Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104737
Revised: January 14, 2025
Accepted: March 4, 2025
Published online: May 15, 2025
Processing time: 135 Days and 3.7 Hours
In China Banxia Xiexin decoction (BXD) has been used in treating gastric cancer (GC) for thousands of years. BXD has been shown to reverse GC histopathology, but its chemical composition and action mechanism are still unknown.
To investigate the mechanism of BXD against GC based on utilizing transcriptomics and proteomics techniques experiments.
Using the AGS cell line as the model group, the Cell Counting Kit-8 method and Annexin V-AbFluor™ were employed 488/propidium iodide double staining method was used to detect the levels of cell proliferation and apoptosis. Differential expression genes and differentially expressed proteins before and after BXD intervention were detected using RNA-seq and Pro DIA techniques. Key tran
BXD significantly inhibited the proliferation rate and migration rate of GC cells and promoted cell apoptosis. The comprehensive analysis of transcriptomics and proteomics showed that five transcription factors, namely phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, phosphoinositide-3-kinase regulatory subunit 1, AKT serine/threonine kinase 1, heat shock protein 90 alpha family class A member 1, and tumor protein p53, were key factors in BXD-mediated anti-cancer therapy and participated in the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway. In vitro experiments were conducted using LY294002, an inhibitor of the PI3K/AKT signaling pathway, to validate the expression of five transcription factors at the protein and mRNA levels. In vivo experiments have shown that BXD inhibits tumor growth and suppresses the expression of the PI3K/AKT signaling pathway.
Transcriptomic and proteomic analysis showed that BXD inhibited tumor growth and slowed cancer progression by suppressing five factors in the PI3K/AKT signaling pathway, including phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, phosphoinositide-3-kinase regulatory subunit 1, and AKT serine/threonine kinase 1.
Core Tip: Based on the clinical efficacy of traditional Chinese medicine Banxia Xiexin decoction in the prevention and treatment of gastric cancer, this paper explored the key genes and proteins in gastric cancer cells treated by Banxia Xiexin decoction using whole transcriptomics sequencing and proteomics sequencing technologies. The signaling pathway was explored, and we verified its molecular mechanism by combining in vivo and in vitro experiments.
- Citation: Zu GX, Tang JQ, Huang HL, Han T. Validation and analysis of key factors of Banxia Xiexin decoction against gastric cancer. World J Gastrointest Oncol 2025; 17(5): 104737
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104737.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104737
Gastric cancer (GC) has the fifth highest incidence rate and is the second-leading cause of cancer-related deaths worldwide[1,2]. As one of the most common malignant tumors in the digestive system, GC ranks second in the incidence and mortality of malignant tumors in China and first in the world, seriously endangering the lives and health of the people. The pathogenesis of GC is not yet completely clear, but the incidence and mortality of GC in China are increasing year by year, which may be due to the dietary habits and the low rate of early diagnosis and detection[3]. For the treatment of GC, the National Comprehensive Cancer Network[4] recommends a strategy of multidisciplinary par
As a traditional Chinese formula first recorded in the Treatise on Typhoid Fever, Banxia Xiexin decoction (BXD) has the efficacy of balancing cold and heat, eliminating nodules, and treating spleen and gastric disorders. It is currently effective in precancerous lesions of the stomach, GC, and related gastrointestinal disorders. Compared with Western medicine, the treatment of GC by BXD is mainly reflected in reversing the changes of chronic atrophic gastritis, slowing down the adverse reactions after GC surgery, and improving the quality of life of the patients and prolonging their survival years[6-8]. To further understand the specific mechanism of the role of BXD in GC, it was studied in detail and in depth using scientific research tools such as high-throughput sequencing and bioinformatics.
Transcription factors are DNA-binding proteins that can interact specifically with eukaryotic genes and have both activating and repressing effects on gene transcription. They have important regulatory roles in processes such as biological growth and development and adversity defense responses[9]. Transcriptome sequencing technology can reveal the expression of genes involved in the development of GC disease and important biological processes during treatment from the overall level of genes[10]. By integrating and analyzing the key factors and their downstream gene expression, it can penetrate the law and essence of organism physiology and pathology at the protein and gene level and reveal the mutual regulatory roles and correlations between the two, which can more accurately grasp the roles of transcription factors and genes[11]. At present, the integration and analysis of proteomics and transcriptomics data has become a trend in genomics research.
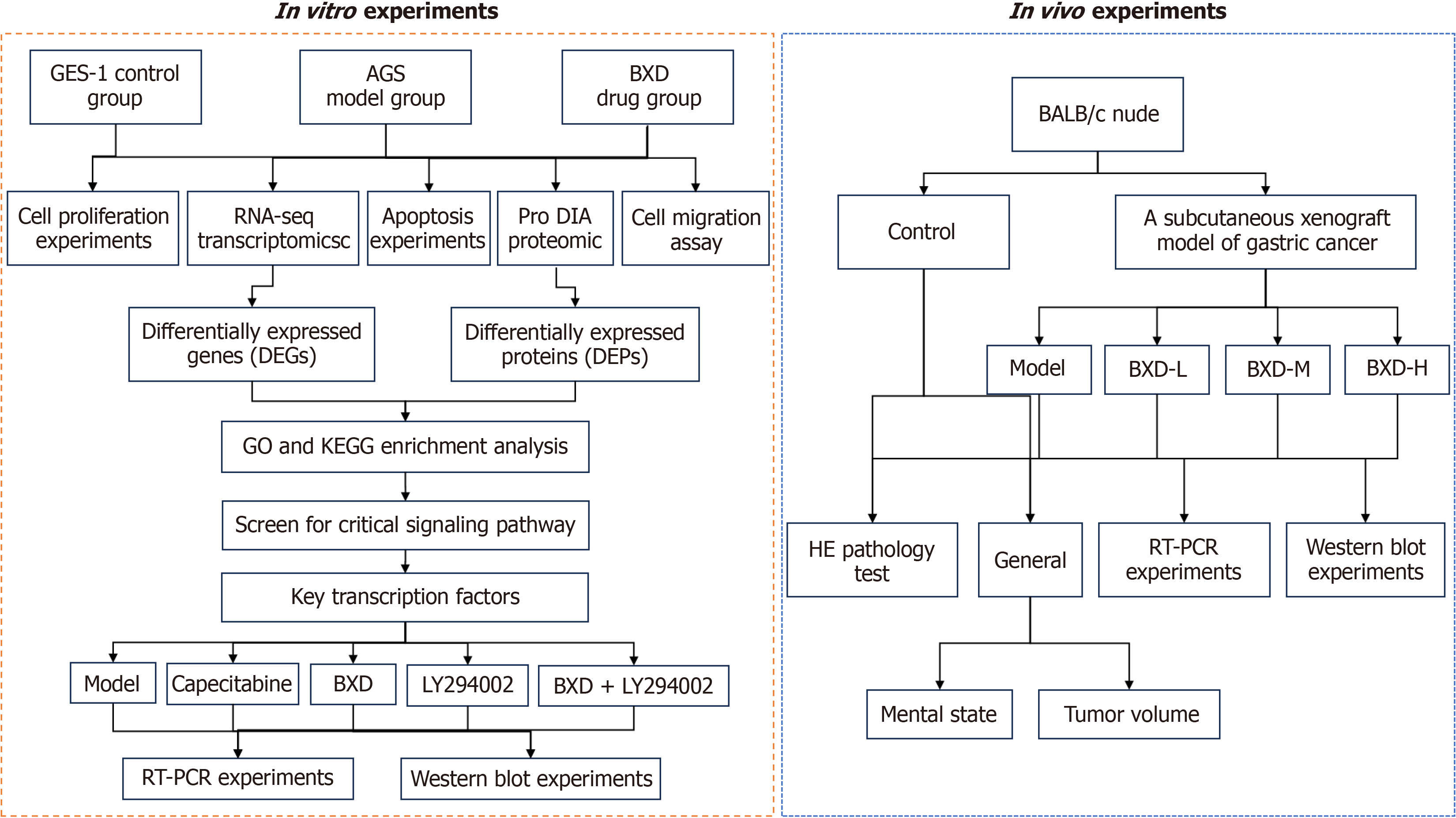
Phosphatidylinositol 3-kinase (PI3K) was found to be an intracellular phosphatidylinositol kinase with the function of activating AKT[12]. In recent years, studies have shown that BXD has a significant effect on the PI3K-AKT signaling pathway in a variety of disease models including cell proliferation, survival, metabolism, and growth, etc. Therefore, understanding the regulation of this signaling pathway by BXD is an important basis for understanding its therapeutic effects. Therefore, the aim of this study was to discuss the mechanism of action of BXD-containing serum to improve GC cells, to systematically explore the key transcription factors of BXD against GC damage by adopting the strategy of combining transcriptomics and proteomics, to reveal the connotations and patterns reflected by the data, and to combine the in vivo and in vitro experiments to reveal the essence of GC and the potential key targets of the therapeutic process of GC so as to provide the development of the drugs for the treatment of GC. We aimed to provide a reference for the development of drugs to treat GC. Figure 1 shows the general idea and flow chart of this experiment.
Six-week-old specific pathogen free grade Wistar rats, 60 rats, body mass (180-220 g) and 60 specific pathogen free grade male BALB/c nude rats, 16 ± 2 g, 4 to 5 weeks old were provided by Beijing Viton Lever Animal Laboratory Technology Co., Ltd, License No. SCXK (Beijing) 2021-0006. They were housed in the Animal Experimentation Center of Shandong University of Traditional Chinese Medicine, Husbandry. The animals were kept in the Animal Experiment Center of Shandong University of Traditional Chinese Medicine at a temperature of 26 °C ± 2 °C, a humidity of 50% ± 10%, and a light/dark cycle of 12 h. The animal experiments were started after 7 days of acclimatization. The animal experiments were reviewed by the Ethics Committee for Laboratory Animals of Shandong University of Traditional Chinese Medicine (Approval No. SDUTCM20230828016). The human GC cell line AGS and the normal gastric mucosa cell line GES-1 were purchased from Shanghai Anwei Biotechnology Co. They were cultured and passaged in the Animal Experiment Center of Shandong University of Traditional Chinese Medicine. The culture conditions of AGS were: 89% F12K medium, 10% fetal bovine serum, and 1% penicillin and streptomycin and cultured in a 37 °C and 5% CO2 cell culture box.
The experimental reagents included: (1) 100 MG desalting column (waters, WAT023590); (2) 200 MG desalting column (waters, WAT054945); (3) 20 × PBS buffer (Bioengineering, B548117-0500); (4) 3KD ultrafiltration tube (Millipore, UFC500324); (5) 500 MG desalting column (waters, WAT020805); (6) 50 MG desalting column (waters, WAT054955); (7) 96-well SPE desalting column (ThermoFisher, 60209-001); (8) 96-well SPE desalting column 10 mg (ThermoFisher, 60309-001); (9) Bicinchoninic acid kit (ThermoScientific, 23225); (10) Dithiothreitol Adamas-beta (1064272); (11) Ethylenediaminetetraacetic acid-2Na (Solebo, E1170-100 mL); (12) EStain LG Protein Dye Decolorization and Concentration Kit (Kingsley, L00755C-500); (13) LDS Sample Buffer, 4× (Ltd, 180-8210D); (14) Phenylmethanesulfonyl fluoride (Biyuntian, ST507-10 mL); (15) PTMScan ubiquitination antibody (Cell Signaling, 5562S); (16) Sodium-dodecyl sulfate gel electrophoresis (SDS-PAGE) gel 15% (Kingsley, M00719); (17) SDS lysate (Biyuntian, P0013G); (18) Electrophoresis buffer dry powder (Ltd., BT8100-2002); (19) Chromatography grade acetone (Wokai, 40064485); (20) Chromatography methanol (CNW, CAS. No. 67-56-1); (21) Chromatography water (ThermoScientific, W5-4); (22) Chromatography acetonitrile (ThermoScientific, A998-4); (23) Chromatography isopropanol (Fisher, A451-4); (24) Molecular weight marker for unstained proteins (ThermoScientific, 26610); (25) Trypsin (Wallis, HLS TRY001C); (26) Mass spectrometry methanol (ThermoScientific, A456-4); (27) Mass spectrometry water (ThermoScientific, W6-4) ); (28) VAHTS mRNA Capture Beads (Vazyme, N401-02); (29) VAHTS Stranded mRNA-seq Library Prep Kit for Illumina V2 (Vazyme, NR612-02); (30) VATHS DNA Clean Beads (Vazyme, N411-03-AA); and (31) NGS dsDNA HS Assay Kit (ABP-Bioscience, FP002).
The experimental instruments included: (1) Q Exactive Mass Spectrometer (ThermoFisher); (2) Easy-nLC 1200 Liquid Phase System (ThermoFisher); (3) Ultimate 3000 Liquid Phase System (ThermoFisher); (4) Q Exactive plus Mass Spectrometer (ThermoFisher); Nanoelute liquid phase (Bruker); (5) Instantaneous centrifuge (yooning); multifunctional cryomill (Shanghai Wanbai Biotechnology Co., Ltd., Wanbai 96E); (6) Digital gel image analyzer (Shanghai Tianneng Science and Technology Co., Ltd., Tanon-1600); (7) Instantaneous tabletop high-speed centrifuge (Jiangsu Keran Instruments Co., Ltd., TCL-16A); (8) Constant temperature shaker (Shanghai Yiheng Scientific Instruments, THZ-300C); (9) Low-speed centrifuge (Shanghai Hayabusa Instrument Co., Ltd., T6); (10) SDS-PAGE gel electrophoresis instrument (Beijing Liuyi Instrument Factory, DYY-6C); (11) Thinking rotary mixer (BF-1100 Mojibell); (12) High pH separation liquid chromatograph Agilent (Agilent 1100 series); (13) High pH separation Liquid Chromatograph Agilent (Agilent 1200 series); (14) Eppendorf Centrifuge (Eppendorf, 5430R); (15) 0.22 μm cellulose acetate membrane (gel matrix filter) (Agilent, 5185-5990); (16) EStain LG Protein Stainer (Nanjing Kingsley, L00760C); (17) Benchtop centrifuge (Eppendorf, Centrifuge 5418R); (18) PCR instrument (Bio-rad, MyCycler); (19) Magnetic stand (Invitrogen, Magnetic stand-96); and (20) Bioanalyzer (Aglient, 2100).
BXD included Banxia (2301002), Huangqin (2030402), Huanglian (230401), Renshen (2305004), Gancao (230303), Ganjiang (230404), and Dazao (230301). The above herbs were purchased from Anguo Anxing Traditional Chinese Medicine Drinking Tablets Co., Ltd., and all the herbs were accompanied with quality inspection reports, which were in accordance with the requirements of the Pharmacopoeia of the People’s Republic of China. Each tablet contained 0.5 g of ca
Fifty rats were divided into five groups, which were the control group (10 rats), the capecitabine group (10 rats), the low-dose group of BXD (10 rats), the medium-dose group of BXD (10 rats), and the high-dose group of BXD (10 rats). The rats in the BXD groups were gavaged with 3 mL of saline daily for 7 consecutive days at a concentration of 16.25 g/kg (low dose), 32.5 g/kg (medium dose), and 65 g/kg (high does) of BXD, and the rats in the capecitabine group were gavaged with 3 mL of saline daily for 7 consecutive days at a concentration of 0.42 g/kg of capecitabine in the control group. Blood was collected from the abdominal aorta after anesthesia administration of 2% pentobarbital sodium (0.3 mL/100 g) 120 min after the end of the gastric gavage. The blood was collected from the abdominal aorta. The whole blood was placed in a refrigerator at 4 °C for 2 h and then centrifuged at 3500 r/minute for 15 min to collect the upper serum layer. This layer was filtered with a 0.22 μm microporous membrane to remove bacteria and then stored in separate packages at -80 °C for subsequent in vitro experiments.
The normal gastric mucosa cell line GES-1 and the GC cell line AGS were cultured with 10% fetal bovine serum and 1% penicillin-streptomycin in F12K medium in a constant temperature incubator. They were digested and passaged with 0.25% trypsin when the cells in the culture flask reached 80%.
AGS cells in logarithmic growth phase were inoculated into 96-well plates with 5 × 103 cells/well and incubated in a 37 °C and 5% CO2 cell culture incubator for 24 h. The old medium was discarded. According to the grouping setup, 100 μL/well of BXD serum-containing medium for the low, medium, and high dose groups were added. Then 10 μL of Cell Counting Kit-8 (CCK-8) solution was added to each well after incubating them for 24 h or 48 h. After incubation for 2 h, the optical density (OD) value at 450 nm was detected by an enzyme labeling instrument. Cell proliferation rate = (OD value of experimental group - OD value of solvent control group)/(OD value of blank group - OD value of solvent control group) × 100%.
AGS cells in logarithmic growth phase were inoculated in 96-well plates at 5 × 103/well. After the cells were attached to the wall, the control and BXD groups were cultured for 24 h and 48 h, respectively. Then 10 μL of CCK-8 solution was added to each well, and the cells were cultured for 2 h. The OD value was determined, and the cell proliferation ability was calculated by using an enzyme labeling instrument at the wavelength of 450 nm.
AGS cells were inoculated on cell crawls, and the cells were observed under the microscope to be completely adhered to the wall. They were incubated for 24 h after drug administration according to the experimental groups and washed twice with PBS. Then 100 μL of 1× Annexin V binding buffer, 5 μL of Annexin V-AbFluor™ 488, and 2 μL of propidium iodide (PI) were added to each well and incubated at room temperature and protected from light for 15 min. They were washed with 1× Annexin V binding buffer and with 1× Annexin V-AbFluor™ 488/PI. They were incubated with Annexin V binding buffer for 15 min at room temperature and protected from light. Then the cells were washed twice with 1× Annexin V binding buffer and observed and analyzed under a fluorescence microscope using appropriate filters.
AGS cells in logarithmic growth phase were selected and inoculated in 6-well culture plates (2 × 105 cells). A scratch was made with a 200 μL pipet tip. After the scratching was completed, the floating cells were washed with PBS, and photos were taken to record the position of the scratches. The cells were cultured in a constant temperature incubator for intervals of 24 h according to the experimental grouping to observe and record the migration of cells under the mi
The RNA-seq transcriptome sequencing method was performed by referring to the methods reported in the literature, and RNA in the GES-1, AGS, and BXD groups was extracted using RNAiso plus lysate. RNA purity was characterized and quantified using a NanoDrop 2000 spectrophotometer (ThermoScientific, United States). RNA integrity was assessed using an Agilent 2100Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States). Transcriptome libraries were constructed using the VAHTSUniversalV5RNA-seqLibraryPrep kit according to the instructions. The libraries were sequenced and analyzed using the llumina Novaseq 6000 sequencing platform. Fastp[13] software was used to process the rawreads in fastq format, and cleanreads were obtained after removing low-quality reads for subsequent data analysis. Reference genome comparison was performed using HISAT2[14] software with gene expression (FPKM)[15] calculation, and reads counts (counts) for each gene were obtained by HTSeq-count[16]. RNA (1 μg) from each sample was used for library construction, and three biological replicates were performed for each group. The level of significance and folds of difference were used to set the screening conditions as P < 0.05 and |log2 fold change|≥ 1 for differential expression genes (DEGs) screening. The DEGs were analyzed for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment using Metascape. GO and KEGG entries with significant (P < 0.05) enrichment were mapped.
The experiments were performed using a high-resolution mass spectrometer for Pro DIA quantitative proteomics analysis, and the original image files generated by Orbitrap Elite were subjected to database searches using Spectronaut Pulsar 18.4 (Biognosys) software to screen the data according to the protein false discovery rate < 0.01 and to obtain highly reliable qualitative and quantitative results. The total proteins in GES-1 and AGS were extracted, and a portion was taken out for protein concentration determination and SDS-PAGE. Another portion was taken for trypsin digestion, and after desalting the digested peptides, the samples were identified by liquid chromatography-tandem mass spe
Growth-stage AGS cells were adjusted to a cell concentration of 6 × 107 cells/mL and injected into the right axillary area of nude mice. After inoculation of the cells, the growth of the mice was observed. After the tumor grew to a certain volume, the long diameter (A) and short diameter (B) of the tumor of the mice were measured every week. The volume size of the tumor was estimated according to the formula V = AB2/2.
One week after the inoculation of AGS cells, the volume of mice axillary subcutaneous tumors was calculated by vernier calipers, and when the volume of mice tumors was more than 100 mm3, they were randomly divided into the control group, capecitabine group, BXD low-dose group, BXD middle-dose group, and BXD high-dose group. The capecitabine was administered by gavage at a dosage of 0.42 g/kg, and BXD capecitabine was administered at a dose of 0.42 g/kg by gavage, and BXD was administered at a dose of 16.25 g/kg, 32.5 g/kg, 65 g/kg, and 0.2 mL/pc/day by gavage, res
At the end of the last administration, blood was collected from the eyeballs of the tumor-bearing mice, and the supernatant was centrifuged at 3500 rpm for 15 min after standing for 2 h. The supernatant was then transferred to a new 1.5 mL tube and stored in the refrigerator at -80 °C. After the mice were sacrificed, the tumor tissues of each group of mice were peeled off, and the ulcerated part of the surface of the tumor tissues was separated. The tumors were cleaned up and photographed at the place of reorganization of the light to compare the size of the tumors.
General condition and body weight: The mental state, activity, response to external stimuli, eating, drinking, sleeping, and other living conditions of the nude mice were observed. The body weight was recorded once every 2 days.
Tumor volume and weight: After the last drug administration, the tumor was measured at the long and short diameters with vernier calipers. The volume and weight of the tumor was recorded.
Nude mice tumors were stained according to the conventional hematoxylin and eosin staining procedure. The stages were dewaxing, water washing, hematoxylin staining, flow washing, hydrochloric acid alcohol, water washing, blue promoting solution back to blue, flow washing, 0.5% eosin solution staining, distilled water slightly washed, and then 80% ethanol, 95% ethanol, xylene (I) and (II), and finally sealed with neutral gum, and collected and analyzed in a graphic manner.
According to the results of transcriptomics and proteomics, six groups of cells containing LY294002, an inhibitor of PI3K/AKT signaling pathway, and BXD + LY294002 cells were collected after culture. Total protein was extracted from them by adding RIPA. Tumor tissues were collected from each group, and total protein was extracted from them by adding RIPA after homogenization. Protein content was determined by the bicinchoninic acid method, and 30 μg of each sample was added and separated by SDS-PAGE gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. The primary antibody dilution was used to dilute each polyantibody [phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), AKT serine/threonine kinase 1 (AKT1), phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1), tumor protein p53 (TP53), heat shock protein 90 alpha family class A member 1 (HSP90AA1)] 1:1000. The PVDF membrane was incubated with the dilution solution containing primary antibody at 4 °C overnight. The secondary antibody was added at a dilution of 1:5000, incubated at room temperature on a shaker, and treated with enhanced chemiluminescence developer. The gray value of the protein bands was analyzed by Image-J. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference to calculate the expression intensity of relevant proteins in tumor tissues of each group.
Tumors and cells of each experimental group were collected, and the total RNA was extracted according to the Steady Pure Rapid RNA Extraction Kit of Acres Biologicals, followed by reverse transcription to synthesize cDNA. cDNA was analyzed by reverse transcription PCR (RT-PCR), and the endogenous reference was selected as glyceraldehyde-3-phosphate dehydrogenase. The specific primers and their sequences are shown in Table 1. The reaction conditions were denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. The relative expression of target genes was measured by the 2-ΔΔct method.
| Primer name | Upstream primer sequence (5’-3’) | Downstream primer sequences (5’-3’) |
| AKT1 | GCTCAGCCCACCCTTCAAG | GCTGTCATCTTGGTCAGGTGGT |
| PIK3CA | TAGTGTCCGGGAAAATGGCT | GGCATGCTCTTCGATCACAG |
| TP53 | GCTGCTCAGATAGCGATGGTCT | CAACCTCAGGCGGCTCATAG |
| HSP90AA1 | GAAGGAATTTGAGGGGAAGACTTTA | TGCCATGTAACCCATTGTTGAG |
| PIK3R1 | AGCAACCTGGCAGAATTACG | GCTGCTGGAATGACAGGATT |
| GAPDH | GAAGGTCGGAGTCAACGGAT | CCTGGAAGATGGTGATGGG |
All data were analyzed using SPSS22.0 software, and the measurement data were expressed as mean ± SD. A normal test was performed when comparing the data between two groups, and if it did not conform to normal distribution, a non-parametric test was performed using the Kruskal-Wallis test. If it conformed to normal distribution, the two independent sample t-test was used, and in the case of comparison of data between multiple groups, one-way analysis of variance was used. The LSDTEST test was used when comparing between multiple experimental groups and one control group. Statistical significance was assumed to be P < 0.05 if the test level was α = 0.05. All statistical plots were drawn with GraphPadPrism 8.0 software.
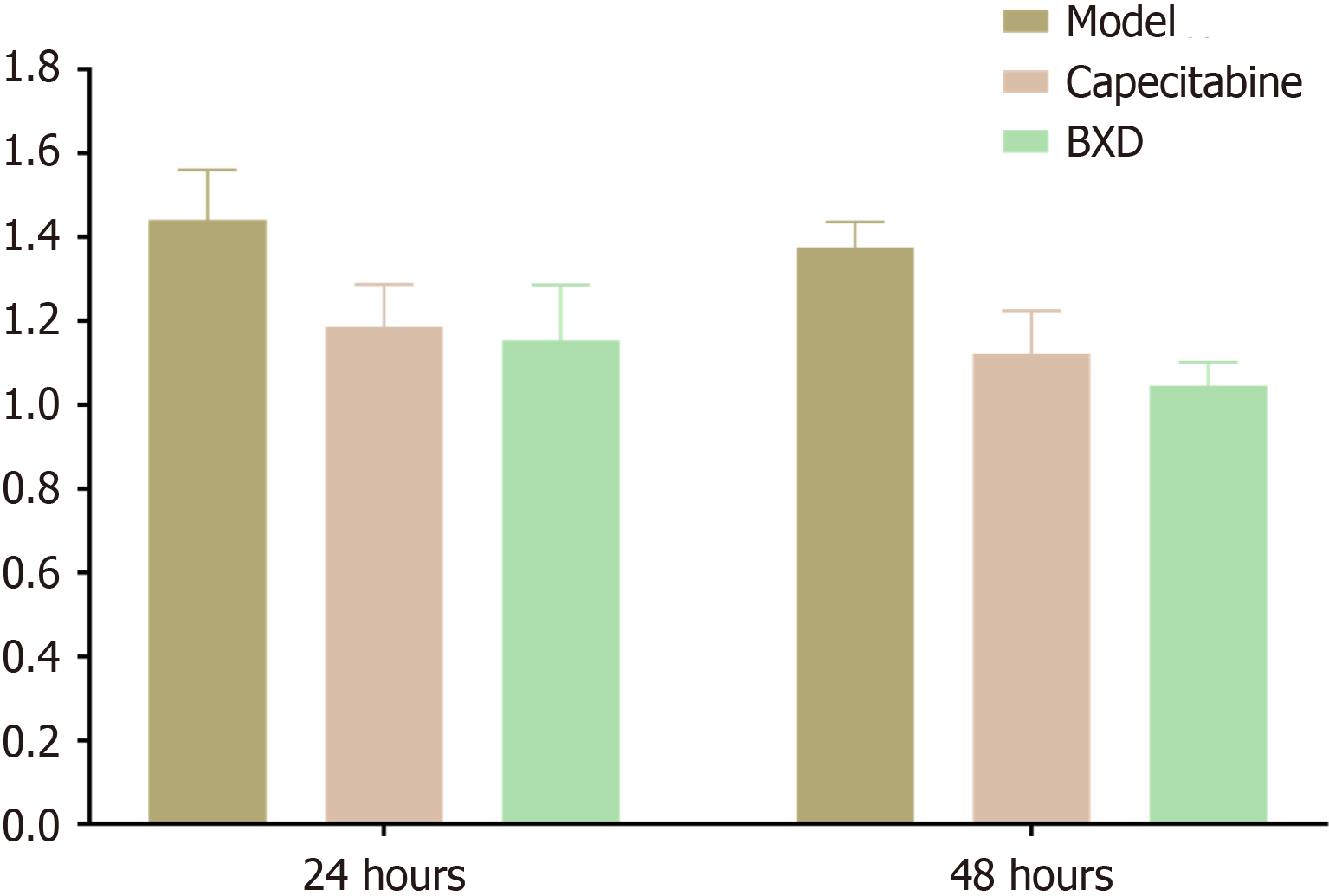
CCK-8 results showed that different concentrations of BXD-containing serum were able to reduce the proliferation rate of GC cells and promoted the inhibition of the cell proliferation rate as the concentration of BXD increased. The IC50 of BXD-containing serum in culture at 24 h and 48 h was 20% as calculated by the transplanted tumors (Table 2). In the subsequent experiments, 20% BXD serum was used.
Compared with the control group, the capecitabine group and the BXD group significantly inhibited the proliferative activity of GC cells at different incubation times (P < 0.01) (Figure 2).
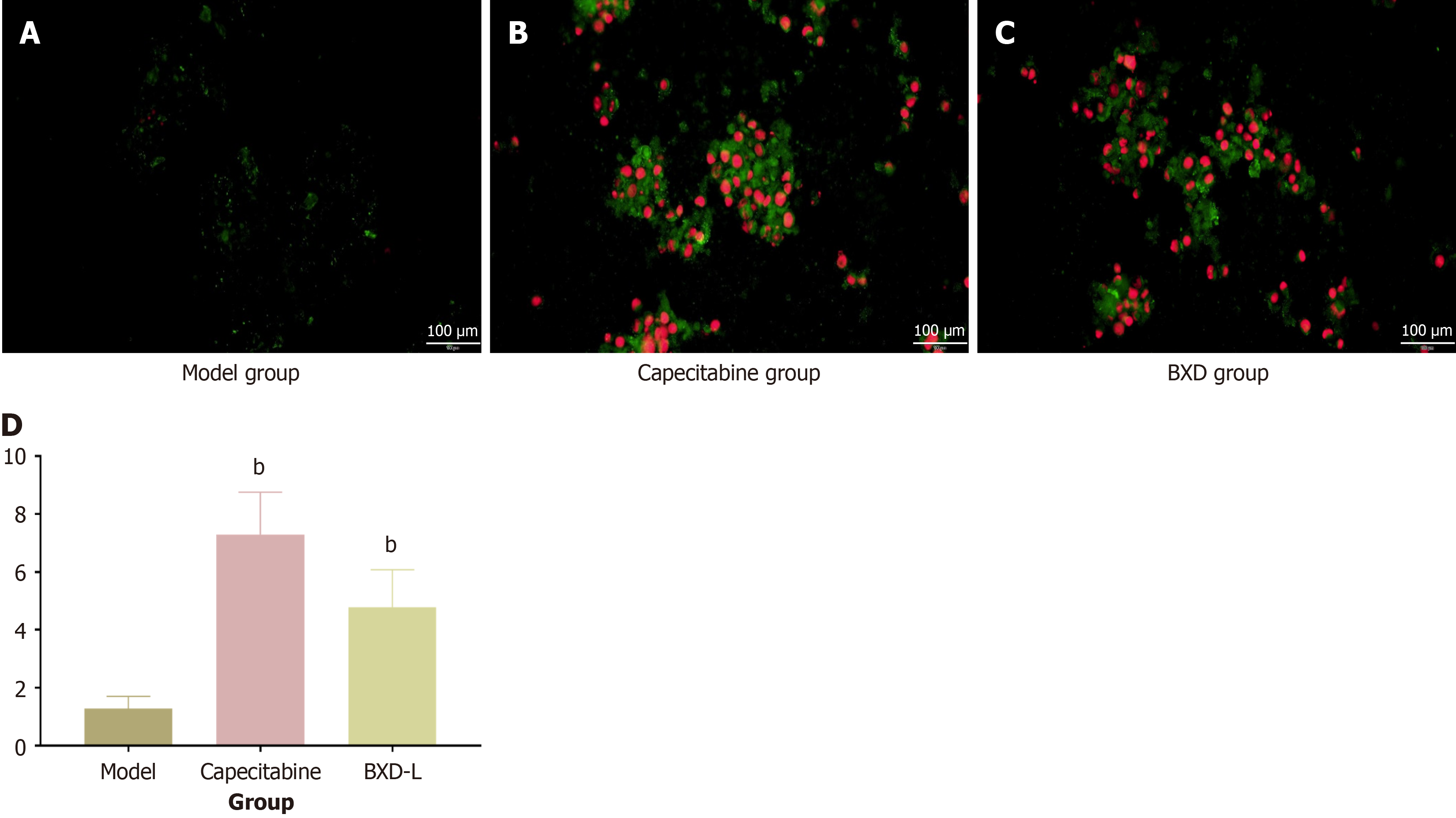
The apoptosis results showed that both BXD and capecitabine promoted the apoptosis rate of GC cells. Unlike capecitabine, BXD was more focused on early apoptosis with green fluorescence, and capecitabine was more potent in late apoptosis of GC cells. The results showed that the average OD of cells in the model group was 0.036 ± 0.019 (/pixel). The capecitabine group was 0.187 ± 0.013 (/pixel), and the BXD group was 0.186 ± 0.014 (/pixel). All groups promoted apoptosis of AGS cells (Figure 3).
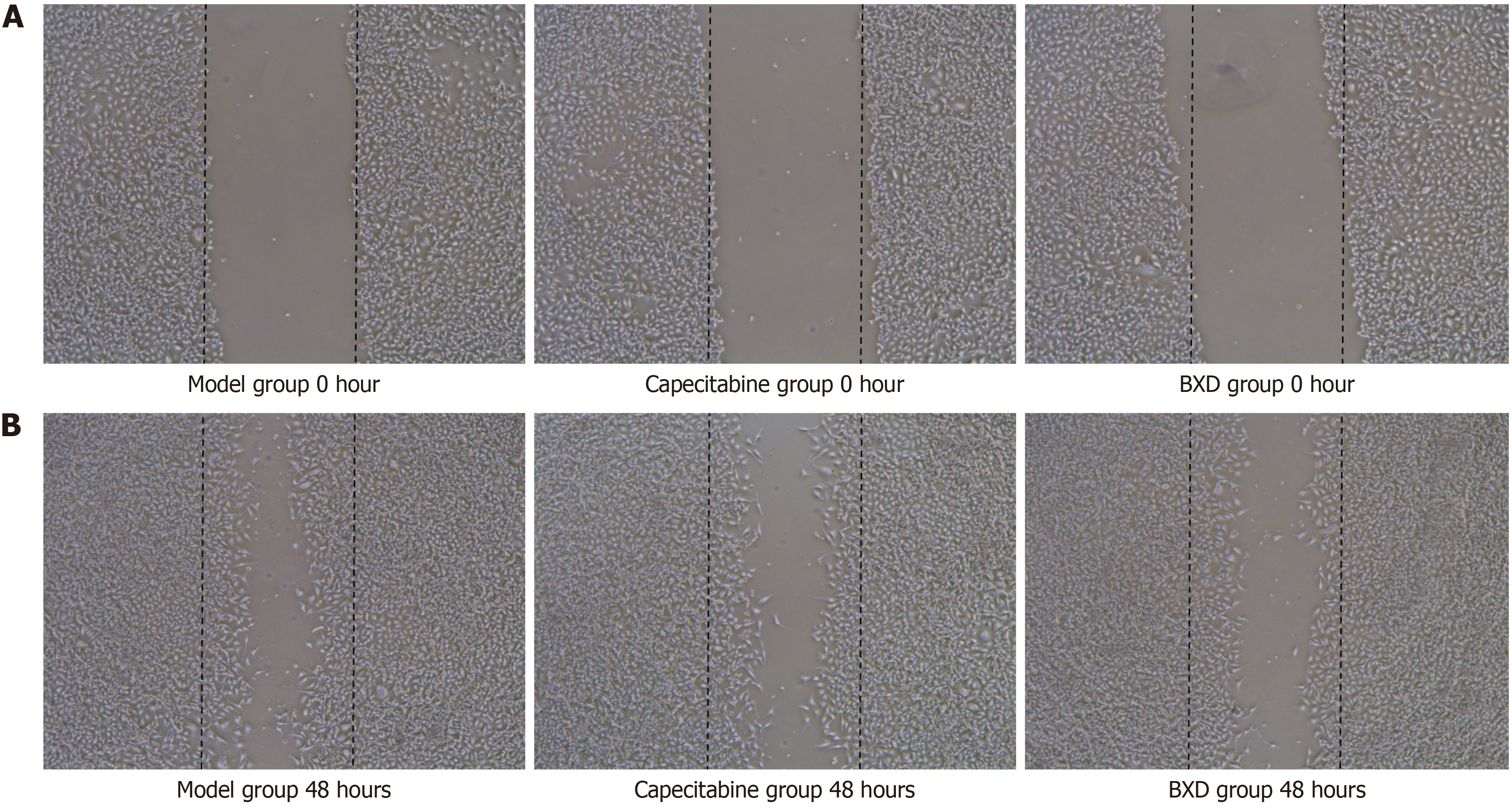
The results of the cell migration assay showed that both capecitabine and BXD could inhibit the migration rate of GC cells, and the difference was statistically significant (P < 0.05). The specific results are shown in Figure 4.
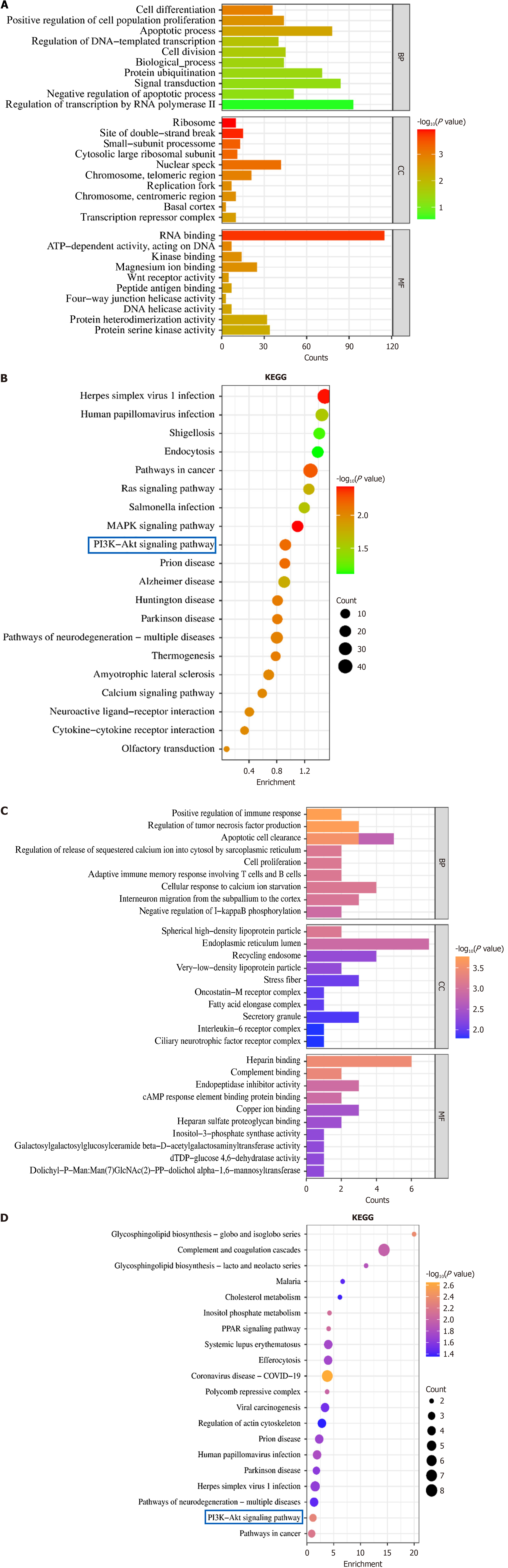
Based on the EBSeq algorithm, the DEGs in each group were screened out. In total, 1210 genes were differentially expressed in the BXD group compared with the control group (376 significantly upregulated and 834 significantly downregulated). In order to further analyze the biological processes involved in DEGs after BXD intervention, GO and KEGG enrichment analyses of DEGs after BXD intervention were performed using Metascape. GO enrichment analysis showed that the entries were bidirectional regulation of transcription by RNA polymerase II, signaling, apoptosis, cell division, DNA damage response, regulation of cell population proliferation, cellular differentiation, and inflammatory response. After BXD intervention, DEGs were mainly enriched in the stimulation of cancer pathways, neuroactive ligand-receptor interactions, PI3K-AKT signaling pathway, mitogen-activated protein kinase signaling pathway, cytokine-cytokine receptor interactions, calcium signaling pathway, and Ras signaling pathway, etc. The results of the GO and KEGG analysis are shown in Figure 5A and B, indicating that BXD intervenes in the recovery process of GC by regulating cell proliferation, apoptosis, signaling pathways and other biological processes closely related to the development of GC.
According to the proteomics calculation method, the DEPs of each group were screened out. Compared with the control group, the DEPs in the BXD group were 748 (430 significantly upregulated and 318 significantly downregulated). In order to further analyze the biological processes involved in DEPs after BXD intervention, GO and KEGG enrichment analyses of DEPs after BXD intervention were performed using Metascape. The GO enrichment analysis showed entries for bidirectional regulation of tumor necrosis factor, signaling, apoptosis clearance process, immune response of T and B cells, cell proliferation regulation, and cell migration response. After BXD intervention, DEPs were mainly enriched in stimulating the cancer pathway, PI3K-AKT signaling pathway, cell phagocytosis pathway, viral carcinogenesis, etc., which indicated that BXD intervened in the recovery process of GC by regulating the biological processes of cell proliferation, apoptosis, immune response, signaling pathway, and so on (Figure 5C and D).
The integrated analysis of RNA-seq and Pro DIA revealed that BXD intervenes in GC cells mainly through the PI3K-AKT signaling pathway, including five key factors such as PIK3CA, PIK3R1, AKT1, HSP90AA1, and TP53, suggesting that these five may be the key factors in anti-GC development mediated by BXD.
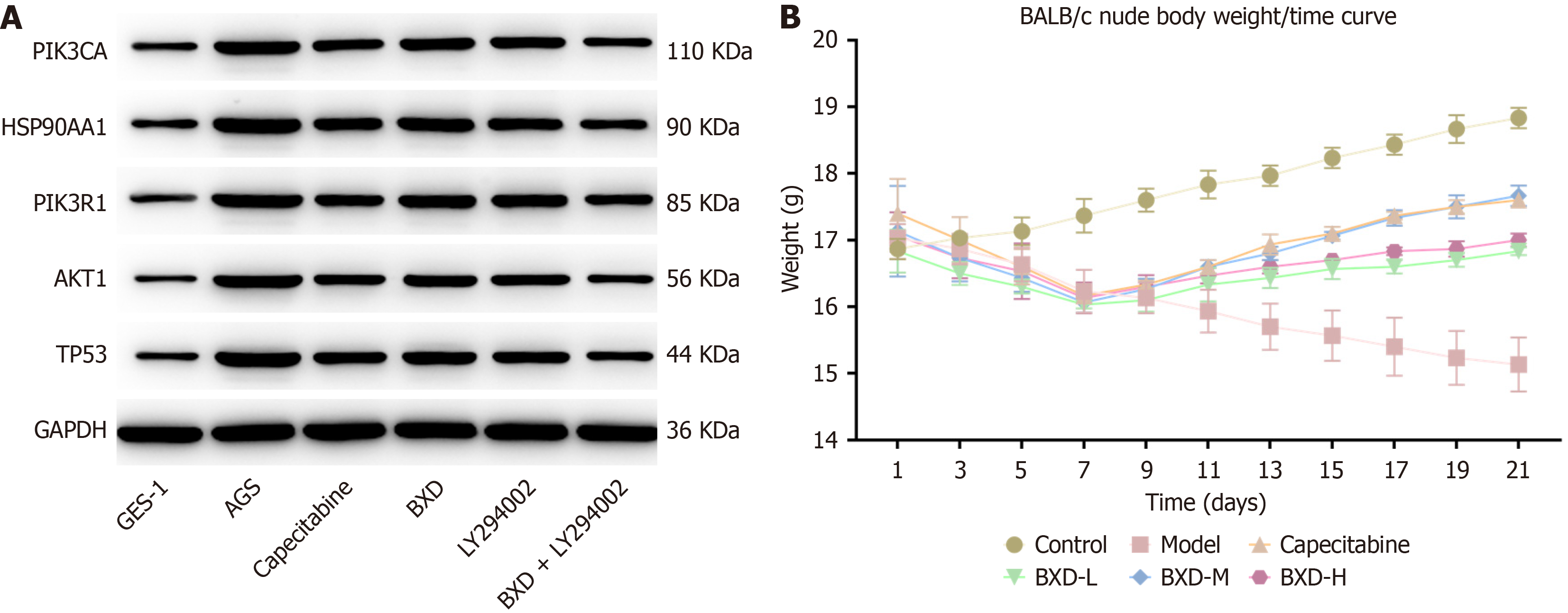
The expression of the five key factors was measured using western blot and RT-PCR. Western blot results showed that compared with the control group both the capecitabine group and the DXB group had significantly reduced protein expression levels of PIK3CA, PIK3R1, HSP90AA1, TP53 and AKT1 in the tumor tissues (Figure 6A, Table 3). RT-PCR results showed that compared with the control group, the capecitabine group had significantly reduced levels of PIK3CA, PIK3R1, HSP90AA1, AKT1, and TP53 mRNA expression in tumor tissues (Table 4).
| Group | PIK3CA | HSP90AA1 | PIK3R1 | AKT1 | TP53 |
| GES-1 | 0.27 ± 0.007 | 0.29 ± 0.05 | 0.22 ± 0.002 | 0.21 ± 0.005 | 0.24 ± 0.03 |
| AGS | 0.82 ± 0.036b | 0.96 ± 0.029b | 0.87 ± 0.014b | 0.9 ± 0.085a | 0.85 ± 0.032b |
| Capecitabine | 0.45 ± 0.05c | 0.57 ± 0.018d | 0.54 ± 0.01d | 0.48 ± 0.03c | 0.55 ± 0.033c |
| BXD | 0.59 ± 0.05 | 0.72 ± 0.04c | 0.73 ± 0.021c | 0.63 ± 0.007 | 0.63 ± 0.063 |
| LY294002 | 0.57 ± 0.015c | 0.64 ± 0.039c | 0.66 ± 0.005d | 0.57 ± 0.013 | 0.61 ± 0.006c |
| BXD + LY | 0.37 ± 0.023d | 0.42 ± 0.006d | 0.47 ± 0.009d | 0.34 ± 0.022c | 0.37 ± 0.028d |
| Group | PIK3CA | HSP90AA1 | PIK3R1 | AKT1 | TP53 |
| GES-1 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| AGS | 1.97 ± 0.011b | 3.73 ± 0.86a | 5.75 ± 0.77b | 1.86 ± 0.09b | 4.66 ± 2.11a |
| Capecitabine | 0.44 ± 0.05d | 1.26 ± 0.11 | 2.81 ± 1.41 | 0.47 ± 015d | 0.13 ± 0.01c |
| BXD | 0.85 ± 0.09c | 1.34 ± 0.19 | 0.55 ± 0.31c | 0.91 ± 0.20d | 0.21 ± 0.09c |
| LY294002 | 1.61 ± 0.22 | 2.01 ± 0.55 | 0.52 ± 0.08d | 0.84 ± 0.03d | 0.27 ± 0.16c |
| BXD + LY | 0.14 ± 0.11d | 1.11 ± 0.05 | 0.46 ± 0.35d | 0.88 ± 0.17d | 0.17 ± 0.02c |
The mice in the control group showed a steady increase in body weight growth, followed by a gradual stabilization, and gradually wasted away with the prolongation of the modeling time. The mice in the capecitabine group and the mice in the BXD group showed an increasing trend in body weight after the administration of the drug, and the effect was most obvious in the Western drug group and the BXD medium-dose group with the prolongation of the administration of the drug(Figure 6B). The results of 14 consecutive days of administration showed that different concentrations of BXD and capecitabine had inhibitory effects on mouse GC transplanted tumors, in which the tumor volume inhibition rate of capecitabine was 32.29%, that of the BXD low dose group was 41.43%, that of the BXD middle dose was 31.23%, and that of the BXD high dose group was 35.64%. The inhibitory effect on GC in the BXD middle dose group was the most effective.
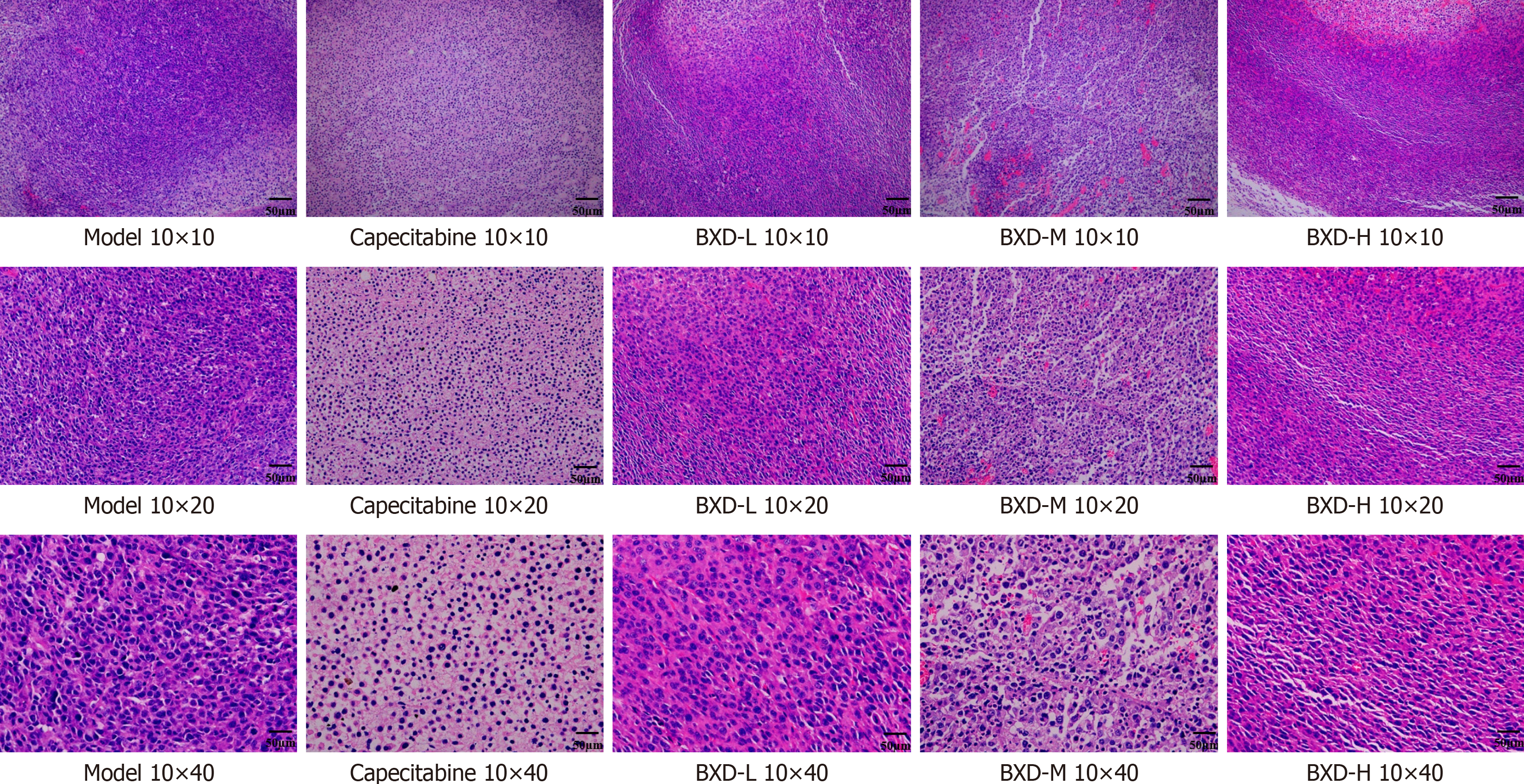
Hematoxylin and eosin staining of tumor tissue sections were detected by light microscopy. The tumor cells transplanted in the subcutaneous model group were more numerous and tightly arranged, with a larger volume, deeply stained nuclei, obvious tumor cell anomaly, abundant mesenchymal blood vessels, and fibrous agglomerations. Tumor cells were more abundant in the low-dose group, with different cell sizes, relatively smaller size, large and deeply stained nuclei, abundant interstitial blood vessels of some cells with nuclear solidification, and increased collagen fibers around the tumor cells. In the other dosing groups, tumor cells were reduced in number, loosely arranged, with an obvious reduction in cell size, and smaller nuclear condensation. Intercellular clefts were seen in the cells of the capecitabine group and the BXD middle-dose group. The number of mesenchymal cells decreased, collagen fibers increased, and apoptotic necrotic cells were seen scattered in the tissues (Figure 7).
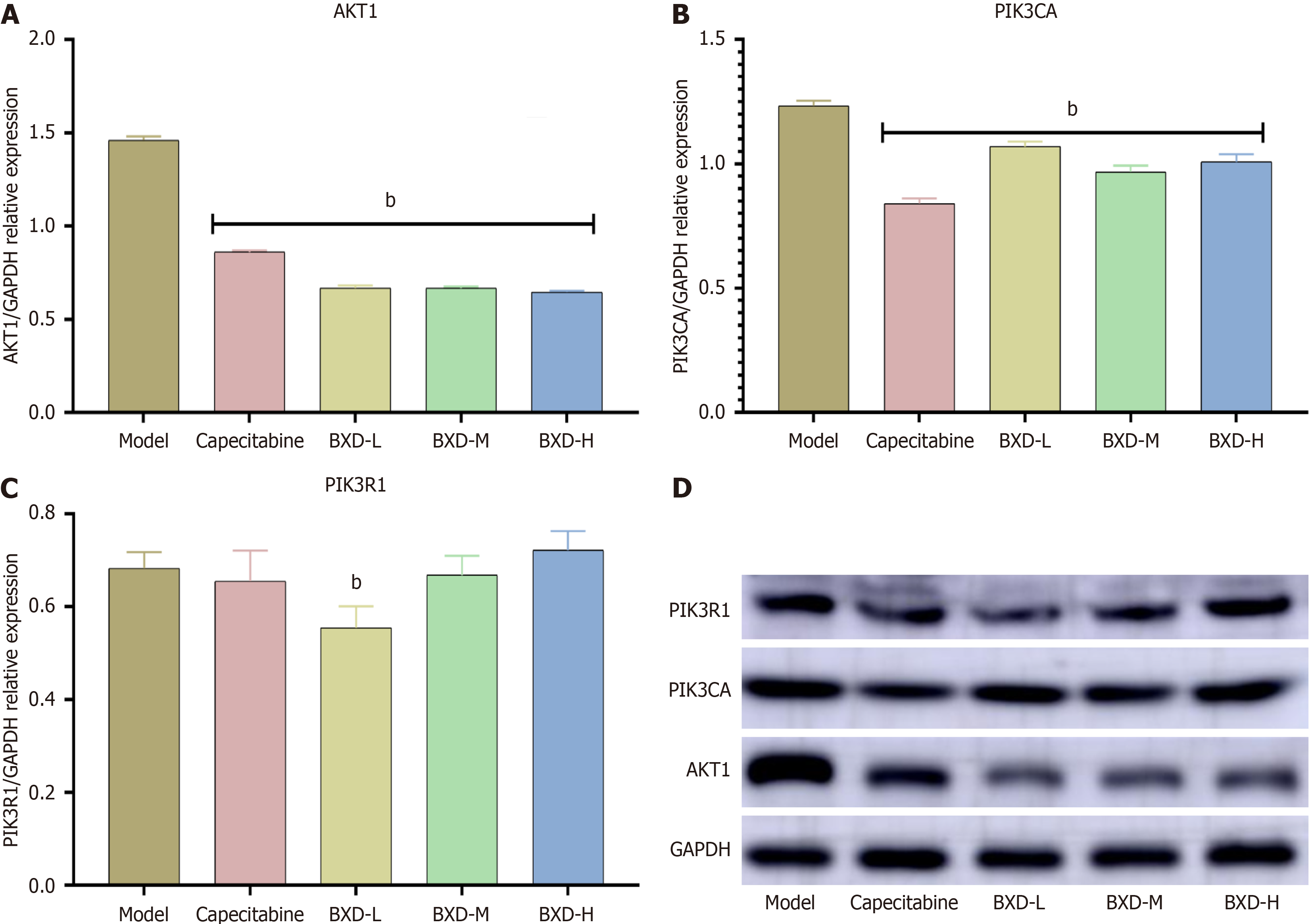
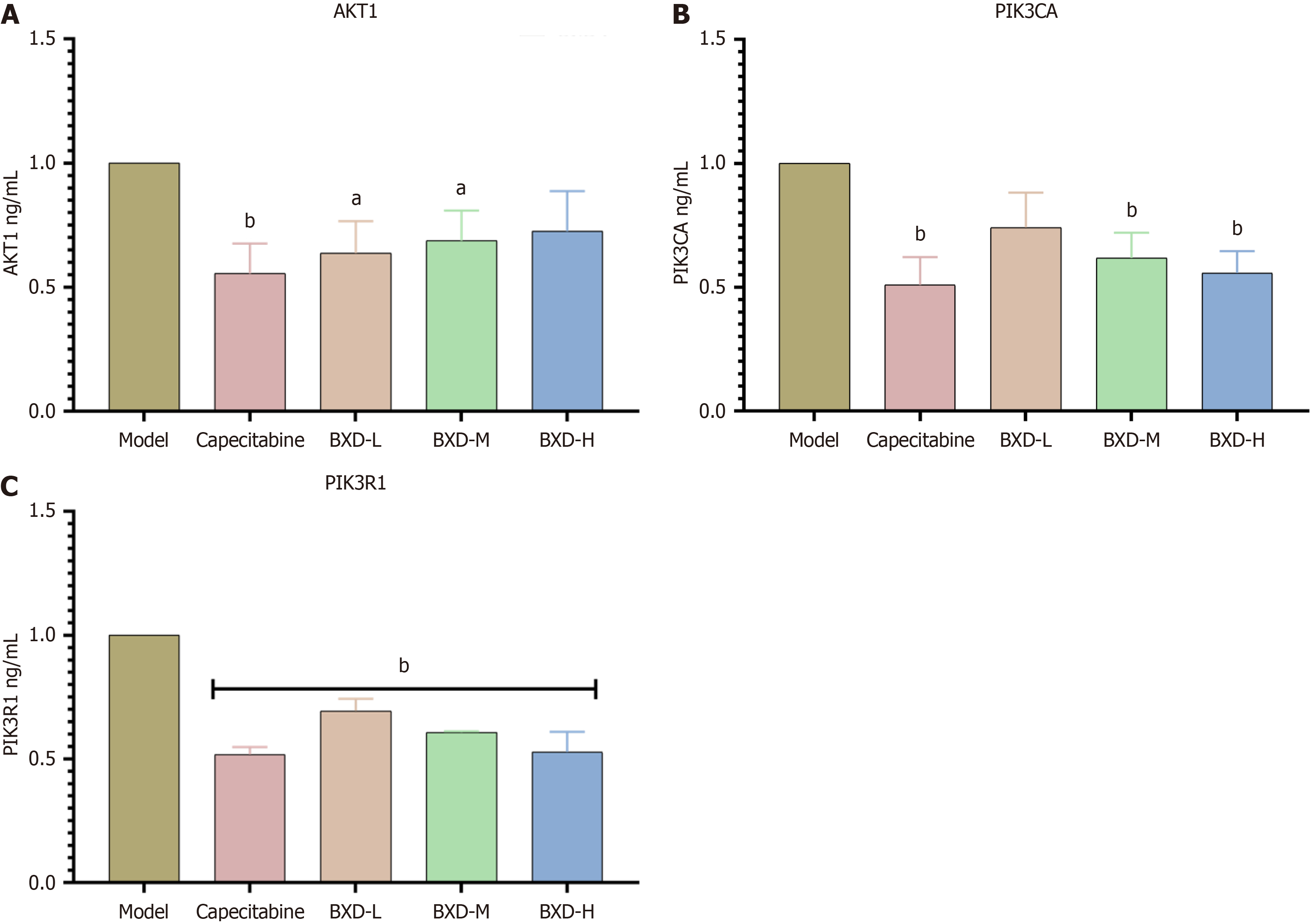
Compared with the control group, the capecitabine group and the low, medium, and high dose groups of BXD had significantly reduced protein expression levels of PIK3CA and AKT1 in the tumor tissues (P < 0.01). The low dose group had significantly reduced expression of the PIK3R1 protein (P < 0.01) (Figure 8).
Compared with the control group, the capecitabine group had significantly reduced levels of PIK3CA, AKT1, and PIK3R1 mRNA expression in tumor tissues (P < 0.01) (Figure 9).
GC, as the malignant tumor with the highest morbidity and mortality rate in China, involves the participation of multiple signaling pathways and multiple gene variants in its development process. It is characterized by high invasiveness and heterogeneity. Exploring the complex pathogenesis of GC and searching for the molecular mechanisms and effective treatments during its development process are the main research directions currently.
BXD is a traditional Chinese herbal compound formula, in which Banxia is one of the main herbs in BXD. It has the effects of drying dampness, resolving phlegm, lowering rebelliousness, and stopping vomiting. Modern pharmacology shows that Banxia can increase gastrointestinal motility and improve loss of appetite. Huanglian is also an important ingredient in BXD and has the effect of clearing heat and removing toxins. It has a certain alleviating effect on the inflammation that may exist in stomach cancer. Huanglian is able to inhibit the growth of stomach cancer cells and reduce the discomfort caused by infection. Ganjiang can assist in enhancing the function of the spleen and stomach and promote appetite, thus improving the symptoms of loss of appetite in stomach cancer patients. Ganjiang also has certain anti-inflammatory effects, which can reduce inflammation of the stomach. Renshen has the effects of improving immunity, fatigue, and loss of appetite.
Li et al[17] found in a clinical study that BXD could alleviate the symptoms of diarrhea, abdominal pain, nausea, vomiting, and loss of appetite in patients with GC with damp-heat spleen and stomach and improve the quality of life of the patients, but the study of the molecular mechanism of action was not involved[18]. Therefore, based on bioinformatics and multiomics technology, we conducted preliminary predictions of BXD treatment of GC at the gene level and protein level and investigated the pharmacodynamics of BXD on GC transplantation tumors by in vivo and in vitro experiments. We explored the molecular pathways of action of BXD for GC and analyzed the corresponding changes of BXD treatment of GC.
In this study, the therapeutic effects of BXD were found using transcriptomics and proteomics analysis, and BXD may act through AKT1, PIK3CA, HSP90AA1, TP53, and PIK3R1-mediated PI3K-AKT signaling pathway. The results of in vitro experiments showed that BXD dose-dependently inhibited the proliferative activity of AGS cells. Furthermore, BXD and the PI3K inhibitor LY294002 had the ability to inhibit the proliferation and migration level of GC cells and promote the apoptosis expression level of GC cells. Meanwhile, the results of the western blot and RT-PCR showed that each administration group reduced the expression levels of key mRNAs and proteins on the PI3K-AKT signaling pathway to varying degrees, thus serving to delay the development of GC.
The PI3K-AKT signaling pathway is an important intracellular signaling network that is widely present in eukaryotic cells. It plays an important role in cell proliferation, survival, metabolism, and other key biological processes. In GC cells, the PI3K-AKT signaling pathway often shows abnormal activation, which is closely related to tumorigenesis and progression. Mutations in the PIK3CA gene are commonly found in GC patients, leading to sustained activation of PI3K, which triggers an increase in the phosphorylation of AKT. Factors in the tumor microenvironment (e.g., growth factors) can also activate the PI3K-AKT signaling pathway, promoting cancer cell proliferation and survival[19,20].
AKT, also known as protein kinase B, has three isoforms, AKT1, AKT2 and AKT3, of which AKT1 regulates tumor cell proliferation and apoptosis[21,22]. In GC tissues, the expression of AKT1 usually shows upregulation. This upregulation is closely related to the malignancy degree and prognosis of GC. It has been shown that overexpression of AKT1 is associated with enhanced proliferative ability, invasiveness, and tolerance to chemotherapeutic agents in tumor cells. Specifically, AKT1 promotes the growth and survival of cancer cells by promoting cell cycle progression and inhibiting apoptosis.
PIK3CA is an oncogene located at 3q26.3, and it is now generally accepted that mutations in PIK3CA play an important role in the development of a variety of solid tumors, such as colon[23], brain, breast, lung, and GC[24]. However, in GC cells, the mutation frequency of the PIK3CA gene is 4.3%-16.0%, and the mutation of the PIK3CA gene plays an obvious role in the clinicopathological features of GC and the development of GC. It was found that PIK3CA gene amplification can promote cell growth and proliferation, which ultimately leads to tumorigenesis, and is an important component of the PI3K/AKT signaling pathway[25]. Therefore, it can be inferred that the upregulation of PIK3CA expression in gastric tumor tissues promotes the phosphorylation of downstream AKT, which in turn activates downstream apoptotic and proliferative factors and contributes to the process of GC development[26,27].
PIK3R1 contains 86102 base pairs (bp) and consists of 16 exons[28]. The PIK3R1 gene undergoes variable splicing, giving rise to several isoforms, mainly p85α and p55α. These isoforms play different roles under different physiological and pathological conditions, which makes the function of PIK3R1 more diverse[29]. PIK3R1 is able to interact with insulin receptor substrate proteins and growth factors, thereby participating in cellular signaling[30]. Studies have shown that dysfunction of PIK3R1 may lead to aberrant activation of the PI3K pathway, which in turn promotes the proliferation and survival of tumor cells[28,31]. By regulating the PI3K signaling pathway, PIK3R1 affects the proliferation migration and invasion ability of GC cells. These alterations in cell behavior are closely related to cancer metastasis and recurrence, and thus PIK3R1 plays an important role in the progression of GC.
In this study, the effect of BXD on PI3K/AKT signaling pathway was determined. The protein expression in the tumor tissues of animals was detected by western blot. By comparing the protein expression of AKT1, PIK3CA, and PIK3R1 with that of the control group, it was found that BXD significantly decreased protein expression in the high, medium, and low dose groups. The results of RT-PCR showed that the gene expression of PIK3CA, AKT1, and PIK3R1 was significantly decreased in each dose group compared with the control group. The capecitabine group also had significant inhibitory effects on the expression of proteins and genes in the PI3K/AKT signaling pathway.
BXD can inhibit the proliferation and migration of GC cells and induce apoptosis expression by inhibiting the PI3K/AKT signaling pathway. In vitro and in vivo experimental evidence for the treatment of GC and the GC transplantation tumor model provided a scientific basis for the inhibitory effect of BXD on the malignant biological behavior of GC. In this study, we showed that BXD has a significant protective effect on gastric mucosal damage in GC. Through the integrated analysis of transcriptomics and proteomics, we identified PIK3CA, AKT1, PIK3R1, HSP90AA1, and TP53 key transcription factors on the PI3K/AKT signaling pathway, which revealed the potential key targets for GC treatment and provided a reference to the development of targeting strategies for gastric mucosal protection. The results will provide a reference for the development of targeted strategies for gastric mucosa protection.
The authors thank Experimental Center, Shandong University of Traditional Chinese Medicine in cellular, animal feeding, and molecular imaging techniques to assist with tissue processing and staining.
| 1. | Hinz N, Jücker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 2. | 2. Sun X, Xue D, Zhang K, Jiang F, Li D. Acrid-release and bitter-downbearing therapy and banxia xiexin decoction regulate Wnt/β-catenin pathway, inhibit proliferation and invasion, and induce apoptosis in gastric cancer cells. Am J Transl Res. 2021;13:6211-6220. [PubMed] |
| 3. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 952] [Article Influence: 317.3] [Reference Citation Analysis (0)] |
| 4. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Chu F, Lin J, Li Y, Johnson N, Zhang J, Gai C, Su Z, Cheng H, Wang L, Ding X. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J Ethnopharmacol. 2021;279:114399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Dai X, Yu Y, Zou C, Pan B, Wang H, Wang S, Wang X, Wang C, Liu D, Liu Y. Traditional Banxia Xiexin decoction inhibits invasion, metastasis, and epithelial mesenchymal transition in gastric cancer by reducing lncRNA TUC338 expression. Heliyon. 2023;9:e21064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Zhu YJ, Wu XY, Wang W, Chang XS, Zhan DD, Diao DC, Xiao J, Li Y, Ma D, Hu M, Li JC, Wan J, Wu GN, Ke CF, Sun KY, Huang ZL, Cao TY, Zhai XH, Chen YD, Peng JJ, Mao JJ, Zhang HB. Acupuncture for Quality of Life in Gastric Cancer Patients Undergoing Adjuvant Chemotherapy. J Pain Symptom Manage. 2022;63:210-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl Oncol. 2022;18:101364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 9. | Feng Y, Ren Y, Zhang X, Yang S, Jiao Q, Li Q, Jiang W. Metabolites of traditional Chinese medicine targeting PI3K/AKT signaling pathway for hypoglycemic effect in type 2 diabetes. Front Pharmacol. 2024;15:1373711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Carino A, Graziosi L, Marchianò S, Biagioli M, Marino E, Sepe V, Zampella A, Distrutti E, Donini A, Fiorucci S. Analysis of Gastric Cancer Transcriptome Allows the Identification of Histotype Specific Molecular Signatures With Prognostic Potential. Front Oncol. 2021;11:663771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Li X, Zheng NR, Wang LH, Li ZW, Liu ZC, Fan H, Wang Y, Dai J, Ni XT, Wei X, Liu MW, Li K, Li ZX, Zhou T, Zhang Y, Zhang JY, Kadeerhan G, Huang S, Wu WH, Liu WD, Wu XZ, Zhang LF, Xu JM, Gerhard M, You WC, Pan KF, Li WQ, Qin J. Proteomic profiling identifies signatures associated with progression of precancerous gastric lesions and risk of early gastric cancer. EBioMedicine. 2021;74:103714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884-i890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4558] [Cited by in RCA: 13861] [Article Influence: 2310.2] [Reference Citation Analysis (0)] |
| 14. | Huang B, Liu J, Ding F, Li Y. Epidemiology, risk areas and macro determinants of gastric cancer: a study based on geospatial analysis. Int J Health Geogr. 2023;22:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Liao Y, Gui Y, Li Q, An J, Wang D. The signaling pathways and targets of natural products from traditional Chinese medicine treating gastric cancer provide new candidate therapeutic strategies. Biochim Biophys Acta Rev Cancer. 2023;1878:188998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 16. | Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12013] [Cited by in RCA: 14966] [Article Influence: 1360.5] [Reference Citation Analysis (0)] |
| 17. | Li Y, Li L, Wang X, Huang H, Han T. Determining the Mechanism of Banxia Xiexin Decoction for Gastric Cancer Treatment through Network Analysis and Experimental Validation. ACS Omega. 2024;9:10119-10131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012;18:3745-3751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Deng YQ, Gao M, Lu D, Liu QP, Zhang RJ, Ye J, Zhao J, Feng ZH, Li QZ, Zhang H. Compound-composed Chinese medicine of Huachansu triggers apoptosis of gastric cancer cells through increase of reactive oxygen species levels and suppression of proteasome activities. Phytomedicine. 2024;123:155169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Furney SJ, Higgins DG, Ouzounis CA, López-Bigas N. Structural and functional properties of genes involved in human cancer. BMC Genomics. 2006;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ding MR, Qu YJ, Hu B, An HM. Signal pathways in the treatment of Alzheimer's disease with traditional Chinese medicine. Biomed Pharmacother. 2022;152:113208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 22. | Gupta I, Gaykalova DA. Unveiling the role of PIK3R1 in cancer: A comprehensive review of regulatory signaling and therapeutic implications. Semin Cancer Biol. 2024;106-107:58-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Ye B, Jiang LL, Xu HT, Zhou DW, Li ZS. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis. Int J Immunopathol Pharmacol. 2012;25:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9910] [Cited by in RCA: 14941] [Article Influence: 1494.1] [Reference Citation Analysis (0)] |
| 25. | Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, Guo Y, Clark S, Walters RG, Chen Y, Pei P, Lv J, Yu C, Jeske R, Waterboer T, Clifford GM, Franceschi S, Peto R, Hill M, Li L, Millwood IY, Chen Z; China Kadoorie Biobank Collaborative Group. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. 2021;6:e888-e896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 26. | Tsay A, Wang JC. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. Int J Mol Sci. 2023;24:12665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 27. | Feng X, Xue F, He G, Ni Q, Huang S. Banxia xiexin decoction affects drug sensitivity in gastric cancer cells by regulating MGMT expression via IL6/JAK/STAT3mediated PDL1 activity. Int J Mol Med. 2021;48:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64411] [Article Influence: 16102.8] [Reference Citation Analysis (176)] |
| 29. | Gu C, Zhang Q, Li Y, Li R, Feng J, Chen W, Ahmed W, Soufiany I, Huang S, Long J, Chen L. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front Med (Lausanne). 2022;9:900809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 30. | Tharin Z, Richard C, Derangère V, Ilie A, Arnould L, Ghiringhelli F, Boidot R, Ladoire S. PIK3CA and PIK3R1 tumor mutational landscape in a pan-cancer patient cohort and its association with pathway activation and treatment efficacy. Sci Rep. 2023;13:4467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1015] [Cited by in RCA: 1137] [Article Influence: 81.2] [Reference Citation Analysis (0)] |