Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.106278
Revised: March 8, 2025
Accepted: March 17, 2025
Published online: May 15, 2025
Processing time: 83 Days and 23.7 Hours
One of the main causes of cancer-related morbidity and mortality globally is hepatocellular carcinoma (HCC). At every stage of the disease, HCC may now be treated using a variety of therapy techniques. Nevertheless, despite the abun
Core Tip: One of the main causes of cancer-related morbidity and mortality globally is hepatocellular carcinoma (HCC). When assessing prognosis and tracking tumor metastases or recurrence, prognostic indicators are crucial. There are many prognostic markers in HCC. We mainly focused on newly reported prognostic markers in the pathogenesis of HCC. Further, we highlighted how these prognostic markers correlated to clinical parameters such as tumor node metastasis, tumor diameter, differentiation, hepatocirrhosis, vascular invasion, and others in HCC. Therefore, the identification of specific prognostic biomarkers of HCC helps to provide a great opportunity to improve the prognosis in patients with HCC and provide therapeutic targets.
- Citation: Rafaqat S, Noshair I, Shahid M, Bibi S, Hafeez R, Hamid H. Correlation between prognostic markers and clinical parameters in hepatocellular carcinoma: Pathophysiological aspects to therapeutic targets. World J Gastrointest Oncol 2025; 17(5): 106278
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/106278.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.106278
Around the world, hepatocellular carcinoma (HCC) is a liver primary tumor and one of the most common malignancies. Of patients with cirrhosis, over 85% develop HCC[1]. The prevalence of primary liver cancer is predicted to increase during the next 20 years. To display worldwide cancer statistics, Rumgay et al[2] took data from the GLOBOCAN 2020 web-based platform. Between 2020 and 2040, they projected that the number of liver cancer fatalities would rise by 56.4%, and the incidence would rise by 55.0%. Consequently, primary liver cancer has always presented a serious risk to world health, both now and in the future. Etiologically, HCC often arises in chronic carriers of the HBV, particularly in sub-Saharan Africa and East Asia where HBV is widespread[3].
However, significant variations have been seen among nations. The majority of HCC cases are found in Asia, with a high frequency in numerous nations, especially in East Asia (more than 20 cases per 100000 population). The incidence, for instance, is 35 per 100000 in China, 49 per 100000 in Korea, 29 per 100000 in Japan, and 99 per 100000 in Mongolia. The rates are similarly high in Thailand and Hong Kong[4,5]. As viral to nonviral causes of HCC have changed over time, the burden of the disease has moved from the low-to-moderate sociodemographic index areas to the high sociodemographic index regions[6].
The primary risk factor for the development of HCC is liver cirrhosis (LC). Viral [chronic hepatitis B (CHB) and hepatitis C], toxic (alcohol and aflatoxins), metabolic (diabetes, nonalcoholic fatty liver disease, hereditary hemochromatosis), and immune-related (primary biliary cirrhosis and autoimmune hepatitis) are the main recognized risk factors for HCC[7]. Recently, the shifting distribution and natural history of HBV and hepatitis C virus infection have been blamed for the regional diversity in HCC incidence[8].
Tumor size, nodule count, positive surgical margin, microvascular invasion (MVI), tumor node metastasis (TNM) stage, and Edmondson-Steiner grade were among the risk variables that may have been substantially linked to early HCC recurrence. Many more patients can prevent early recurrence and hence live longer with effective risk surveillance of HCC development and curative resection[3].
Since cirrhosis represents advanced liver damage and significantly alters normal liver physiology, the presence of cirrhosis in a patient can significantly affect the effectiveness of certain prognostic markers by potentially distorting their values and making them less accurate in predicting patient outcomes. This is especially true when the marker heavily relies on liver function. As a result, a marker may not accurately reflect the overall health status of a patient with cirrhosis compared to without liver disease. The presence of other medical conditions in a patient with cirrhosis can further impact the accuracy of prognostic markers. HCC risk factors have a significant impact on the prognosis by influencing the stage at which the cancer is diagnosed. For example, cirrhosis, heavy alcohol use, CHB or hepatitis C infection, large tumor size, vascular invasion, and advanced stage all contribute to a poorer prognosis because they increase the likelihood of metastasis and limit treatment options. Early detection in patients with known risk factors is essential for better outcomes[9].
The clinical, pathological, and prognostic features of HCC vary, making it a heterogeneous malignancy. Numerous clinicopathological factors, such as serum alpha-fetoprotein (AFP) level, tumor size and focality, lymphovascular invasion, pathological stage and grade, and background liver, influence the prognosis of HCC[10]. Aflatoxin exposure, cirrhosis, heavy alcohol use, and CHB or hepatitis C infection are risk factors for HCC that have a substantial impact on prognosis by raising the chance of developing advanced-stage tumors at diagnosis. This results in worse treatment outcomes and lower survival rates because of the advanced disease progression and compromised liver function; in other words, the more significant the risk factors, the worse the prognosis for the patient with HCC.
Emerging markers for HCC that could potentially improve survival rates by enabling early diagnosis and targeting specific therapeutic pathways include Glypican-3 (GPC3), Golgi protein-73 (GP73), microRNAs, des-gamma-carboxy prothrombin (DCP), fucosylated GP73, and certain circulating extracellular vesicles, which can provide insights into the molecular profile of the tumor and identify potential therapeutic targets. Numerous signaling pathways are dysregulated in HCC, leading to unchecked cell division, metastasis, and HCC recurrence[11].
Treatment potential is also offered by pathways related to cell differentiation, telomere control, epigenetic change, and stress response, in addition to the often modified and therapeutically targeted receptor tyrosine kinase pathways in HCC. To achieve therapeutic breakthroughs in the management of HCC, it is essential to investigate the major signaling pathways and their inhibitors. Tyrosine kinase inhibitors, immune checkpoint inhibitors (ICIs), and combination regimens are now the main treatment modalities for advanced HCC. ICIs and tyrosine kinase inhibitors, anti-vascular endothelial growth factor (VEGF) treatments, and combinations of two immunotherapy regimens are being studied in current research. It is anticipated that the findings of these studies will completely transform the treatment of HCC at every stage[11].
Even in nations with adequate medical resources, HCC surveillance is significantly underused, even though early identification and surveillance improve the likelihood of possibly curative therapy. Liver transplantation, surgical resection, or local ablation are all curative options for early-stage HCC. The features of the tumor, the degree of underlying liver dysfunction, age, other medical comorbidities, the availability of medical resources, and local knowledge all influence the choice of treatment. Patients with intermediate-stage cancer are treated with catheter-based locoregional therapy. Patients with advanced-stage HCC respond well to therapy with kinase inhibitors and ICIs. Within the next few years, there is hope for a significant decrease in the global burden of HCC through the rational implementation of prevention, the achievement of global targets for the eradication of viral hepatitis, and advancements in HCC surveillance and treatment[12].
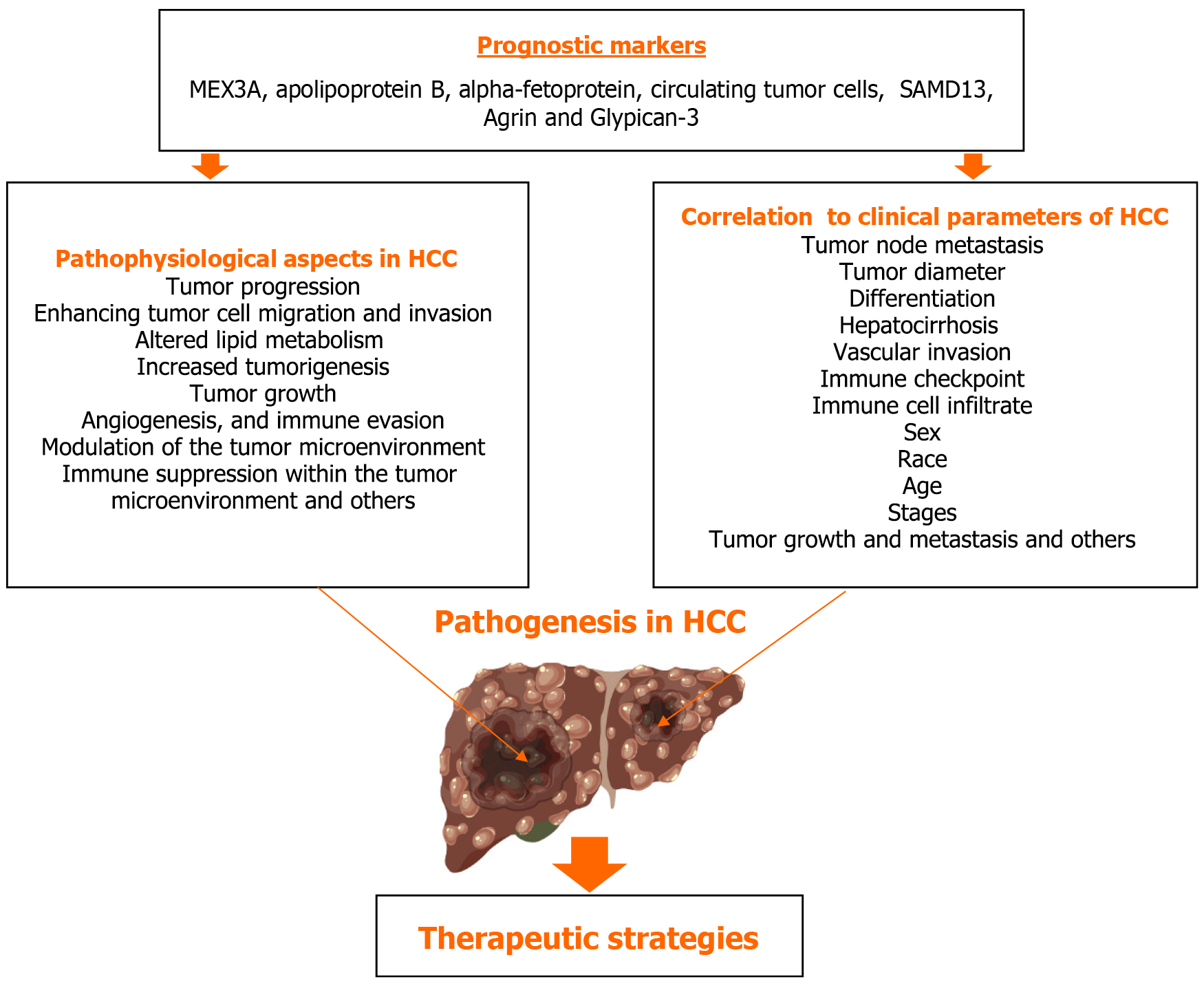
Nevertheless, despite the abundance of effective therapeutic choices, the prognosis for people with HCC is still typically dismal. When assessing prognosis and tracking tumor metastases or recurrence, prognostic indicators are crucial. The pathophysiology of HCC involves several prognostic indicators. However, we primarily concentrate on recently identified prognostic markers such MEX3A, apolipoprotein B (APOB), AFP, circulating tumor cells (CTCs), SAMD13, Agrin, and GPC3. Additionally, we emphasized the correlation between these prognostic indicators and clinical characteristics including TNM, tumor diameter, differentiation, hepatocirrhosis, vascular invasion, and others in HCC, as illustrated in Figure 1.
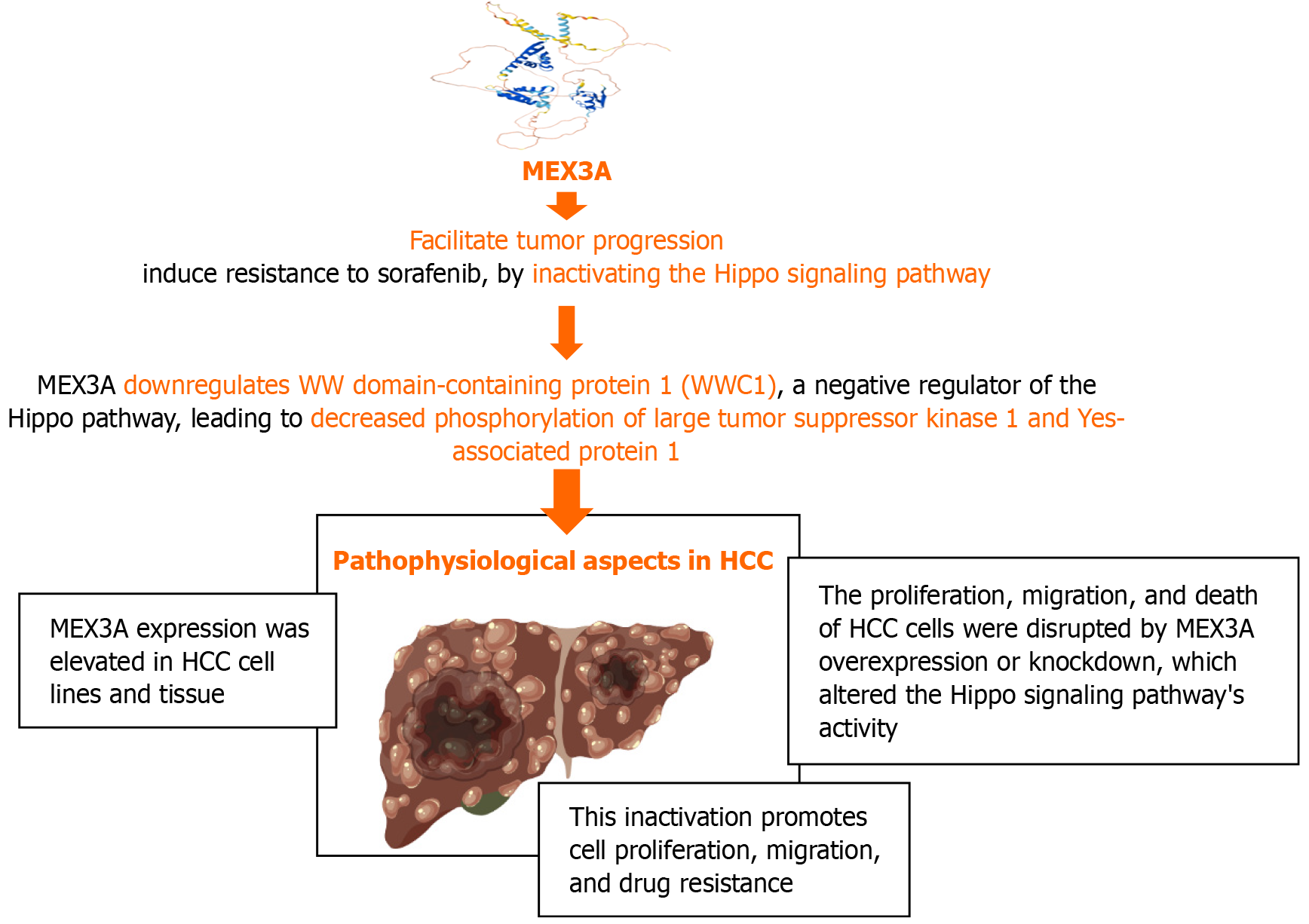
MEX3A is an RNA-binding protein that regulates gene expression at the post-transcriptional level. Members of the RNA-binding protein family MEX3 are evolutionarily conserved. MEX3A, MEX3B, MEX3C, and MEX3D are the four homologous isoforms of the Mex-3 RNA binding family. A ubiquitin E3 ligase RING domain and two K homology domains make up each of the four Mex-3 proteins. This family has a significant impact on the onset and spread of cancer, positively regulating stem cell and immunotherapy resistance, migration, and proliferation[13]. The molecular processes behind MEX3A and its pathophysiological characteristics in HCC are explained in Figure 2.
In liver cancer, MEX3A is upregulated, and this increases with histological grade. MEX3A shows a modest capacity for liver cancer diagnosis. A strong correlation was found between low survival and high expression of MEX3A. The prognosis of liver cancer may be predicted independently by MEX3A expression[14]. The methylation level of the MEX3A promoter was much lower in patients with HCC compared with patients with CHB and healthy individuals. MEX3A mRNA levels in patients with HCC were much greater than those in healthy controls and patients with CHB. HCC can be identified early on using a non-invasive biomarker for the presence of MEX3A promoter hypomethylation[15].
RNA-binding protein MEX3A has a significant role in the development and spread of tumors. MEX3A may promote the growth of HCC and reduce sorafenib sensitivity, by deactivating Hippo signaling. Targeting MEX3A may be a potential treatment approach for HCC[16].
MEX3A is an RNA-binding protein that interacts with the Hippo signaling system and has been linked to the development of hepatocellular cancer. MEX3A is elevated in HCC tissues and cell lines and promotes migration, proliferation, and resistance to the chemotherapeutic medication sorafenib. By reducing the phosphorylation of LATS1 and YAP1, MEX3A mechanistically inhibits WWC1, a recognized Hippo pathway activator, which in turn deactivates Hippo signaling. This inactivation helps HCC tumors grow and spread more quickly[16].
Beyond HCC, MEX3A has been shown to influence other oncogenic pathways in different cancers. In breast cancer, MEX3A activates the RhoA/ROCK1/LIMK1 signaling pathway, enhancing cell proliferation and migration. In endometrial carcinoma, MEX3A interacts with DVL3 to stabilize Wnt/β-catenin signaling and promote tumor progression[17]. These findings suggest that the role of MEX3A in cancer progression is context-dependent, affecting various signaling pathways across different cancer types[18]. While the interaction between MEX3A and the Hippo pathway is established in HCC, its potential involvement with other oncogenic signaling pathways in this cancer type requires further investigation.
The post-transcriptional and post-translational regulation of MEX3A likely is the cause of their pro-oncogenic properties. As a result, they have an impact on signaling pathways, cell migration, proliferation, and maybe tumor immune evasion. Numerous studies examining the role of MEX3A in carcinogenesis have been released in recent months. This improved our understanding of the role MEX3A plays in xenograft and in vitro investigations of oncogenic phenotypes, but there is still a dearth of especially thorough mechanistic research on post-transcriptional gene control[19].
To our knowledge, there is a lack of publicly available data to identify putative mRNA targets and their respective binding sites as well as putative target proteins that are ubiquitinated by MEX3 proteins. These analyses would help to identify a broader spectrum of MEX3-affected signaling pathways and to shed light on possible paralog-specific functions. As a result, even though the number of genes affected by the MEX3 protein is increasing, few reports describe or distinguish between post-transcriptional and post-translational control[19].
One of the main components of low-density lipoprotein (LDL) and very LDL, APOB is a protein that transports lipids such as cholesterol and triglycerides in the blood. The formation and progression of several human tumor types are greatly impacted by APOB. It demonstrated a significant drop in APOB expression levels in those with HCC. Furthermore, APOB expression and immune checkpoint gene expression were shown to be significantly correlated as was immune cell infiltration. Several clinicopathological characteristics were also correlated with the levels of APOB expression in HCC tissues[20].
APOB may be an important new prognostic indicator for individuals with HCC who had liver resection for treatment. Additionally, due to the connection between APOB and residual liver function, increased APOB levels may be a sign of a poorer prognosis for individuals with HCC[21]. The use of comparative genomics, which integrates genomic data from human and mouse HCC tumors, demonstrates that loss of APOB in HCC is strongly linked to poor survival of patients with HCC[22].
APOB may regulate several genes involved in the formation of HCC, and its inactivation is linked to poor outcomes in patients with HCC[23]. In short, in individuals with CHB, a low blood ApoB/ApoA1 ratio was associated with a higher risk of LC and HCC development. Patients with LC and HCC at different stages showed a significant difference in the serum ApoB/ApoA1 ratio, and AFP-assisted diagnosis demonstrated the capacity to improve the specificity and detection effectiveness of AFP for HCC and AFP-negative HCC. To identify high-risk individuals for LC or HCC and to enable early diagnosis and treatment of these conditions, the blood ApoB/ApoA1 ratio in patients with CHB may be helpful[24].
Reduced APOB expression in HCC is linked to a poorer prognosis. This downregulation is associated with decreased infiltration of tumor-suppressive immune cells and decreased expression of immunological checkpoints, suggesting that APOB may modulate immune cell dynamics to influence the tumor microenvironment (TME)[20].
Compared to normal liver tissues, HCC tissues have noticeably lower levels of APOB expression. Different immune cell infiltration levels are linked to this downregulation: APOB expression has an inverse relationship with CD4+ T cell, dendritic cell, and B cell infiltration levels. This implies a correlation between increased infiltration of these immune cells into the TME and decreased APOB levels. Higher APOB levels may encourage the presence of these cytotoxic T cells within the tumor as seen by the positive connection found between APOB expression and CD8+ T cell infiltration. The intricate function of APOB in regulating immune responses in HCC is highlighted by these findings. APOB expression and immune checkpoint molecules, which can inhibit immunological function and promote tumor immune evasion, are significantly correlated[20].
In HCC, the exact mechanisms through which APOB affects immune cell infiltration and tumor growth remain unclear. The observed associations with immune checkpoint expression and immune cell infiltration imply that APOB might alter the TME, which could have an impact on tumor immunity and immunotherapy compliance. Given these correlations, more investigation is necessary to examine the potential therapeutic benefits of APOB pathway targeting. To improve anti-tumor immune responses and clinical outcomes for patients with HCC, it may be possible to modify APOB expression or function. The expression of APOB in HCC is closely associated with immune cell infiltration patterns and has potential as a therapeutic target as well as a prognostic index. Therapies targeting APOB or its lipid metabolism pathways hold promise for enhancing immune responses in HCC, particularly in combination with ICIs. Statins have been linked to reducing HCC progression and may enhance checkpoint inhibitor responses. Studies suggest that reducing lipid overload in immune cells can make tumors more susceptible to PD-1/PD-L1 blockade. Inhibiting APOB-related pathways may synergize with immunotherapy by improving T cell infiltration and cytotoxicity. However, further clinical studies are needed to validate these strategies.
The prognosis for patients with HCC is negatively correlated with reduced APOB expression. Disease-free survival, progression-free survival (PFS), and overall survival (OS) are all negatively correlated with low APOB levels. Larger and more progressed tumors are among the more aggressive tumor characteristics seen in patients with downregulated APOB. Immunotherapy responses may be impacted by APOB regulation, which may affect immune checkpoint function.
APOB and MEX3A could contribute to better risk stratification in patients with chronic hepatitis, cirrhosis, and HCC by identifying those at higher risk of malignant transformation, disease progression, and treatment resistance. The development of cirrhosis and HCC is facilitated by the advancement of fibrosis and elevated liver inflammation, both of which are linked to low APOB levels. Reduced APOB expression is associated with increased immune evasion and a more immunosuppressive TME in individuals with cirrhosis. APOB may be utilized to find cirrhosis individuals at high risk who might benefit from metabolic or early immunotherapy treatments to lower their likelihood of developing HCC. Early on in the progression from cirrhosis to HCC, MEX3A overexpression occurs. Patients with MEX3A-positive cirrhosis may be at increased risk for aggressive, treatment-resistant HCC and may benefit from more frequent monitoring and focused treatment.
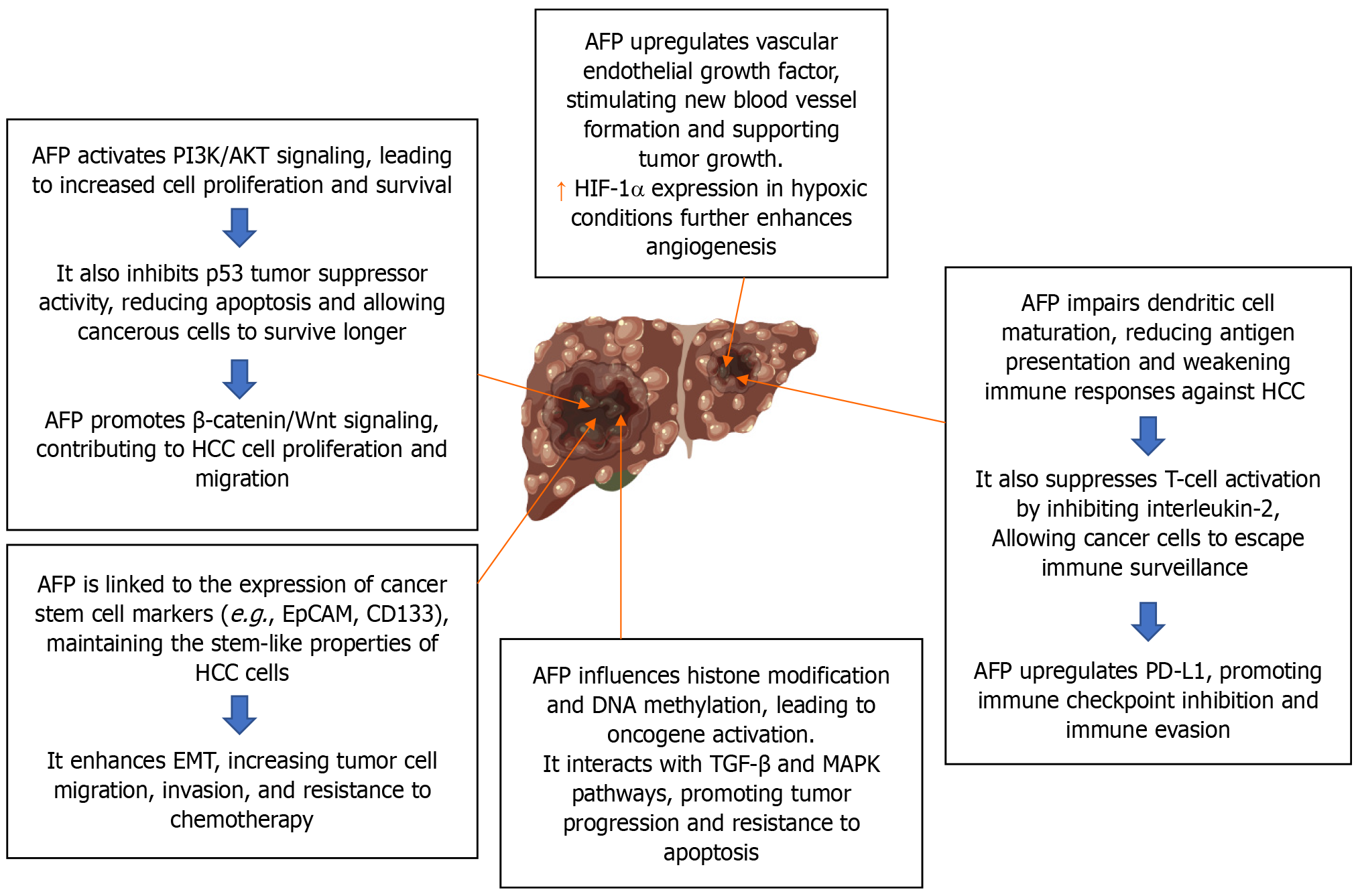
During the first trimester of pregnancy, the fetal liver and yolk sac manufacture the 70 kD glycoprotein known as AFP. The main molecular pathways behind the role of AFP in HCC are explained in Figure 3. The tumor marker serum AFP is often used for HCC screening, diagnosis, and therapy monitoring. Investigations on the nature and behavior of this normal AFP HCC (N-HCC) have been limited to this point. Hepatitis virus infections and cirrhosis were less common in patients with N-HCC who were also somewhat older. Generally, their tumors were well-differentiated, smaller, and less vascularly invaded. The carcinogenesis of N-HCC may differ from that of normal HCC[25].
Those in the high-normal AFP group had smaller tumors, more multiple tumors, and a lower liver functional reserve than those in the low-normal AFP group. It revealed that the following independent prognostic variables were associated with poor OS: Advanced albumin-bilirubin grades; vascular invasion; tumor size ≥ 5 cm; non-curative treatment methods; and the absence of antiviral medication. It also revealed no association between OS and serum AFP levels. Patients with HCC who had normal blood AFP levels had different outcomes depending on factors such as liver functional reserve, antiviral medication, tumor size, vascular invasion, and treatment methods[26].
Acute and chronic liver diseases as well as cirrhosis are frequently associated with elevated AFP levels, which may also indicate the degree of hepatic damage and subsequent regeneration[27]. In addition to the standard morphological parameters such as tumor size and several nodules, AFP is well-positioned to be included in composite criteria for liver transplantation, which take into account surrogates of tumor biology[28].
The effectiveness of therapy for HCC has been demonstrated to be correlated with AFP levels. Prognosis and treatment response can be better understood by looking at baseline AFP levels as well as variations in AFP over time. Higher pretreatment AFP levels are linked to worse disease-free survival and OS in patients with HCC. According to a meta-analysis, individuals with HCC who had elevated pretreatment blood AFP-L3% levels had a poor prognosis[29].
Reduced response rates to ICIs have been associated with elevated baseline AFP levels (≥ 400 ng/mL). When treated with ICIs, patients with AFP ≥ 400 ng/mL had an increased risk of disease progression and mortality, according to a meta-analysis and retrospective cohort research. Improvements in treatment results are linked to a decrease in AFP levels. According to one trial, patients taking ICIs who experienced an early AFP response (defined as a reduction of more than 20% during the first 4 weeks of treatment) had longer progression-free survival and OS rates as well as greater objective response rates[30]. A similar correlation was shown between an early AFP response and better treatment effectiveness and survival outcomes in patients receiving antiangiogenic therapy[30].
Due to its high recurrence rate and poor prognosis, HCC is a major type of primary liver cancer and a major cause of cancer-related deaths worldwide. AFP is a biomarker for the diagnosis of HCC, and many patients with HCC have elevated serum expression levels of AFP, which is a risk factor for HCC progression. Numerous studies have shown that AFP functions as an immune suppressor, and that it can promote malignant transformation during HCC development and may be involved in the process of multidrug resistance in patients with liver cancer. It discusses drug resistance mechanisms during HCC drug treatment and examines the relationship between the mechanism of AFP in HCC development and progression and HCC drug resistance[31].
AFP is one of the most widely used biomarkers for HCC, with roles in screening, diagnosis, prognosis, and treatment response monitoring. However, its utility varies across different stages of HCC progression. In those who are at risk for developing HCC, AFP > 20 ng/mL may be a sign of early-stage HCC [(Barcelona Clinic Liver Cancer (BCLC) 0 & A: Very early/early stage]. Small tumors with high AFP levels may have a more aggressive tumor biology. AFP levels (> 200-400 ng/mL) are increasingly prevalent in intermediate-stage HCC (BCLC B: Locally advanced, no extrahepatic spread) as tumors enlarge. AFP levels that are noticeably higher (> 400-1000 ng/mL) in advanced-stage HCC (BCLC C: Metastatic or macrovascular invasion) frequently signify advanced illness. Greater AFP levels are associated with vascular invasion, metastasis, and a worse survival rate, making them a strong predictor of a bad prognosis. As a result of the tumor burden, AFP levels may continue to grow in end-stage HCC (BCLC D: Terminal stage, poor liver function/performance status). It might influence choices about palliative care since patients with elevated AFP can have poorer prognoses and lower quality of life[27].
In clinical settings, AFP has been widely used as a biomarker for HCC diagnosis. The sensitivity of combined serum AFP and ultrasonography only ranges from 40% to 65%, according to current research, which indicates that poor sensitivity and specificity are difficulties in the diagnosis of HCC, particularly for early-stage HCC. Therefore, it is still to be determined if serum AFP is useful in predicting metastases in small HCC.
In late-stage metastatic HCC, AFP is more significant than in early-stage HCC. The diagnostic utility of AFP is limited by its low sensitivity (approximately 40%-60%) in early-stage HCC, despite its usage for screening and monitoring in patients with high risk. AFP, on the other hand, is highly predictive of aggressive disease and treatment response since it is markedly raised in advanced/metastatic HCC (approximately 70%-85% sensitivity)[32,33].
Although AFP is a commonly utilized biomarker for HCC, the stage of the disease affects both its diagnostic sensitivity and specificity. Tumor load, liver function, and underlying liver disease (cirrhosis, hepatitis B/C, and nonalcoholic fatty liver disease, or nonalcoholic fatty liver disease/non-alcoholic steatohepatitis) all affect how well it performs. AFP in early-stage detection and screening for HCC has a 40%-60% sensitivity and 80%-90% specificity. AFP in HCC at intermediate stage has a 60%-70% sensitivity and 70%-80% specificity. In advanced HCC, AFP has a sensitivity of 70%-80% and a specificity of 60%-75%. Also, end-stage HCC has an 80%-85% sensitivity and 50%-65% specificity[27,34].
Although AFP is still a crucial biomarker for HCC, the disease phases affect how well it can detect cancer. Although it has to be used in conjunction with imaging for early diagnosis, it is most helpful for prognosis and therapy selection in intermediate and advanced HCC. Multibiomarker panel developments in the future (e.g., AFP + DCP + GPC3) might improve risk stratification and HCC screening. The ability of AFP, a well-known biomarker in HCC, to forecast how effectively systemic treatments like immunotherapy and sorafenib will work has been studied. The response rate to ICIs such as nivolumab or atezolizumab + bevacizumab is often lower in patients with elevated AFP. Increased M2 macrophages (protumor activity) and minimal T cell infiltration are hallmarks of an immunosuppressive TME linked to high AFP. A notable decrease in AFP could suggest that the medication is working, but consistently elevated levels might call for changing the course of treatment.
In patients with HCC, a very high AFP level of more than 10000 at diagnosis is linked to a much lower OS at 1 month and 6 months, even with better treatment choices. These patients are also more likely to develop distant metastatic illness[35]. In terms of sensitivity and specificity, serum AFP levels demonstrated high accuracy in diagnosing HCC. Whether AFP was used alone or in conjunction with ultrasonography, its threshold of 400 ng/mL was superior to that of 200 ng/mL[36].
Serum AFP levels were considerably greater in patients with HCC, with bigger tumor sizes, with liver cancer with cirrhosis of the liver, with portal vein thrombosis, with metastases, with high Child-Pugh score, with high BCLC stage, and with an advanced clinical stage. Serum AFP levels were also noticeably higher in individuals with high HBV DNA levels who were also hepatitis B surface antigen and hepatitis B e antigen positive. Higher levels of protein induced by vitamin K absence or antagonist-II, alanine aminotransferase (ALT), aspartate aminotransferase, alpha-l-fucosidase, gamma-glutamyl transpeptidase (γ-GT), γ-GT/ALT, direct bilirubin, indirect bilirubin, fibrinogen, and D-dimer were all elevated in patients with elevated serum AFP levels. White blood cell, neutrophil, monocyte, platelet, and neutrophil-to-lymphocyte ratios were all greater in patients who tested positive for AFP[37].
Monitoring AFP levels before and during treatment can aid in assessing prognosis and tailoring therapeutic strategies for patients with HCC. However, while AFP is a useful biomarker, treatment decisions should also consider other clinical factors and biomarkers.
Cancer cells that separate from a tumor and enter the circulation are known as CTCs. They can spread to other areas of the body and lead to the growth of additional tumors. Active research has been done on the function of CTCs in predicting prognosis in patients with HCC. It is uncertain how well they predict early progression recurrence (EPR), though. MVI, satellite nodules, and a total-CTC (T-CTC) count of more than 5/5 mL were found to be independent risk factors for EPR. T-CTC was more effective than the other markers for predicting EPR. For patients with HCC, the preoperative CTCs were used as an EPR prognostic predictor. For predicting EPR following hepatectomy, the combination models comprising T-CTCs, MVI, and satellite nodules performed the best[38].
Patients with metastases had more portal vein CTCs than those without metastases. The CTC count was a risk factor for HCC metastasis. The CTC count had an 82.93% sensitivity and a 52.38% specificity in predicting the metastasis of HCC. Significant correlations were seen between the CTC count and MVI, vascular invasion, and tumor size. The greatest significant capacity to predict metastasis was demonstrated by a threshold CTC level of 7. The presence of vascular invasion and HCC metastases were tightly associated. The CTC count in the portal vein before therapy was associated with vascular invasion and may play a role in the metastasis of HCC. Unfortunately, due to inadequate specificity, the capacity of the CTC count to predict HCC metastasis was constrained[39].
Preoperative and postoperative CTCs were more successful than AFP in predicting prognosis and supervising recurrence, suggesting that CTCs may serve as a biomarker for the clinical treatment of HCC[40].
The protein known as SAMD13 is encoded by the SAMD13 gene and is distinguished by the presence of a sterile alpha motif domain that is involved in protein-protein interactions. SAMD13 may be a prognostic biomarker for some cancers, especially HCC, where high expression levels may be linked to a poor prognosis. In addition to offering new insights into tumor immunology in HCC, SAMD13 may be a unique predictive biomarker for HCC diagnosis[41].
During embryogenesis, the formation of the neuromuscular junction is the most well-characterized function of the big proteoglycan Agrin. The role of Agrin in acetylcholine receptor aggregation during synaptogenesis is the basis for its name. Agrin immunohistochemistry was also shown to be useful in differentiating between benign parenchymal lesions and HCCs. Here, the expression of Agrin in primary liver tumors and metastatic liver carcinomas was compared[42]. Using both quantitative and qualitative approaches, immunohistochemistry for Agrin was carried out on 25 HCC, 16 intrahepatic cholangiocellular carcinoma (CCC), 20 colorectal cancer metastasis (CRCm), and 18 pancreatic ductal carcinoma metastasis tissues. Sections that had been frozen were subjected to double immunofluorescent labelling with Agrin/CD34. The microvessels of HCCs showed significant Agrin immunostaining, regardless of tumor grade[42].
In contrast to HCC, Agrin immunostaining was seen in the basement membranes surrounding tumor cell pseudoglandules but was weak or almost nonexistent in the CD34-positive microvessels of CCC, CRCm, and pancreatic ductal carcinoma metastases. The basement membranes of Grade III CCCs retained Agrin, while poorly differentiated metastatic adenocarcinomas exhibited little Agrin. The levels of Agrin mRNA were greatest in CCC and lower in HCC and CRCm, but they remained elevated. Based on microvascular Agrin tagging, HCCs were confidently recognized. Therefore, in situations with troublesome liver cancer, Agrin immunohistochemistry may help decide if the disease is primary or metastatic[42].
GPC3, a member of the glypican family that binds to the cell surface using a glycosylphosphatidylinositol anchor, is increased in the serum of many patients with HCC and overexpressed in HCC cases. Expression of GPC3 promotes the development and spread of HCC. Additionally, several distinct antibody types that target GPC3 have been created[43].
Mechanistic research has demonstrated that GPC3 contributes to the development of HCC by attaching itself to growth factors and Wnt signaling proteins. Molecular imaging and therapeutic intervention have also been employed to target GPC3 in HCC. To date, several immunotherapeutic protocols targeting GPC3 have been developed, including the use of humanized anti-GPC3 cytotoxic antibodies, treatment with peptide/DNA vaccines, immunotoxin therapies, and genetic therapies[44].
GPC3-targeted magnetic resonance imaging, positron emission tomography, and near-infrared imaging have also been studied for early HCC detection. GPC3 has a high sensitivity for detecting HCC. However, it is less sensitive for detecting fibrolamellar and highly differentiated HCC. Due to the significant manifestation of cirrhotic nodules, GPC3 should be used with caution in biopsy specimens. Due to its better sensitivity than Hep Par 1, GPC3 can be particularly helpful in identifying poorly differentiated HCC[45].
GPC3 positivity in tumor cells and serum GPC3 levels have been shown to have predictive relevance in patients with HCC[46]. In the differential diagnosis between LC and HCC, serum GPC3 performs worse than AFP. But GPC3 and AFP together performed significantly better[47]. The pathophysiological aspects of prognostic markers in HCC are explained in detail in Table 1.
| Prognostic markers | Pathophysiological aspects in HCC | Ref. |
| MEX3A | Explained in Figure 1 | [16] |
| APOB | APOB appears to play a crucial role in HCC pathophysiology by regulating lipid metabolism and influencing tumor progression | [22] |
| APOB depletion causes a change in the balance of lipid metabolism that favors tumor development | ||
| AFP | AFP is an important component of the TME, which promotes the development of HCC, and several studies have shown that TME is essential to the malignant transformation of HCC | [55,31,69] |
| Many individuals with HCC have elevated blood AFP expression levels, and elevated AFP levels over time are associated with an increased risk of HCC development | ||
| AFP is an immune suppressor that can encourage malignant transformation during the development of HCC and may have a role in the MDR process in liver cancer patients | ||
| High expression of AFP is closely associated with the onset and spread of HCC, making it a valuable biomarker for HCC | ||
| Through PI3K/Akt signaling pathway activation, AFP can promote LCSC growth in the TME. Furthermore, AFP can promote the development of HCC by upregulating the expression of genes linked to LCSCs | ||
| Furthermore, by interacting with macrophage receptors and causing tumor immune escape, AFP can preemptively interfere with macrophage-mediated phagocytosis of tumor cells | ||
| Concurrently, AFP may weaken the function of DCs, CAFs, endothelial cells, mesenchymal stem cells, NK cells, and macrophages | ||
| It may also hinder the capacity of cytotoxic T lymphocytes to eradicate tumor cells, which would aid in tumor immune evasion | ||
| Higher AFP levels and higher VEGFR2 staining were linked to worse progression-free survival and overall survival. The sole independent predictor of progression-free survival and overall survival was AFP level | ||
| CTCs | The CTC count was a risk factor for HCC metastasis. Significant correlations were seen between the CTC count and MVI, vascular invasion, and tumor size | [39,61] |
| CTCs, which are released into the circulation from both primary and metastatic lesions, offer important insights into the development and spread of cancers | ||
| CTCs facilitate EMT, which leads to tumor metastasis. Stem-like characteristics and CTC clusters increase the likelihood of metastasis and aggression | ||
| The characteristics of comparable primary tumors are mimicked by CTCs, which maintain their heterogeneity | ||
| Consequently, single-cell-based analysis is crucial for learning about the biology and heterogeneity of tumors | ||
| SAMD13 | The expression of SAMD13 in HCC samples was much greater than in normal liver tissue; it was also higher in initial tumors, different cancer stages and tumor grades, and the presence of nodal metastases | [41] |
| Additionally, a worse prognosis was linked to higher SAMD13 expression | ||
| The expression of SAMD13 was positively connected with B cells, neutrophils, macrophages, DC, CD8+ T cells, and CD4+ T cells | ||
| Agrin | Agrin promotes movement, oncogenic signaling, and cellular proliferation | [64,65,72] |
| Through prolonged focal adhesion integrity, the extracellular matrix sensor activity of Agrin mechanistically offers oncogenic cues to govern Arp2/3-dependent ruffling, invadopodia formation, and EMT, all of which contribute to the development of liver tumors | ||
| Agrin signaling also forms a crucial oncogenic axis through Lrp4-MuSK | ||
| Antibodies that target Agrin have been shown to decrease tumor development and oncogenic signaling in vivo | ||
| The liver tumor microenvironment plays a key role in promoting hepatocarcinogenesis, and Agrin, a secreted proteoglycan, is often overexpressed in HCC | ||
| In the portal regions of the normal liver, Agrin was seen by immunohistochemistry around the bile ducts and blood arteries | ||
| While Agrin was deposited in the neovascular basement membrane of HCCs, it was not expressed in the hepatic lobules | ||
| While immunostaining caused Agrin to fragment, diminish, or even vanish in less differentiated regions and locations of infiltration, it was plentiful in the tumor-specific basement membrane in well-differentiated CCs | ||
| Even more, Agrin was expressed in CC samples. Immunoblotting validated these results | ||
| As a component of the newly created vasculature, our findings suggest that Agrin may be crucial to neoangiogenesis in human HCC | ||
| However, Agrin may have a role in the development of tumors in CC | ||
| GPC3 | Through its interaction with molecules including growth factors and Wnt signaling proteins, GPC3 contributes to the advancement of HCC | [44,67] |
| Proliferation, metastasis, apoptosis, and EMT are all significant cellular processes that GPC3 influences via a variety of signaling pathways, including Wnt, IGF, YAP, and Hedgehog |
Tissues showed increased MEX3A expression in both early and later stages of HCC. In contrast to the peripheral liver tissues, MEX3A was as anticipated substantially expressed in HCC tissue. In comparison to non-tumor hepatic cells, the HCC cell line had a greater amount of MEX3A protein. The HCCLM3 and MHCC97H cells showed the two largest increases, while the Huh7 cells showed the smallest. Utilizing a subcutaneously implanted tumor model in nude mice, the proliferative impact of MEX3A was confirmed. The tumor nodes were gathered 4 weeks after the transplant. Mice who received transplants of sh-MEX3A downregulated HCCLM3 and had smaller tumors in terms of both weight and volume than the negative control. Accordingly, MEX3A might encourage HCC development and proliferation[16].
The expression of MEX3A mRNA in liver cancer was described in another study, which also showed that it was related to some clinical characteristics such as age, gender, histologic grade, TNM stage, vital status, and radiation therapy. Together with residual tumors and T stage, MEX3A was found to be an independent risk factor for the prognosis of liver cancer. It is interesting to note that MEX3A expression elevated with increasing histological grade, indicating a possible link between MEX3A and tumor growth[14].
When MEX3A methylation and mRNA levels in peripheral blood mononuclear cells are detected in conjunction with blood AFP, the diagnostic potential of AFP can be greatly enhanced. AFP did not connect with either mRNA levels or MEX3A promoter methylation levels. Despite AFP, findings imply that MEX3A promoter methylation levels and mRNA levels might be employed as non-invasive diagnostic indicators for HCC[15].
The degree of mRNA expression has a negative correlation with the MEX3A percentage of the methylated reference score. In patients with HCC, there was a strong correlation between the mRNA level and ALT and between the MEX3A percentage of the methylated reference score and age. Liver cancer development was also independently influenced by the amounts of mRNA and MEX3A promoter methylation. mRNA levels and MEX3A promoter methylation were more effective at differentiating HCC from CHB than AFP. This substantially enhanced the area under the receiver operating characteristic curve by combining mRNA levels with AFP and MEX3A methylation levels. When comparing patients with HCC with ascites to those without, it found that they had a greater level of Mex3a promoter methylation. This might be because MEX3A can influence prognosis and increase tumor growth[15].
When used in conjunction with AFP, the serum apolipoprotein B/apolipoprotein A1 (ApoB/ApoA1) ratio demonstrated the capacity to improve the specificity and detection effectiveness of AFP for HCC and AFP-negative HCC. In patients with CHB, the serum ApoB/ApoA1 ratio was negatively correlated with the incidence of LC and HCC in the corresponding logistic regression model[24].
Three strong subclasses of HCC (termed S1, S2, and S3), were found using a meta-analysis of gene expression patterns from 603 patient samples with HCC. These subclasses were linked to several factors, such as tumor size, degree of cellular differentiation, and serum AFP levels. It has been demonstrated that high AFP serum levels are connected to the S2 subclass, which is associated with big tumors, and the G1, G2, and G3 subclasses, which are distinguished by strong proliferation and chromosomal instability[48,49].
The serum AFP levels did not substantially differ between patients with HCC with and without LC. Nevertheless, they were considerably higher in patients with tumor sizes ≥ 10 cm than in those with tumor sizes < 10 cm[37]. AFP levels were higher in patients with portal vein thrombosis, metastases, and large tumors than in patients with diffuse or single nodule tumors. They were also higher in patients with high Child-Pugh scores than in those with low scores, and they were higher in patients with high BCLC stages than in those with low stages. Likewise, the AFP levels of patients in more advanced clinical stages were greater than those in lower stages. Serum AFP levels were not significantly correlated with tumor differentiation[37]. Hematological indices and the HBV DNA viral load are particularly novel metrics that are linked to the serum AFP status in HCC[37].
CTCs have been demonstrated to be a proxy for the prognosis and development of cancer. CTCs were identified by an immunoaffinity-based technique that used mucin 1 and epithelial cell adhesion molecule. Patients with BCLC stages A, B, and C had detection rates of 65.4%, 77.3%, and 96.0%, respectively, using CTCs. Patients with HCC who have more peripheral CTCs had worse survival rates and more aggressive tumor characteristics. CTCs may develop into a unique predictive biomarker for HCC[50].
A favorable correlation was found between tumor malignancy and high recurrence risk and both the overall CTC quantity and the mesenchymal CTC (M-CTC) percent. With respective sensitivities of 80.95% and 90.48% and specificities of 74.12% and 84.71%, preoperative total CTC number and M-CTC percent could reliably differentiate relapse patients from those without relapse. M-CTC percent and preoperative T-CTC levels are both strongly associated with worse recurrence-free survival and can be considered separate risk factors for HCC with early recurrence. Compared to AFP, overall CTC and M-CTC numbers were more effective in HCC longitudinal monitoring[40].
Comparing the HCC group categorized as stage I to stage III to the matching normal group, SAMD13 expression was considerably elevated based on tumor stage. The HCC group showed a substantial rise in SAMD13 levels across all tumor grades, regardless of tumor grade. Additionally, patients with fibrolamellar carcinoma and HCC types showed increased expression of SAMD13. Furthermore, in all other HCC categories such as gender, age, and race SAMD13 expression was statistically significant. SAMD13 and clinical indicators in patients with HCC showed a substantial correlation[51].
SAMD13 expression and the clinicopathological features of HCC are related. The following clinical characteristics showed a negative correlation with a higher SAMD13 expression: Sex; race; age; and stage. In males, stage I + II, stage II, stage II + III, stage III, grade II, grade III, Asian race, alcohol use, hepatitis virus, and higher SAMD13 expression were associated with worse OS[41].
Findings demonstrated a correlation between SAMD13 expression and the prognosis of HCC. The value of presurgery ApoB was shown to be somewhat linked with tumor size. Furthermore, it was discovered that the blood levels of LDL-cholesterol and APOB had a strong correlation[21].
Clinicopathological research showed that high-grade tumor type and advanced HCC stage were substantially correlated with SAMD13 overexpression. Simultaneously, elevated SAMD13 expression led to immunotherapy effectiveness and correlation with different immunological markers in the immune cell subsets via TIMER databases. SAMD13 was substantially linked to prognosis, according to a methylation study. Moreover, the cell cycle, transcription, and epigenetic regulation were linked to a six-hub gene signature linked to a poor prognosis; this research may bolster the link between SAMD13 expression and drug resistance[51]. Immune cell infiltration in HCC is correlated with SAMD13 expression[41].
Tumor growth and metastasis were significantly correlated with Agrin expression. Compared with patients who were negative for Agrin, patients with HCC who were positive for Agrin had a significantly worse recurrence-free survival rate. Moreover, a Cox regression study verified that tumor size, metastasis, MVI, and Edmondson-Steiner grade all had independent correlations with HCC survival. The development of HCC may be significantly influenced by Agrin upregulation. Agrin may be employed as a prognostic indicator to predict the outcomes of patients with HCC[52].
The overall therapeutic strategies are explained in Table 2. The most frequent kind of cancer is HCC, and liver cancer is the second most common cause of cancer-related fatalities. Consequently, molecular targets are desperately needed for both the development of new treatment strategies and the early identification of HCC. HCC differs from other malignancies in that its prognosis depends on the stage of the tumor and the severity of the underlying liver disease. Curative options including surgical excision and liver transplantation are only accessible in the first stages.
| Prognostic markers | Therapeutics in HCC | Ref. |
| MEX3A | In HCC, MEX3A could be a new regulator of the Hippo signaling pathway and a possible target for treatment | [16,54] |
| A novel treatment approach for progressive and sorafenib-resistant HCC is provided by the combined data, which further clarifies the possible role and mechanism of MEX3A in HCC | ||
| Via an antiangiogenic mechanism rather than by specifically targeting HCC cells, dovitinib selectively suppresses HCC development and metastasis | ||
| MEX3A inhibition could enhance the efficacy of standard HCC treatments such as sorafenib, lenvatinib, or immunotherapy | ||
| Combining MEX3A-targeting strategies with PD-L1 inhibitors could improve immune response against HCC | ||
| APOB | Not determined | |
| AFP | According to recent research, AFP inhibition significantly suppresses the malignant tendencies of HCC cells, causing cancer cell death and preventing their invasion, metastasis, and proliferation | [55,69] |
| For this reason, AFP is essential for the malignant development of HCC | ||
| In the development, diagnosis, and management of HCC, AFP is a two-edged sword | ||
| Assessing AFP levels in clinical practice is crucial for the early identification, diagnosis, and management of HCC | ||
| Medical practitioners can guide clinical decision-making by tracking changes in AFP levels, which allows them to track the course of the disease and the effectiveness of treatment | ||
| Additionally, physicians can employ AFP vaccinations to produce CD8+ T lymphocytes that are specific to AFP and destroy cancer cells | ||
| Furthermore, AFP and immunotherapy together can increase the effectiveness of treatment | ||
| Evidence for the negative predictive impact of increased baseline AFP raises the possibility that AFP plays a part in primary resistance to sorafenib treatment | ||
| CTCs | Moreover, CTCs are excellent choices for creating preclinical models (particularly 3D organoid cultures) for drug screening, disease modeling, genome editing, tumor immunity research, and the creation of organ-like biobanks | [61,62] |
| According to recent research, CTCs are promising candidates for early diagnosis, assessing the prognosis of metastases or recurrences, and possibly serving as a possible target for therapy in patients with HCC | ||
| In the future, it will be used as a novel indication in therapeutic settings | ||
| SAMD13 | The potential function of SAMD13 as a therapeutic target and a promising biomarker for prognosis in HCC is highlighted by the features of its involvement in patients with HCC utilizing a variety of bioinformatics techniques | [51] |
| While SAMD13 shows promise as a prognostic indicator, its potential as a therapeutic target in HCC requires further investigation | ||
| Understanding the precise biological functions of SAMD13 in HCC progression and its interactions within the tumor microenvironment is essential | ||
| Agrin | Agrin is often overexpressed, has a significant role in the carcinogenic characteristics of HCC, and is a desirable target for antibody treatment | [64] |
| Incorporating Agrin inhibitors with existing treatments, such as tyrosine kinase inhibitors or immunotherapies, may enhance overall therapeutic efficacy | ||
| This combination strategy could potentially overcome resistance mechanisms and improve patient outcomes | ||
| GPC3 | GPC3 knockdown by siRNA increased the suppression effects of curcumin on Wnt/β-catenin signaling | [43,66,73] |
| Small molecules that interfere with GPC3-mediated signaling pathways are an appealing therapeutic strategy that may be used in HCC patients; however, as of yet no small molecules that target GPC3 have been developed or tested in HCC patients. Small-molecule GPC3 drugs may provide greater efficacy, stability, and safety for the treatment of HCC | ||
| Further research is required to develop GPC3 targeting small molecular compounds | ||
| Numerous clinical trials involving GPC3 are currently underway, and several novel GPC3-targeted therapeutic approaches, such as the GPC3 vaccine, anti-GPC3 immunotoxin, combined therapy with immune checkpoint blockades, and chimeric antigen receptor T or NK cells, have recently surfaced with promising outcomes | ||
| Current research on GPC3 expression in human HCC, GPC3 regulation molecular mechanisms, and GPC3-targeting antibodies | ||
| Furthermore, several immunotherapies that target GPC3 have been created, such as chimeric-antigen-receptor-modified cells, anti-GPC3 immunotoxins, and GPC3 vaccines | ||
| After summarizing and evaluating the physicochemical characteristics and structure of GPC3 molecules, the authors go over their biological roles and clinical diagnostic uses before investigating GPC3-based diagnosis and therapy approaches |
However, in recent years, systemic and locoregional treatment for advanced HCC has made tremendous strides. Three key proangiogenic factors involved in hepatocarcinogenesis such as VEGF, basic fibroblast growth factor, and platelet-derived growth factor (PDGF) are implicated in the neovascular, invasive, and metastatic potentials of HCC. VEGF is elevated during hepatocarcinogenesis and is found in dysplastic nodules. It also corresponds with histological grades[53].
The current first-line treatment for advanced or recurrent HCC is sorafenib, an inhibitor of several kinases, including Raf-1 and VEGF receptor (VEGFR). Although individuals see a little improvement in survival, they eventually become resistant to the medication. When considered collectively, clarifying the possible role and mechanism of MEX3A in HCC will offer a fresh approach to treating progressive and sorafenib-resistant HCC[16].
Dovitinib is an inhibitor of receptor tyrosine kinase that targets receptors for PDGF receptor β (PDGFRβ), fibroblast growth factor receptors, and VEGFRs. Clinical studies with dovitinib are presently being conducted to treat HCC. A model of orthotopic HCC showed considerable suppression of tumor development and lung metastasis. Only PDGFR-β, one of the recognized dovitinib targets, was expressed in two HCC cell lines, according to immunoblotting, whereas PDGFR-β, fibroblast growth factor receptor 1, and VEGFR2 were expressed in four out of five endothelial lines. Inhibiting endothelial cell proliferation and motility at a pharmacologically relevant dose of 0.04 μmol/L, dovitinib was unable to do the same for HCC cells. It demonstrated how dovitinib inhibited proliferation and induced apoptosis in HCC cells, therefore dramatically reducing the microvessel density of xenograft tumors. Through an antiangiogenic mechanism rather than by directly targeting HCC cells, dovitinib selectively suppresses HCC development and metastasis[54].
APOB functions as a tumor suppressor in HCC by maintaining lipid homeostasis and preventing lipid accumulation that could fuel tumor progression. Reduced APOB levels have been correlated with poor prognosis and aggressive tumor behavior. APOB-targeting strategies may be combined with immunotherapy or kinase inhibitors to enhance treatment efficacy in patients with HCC. Future studies should explore how APOB modulation interacts with current standard-of-care treatments, such as sorafenib.
Comprehending the clinical pathological features of patients with low and high blood AFP levels may aid in the prognostication and clinical management of this fatal condition[37]. AFP is therefore essential to the diagnosis and treatment of HCC. It is a marker that can be utilized for liver cancer follow-up, diagnosis, and early screening. AFP levels may also be utilized to forecast the prognosis and assess the therapy impact[55]. Although there are limitations and difficulties with AFP, patients with an AFP-positive status can benefit greatly from treating HCC by focusing on the AFP-TME interaction[56].
By blocking the activity of AFP or preventing it from interacting with TME, liver cancer cells and the TME can no longer communicate, which will stop tumor development and spread. This method could offer a fresh way to treat HCC. Second, the relationship between liver cancer cells and the immunological microenvironment is also influenced by AFP[57].
HCC cells may be shielded from the immunological response by immune evasion facilitated by AFP overexpression. By boosting immune identification and responsiveness, targeting AFP may help reduce immune evasion and increase the effectiveness of immunotherapy. Focusing on the AFP-TME interaction in HCC can hinder tumor development, invasion, and immune evasion by severing the connection between liver cancer cells and the TME[55]. Research is looking into ways to use AFP fragments or antibodies to block its activity and possibly treat HCC, but more work is required to fully realize its therapeutic potential. Many solid tumors have been found to have drug-resistant molecular expression patterns and mutation profiles[58].
CTC analysis offers the ability to identify the patient group most likely to react to particular treatments and in this context enables the identification of therapeutic targets and resistance mechanisms to cancer therapies at the DNA, RNA, and protein levels. To give quantitative information on sorafenib-related targets, Li et al[58] developed a new method that simultaneously detects phosphorylated ERK (pERK) and Akt (pAkt) in HCC CTCs. This work showed that when characterizing pERK/pAkt, CTCs may be used in place of tumor tissues. The most sorafenib-sensitive cells, pERK+/pAkt-CTCs, may be used as a stand-alone PFS predictor in patients with sorafenib-treated HCC. A new age in immunotherapy has been ushered in by ICIs, which have produced remarkable long-term remissions in certain patients with a variety of tumor types. However, this method only works for a small percentage of patients, and many of them have serious adverse effects[59].
Consequently, it is essential to track the expression of the biological targets of ICI therapy, including PD-L1. PD-L1 + CTC assessment was the first to distinguish between patients with HCC with advanced/metastatic illness and those with early-stage disease[60]. Three of the six patients with PD-L1 + CTCs showed a strong response to anti-PD1 treatment. The fact that only one out of three non-responders contained PD-L1 + CTCs did raise the possibility that these cells might indicate how well immunotherapy would work[61].
Early identification, tumor staging, recurrence and prognosis assessment, and even the prediction of drug resistance to targeted treatment and immunotherapy in patients with HCC are all greatly aided by the discovery of CTCs. However, because of several issues, including a lack of standardized technical processes, low detection efficiency, the extremely low frequency of CTCs in blood, and the lack of external validation in many clinical trials, CTC detection is not commonly employed in the clinical application of HCC[58].
CTC detection will be a breakthrough in HCC clinical care if technological advancements can overcome their limitations in the future. This is especially true for early diagnosis, which offers the chance for curative surgery before metastasis. Numerous pathological activities of CTCs, including tumor cell migration and shedding, vascular invasion, and distant implantation, are significantly influenced by epithelial-to-mesenchymal transition (EMT). Further research into the processes of EMT in CTCs is necessary to get a better understanding of the clinical management of HCC[62].
The peripheral blood contains CTCs, which are cells discharged from the main tumor that can infiltrate and grow remotely through blood circulation. Currently, the three primary categories of CTC separation and detection techniques are physical, biological, and microfluidic chip-based techniques. For HCC early screening and diagnosis, monitoring CTC count and cell phenotype is crucial. The clinical stage of HCC and the clinical effectiveness of therapy are also correlated with the CTC count and cell phenotype. In patients with HCC, the number of CTCs is also strongly associated with postoperative recurrence, PFS, and OS[63].
While SAMD13 shows promise as a prognostic indicator, its potential as a therapeutic target in HCC requires further investigation. Understanding the precise biological functions of SAMD13 in HCC progression and its interactions within the TME is essential. Future research should focus on elucidating these mechanisms to determine whether targeting SAMD13 could offer therapeutic benefits for patients with HCC[51].
The role of Agrin in carcinogenesis suggests that it might be a good target for treatment in HCC. As a result, two antibodies that target Agrin (D2 and MAb5204) were identified. Their antigenic epitopes identify both the full-length protein and the C-terminal portion of rat Agrin, which is 20 kDa. In differentiated muscle C2C12 and MHCC-LM3 cells, Agrin antibodies significantly reduced Agrin-induced MuSK (muscle-specific tyrosine kinase) phosphorylation, indicating their functional blocking properties. Accordingly, both antibodies considerably decreased cell migration by around 77% as compared to the isotype control[64].
Agrin antibodies upregulated E-cadherin without changing GPC3 levels, but they significantly inhibited the expression of pY397FAK, pAKT (Ser473), N-cadherin, and vimentin. In a xenograft model, it investigated the inhibitory effect of MAb5204 on tumor development because it showed stronger in vitro effects. Treatment with the MAb5204 antibody inhibited tumor development by approximately 40% [64]. MAb5204 antibody therapy appears to inhibit tumor growth, as evidenced by the consistent 42% decrease in proliferation (Ki67) and 87% increase in apoptosis (cleaved caspase-3) in treated tumors[64].
Through the Lrp4-MuSK signaling axis, Agrin, an extracellular matrix sensor that controls cell proliferation, motility, invasiveness, focal adhesion integrity, and EMT, promotes liver carcinogenesis. Short hairpin RNA-mediated Agrin depletion or function-blocking antibodies inhibited liver tumorigenesis and oncogenic signaling in vivo. Together, results demonstrate that Agrin overexpression plays a part in HCC and point to it as a promising therapeutic target[64].
Despite being suggested as biomarkers for HCC, AFP and GPC3 have little predictive value for predicting the course of the illness. It indicates that among individuals with HCC, high serum Agrin levels are linked to a poor prognosis and performance. According to multivariate Cox regression models, secreted Agrin is a more accurate prognostic predictor than AFP, which has a strong correlation with other secreted biomarkers (such as interleukin 6). The clinical utility of Agrin in the diagnosis and prognosis of HCC is demonstrated by this work collectively[65].
As a target of in vivo imaging, it can precisely identify the liver cancer before treatment as well as diagnose the stage of cancer and surgical margins by merging AI technology in the picture group and non-invasively detecting tumor recurrence and the curative effect following surgery. Secretory GPC3 offers a trustworthy new target for early tumor detection and non-invasive screening. A more precise and efficient method of treating liver cancer is through adoptive immunotherapy and antibody-based medications made from GPC3 antibodies[66].
Current research shows promise in halting tumor development and enhancing clinical results through immunotherapies such as chimeric antigen receptor T cell treatments and monoclonal antibodies. GPC3 has a variety of functions in carcinogenesis, including influencing glucose metabolism, tumor-associated macrophages, and EMT, all of which accelerate the development of HCC. Because GPC3 is involved in important tumorigenic processes and is re-expressed in HCC, it is a valuable biomarker for early detection and a target for therapeutic intervention. To fully use the diagnostic and therapeutic potential of GPC3 in the therapy of HCC, more study is necessary[67].
The main ways that anti-GPC3 antibodies kill cancer cells are by complement-dependent cytotoxicity and/or antibody-dependent cellular cytotoxicity. Given that GPC3 Ab is linked to immunological responses, a combination of ICIs and regimens has also been studied. Furthermore, some novel strategies for GPC3-targeting treatment have surfaced recently. This review covered the most current findings on the role of GPC3 in the development of HCC and therapeutic applications that target GPC3. It also presented the findings of recent clinical studies that target GPC3 in HCC[46].
Immunotherapy targeting GPC3 and its affiliated proteins is being investigated because these new biomarkers may hold potential for the detection and treatment of HCC and other diseases in which GPC3 may be overexpressed. Studies have reported that overexpression of GPC3 in HCC predicts a poorer prognosis, which further begs the question of the role of GPC3 in the regulation and progression of HCC. Given the current gap in the literature, we think that more research on the function, structure and domains, cellular localization, and other subfields of GPC3 is necessary to assess the protein overall and its role in the study of HCC[68].
The following are emerging markers for HCC that may increase survival rates by facilitating early diagnosis and focusing on particular therapeutic pathways: GPC3; GP73; microRNAs; DCP; fucosylated GP73; and some circulating extracellular vesicles. These markers can reveal information about the molecular makeup of the tumor and suggest possible targets for treatment.
Ineffective diagnostic and prognostic assessment techniques have caused a steady rise in death rates in patients with liver cancer. It is critically necessary to develop new molecular markers that help guide prognosis and increase survival rates for patients with liver cancer. Further validation of these results and the establishment of MEX3A as a new prognostic tool for patients with liver cancer would require further clinicopathological data and associated clinical tissue samples[14]. MEX3A plays a crucial role in promoting HCC progression by enhancing tumor growth, metastasis, and drug resistance. Targeting MEX3A with RNA-based therapies, small molecules, or gene-editing technologies could provide a novel therapeutic approach. However, further preclinical and clinical studies are needed to validate its potential as a treatment target.
While still an emerging field, targeting APOB could provide novel therapeutic approaches for liver cancer. More research is needed to clarify the mechanisms and develop clinically viable strategies. The most widely utilized biomarker in the treatment of HCC is AFP, despite its subpar efficacy as a screening, diagnostic, and prognostic marker for the disease[28].
Even with its substantial age, there are still unanswered problems about the usefulness of AFP in HCC that need to be answered: What are the ideal AFP cutoff levels for the diagnosis, prognosis, and monitoring of HCC? Which AFP-L3 or DCP combos with other biomarkers can greatly enhance the performance of AFP in the different HCC settings? Can AFP be used to track how well more current drugs like nivolumab or regorafenib are working? What part, if any, does it play in the growth of tumors[28]?
AFP has a significant regulatory function in the TME and is a marker for HCC. The present function of AFP in the TME of HCC is not entirely known. For the early detection and treatment of HCC, a better understanding of the function of AFP in the TME is therefore crucial[55].
Future and ongoing studies on single-cell analysis, molecular profiling, CTC capture technologies, and preclinical models could significantly enhance CTC testing for early diagnosis, successful therapy, and efficient prognosis management of HCC[61]. Patients with AFP-positive and AFP-negative HCC have notable variations in clinical pathological features, which might be useful for prognostication and treatment[37]. Another study demonstrated that increased baseline AFP had a negative predictive function and raised the possibility that AFP plays a part in primary resistance to sorafenib treatment in patients with advanced HCC[69].
Many questions and technical issues need to be resolved before this technique truly influences clinical practice recommendations, even if the dynamic assessment of CTCs in patients with HCC may help with decision-making. To prevent HCC recurrence following surgery or to impede tumor progression and extrahepatic spreading, more research is required to determine the best combination of surface markers, boost the effectiveness of CTC expansion ex vivo, or even target CTCs as a possible therapeutic approach[70].
Early-stage HCC diagnostic and prognostic applications show promise with CTCs and associated technologies. There are still a lot of issues that need to be resolved even though various cutting-edge technologies are now utilized in clinics to identify CTCs. These technologies might be used with additional information from bioinformatics and annotated databases to effectively track and identify CTCs in the blood and to estimate risk in this context as a new diagnostic method[71].
Furthermore, combining CTC and cell-free DNA detection techniques may be a viable strategy for early diagnosis. Current CTC biomarker panels need to be significantly improved in terms of sensitivity and specificity. The analysis of simultaneous profiles of multimarker panels and the provision of thorough coverage of the extremely diverse cancer cell subpopulations do need new ultra-high-throughput quantification techniques. Due to these drawbacks, a thorough multidisciplinary strategy and accurate tools for processing big data are required. Machine learning should help with CTC-related evaluations and verify viewpoint findings[71].
Future research should focus on elucidating mechanisms to determine whether targeting SAMD13 could offer therapeutic benefits for patients with HCC[51].
Another study suggested that Agrin may be crucial to neoangiogenesis in human HCC. However, Agrin may have a role in the development of tumors in cholangiocarcinoma[72]. Agrin plays a critical role in the pathogenesis of HCC by promoting tumor growth, migration, and angiogenesis. Its overexpression in HCC and involvement in key oncogenic pathways make it a compelling target for therapeutic intervention. Ongoing research into Agrin-targeted therapies holds promise for developing more effective treatment strategies for patients with HCC.
Numerous clinical trials involving GPC3 are currently underway, and several novel GPC3-targeted therapeutic approaches, such as the GPC3 vaccine, anti-GPC3 immunotoxin, combined therapy with immune checkpoint blockades, and chimeric antigen receptor T or natural killer (NK) cells, have recently surfaced with promising outcomes. Current research is focused on GPC3 expression in human HCC, GPC3 regulation molecular mechanisms, and GPC3-targeting antibodies[73]. In clinical applications, GPC3 still faces several technological obstacles despite this. It is not yet clear how GPC3 contributes to the development of liver cancer. The significance of GPC3 in controlling the Wnt and Hedgehog signaling pathways is well known. However, it is still unknown if GPC3 can control cell differentiation in cancer and embryonic development[66].
Since GPC3 is a membrane-anchored protein, it does not have the necessary domain to send intracellular signals, hence its role on the cell surface is probably related to intercellular or cell-to-matrix interactions. Important pathophysiological processes may also be regulated by the interaction of GPC3 with other cells in the microenvironment. GPC3 may also be involved in the formation of a lipid raft rich in cholesterol and glycosphingolipid, and the small G protein on the cytoplasmic surface of the lipid raft may be involved in the transduction of GPC3 downstream signaling. Perhaps GPC3 is a “chameleon molecule” whose biological activity is dictated by the “membrane-matrix” biological interaction. The molecular makeup at this contact dictates the biological role of GPC3. Additionally, these theories require confirmation[66].
Research indicates that the anti-GPC3 medications are not enough to cure HCC on their own. To use GPC3 as a combination therapy approach with immunotherapeutic agents and anti-cancer medications, more research is thus required to support the evidence gathered. In addition, it is necessary to determine the best way to control GPC3 expression to prevent the formation of drug-resistant clones[67].
We conclude that the prognostic markers MEX3A, APOB, AFP, CTCs, SAMD13, Agrin, and GPC3 play a significant role in the pathogenesis of HCC. The pathophysiological roles of these prognostic markers in HCC are diverse, ranging from tumor proliferation and metastasis to immune evasion and metabolic reprogramming. Their prognostic value is critical for predicting patient outcomes, guiding treatment decisions, and identifying novel therapeutic targets.
| 1. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 2. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1058] [Article Influence: 352.7] [Reference Citation Analysis (0)] |
| 3. | Qu LS, Liu JX, Zhu J, Lu CH. Risk Factors for Prognosis of Hepatocellular Carcinoma After Curative Resection In Patients with Low Hepatitis B Viral Load. Ann Hepatol. 2017;16:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, Castagnetta LA. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 456] [Cited by in RCA: 500] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 6. | Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, Ngeow J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164:766-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 269] [Reference Citation Analysis (0)] |
| 7. | Parikh S, Hyman D. Hepatocellular cancer: a guide for the internist. Am J Med. 2007;120:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: epidemiology and pathogenic role of viral factors. J Chin Med Assoc. 2007;70:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4264] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 10. | Wang X, Wang Z, Wu L. Combined measurements of tumor number and size helps estimate the outcome of resection of Barcelona clinic liver cancer stage B hepatocellular carcinoma. BMC Surg. 2016;16:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Zheng J, Wang S, Xia L, Sun Z, Chan KM, Bernards R, Qin W, Chen J, Xia Q, Jin H. Hepatocellular carcinoma: signaling pathways and therapeutic advances. Signal Transduct Target Ther. 2025;10:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2893] [Article Influence: 482.2] [Reference Citation Analysis (17)] |
| 13. | Huang L, Malu S, McKenzie JA, Andrews MC, Talukder AH, Tieu T, Karpinets T, Haymaker C, Forget MA, Williams LJ, Wang Z, Mbofung RM, Wang ZQ, Davis RE, Lo RS, Wargo JA, Davies MA, Bernatchez C, Heffernan T, Amaria RN, Korkut A, Peng W, Roszik J, Lizée G, Woodman SE, Hwu P. The RNA-binding Protein MEX3B Mediates Resistance to Cancer Immunotherapy by Downregulating HLA-A Expression. Clin Cancer Res. 2018;24:3366-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Yang D, Jiao Y, Li Y, Fang X. Clinical characteristics and prognostic value of MEX3A mRNA in liver cancer. PeerJ. 2020;8:e8252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Yang JR, Tian YX, Li JE, Zhang Y, Fan YC, Wang K. Mex3a promoter hypomethylation can be utilized to diagnose HBV-associated hepatocellular carcinoma: a randomized controlled trial. Front Pharmacol. 2024;15:1325869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Fang S, Zheng L, Chen X, Guo X, Ding Y, Ma J, Ding J, Chen W, Yang Y, Chen M, Zhao Z, Tu J, Ji J. MEX3A determines in vivo hepatocellular carcinoma progression and induces resistance to sorafenib in a Hippo-dependent way. Hepatol Int. 2023;17:1500-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Yan L, Li H, An W, Wei W, Zhang X, Wang L. Mex-3 RNA binding MEX3A promotes the proliferation and migration of breast cancer cells via regulating RhoA/ROCK1/LIMK1 signaling pathway. Bioengineered. 2021;12:5850-5858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Yang P, Zhang P, Zhang S. RNA-Binding Protein MEX3A Interacting with DVL3 Stabilizes Wnt/β-Catenin Signaling in Endometrial Carcinoma. Int J Mol Sci. 2022;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lederer M, Müller S, Glaß M, Bley N, Ihling C, Sinz A, Hüttelmaier S. Oncogenic Potential of the Dual-Function Protein MEX3A. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Lin Z, Ji X, Tian N, Gan Y, Ke L. APOB is a potential prognostic biomarker in hepatocellular carcinoma. Discov Oncol. 2024;15:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Yan X, Yao M, Wen X, Zhu Y, Zhao E, Qian X, Chen X, Lu W, Lv Q, Zhang L, Lu F. Elevated apolipoprotein B predicts poor postsurgery prognosis in patients with hepatocellular carcinoma. Onco Targets Ther. 2019;12:1957-1964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Lee G, Jeong YS, Kwak MJ, Dowon K, Lee J, Young YS. Abstract 3474: Loss of function mutations in APOB and their clinical significance in hepatocellular carcinoma. Cancer Res. 2019;79:3474-3474. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Lee G, Jeong YS, Kim DW, Kwak MJ, Koh J, Joo EW, Lee JS, Kah S, Sim YE, Yim SY. Clinical significance of APOB inactivation in hepatocellular carcinoma. Exp Mol Med. 2018;50:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Cai X, Peng S, Xiao X, Huang Z, Zhang P. Serum ApoB/ApoA1 ratio in patients with CHB and the occurrence of HBV related cirrhosis and HBV related hepatocellular carcinoma. Sci Rep. 2024;14:10996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Lee CW, Tsai HI, Lee WC, Huang SW, Lin CY, Hsieh YC, Kuo T, Chen CW, Yu MC. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Fu CC, Wei CY, Chu CJ, Lee PC, Huo TI, Huang YH, Chao Y, Hou MC, Wu JC, Su CW. The outcomes and prognostic factors of patients with hepatocellular carcinoma and normal serum alpha fetoprotein levels. J Formos Med Assoc. 2023;122:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, Lau DT. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 2022;28:216-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (11)] |
| 28. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 397] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 29. | Cheng J, Wang W, Zhang Y, Liu X, Li M, Wu Z, Liu Z, Lv Y, Wang B. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: systematic review and meta-analysis. PLoS One. 2014;9:e87011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Li W, Liu K, Chen Y, Zhu M, Li M. Role of Alpha-Fetoprotein in Hepatocellular Carcinoma Drug Resistance. Curr Med Chem. 2021;28:1126-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015;19:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Parikh ND, Mehta AS, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2020;29:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Gupta S, Kutty G, Jagar P, Jain P, Lad T. Very High Alpha-Fetoprotein (Afp): a Poor Prognostic Indicator in Hepatocellular Carcinoma in the Modern Era. Ann Oncol. 2013;24:iv67. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, Cao X, Han M, Du H, Ye Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2020;15:e0228857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Chi X, Jiang L, Yuan Y, Huang X, Yang X, Hochwald S, Liu J, Huang H. A comparison of clinical pathologic characteristics between alpha-fetoprotein negative and positive hepatocellular carcinoma patients from Eastern and Southern China. BMC Gastroenterol. 2022;22:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (23)] |
| 38. | Lu Z, Ni H, Yang X, Tan L, Zhuang H, Mo Y, Wei X, Qi L, Xiang B. Prognostic potential of preoperative circulating tumor cells to predict the early progression recurrence in hepatocellular carcinoma patients after hepatectomy. BMC Cancer. 2023;23:1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | Zhao X, Zhao J, Tao L, Pan Y, Yang L, Zhang X, Yuan J, Zhu H. Significance of circulating tumor cells in the portal vein regarding metastases and vascular invasion in hepatocellular carcinoma patients. J Gastrointest Oncol. 2021;12:3050-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Zhao L, Zheng Z, Liu Y, Liu F, Li X, Wu Z. The mesenchymal circulating tumor cells as biomarker for prognosis prediction and supervision in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149:6035-6048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Kim H, Seo CW, Lee J, Han SJ, Kim J. SAMD13 as a Novel Prognostic Biomarker and its Correlation with Infiltrating Immune Cells in Hepatocellular Carcinoma. Biomed Sci Letters. 2022;28:260-275. [DOI] [Full Text] |
| 42. | Somorácz A, Tátrai P, Horváth G, Kiss A, Kupcsulik P, Kovalszky I, Schaff Z. Agrin immunohistochemistry facilitates the determination of primary versus metastatic origin of liver carcinomas. Hum Pathol. 2010;41:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Shih TC, Wang L, Wang HC, Wan YY. Glypican-3: A molecular marker for the detection and treatment of hepatocellular carcinoma(☆). Liver Res. 2020;4:168-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Zhou F, Shang W, Yu X, Tian J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38:741-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 45. | Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 47. | Xu D, Su C, Sun L, Gao Y, Li Y. Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis. Ann Hepatol. 2019;18:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-7392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 942] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 49. | Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, Nault JC, Zucman-Rossi J. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 50. | Prasoppokakorn T, Buntho A, Ingrungruanglert P, Tiyarattanachai T, Jaihan T, Kulkraisri K, Ariyaskul D, Phathong C, Israsena N, Rerknimitr R, Treeprasertsuk S, Chaiteerakij R. Circulating tumor cells as a prognostic biomarker in patients with hepatocellular carcinoma. Sci Rep. 2022;12:18686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Yoo W, Kim S, Noh K. SAMD13 serves as a useful prognostic biomarker for hepatocellular carcinoma. Eur J Med Res. 2023;28:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Zhang QJ, Wan L, Xu HF. High expression of agrin is associated with tumor progression and poor prognosis in hepatocellular carcinoma. Math Biosci Eng. 2019;16:7375-7383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Chen ZY, Shi M, Peng LX, Wei W, Li XJ, Guo ZX, Li SH, Zhong C, Qian CN, Guo RP. Dovitinib preferentially targets endothelial cells rather than cancer cells for the inhibition of hepatocellular carcinoma growth and metastasis. J Transl Med. 2012;10:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Lu Y, Lin B, Li M. The role of alpha-fetoprotein in the tumor microenvironment of hepatocellular carcinoma. Front Oncol. 2024;14:1363695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 56. | Lurje I, Hammerich L. The suppressive tumor microenvironment of AFP-positive hepatocellular carcinoma and its therapeutic implications. Transl Gastroenterol Hepatol. 2024;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 57. | Munson PV, Adamik J, Butterfield LH. Immunomodulatory impact of α-fetoprotein. Trends Immunol. 2022;43:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 58. | Li J, Shi L, Zhang X, Sun B, Yang Y, Ge N, Liu H, Yang X, Chen L, Qian H, Wu M, Yin Z. pERK/pAkt phenotyping in circulating tumor cells as a biomarker for sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncotarget. 2016;7:2646-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 60. | Winograd P, Hou S, Court C, Sadeghi S, Finn R, Dipardo B, Li Q, Kaldas F, Busuttil R, Tomlinson J, Tseng H, Agopian V. Evaluation of hepatocellular carcinoma circulating tumor cells expressing programmed death-ligand 1. HPB. 2018;20:S2-S3. [DOI] [Full Text] |
| 61. | Zhang Q, Rong Y, Yi K, Huang L, Chen M, Wang F. Circulating tumor cells in hepatocellular carcinoma: single-cell based analysis, preclinical models, and clinical applications. Theranostics. 2020;10:12060-12071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 62. | Hua Y, Dong J, Hong J, Wang B, Yan Y, Li Z. Clinical applications of circulating tumor cells in hepatocellular carcinoma. Front Oncol. 2022;12:968591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 63. | Wan C, Zhou B. Research progress on circulating tumor cells of hepatocellular carcinoma. J Interv Med. 2021;4:181-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 64. | Chakraborty S, Lakshmanan M, Swa HL, Chen J, Zhang X, Ong YS, Loo LS, Akıncılar SC, Gunaratne J, Tergaonkar V, Hui KM, Hong W. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun. 2015;6:6184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 65. | Kapoor A, Bayat Mokhtari R, Sonti SS, Patel R, George A, Attwood K, Iyer R, Chakraborty S. Circulatory Agrin Serves as a Prognostic Indicator for Hepatocellular Carcinoma. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer. 2020;11:2008-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 67. | Devan AR, Nair B, Pradeep GK, Alexander R, Vinod BS, Nath LR, Calina D, Sharifi-Rad J. The role of glypican-3 in hepatocellular carcinoma: Insights into diagnosis and therapeutic potential. Eur J Med Res. 2024;29:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 68. | Montalbano M, Georgiadis J, Masterson AL, McGuire JT, Prajapati J, Shirafkan A, Rastellini C, Cicalese L. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma (Review). Oncol Rep. 2017;37:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Negri F, Gnetti L, Pedrazzi G, Silini EM, Porta C. Sorafenib and hepatocellular carcinoma: is alpha-fetoprotein a biomarker predictive of tumor biology and primary resistance? Future Oncol. 2021;17:3579-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Espejo-Cruz ML, González-Rubio S, Zamora-Olaya J, Amado-Torres V, Alejandre R, Sánchez-Frías M, Ciria R, De la Mata M, Rodríguez-Perálvarez M, Ferrín G. Circulating Tumor Cells in Hepatocellular Carcinoma: A Comprehensive Review and Critical Appraisal. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Salehi M, Lavasani ZM, Keshavarz Alikhani H, Shokouhian B, Hassan M, Najimi M, Vosough M. Circulating Tumor Cells as a Promising Tool for Early Detection of Hepatocellular Carcinoma. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 72. | Batmunkh E, Tátrai P, Szabó E, Lódi C, Holczbauer A, Páska C, Kupcsulik P, Kiss A, Schaff Z, Kovalszky I. Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Hum Pathol. 2007;38:1508-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Zheng X, Liu X, Lei Y, Wang G, Liu M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front Oncol. 2022;12:824208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |