444 |
| Number of tumors (no/yes: 0/1) | 0.500 | 0.365 | 0.784 | 0.574 | 0.149 | 0.450 |
| Portal vein thrombosis (no/yes: 0/1) | 0.500 | 0.703 | 0.489 | 0.596 | 0.192 | 0.608 |
| Duration of surgery (minutes) | 97.500 | 0.932 | 0.352 | 0.606 | 0.284 | 0.091 |
| AFP (ng/mL) | 74.000 | 0.986 | 1.000 | 0.986 | 0.986 | 0.993 |
| AST (U/L) | 45.500 | 0.581 | 0.705 | 0.605 | 0.286 | 0.601 |
| PA (mg/L) | 168.25 | 0.662 | 0.602 | 0.625 | 0.264 | 0.329 |
| FIB (g/L) | 3.215 | 0.473 | 0.795 | 0.617 | 0.268 | 0.551 |
| NLR | 4.495 | 0.351 | 0.920 | 0.610 | 0.271 | 0.486 |
| F-NLR (0/1/2) | 0.500 | 0.797 | 0.443 | 0.645 | 0.240 | 0.648 |
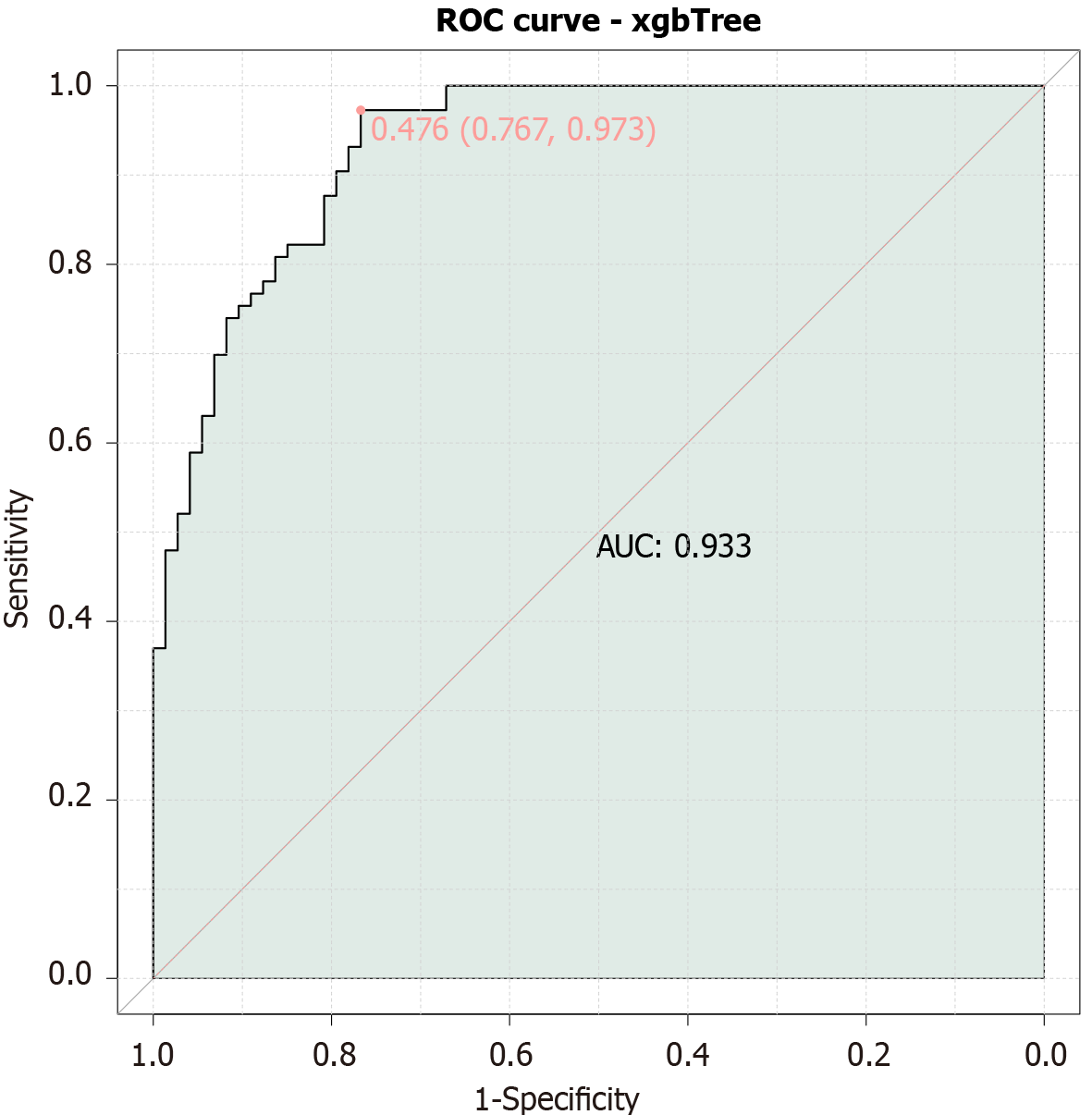
By using the threshold value of AUC of 0.6, we combined three parameters, namely, F-NLR, AFP, and PA, which had relatively high AUC values, to construct an integrated predictive model for the prognosis of patients with primary liver cancer undergoing TACE (Figure 4). The combined model achieved an AUC of 0.933, indicating that F-NLR, AFP and PA collectively provide a highly valuable tool for prognostic prediction in these patients.
The findings provide significant insights into the prognostic value of the combined assessment of the F-NLR score and serum AFP levels in patients with primary liver cancer undergoing TACE. Understanding the mechanisms underlying the use of F-NLR, AFP and PA as prognostic indicators hinges on their biological relevance to liver cancer pathology and systemic response to neoplastic growth. The F-NLR score, which combines FIB and NLR, is particularly informative. Elevated FIB levels can contribute to cancer progression by promoting angiogenesis, cell proliferation, and metastasis through intricate biomolecular pathways involving fibroblast growth factors and integrin-mediated signaling pathways[20,21]. The FIB component of the score is a known acute-phase reactant that is often elevated in response to systemic inflammation, a common occurrence in cancer patients[22]. Increases in FIB can reflect a hypercoagulable state, which is frequently observed in malignancies and is associated with poor prognosis because of enhanced tumor dissemination potential[23].
NLR indicates the inflammatory response of the host[24]. Neutrophilia and relative lymphopenia can be interpreted as the body’s response to tumor-induced systemic inflammation[25]. This inflammation can facilitate a microenvironment conducive to tumor progression and immune escape[26]. Elevated NLR values in the poor prognosis group suggest an inefficient anti-tumor immune response possibly because of neutrophil-mediated suppression of cytotoxic lymphocytes and natural killer cell activity[27]. The interplay of systemic inflammation and immune surveillance circumvents the normal cellular safeguards against tumor growth, resulting in an unfavorable prognostic outcome[28].
Serum AFP, a well-established marker for HCC, reflects tumor burden and biological behavior[29]. Elevated preoperative AFP levels in patients with poor prognosis suggest high tumor activity and aggressiveness[30]. The exceptionally high AUC (0.986) for AFP underscores its utility as a standalone prognostic marker, while its integration with F-NLR and PA further enhances predictive accuracy. AFP is involved in complex molecular interactions that inhibit immune cell functions, promote angiogenesis, and facilitate cancer cell proliferation and survival through the mitogen-activated protein kinase/extracellular signal-related kinase and phosphoinositide 3-kinase/AKT pathways[31]. The relatively higher preoperative and postoperative AFP levels in the poor prognosis group may reflect aggressive cancer phenotypes that do not respond well to TACE.
PA, a marker of nutritional status and hepatic synthetic function, emerged as another significant prognostic factor in our analysis. Patients with liver cancer often experience malnutrition because of cancer-related cachexia, hepatic dysfunction, or treatment side effects. Low PA levels may not only reflect poor nutritional status but also correlate with impaired liver function and systemic inflammation, both of which can significantly affect prognosis. In the context of TACE, sufficient hepatic function is crucial for those undergoing and recovering from treatment. Hence, PA, by reflecting nutritional and hepatic status, can provide valuable insights into a patient’s ability to tolerate and respond to therapy[32,33].
Additionally, it is important to note the significance of hepatitis C infection in the poor prognosis group. Hepatitis C infection can exacerbate liver damage and lead to more aggressive tumor biology, potentially contributing to poorer outcomes following TACE. Future studies should explore the impact of viral hepatitis on prognosis in greater detail to better understand its role in liver cancer progression.
From a therapeutic and clinical perspective, these findings have practical implications. The ability to preoperatively stratify patients based on F-NLR, AFP, and PA could allow for personalized therapeutic approaches. For instance, patients identified as having a poor prognosis because of high scores could be considered for adjunctive treatments or close surveillance to mitigate the risk of progression post-TACE. Furthermore, these biomarkers could serve as targets for therapeutic interventions aimed at modulating inflammatory and immune responses. Drugs that alter FIB levels or inhibit neutrophil-mediated immunosuppression could theoretically improve outcomes in patients identified at high risk.
This study uniquely integrates three distinct biomarkers-F-NLR, AFP, and PA-offering a comprehensive prognostic tool. While each of these markers is individually recognized for its prognostic significance, their combined use represents a novel approach to leveraging their complementary strengths. By integrating these markers, we have not only enhanced the granularity of prognostic predictions but also paved the way for more personalized therapeutic strategies. This combination enables clinicians to tailor treatment plans according to a broader spectrum of biological and clinical parameters, potentially improving patient outcomes and reducing the risk of adverse events. Importantly, this integrated approach underscores the importance of considering multiple facets of cancer pathology and systemic response, providing a more holistic perspective on patient management.
The high AUC values of the combined model (0.933) indicate excellent predictive power. The clinical applicability of this model is promising. By preoperatively stratifying patients based on their biomarker profiles, clinicians can tailor treatment plans more effectively. For example, patients with high-risk scores may benefit from more aggressive therapies or adjunctive treatments, such as targeted therapies or immunotherapy, in addition to TACE. The model also enables early identification of patients who are at higher risk of disease progression post-TACE, allowing for closer monitoring and timely interventions to manage complications and improve outcomes. The simplicity of integrating routine blood tests (AFP, PA) with readily available inflammatory markers (F-NLR) makes this model practical for widespread adoption, avoiding the need for invasive procedures or costly imaging techniques, thereby reducing both patient discomfort and healthcare costs. Incorporating this model into clinical workflows can streamline decision-making processes, enabling oncologists to make evidence-based recommendations that are tailored to an individual's risk profile, leading to more efficient use of resources and better patient outcomes. Despite these advantages, it is important to note that further validation in larger, multicentric studies is necessary to confirm the generalizability and robustness of the model across diverse populations. Additionally, incorporating additional biomarkers or clinical parameters could potentially refine the predictive power of the model even further.
These insights should be interpreted within the study's limitations, such as its retrospective design, which might introduce selection and information biases. Despite thorough statistical adjustments and robust validation, confounding factors cannot be entirely ruled out. The patient cohort was limited to a single institution, which could affect the generalizability of the findings. Future multicentric prospective studies could help validate and expand the findings by potentially incorporating additional biomarkers or clinical parameters to refine the predictive power.
This study underscores the prognostic importance of combining F-NLR, AFP, and PA for patients with primary liver cancer undergoing TACE. Each marker offers distinct insights into the pathophysiological processes at play, and their combined use significantly enhances the prognostic granularity available to clinicians. This integrated approach offers a promising pathway towards personalized treatment regimens that align with the burgeoning field of precision oncology. By harnessing the biological insights afforded by these biomarkers, clinicians can navigate the complexities of cancer treatment, potentially improving outcomes through tailored therapeutic strategies and vigilant monitoring of high-risk patients.
| 1. | Du D, Zhang Z, Wang X, Ma M, Wu N. Retrospective Analysis of Aberrant Hepatic Artery in 1250 Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Ann Ital Chir. 2024;95:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Saeed KM, Aftab A, Bakar MA, Iqbal J. Incidence of acute kidney injury and assessment of its associated risk factors in patients undergoing transarterial chemoembolisation for hepatocellular carcinoma. J Pak Med Assoc. 2022;72:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Kawaguchi Y, Chiang YJ, Velasco JD, Tzeng CD, Vauthey JN. Long-term outcomes in patients undergoing resection, ablation, and trans-arterial chemoembolization of hepatocellular carcinoma in the United States: a national cancer database analysis. Glob Health Med. 2019;1:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Duan H, Zhang J, Wang P, Zhang J, Jiang J. Association between nutritional status and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization. Nutr Hosp. 2023;40:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Wang L, Wen X, Zhuang L, Fang K, Shen J. Adjuvant Transarterial Chemoembolization for Patients with Intrahepatic Cholangiocarcinoma after Surgical Resection: A Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2022;31:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kumar V, Shah M, Gala D, Singh MK, Jeanty H, Thomas R, Forlemu AN, Gayam VR, Etienne D. Hepatic Dystrophic Calcification Secondary to Transarterial Chemoembolization: Case Report and Review of Literature. Cureus. 2023;15:e35765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Jiang H, Chen Y, Liao H, Gu Y, Meng X, Dong W. Operator radiation dose during trans-hepatic arterial chemoembolization: different patients' positions via transradial or transfemoral access. Diagn Interv Radiol. 2022;28:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Hu YD, Zhang H, Tan W, Li ZK. Impact of hepatectomy and postoperative adjuvant transarterial chemoembolization on serum tumor markers and prognosis in intermediate-stage hepatocellular carcinoma. World J Gastrointest Surg. 2023;15:2820-2830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Li T, Zhao J, Zhang S, Wang H, Sun L, Hu J. Efficacy and safety of apatinib and transcatheter arterial chemoembolization as second-line therapy for advanced hepatocellular carcinoma: A retrospective cohort study. J Cancer Res Ther. 2023;19:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Li J, Wang N, Shi C, Liu Q, Song J, Ye X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J Cancer Res Ther. 2021;17:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Risaliti M, Bartolini I, Campani C, Arena U, Xodo C, Adotti V, Rosi M, Taddei A, Muiesan P, Amedei A, Batignani G, Marra F. Evaluating the best treatment for multifocal hepatocellular carcinoma: A propensity score-matched analysis. World J Gastroenterol. 2022;28:3981-3993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Balducci D, Montori M, De Blasio F, Di Bucchianico A, Argenziano ME, Baroni GS, Scarpellini E. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in Treating Portal Hypertension in Patients with Hepatocellular Carcinoma. Medicina (Kaunas). 2023;59:1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Sideris GA, Tsaramanidis S, Vyllioti AT, Njuguna N. The Role of Branched-Chain Amino Acid Supplementation in Combination with Locoregional Treatments for Hepatocellular Carcinoma: Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15:926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Krieg S, Essing T, Krieg A, Roderburg C, Luedde T, Loosen SH. Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019. Cancers (Basel). 2022;14:2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Ilagan CH, Goldman DA, Gönen M, Aveson VG, Babicky M, Balachandran VP, Drebin JA, Jarnagin WR, Wei AC, Kingham TP, Abou-Alfa GK, Brown KT, D'Angelica MI. Recurrence of Hepatocellular Carcinoma After Complete Radiologic Response to Trans-Arterial Embolization: A Retrospective Study on Patterns, Treatments, and Prognoses. Ann Surg Oncol. 2022;29:6815-6826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Yan H, Chen S, Qiong Y, Cai L. Preoperative prealbumin-to-fibrinogen ratio to predict survival outcomes in hepatocellular carcinoma patients after hepatic resection. J Med Biochem. 2022;41:290-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Xu L, Zhao D, Tian P, Ding J, Jiang Z, Ni G, Hou Z, Ni C. Development and Validation of a Prognostic Model for Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma Based on Preoperative Serum Prealbumin. J Hepatocell Carcinoma. 2023;10:2239-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3302] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 19. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Zhang L, Yang C, Wu Z, Dai Z, Chen M, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 197] [Reference Citation Analysis (0)] |
| 20. | Ananchuensook P, Sriphoosanaphan S, Suksawatamnauy S, Siripon N, Pinjaroen N, Geratikornsupuk N, Kerr SJ, Thanapirom K, Komolmit P. Validation and prognostic value of EZ-ALBI score in patients with intermediate-stage hepatocellular carcinoma treated with trans-arterial chemoembolization. BMC Gastroenterol. 2022;22:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Gao FL, Wang Y, Huang XZ, Pan TF, Guo JH. I-125 seeds brachytherapy with transcatheter arterial chemoembolization for subcapsular hepatocellular carcinoma. BMC Gastroenterol. 2022;22:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 22. | Yang XY, Deng JB, An TZ, Zhou S, Li JX. Tumor enhancement ratio with unenhanced imaging is an independent prognostic factor for patients with hepatocellular carcinoma after transarterial chemoembolization. J Int Med Res. 2021;49:3000605211058367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Mendiratta-Lala M, Masch WR, Shampain K, Zhang A, Jo AS, Moorman S, Aslam A, Maturen KE, Davenport MS. MRI Assessment of Hepatocellular Carcinoma after Local-Regional Therapy: A Comprehensive Review. Radiol Imaging Cancer. 2020;2:e190024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9:e003311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 25. | Ogasawara S, Koroki K, Kanzaki H, Kobayashi K, Kiyono S, Nakamura M, Kanogawa N, Saito T, Kondo T, Nakagawa R, Nakamoto S, Muroyama R, Chiba T, Kato N. Changes in therapeutic options for hepatocellular carcinoma in Asia. Liver Int. 2022;42:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Tak KY, Jang B, Lee SK, Nam HC, Sung PS, Bae SH, Choi JY, Yoon SK, Jang JW. Use of M2BPGi in HCC patients with TACE. J Gastroenterol Hepatol. 2021;36:2917-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Choi Y, Jeong YS, Hwang JS, Kim HC, Chung JW, Choi JW. C-Arm Computed Tomographic Image Fusion for Repetitive Transarterial Chemoembolization of Hepatocellular Carcinoma. J Comput Assist Tomogr. 2023;47:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Piron L, Cassinotto C, Guiu B. [Interventional radiology of liver tumors]. Presse Med. 2019;48:1156-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Restrepo CR, Tabori NE, Sabri SS, Horton KM, Sivananthan G. Prospective Study of Radial Artery Occlusion Following Transradial Arterial Access during IR Procedures. J Vasc Interv Radiol. 2022;33:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Egger ME, Armstrong E, Martin RC 2nd, Scoggins CR, Philips P, Shah M, Konda B, Dillhoff M, Pawlik TM, Cloyd JM. Transarterial Chemoembolization vs Radioembolization for Neuroendocrine Liver Metastases: A Multi-Institutional Analysis. J Am Coll Surg. 2020;230:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Jia KF, Wang H, Yu CL, Yin WL, Zhang XD, Wang F, Sun C, Shen W. ASARA, a prediction model based on Child-Pugh class in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Hepatobiliary Pancreat Dis Int. 2023;22:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Lei K, Wang JG, Li Y, Wang HX, Xu J, You K, Liu ZJ. Prognostic value of preoperative prealbumin levels in patients with unresectable hepatocellular carcinoma undergoing transcatheter arterial chemoembolisation. Heliyon. 2023;9:e18494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Huo RR, Liu HT, Deng ZJ, Liang XM, Gong WF, Qi LN, You XM, Xiang BD, Li LQ, Ma L, Zhong JH. Dose-Response Between Serum Prealbumin and All-Cause Mortality After Hepatectomy in Patients With Hepatocellular Carcinoma. Front Oncol. 2020;10:596691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |