Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105417
Revised: March 22, 2025
Accepted: April 22, 2025
Published online: May 15, 2025
Processing time: 111 Days and 4.7 Hours
As a heterogeneous group of lesions, pancreatic cystic lesions (PCLs) vary enormously in malignant potential, mandating different treatment strategies. Despite significant advances in diagnostic imaging and laboratory tests, the accurate diagnosis of PCLs remains challenging, leading to overtreatment or delayed/missed surgical timing in patients with PCLs.
We present a case of a 64-year-old female patient in whom an asymptomatic, incidental cystic mass was found in the pancreatic tail on a routine abdominal ultrasound. After a comprehensive work-up with laboratory examinations, contrast-enhanced computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography, a pancreatic pseudocyst was suspected. Subsequent endoscopic ultrasound with fine-needle aspiration and needle-based confocal laser endomicroscopy supported a benign diagnosis. Follow-up computed tomography and magnetic resonance imaging examinations five months later showed significant cyst shrinkage without any abnormalities. However, three years after being lost to follow-up, the patient was readmitted and diagnosed with pancreatic adenocarcinoma with multiple metastases, suggesting that the initial lesion was a mucinous cystic neoplasm misdiagnosed as a pan
Comprehensive integration of all available information (e.g., cyst features, abnormal imaging findings, cyst biochemistry, clinical history, and patient demographics) rather than over-reliance on imaging or endoscopic findings is pivotal to diagnosing PCLs, and patients with concerning features should undergo strict surveillance.
Core Tip: Advances in imaging are resulting in more incidental diagnoses of pancreatic cystic lesions. However, they can be challenging to subclassify, and their malignant potential varies, requiring different treatment approaches. Endoscopic ultrasound and ancillary methods like fine-needle aspiration and needle-based confocal laser endomicroscopy are significantly improving the sensitivity and accuracy of diagnosis. However, in this case, all these methods failed to identify subsequent malignant transformation, emphasizing the importance of attention to detail and strict and close surveillance where clinical uncertainty exists.
- Citation: Yan ZY, Shi W, Guo T, Yang AM. Mucinous cystic neoplasm mimicking pancreatic pseudocyst and progressing to adenocarcinoma: A case report. World J Gastrointest Oncol 2025; 17(5): 105417
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105417.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105417
Pancreatic cystic lesions (PCLs) are a heterogeneous group of cystic lesions of the pancreas with distinct biological behaviors and malignant potential; of these, pseudocysts (PCs) are the most common, and they often have non-specific symptoms and can resolve spontaneously. Endoscopic transluminal drainage is only recommended for symptomatic or persistent PCs, given their benign behavior[1,2]. By contrast, pancreatic cystic neoplasms (PCNs) are thought to carry a higher risk of malignant transformation, especially mucinous lesions such as mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs), for which surgery is preferred[1]. Thus, the precise risk stratification of PCLs and corresponding close surveillance are pivotal to balancing the benefit-to-risk ratio of pre-emptive surgery vs surgical over-treatment[3]. Although there have been significant advances in diagnostic techniques for PCLs in recent years [including advances in imaging and endoscopy and the emergence of endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) and needle-based confocal laser endomicroscopy (nCLE)], accurate diagnosis and monitoring of PCLs are still very challenging, resulting in over-treatment of patients with PCLs or delays/missed surgical timing[4,5]. Here we report a patient with a MCN that mimicked PC despite comprehensive investigation, and the lesion eventually progressed to advanced adenocarcinoma. In presenting the case, we discuss the salient issues surrounding diagnosing and managing PCLs under the current framework of mainstream guidelines.
A 64-year-old female patient was admitted to our hospital with a four-month history of an incidental pancreatic cystic mass.
During a health checkup four months ago, an incidental cystic mass was found in the pancreatic tail during routine abdominal ultrasound. The patient was asymptomatic, experiencing no discomfort or clinical abnormalities.
The patient had a history of hypertension, which was managed well with amlodipine 5 mg/day.
There was no family history of malignant tumors, psychological illness, or genetic disorders.
On presentation, her vital signs were stable, and the abdominal examination was normal.
All the results of laboratory examinations were within normal limits, including serum blood glucose, amylase, lipase, total and direct bilirubin, carcinoembryonic antigen (CEA), and carbohydrate antigens (CA) 19-9 and 242.
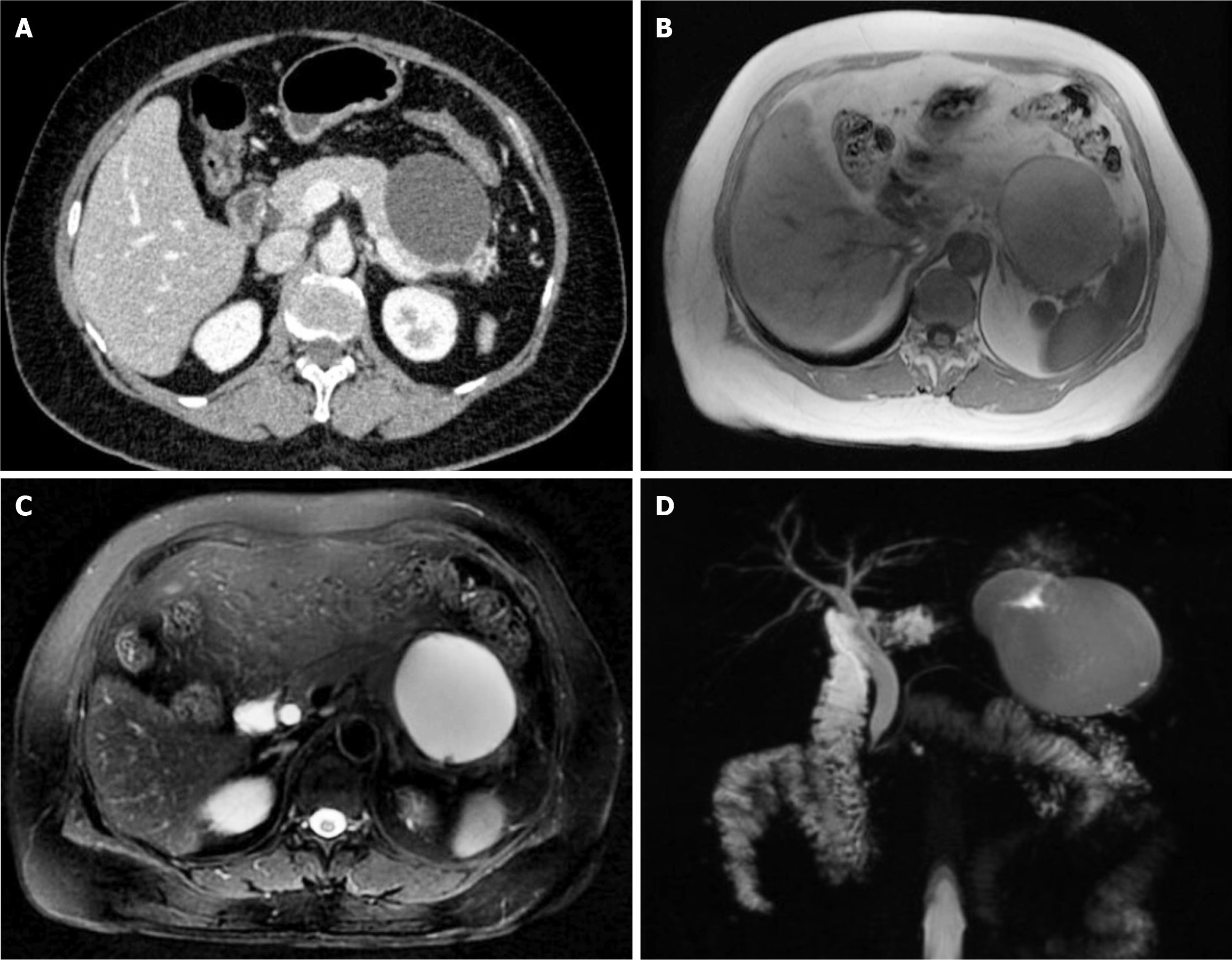
An abdominal contrast-enhanced computed tomography (CT) scan revealed an approximately 7.1 cm × 7.3 cm round, thin-walled, well-defined, and low-density cyst located in the tail of the pancreas and pushing against the stomach wall. There was no communication with the main pancreatic duct nor surrounding pancreatic parenchymal changes. There was no contrast enhancement in multiple phases (Figure 1A). Magnetic resonance imaging (MRI) revealed a unilocular cyst with low and high signal intensities in T1- and T2-weighted images, respectively, covered by a uniform peritubular capsule in the T1-weighted sequence (Figure 1B and C), which was further confirmed by magnetic resonance cholangiopancreatography (MRCP) (Figure 1D). There was no intra-/extra-hepatic bile duct or main pancreatic duct dilations.
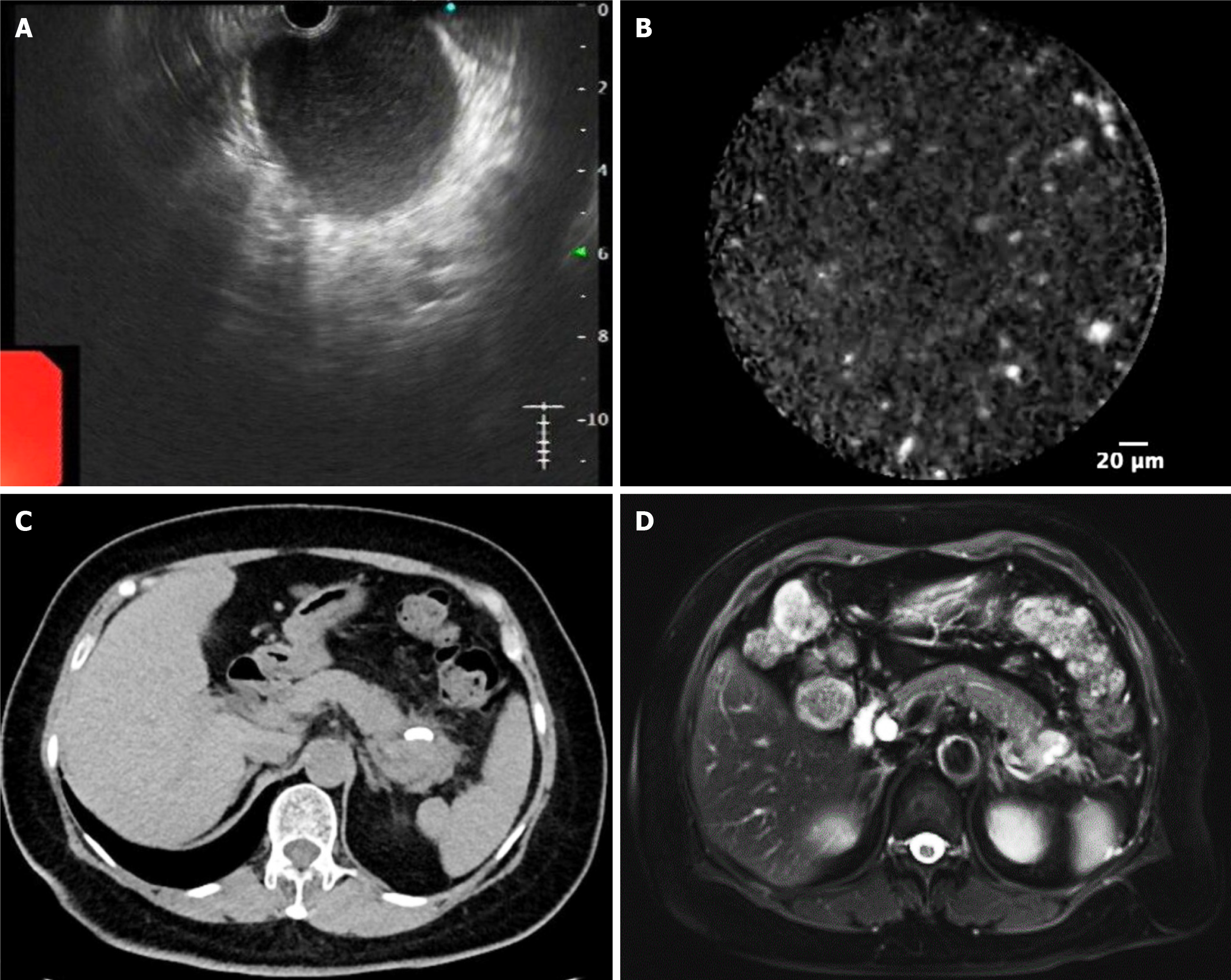
Subsequently, EUS-FNA and nCLE were undertaken, revealing hyperechoic spots on the inner wall without epithelial components (Figure 2A and B). The EUS appearances (uniform echogenicity, no loculation, and wall nodules) and nCLE findings (no clear epithelial components) supported a diagnosis of PC, and as the patient’s history, laboratory tests, and imaging showed no indication of malignancy, there was no indication for biopsy. Thus, plastic stents were placed transgastrically near the upper part of the gastric body under US guidance to treat suspected PC following the Chinese expert consensus on the endoscopic diagnosis and treatment for pancreatic PC, which recommends treatment when the PC has persisted for over four weeks and is > 6 cm in diameter, amongst other criteria[6]. The procedure drained brown and cloudy cystic fluid with an amylase level of 13011.0 U/L, lipase level of > 150000 U/L, CEA level of 922.3 ng/mL, and CA 19-9 Level of 182.7 U/mL. Of note, no atypical cells were found by direct smears and the Thinprep cytologic test.
By integrating all the above information and examinations, the patient was diagnosed with PC.
Given the benign behavior of PC and successful catheterization and drainage, the team opted to observe rather than subject the patient to further aggressive treatment such as surgery.
Follow-up CT and MRI one and five months later indicated significant cyst shrinkage without any signs of recurrence, and the stent was still in place (Figure 2C and D). The patient was subsequently lost to follow-up; no further evaluation was performed. However, the patient presented to the local hospital three years after cyst drainage, complaining of loss of appetite, loathing the smell of greasy foods, and the gradual development of upper abdominal pain radiating to the back. Gastrointestinal endoscopy detected a bleeding gastric ulcer in the gastric fundus (Forrest Ib) distant from the site of stent placement (now not present and presumed dislodged), with histopathological examination of the biopsy revealing moderately differentiated adenocarcinoma. She lost 2.5 kg in weight during this period and was transferred to our hospital for further evaluation. Both physical examination and laboratory examinations were still unremarkable, with CEA, CA19-9, CA242, amylase, lipase, and liver function tests all within normal limits.
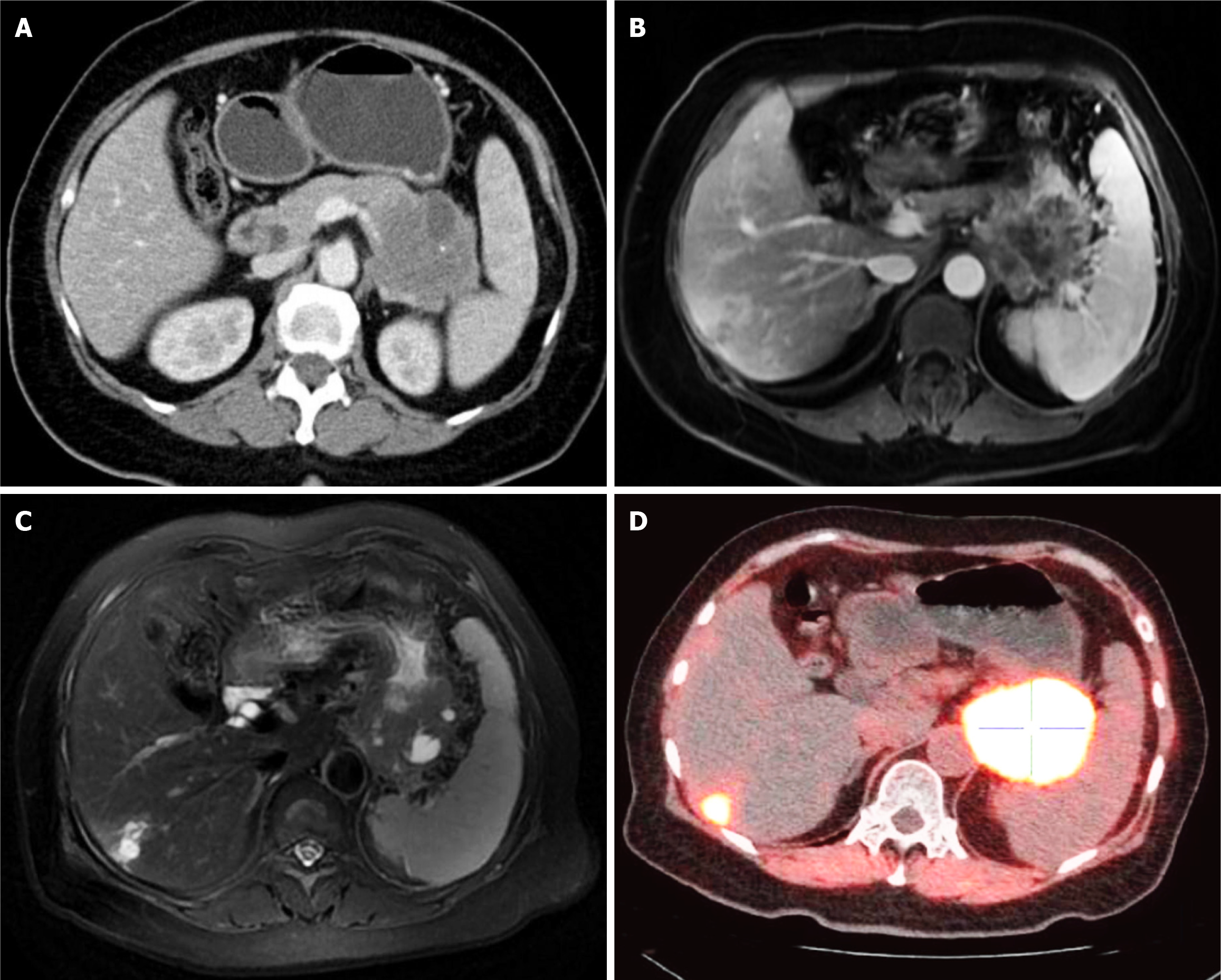
Unexpectedly, the contrast-enhanced CT scan detected an irregular and inhomogeneous contrasted-enhancing cystic mass of approximately 5.6 cm × 6.0 cm between the pancreatic tail and the posterior wall of the gastric fundus, and there were several internal necrotic areas and calcifications (Figure 3A). In addition, numerous abnormal retroperitoneal lymph nodes were present, and the splenic vessels were stenosed. Dynamic contrast-enhancing MRI showed garland-like contrast enhancements of the cystic mass and multiple abnormal lesions in the liver consistent with metastasis (Figure 3B and C). Moreover, fluorine 18 fluorodeoxyglucose- and fluorine 18-labeled fibroblast-activation protein inhibitor-positron emission tomography/CT further verified increased uptake in the cystic mass, especially in the pancreatic tail, as well as multiple liver lesions; both the gastric body and the left adrenal gland were also involved (Figure 3D). After a multidisciplinary team meeting, the patient was ultimately diagnosed with pancreatic adenocarcinoma with multiple metastases, and there were no surgical options. Thus, chemo- and immuno-therapies were recommended despite a high risk of gastric perforation. However, she was discharged without further treatment due to the expense.
Here, we report a case of advanced pancreatic adenocarcinoma that initially presented as MCN but was misdiagnosed as PC after comprehensive examinations that included EUS-guided FNA and nCLE. Of note, even imaging five months after stent insertion still favored PC. PCLs can broadly be subclassified into neoplastic (PCNs) and non-neoplastic lesions. PCNs are a heterogenous group of lesions, with serous cystic neoplasms, MCNs, and IPMNs most commonly encountered in practice[7]. PCs are also the most common type of PCL, accounting for up to 80% of cases[8]. PCs are considered one of the most common complications of pancreatitis, with a few occurring after pancreatic trauma or abdominal surgery. They are localized fluid collections rich in amylase and other pancreatic enzymes, without solid components, surrounded by a well-defined wall of fibrous tissue[2,9,10]. Although the clinical presentation varies greatly in PCs, with a lack of specific symptoms and signs, most patients describe a pancreatitis-related history that helps the diagnosis. However, PCNs, especially MCNs and IPMNs[11], must be distinguished from benign PCLs as they have malignant potential. As advances in imaging are resulting in more incidental discoveries of PCLs[12], it is becoming more important to precisely evaluate and differentiate cysts with the aid of EUS or even invasive FNA to obtain cyst fluid for laboratory and cytopathological examinations. The fluid in pseudocysts commonly have low CEA levels, negative cytology, and high amylase levels, while fluid in MCNs and IPMNs commonly have high CEA levels, atypical epithelial cells on cytological analysis harboring KRAS mutations, mucin, and variable amylase levels[7]. Proactive treatment and follow-up should be provided to high-risk patients to avoid adverse consequences, while patients with PCs should follow more conservative strategies to preserve the physiological functions of the pancreas and minimize treatment-related side effects.
Nevertheless, it remains challenging to distinguish MCNs from benign PCLs. MCNs mainly appear in the pancreatic body or tail and are characterized by unilocular cysts and peripheral mural or septal calcifications. Enhancing mural nodules and thick, irregular walls are associated with higher malignant risk. By contrast, PCs are more commonly round, uniform, fluid-filled masses adjacent to the pancreas visible in T2-weighted MRI or MRCP images[13,14]. Additionally, as a complication of pancreatitis, PCs are often accompanied by pancreatic changes such as pancreatic and/or common bile duct dilatation and calcifications. However, the specificity of the above imaging features is low, and CT is relatively poor at identifying them; even MRI/MRCP is only 55-87% accurate in distinguishing malignant mucinous lesions from benign PCLs[2,15].
Until now, EUS has been an essential diagnostic modality for evaluating the malignant risk of PCLs, especially in cases where the above imaging approaches are equivocal[16]. First, cystic fluid can be aspirated, and CEA > 192 ng/mL supports a diagnosis of mucinous PCL with high sensitivity and specificity, while amylase < 250 U/L effectively excludes PCs[3]. Access to the lesion also allows for cytological or tissue diagnoses, and, as an emerging and excellent addition to EUS-FNA, EUS-nCLE permits real-time microscopic visualization of the intra-cystic epithelium of PCLs to achieve in vivo optical biopsies[17]. Napoleon et al[18] established criteria to discriminate MCNs (a grey band with a thin dark line with or without deep blood vessels) and PCs (a field of bright grey and black particles) using EUS-nCLE, and Feng et al[19] also described a unique pattern of malignant PCLs (dark aggregates of cells) with 94% accuracy and 100% specificity for the diagnosis.
It is worth noting that these advanced approaches failed in the present case. On reviewing the entire diagnosis and treatment of the patient, some features - namely the cyst’s large size, abnormal MRI/MRCP signs, the relatively high cyst CEA level, a lack of pancreatitis history, and female gender - should have raised uncertainty and suggested the possibility of MCN[2,20]. Accordingly, for more precise diagnosis and differentiation of PCLs, all information must be considered and integrated rather than over-relying on imaging or endoscopic findings. Regrettably, after two imaging follow-ups over five months, the patient was lost to follow-up for three years and the opportunity for early detection and surgical cure was missed. Although the modality and manner of surveillance have yet to be agreed upon in different guidelines, the overall principle is that the malignant risk should be weighed against the specific patient’s life expectancy and comorbidities[3,21,22]. Generally, follow-up strategies for MCNs are more rigorous than for IPMNs. According to European guidelines, asymptomatic patients with MCNs < 40 mm without mural nodules should undergo surveillance with CA 19-9, EUS, and/or MRI every six months for one year and then annually thereafter[3]. By contrast, the American Gastroenterological Association and International Association of Pancreatology guidelines are much more aggressive; resection is recommended for all patients with MCNs[21,22]. Therefore, combined with the fact that MCN takes several years to become invasive cancer and the practical clinical considerations[4,23], we recommend close and lifelong surveillance for PCs with concerning features following the surveillance intervals for MCN defined in the European guidelines until patients are fit for surgery. Overly aggressive strategies centered on surgery could result in complications related to dysfunction of the endocrine and exocrine functions of the pancreas, seriously affecting patients’ quality of life. Furthermore, pancreatic surgery carries risks to several organs and tissues[24-26].
Given advances in imaging modalities, the diagnosis and stratification of incidental PCLs are becoming more important. This case re-emphasizes the importance of comprehensively integrating information to precisely diagnose the lesion and carefully balance the benefit-to-risk ratio between surveillance and treatment.
We thank this patient for sharing her medical history and records so that we can learn more about PCNs and better manage other patients in the future. We also express gratitude to all physicians, surgeons, pathologists, radiologists, and medical staff who provided professional advice and invaluable support.
| 1. | Ardeshna DR, Cao T, Rodgers B, Onongaya C, Jones D, Chen W, Koay EJ, Krishna SG. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J Gastroenterol. 2022;28:624-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (6)] |
| 2. | Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol. 2009;15:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 174] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (7)] |
| 3. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 4. | van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Cheesman AR, Zhu H, Liao X, Szporn AH, Kumta NA, Nagula S, DiMaio CJ. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Zhu H, Du Y, Wang K, Li Z, Jin Z. Consensus guidelines on the diagnosis and treatment of pancreatic pseudocyst and walled-off necrosis from a Chinese multiple disciplinary team expert panel. Endosc Ultrasound. 2024;13:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Gupta A, Chennatt JJ, Mandal C, Gupta J, Krishnasamy S, Bose B, Solanki P, H S, Singh SK, Gupta S. Approach to Cystic Lesions of the Pancreas: Review of Literature. Cureus. 2023;15:e36827. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 9. | Pitchumoni CS, Agarwal N. Pancreatic pseudocysts. When and how should drainage be performed? Gastroenterol Clin North Am. 1999;28:615-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sardana NK, Yamaguchi J, Mcfadden DW. Chapter 47 - Acute Pancreatitis. In: Parsons PE, Wiener-Kronish JP. Critical Care Secrets. 5th ed. City of Saint Louis: Mosby, 2013: 336-342. [DOI] [Full Text] |
| 11. | Keane MG, Afghani E. A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. J Clin Med. 2021;10:1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Rimbaș M, Rizzatti G, Larghi A. Diagnostic Approach to Incidentally Detected Pancreatic Cystic Lesions. Curr Treat Options Gastro. 2022;20:20-33. [DOI] [Full Text] |
| 13. | Jeffrey RB. Imaging Pancreatic Cysts with CT and MRI. Dig Dis Sci. 2017;62:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lee LS. Updates in diagnosis and management of pancreatic cysts. World J Gastroenterol. 2021;27:5700-5714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Mohamed E, Jackson R, Halloran CM, Ghaneh P. Role of Radiological Imaging in the Diagnosis and Characterization of Pancreatic Cystic Lesions: A Systematic Review. Pancreas. 2018;47:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Rangwani S, Juakiem W, Krishna SG, El-Dika S. Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review. Diagnostics (Basel). 2023;13:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lévy P, Rebours V. The Role of Endoscopic Ultrasound in the Diagnosis of Cystic Lesions of the Pancreas. Visc Med. 2018;34:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Feng Y, Chang X, Zhao Y, Wu D, Meng Z, Wu X, Guo T, Jiang Q, Zhang S, Wang Q, Yang A. A new needle-based confocal laser endomicroscopy pattern of malignant pancreatic mucinous cystic lesions (with video). Endosc Ultrasound. 2021;10:200-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 20. | Postlewait LM, Ethun CG, McInnis MR, Merchant N, Parikh A, Idrees K, Isom CA, Hawkins W, Fields RC, Strand M, Weber SM, Cho CS, Salem A, Martin RC, Scoggins C, Bentrem D, Kim HJ, Carr J, Ahmad S, Abbott DE, Wilson GC, Kooby DA, Maithel SK. Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms: A Multicenter Study. JAMA Surg. 2017;152:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 22. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 23. | Lawrence SA, Attiyeh MA, Seier K, Gönen M, Schattner M, Haviland DL, Balachandran VP, Kingham TP, D'Angelica MI, DeMatteo RP, Brennan MF, Jarnagin WR, Allen PJ. Should Patients With Cystic Lesions of the Pancreas Undergo Long-term Radiographic Surveillance?: Results of 3024 Patients Evaluated at a Single Institution. Ann Surg. 2017;266:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Tran TC, van Lanschot JJ, Bruno MJ, van Eijck CH. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology. 2009;9:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Das A, Ngamruengphong S, Nagendra S, Chak A. Asymptomatic pancreatic cystic neoplasm: a cost-effectiveness analysis of different strategies of management. Gastrointest Endosc. 2009;70:690-699.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Sharib J, Esserman L, Koay EJ, Maitra A, Shen Y, Kirkwood KS, Ozanne EM. Cost-effectiveness of consensus guideline based management of pancreatic cysts: The sensitivity and specificity required for guidelines to be cost-effective. Surgery. 2020;168:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |