INTRODUCTION
Gallbladder cancer (GBC) has been increasingly recognized as a significant health concern worldwide with a notable increase in its incidence, particularly among high-risk populations. In 2020, there were 115949 newly diagnosed cases of GBC worldwide, with a higher incidence in females than in males[1]. The incidence of GBC exhibits considerable regional variation, with the highest rates reported in South America, South Asia, and East Asia. In East Asia, the incidence rate is 1.4 per 100000 individuals, in contrast to 0.66 per 100000 in Europe and 0.67 per 100000 in North America[2]. Furthermore, there is a growing trend of GBC in younger populations[3]. In China, GBC is relatively more prevalent, with 31114 new cases and 25143 deaths reported in 2022. The incidence among females is notably higher than that among males, with a female-to-male ratio of approximately 2:1[4]. Most GBC cases are sporadic.
The etiology of GBC is multifactorial, with established risk factors including gallstones, chronic cholecystitis, and genetic predispositions. Gallstones, which are prevalent in many populations, have been implicated as a major risk factor because they can lead to chronic inflammation of the gallbladder, setting the stage for malignant transformation[5]. Chronic cholecystitis, characterized by ongoing inflammation, is another critical factor associated with the development of GBC. A chronic inflammatory environment can induce cellular changes that predispose individuals to cancer[6].
Genetic factors have also been identified as significant contributors to GBC risk. For example, gene polymorphisms such as poly (adenosine diphosphate-ribose) polymerase 1 rs1136410 have been linked to an earlier onset of GBC, highlighting the role of genetic susceptibility in the disease[7]. Additionally, environmental factors such as exposure to arsenic in drinking water have been suggested to elevate GBC risk, particularly in endemic regions[8]. Despite these insights into risk factors, the challenge remains that many patients are present with advanced disease due to the lack of early symptoms. The insidious nature of GBC often leads to late diagnosis, which significantly hampers treatment efficacy and overall survival rates. Therefore, a comprehensive understanding of the risk factors, coupled with advancements in diagnostic and therapeutic strategies, is essential for improving patient outcomes. Recent studies have explored novel diagnostic modalities and treatment options that could enhance early detection and improve prognostic outcomes in patients with GBC[9,10].
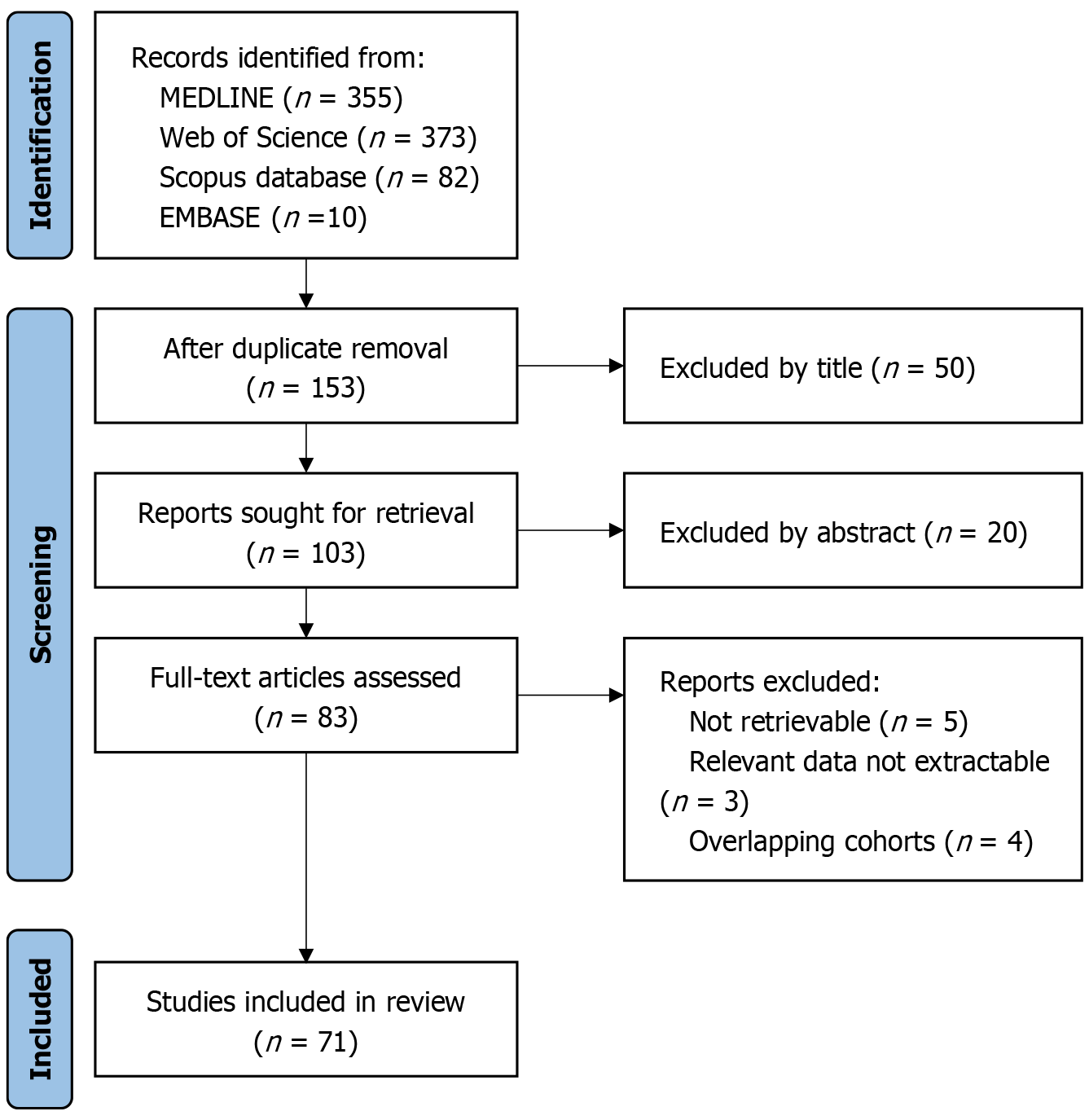
A systematic literature search was conducted utilizing four prominent academic databases: MEDLINE (accessed through the PubMed interface), Web of Science Core Collection, Embase, and Scopus. The screening process adhered to a stringent four-stage protocol: (1) Initial identification of records via database searches; (2) Screening of titles and abstracts; (3) Evaluation of full-text eligibility; and (4) Final inclusion of studies. The methodology, structured to ensure transparency and reproducibility, is thoroughly detailed in Figure 1.
Figure 1
Literature screening flowchart.
RISK FACTORS FOR GBC
Association between gallstones and GBC
Numerous studies have established that gallstones are a significant risk factor for GBC, correlating their presence with malignancy development. The mechanisms underlying this association include chronic inflammation and changes in the gallbladder lining due to stone irritation. Patients with cholelithiasis show histopathological changes in the gallbladder lining, including dysplasia and metaplasia, which may indicate cancer progression[11]. Additionally, research indicates that both the size and quantity of gallstones are related to an increased cancer risk, which means that larger stones may further elevate this risk[12]. A retrospective study showed that patients with gallstones had a higher incidence of GBC, highlighting the need for vigilant monitoring in patients diagnosed with cholelithiasis[13].
Pathological mechanisms of chronic cholecystitis
Chronic cholecystitis is a critical factor that is associated with GBC. Persistent inflammation of the gallbladder wall leads to a series of pathological changes including fibrosis and dysplastic changes in the epithelial lining. These alterations may predispose the tissues to malignant transformation. Inflammatory cytokines and mediators released in response to chronic irritation are believed to play a role in carcinogenesis by promoting cellular proliferation and survival[14]. Additionally, studies have shown that chronic cholecystitis can lead to the development of gallbladder dysplasia, which is a precursor to invasive cancer[11]. Understanding this relationship is complicated by the interplay between inflammation and genetic predisposition, which highlights the need for a thorough understanding of the molecular mechanisms involved.
Genetic susceptibility and family history
Genetic predisposition is increasingly being recognized as a significant risk factor for the development of GBC. A family history of GBC or related malignancies may indicate an inherited genetic susceptibility. Specific genetic mutations and polymorphisms are associated with increased risk, highlighting the importance of genetic screening in high-risk populations[15]. Furthermore, familial aggregation studies have suggested that individuals with a family history of GBC are more likely to develop the disease, underscoring the need for targeted surveillance in these individuals[16]. Understanding the genetic landscape of GBC could lead to better risk assessment and management strategies for affected families.
Other potential risk factors (diet and environment)
Various dietary and environmental factors have been implicated in the risk of developing GBC. Diets high in fat and cholesterol, particularly those low in fiber, have been linked to an increased incidence of gallstones and subsequently, GBC[17]. Environmental exposures, such as certain industrial chemicals and toxins, may also contribute to the risk profile of GBC, although the evidence remains less robust than that for gallstones and chronic inflammation[18]. Lifestyle factors, including obesity and sedentary behavior, further exacerbate this risk, highlighting the multifactorial nature of the etiology of GBC. A comprehensive understanding of these risk factors is essential for developing preventive strategies and risk-reduction initiatives for at-risk populations.
ADVANCES IN DIAGNOSTIC TECHNIQUES FOR GBC
Application of imaging techniques (ultrasound, computed tomography, and magnetic resonance imaging)
Imaging techniques play a crucial role in early detection and diagnosis of GBC. Ultrasound is often the first-line imaging modality because of its accessibility and ability to detect gallbladder wall thickening, a common sign of malignancy. Recent studies have highlighted the effectiveness of high-frame-rate contrast-enhanced ultrasound in differentiating between benign and malignant gallbladder lesions, showing promising results in enhancing diagnostic accuracy[19]. Additionally, computed tomography (CT) and magnetic resonance imaging (MRI) have been utilized because of their superior spatial resolution and their ability to provide detailed anatomical information. A comparative study indicated that both contrast-enhanced CT and MRI are valuable for diagnosing wall-thickening type GBC, with each modality offering unique advantages depending on the clinical scenario[20]. Moreover, the integration of deep learning algorithms into ultrasound imaging has shown potential in improving the detection rates of GBC, making it a promising area for future research[21]. Overall, the combination of these imaging modalities provides a comprehensive approach for GBC diagnosis, enabling earlier and more accurate detection.
Role of endoscopic techniques in GBC diagnosis
Endoscopic techniques, particularly endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS), have emerged as critical tools for diagnosing GBC. EUS offers a high-resolution view of the gallbladder and surrounding structures, allowing the detection of small lesions that may not be visible on other imaging modalities. It is especially useful in assessing the depth of invasion and presence of lymphadenopathy, which are important for staging the disease[22]. Furthermore, ERCP can facilitate the collection of bile for cytological analysis, aiding in the diagnosis of malignancy when imaging findings are inconclusive. The combination of EUS and ERCP enhances the diagnostic accuracy by providing both imaging and tissue sampling capabilities, which are essential for confirming the diagnosis of GBC and planning subsequent treatment strategies[23]. As endoscopic techniques continue to evolve, their role in the diagnosis of GBC is likely to expand, offering clinicians valuable tools for early detection and intervention.
Research progress on biomarkers
The identification of reliable biomarkers for GBC is an area of intense research focus as it can significantly enhance early detection and improve patient management. Recent studies have explored various biological markers, including volatile organic compounds in bile, which have shown promise as potential diagnostic indicators for GBC[24]. Additionally, the quantification of cell-free DNA in bile has been investigated as a non-invasive method for diagnosing suspected GBC, providing a novel approach for early detection[25]. The exploration of mucin markers, such as mucin 5ac, has also gained attention because of their association with biliary tract cancers, suggesting their potential utility in diagnostic applications[26]. As research continues to uncover new biomarkers, their integration into clinical practice could lead to a more accurate and earlier diagnosis of GBC, ultimately improving patient outcomes.
Importance of histopathological examination
Histopathological examination remains the gold standard for definitive diagnosis of GBC. Tissue biopsy, whether obtained through endoscopic techniques or surgical procedures, allows the assessment of cellular morphology and confirmation of malignancy. The importance of accurate histopathological evaluation cannot be overstated, as it not only confirms the diagnosis but also provides critical information regarding tumor type, grade, and stage, which are essential for treatment planning[27]. Moreover, the identification of specific histopathological features can aid in distinguishing GBC from other conditions that may mimic its appearance, such as adenomyomatosis or inflammatory lesions[28]. Advances in pathological techniques such as immunohistochemistry and molecular profiling continue to evolve and promise to enhance the diagnostic accuracy and prognostic stratification of GBC, thereby improving patient management and therapeutic outcomes.
EARLY DIAGNOSIS STRATEGIES FOR GBC
Methods for screening high-risk populations
Early diagnosis of GBC is crucial, especially in high-risk populations, which include individuals with a history of gallstones, chronic cholecystitis, or genetic predispositions. Screening methods for these groups often involve a combination of imaging techniques and biomarker assessment. Ultrasound remains the first-line imaging modality because of its non-invasive nature and effectiveness in detecting gallbladder abnormalities. However, advanced imaging techniques, such as MRI and CT, provide superior details and can help differentiate between benign and malignant lesions[29]. Additionally, the incorporation of serum biomarkers, such as carbohydrate antigen 19-9, has shown promise in enhancing the diagnostic accuracy. Elevated levels of carbohydrate antigen 19-9 have been correlated with gallbladder carcinoma, thus serving as a potential adjunct in screening protocols[30]. Moreover, recent studies have advocated the implementation of targeted screening programs in populations with known risk factors, which could lead to earlier detection and improved outcomes[31].
Importance of multidisciplinary collaboration in early diagnosis
The complexity of GBC necessitates a multidisciplinary approach to enhance its early diagnosis and treatment outcomes. Collaboration between gastroenterologists, radiologists, oncologists, and pathologists is essential for accurate diagnosis and management. Each specialty contributes unique expertise; for instance, gastroenterologists can perform endoscopic evaluations and radiologists can interpret imaging studies that are critical for identifying early-stage disease[32]. Multidisciplinary tumor boards have been shown to improve clinical decision-making, allowing for a comprehensive review of atypically present cases[33]. Furthermore, ongoing education and training among healthcare providers regarding the latest diagnostic modalities and treatment options are vital for maintaining high standards of care in GBC management.
Application prospects of advanced technologies
Liquid biopsy represents a promising advancement in the early detection of GBC. This non-invasive technique involves analyzing circulating tumor cells and cell-free DNA in the bloodstream, offering insights into tumor biology without the need for invasive procedures[34]. Liquid biopsies can potentially identify genetic mutations and other biomarkers associated with GBC, thus facilitating timely intervention[35]. Recent studies have indicated that liquid biopsy may outperform traditional tissue biopsy in certain scenarios, particularly when tumors are located at challenging anatomical sites[36]. Moreover, the ability to monitor treatment responses and detect recurrence through serial liquid biopsies adds a significant advantage to patient management[37]. As research progresses, the integration of liquid biopsy into routine clinical practice could revolutionize the landscape of GBC diagnosis, enabling earlier detection and personalized therapeutic strategies[38].
TREATMENT METHODS FOR GBC
Current status and challenges of surgical treatment
Surgical resection remains the primary treatment modality for GBC, particularly in patients with early-stage disease. The standard approach involves cholecystectomy, often accompanied by liver resection, when the cancer has invaded the adjacent tissues. However, challenges are significant because many patients present with advanced disease at diagnosis, which limits the feasibility of curative surgery. Current literature indicates that despite advancements in surgical techniques, such as laparoscopic methods and robotic surgery, the overall survival rates for GBC remain poor compared to those for other hepatobiliary malignancies[39,40]. Furthermore, complications, such as the bile leak and the need for conversion to open surgery, can hinder outcomes. The complexity of the disease, coupled with the anatomical intricacies of the biliary tree, necessitates a multidisciplinary approach to optimize the surgical outcomes and minimize morbidity. Additionally, the lack of standardized guidelines for the surgical management of GBC poses a challenge, highlighting the need for further research and consensus on the best practices[41,42].
Surgery is highly effective for early-stage GBC and offers the potential for long-term survival. This is particularly evident in cases where radical cholecystectomy is performed, which can lead to satisfactory survival outcomes even in regions with a high incidence of the disease. For instance, a study analyzing patients from an endemic region undergoing radical cholecystectomy for de novo GBC reported a median overall survival of 36 months, with significant survival benefits observed when extended resections were performed in high-volume centers combined with appropriate adjuvant treatments[43].
However, the efficacy of surgical intervention is notably limited in patients with advanced-stage GBC. Factors such as positive lymph node metastasis, hepatic invasion, and advanced tumor stage significantly impede the potential for achieving a cure post-surgery. A multi-center evaluation of GBC patients highlighted that combined surgical resection involving invaded organs and advanced American Joint Committee on Cancer stage were among the factors that virtually precluded a cure, with a very low likelihood of achieving long-term survival[44]. This underscores the need for careful patient selection and potential integration of adjuvant therapies to improve outcomes in advanced-stage GBC.
Comprehensive application of radiotherapy and chemotherapy
The integration of radiotherapy and chemotherapy has proven to be a promising approach for managing GBC, especially when surgical options are limited. Neoadjuvant and adjuvant therapies have demonstrated potential to improve treatment outcomes by reducing tumor burden and preventing recurrence[45]. Gemcitabine-based chemotherapy is a cornerstone in the treatment of advanced GBC and is frequently combined with other agents to enhance its effectiveness. The addition of radiotherapy to chemotherapy regimens has shown potential benefits in terms of local control and overall survival in various cancers, including pancreatic cancer and GBC. For example, a study on the combination of gemcitabine and radiotherapy in patients with locally advanced pancreatic cancer found that the addition of radiation therapy to gemcitabine improved overall survival, although it also increased toxicity[46]. This suggests that a similar approach could be beneficial in advanced GBC, in which local control is crucial because of the aggressive nature of the disease.
Moreover, the combination of gemcitabine with other chemotherapeutic agents, such as cisplatin or oxaliplatin, has been explored in advanced GBC, with some studies indicating that these combinations can lead to a significant tumor response and control of disease progression. For instance, a case report highlighted the efficacy of combining gemcitabine, oxaliplatin, and erlotinib in treating epidermal growth factor receptor-mutated GBC with liver metastasis, resulting in a substantial partial radiological response[47]. This underscores the potential of integrating targeted therapies with standard chemotherapy and radiotherapy to enhance the treatment outcomes in advanced GBC. However, the application of these modalities is often hampered by the inherent resistance of GBC to conventional chemotherapeutic agents, necessitating the development of more effective combinations of these novel agents[48]. Furthermore, the timing and sequence of chemotherapy and radiotherapy remain areas of active investigation, with the goal of maximizing therapeutic efficacy while minimizing adverse effects[49].
Recent advances in targeted and immunotherapy
Recent advancements in targeted therapy and immunotherapy have opened new avenues for GBC treatment. Targeted agents, such as those inhibiting specific molecular pathways involved in tumor growth, have shown promise in early clinical trials, offering hope for improved outcomes in this challenging malignancy[50]. Immunotherapy, particularly immune checkpoint inhibitors, has gained traction as a viable treatment option, and several studies have demonstrated its efficacy in cholangiocarcinoma and GBC[51]. However, challenges remain, including the need for robust clinical trial data to establish the optimal use of these therapies in the context of GBC and the potential for immune-related adverse events that require careful management[52].
An international multi-center study[53] analyzed the transcriptomic data of GBC to classify its molecular subtypes and revealed significant cellular and molecular heterogeneity in GBC. Based on survival times, two subtypes (subtypes 1 and 3) were found to have poor survival outcomes and were associated with adverse pathological features (e.g., pN1, pM1), an immunosuppressive microenvironment (myeloid-derived suppressor cell accumulation, extensive fibrous connective tissue proliferation, and hypoxia), and T lymphocyte dysfunction. In contrast, the better-prognostic subtype (subtype 2) exhibited opposite characteristics. The poorly prognostic subtypes also have a mesenchymal-like phenotype, with upregulation of gene sets related to epithelial-mesenchymal transition and transforming growth factor-β signaling[54]. During epithelial-mesenchymal transition, bidirectional interactions between tumor cells and the surrounding microenvironment facilitate the simultaneous activation of multiple signaling cascades during various transition phases[28]. Moreover, GBC cells can induce tumor immune suppression and promote cancer progression by releasing immunosuppressive cytokines and chemokines. In recent years, there has been an increase in multi-omics molecular classification research on GBC; however, no widely accepted consensus on molecular classification has been established.
The currently available fibroblast growth factor receptor inhibitors, infigratinib and pemigatinib, and the isocitrate dehydrogenase 1 inhibitor ivosidenib, although effective, are limited in their patient populations because of the low prevalence of these gene mutations. HER-2/neu targeted therapies, such as trastuzumab and zanidatamab, face the same issue[55]. Currently, immunotherapy for GBC primarily targets the programmed death-ligand 1 immune checkpoint. A phase II clinical trial showed that combined immunotherapy improves the prognosis of patients with advanced biliary tract cancer, and this approach is more effective than the use of anti-programmed death 1 alone and deserves further exploration[56]. Comprehensive comparisons of efficacy, safety, and quality of life are critical for optimizing GBC management. A multidisciplinary approach integrating surgery, systemic therapy, and biomarker-driven strategies is essential to balance survival benefits with patient-centered outcomes. Prioritizing cost-effective personalized care and addressing access disparities remain urgent challenges.
Exploration of personalized treatment strategies
The exploration of personalized treatment strategies for GBC is increasingly being recognized as a critical component of modern oncology. Given the heterogeneity of GBC, there is a growing emphasis on tailoring therapy based on individual patient characteristics, including genetic and molecular profiles[57]. Advances in genomic sequencing technology have facilitated the identification of specific mutations and alterations that can inform targeted treatment approaches[58,59]. Clinical trials focusing on personalized medicine are underway to validate the efficacy of tailored therapies and to establish guidelines for their implementation in clinical practice. However, the complexity of GBC necessitates a collaborative approach among oncologists, pathologists, and geneticists to ensure that personalized strategies are effectively integrated into routine care[60].
Role of the extracellular matrix in GBC
The extracellular matrix (ECM) plays a crucial role in the pathogenesis and progression of GBC. The ECM is a complex network of proteins and polysaccharides that provides structural and biochemical support to the surrounding cells. In the context of cancer, the ECM is not merely a passive scaffold but also actively participates in tumor progression by influencing cell behavior, including proliferation, migration, and invasion. In GBC, alterations in ECM composition and organization can promote tumorigenesis. A study using quantitative proteomic analysis revealed significant remodeling of ECM proteins in early-stage GBC, highlighting the downregulation of ECM organization pathways and upregulation of neutrophil degranulation pathways[61]. This suggests that ECM degradation may facilitate cancer cell invasion and metastasis by disrupting the normal tissue architecture and promoting a more invasive phenotype[62,63].
Furthermore, genetic variants of matrix metalloproteinases (MMPs), which are enzymes that degrade ECM components, have been associated with increased susceptibility to GBC. Polymorphisms in MMP-2, MMP-7, MMP-9, and their tissue inhibitors have been linked to a higher risk of developing GBC. These findings underscore the importance of ECM remodeling in the pathogenesis of GBC, and suggest that targeting ECM components or their regulators could be a potential therapeutic strategy[64]. Overall, the ECM is a dynamic and integral component of the tumor microenvironment in GBC. ECM remodeling corresponds to the stages of tumor progression and is associated with tumor evolution, with ECM properties potentially stimulating tumor resistance or increasing tumor sensitivity to therapy. Maintaining ECM homeostasis and function in GBC could provide insights into novel diagnostic and therapeutic approaches for aggressive malignancy[65].
Chimeric antigen receptor T-cell challenges in GBC
Chimeric antigen receptor T-cell (CAR-T) therapy has emerged as a revolutionary approach for cancer treatment, particularly for hematological malignancies. However, its application in solid tumors such as GBC presents unique challenges. One of the primary obstacles is the tumor microenvironment, which can inhibit CAR-T cell infiltration and function. Solid tumors often have dense ECM and immunosuppressive cells that create a hostile environment for CAR-T cells, limiting their efficacy[66,67]. Another significant challenge is antigen heterogeneity within solid tumors. Unlike hematological cancers, where target antigens, such as CD19, are uniformly expressed, solid tumors may express target antigens heterogeneously. This heterogeneity can lead to antigen-negative tumor escape, whereby tumor cells that do not express the target antigen survive and proliferate, causing relapse[68].
Moreover, the risk of on-target off-tumor toxicity increases in solid tumors. This occurs when CAR-T cells target antigens that are expressed in healthy tissues, leading to unintended damage. The development of strategies to enhance the specificity of CAR-T cells and reduce off-tumor effects is crucial for improving the safety profile of CAR-T therapies for solid tumors[69]. At present, research on CAR-T cell therapy for GBC is still in its infancy, but there are already some preliminary studies and clinical trials. Researchers are currently exploring potential GBC-related antigens as targets for CAR-T cells, such as the epidermal growth factor receptor and carcinoembryonic antigen. Although there is a lack of large-scale clinical trial data, initial studies have indicated that CAR-T cell therapy may be effective for GBC treatment.
To address these challenges, ongoing research is focused on engineering CAR-T cells with enhanced capabilities, such as the ability to secrete cytokines that modulate the tumor microenvironment, or the use of dual-targeting CARs to overcome antigen heterogeneity. Additionally, combining CAR-T therapy with other treatment modalities, such as checkpoint inhibitors or targeted therapies, is being explored to improve the outcomes in GBC and other solid tumors[70].