Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104229
Revised: February 20, 2025
Accepted: March 31, 2025
Published online: May 15, 2025
Processing time: 153 Days and 0.4 Hours
Cholangiocarcinoma (CCA) is a heterogeneous group of aggressive malignancies arising from the biliary tree. Epidemiological data show an increase in the in
To investigate the recent epidemiology of CCA and gallbladder cancer in Nor
We performed a 17-year (2007-2023) retrospective analysis of hospital discharge records of the Veneto Region. During the period 10778 first hospital admissions for biliary tract cancers in the main or secondary diagnosis were recorded. Data were analyzed by the χ2 test for categorical data and the Student’s t-test for continuous data to assess differences in percentages and averages, respectively. Trends in the age-standardized hospitalization rate were evaluated using Joinpoint regression, estimating annual percentage changes (APC).
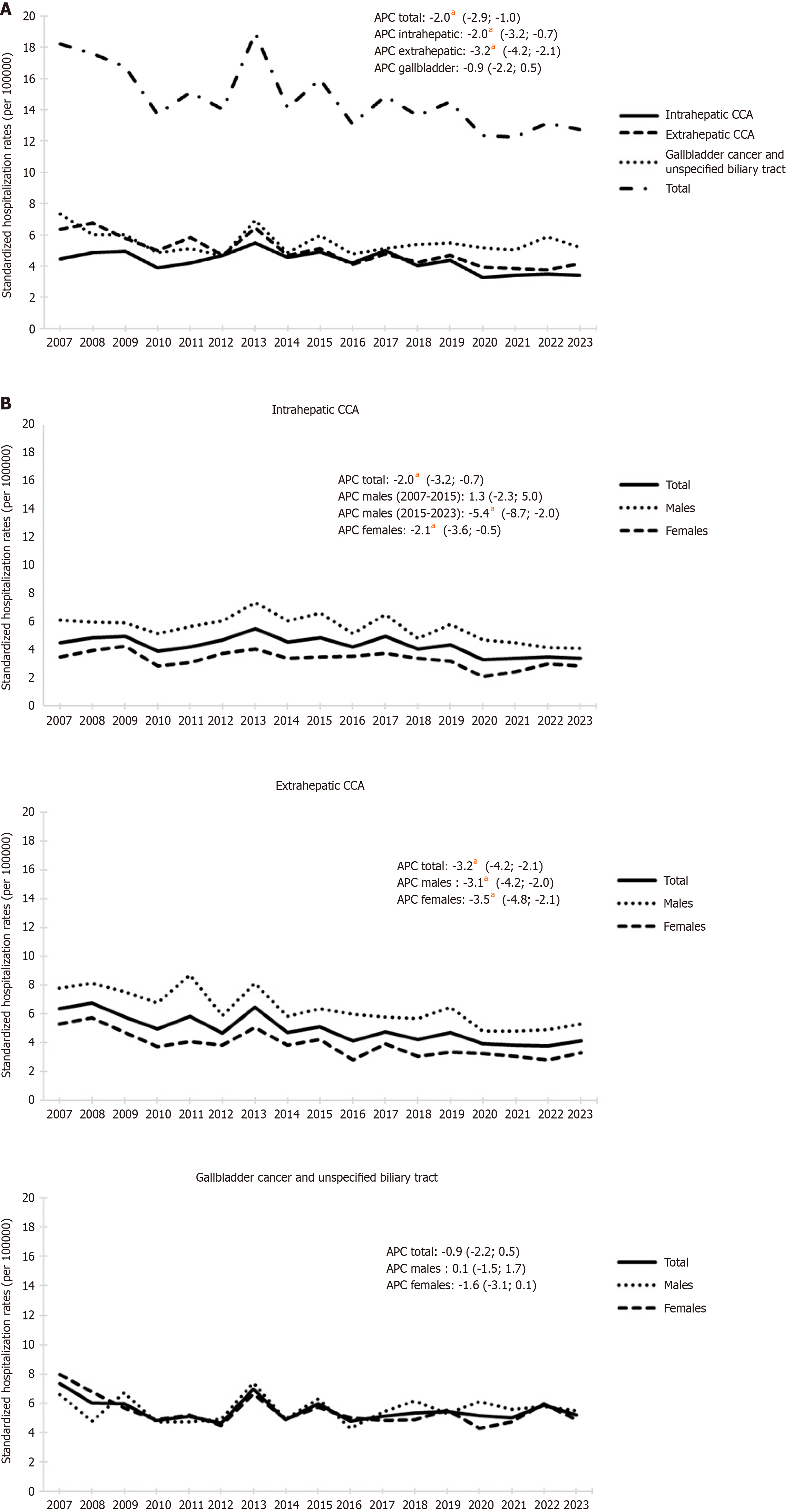
The total number of hospitalizations for biliary tract cancers remained stable over the past 17 years (186 hospitalizations/year for intrahepatic CCA, 211 for extrahepatic CCA, and 237 for gallbladder cancer/unspecified biliary tract). Age-standardized hospitalization rates for intrahepatic and extrahepatic CCA decreased respectively from 4.9 cases to 3.4 per 100000 inhabitants (APC = -2.0, 95% confidence interval: -3.2 to -0.7, P < 0.001) and from 6.7 to 3.8 cases per 100000 inhabitants (APC = -3.2, 95% confidence interval: -4.2 to -2.1, P < 0.001). Instead, hospitalizations for gallbladder cancer remained stable, with an average rate of 5.5 per 100000 inhabitants. Overall, hospitalization rates for biliary tract cancers increased with age in both genders.
Our study reported a decreasing hospitalization rate for CCA and a stable trend for gallbladder cancer over a 17-year period, suggesting a change in the epidemiology of these tumors.
Core Tip: Cholangiocarcinoma (CCA) comprises aggressive biliary malignancies with varying trends in incidence. While intrahepatic CCA has increased in Western countries, extrahepatic CCA shows stable or declining rates, and gallbladder cancer trends remain inconsistent. This study analyzed 17 years (2007-2023) of hospitalization data from Northeast Italy, finding that overall biliary tract cancer hospitalizations remained stable, with decreased rates for intrahepatic and extrahepatic CCA, particularly in males. Gallbladder cancer hospitalizations showed no significant gender differences or trends. Age-related increases in hospitalization rates were noted for all biliary cancers, indicating evolving epidemiology and emphasizing the importance of continued regional monitoring.
- Citation: Baldo V, Cozza A, Grego V, Furlan P, Cozzolino C, Saia M, Cocchio S, Floreani A. Epidemiological trends of cholangiocarcinoma and gallbladder cancer in Northeastern Italy: Administrative analysis over a 17-year period (2007-2023). World J Gastrointest Oncol 2025; 17(5): 104229
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104229.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104229
Cholangiocarcinoma (CCA) is a heterogeneous group of aggressive malignancies arising from cells lining bile ducts, including cholangiocytes and hepatic stem or progenitor cells. Based on their anatomic site of origin, CCAs are classified into intrahepatic CCA, perihilar CCA, and distal CCA. Intrahepatic CCA is further subclassified into small-duct-type and large-duct-type. Perihilar CCA (also known as Klatskin tumor) and distal CCA are together classified as extrahepatic CCA[1,2].
The global incidence rate of CCA varies from the lowest in Israel (0.3/100000) to the peak (85/100000) in Northeastern Thailand[3]. Multiple risk factors accounting for the wide geographical variability in the development of CCA have been identified. Liver parasitic infections due to flukes Opisthorchis viverrini and Clonorchis sinensis, acquired by consuming raw or undercooked cyprinoid fish species harboring an infective meta-cercariae stage, have been identified as a strong risk factor in Korea, China, and Thailand[4]. In Western countries a strong association between primary sclerosing cholangitis (PSC) and CCA has been reported[5]. A recent systematic review and meta-analysis on risk factors has been published, reporting congenital cystic dilatation of the biliary tree as strongly associated with both intrahepatic CCA and ex
Recent decades have seen new epidemiological trends in CCA, characterized by an increased incidence of intrahepatic CCA in Western countries and a stable or decreasing incidence of extrahepatic CCA[7]. However, the majority of data are retrospective and from before the 2000s or in the first decade of the new millennium. In particular, Witjes et al[8] analyzed 809 cases of intrahepatic CCA selected from the Netherlands Cancer Registry between 1989 and 2009 and found that the incidence rates of intrahepatic CCA rose, especially in the group aged 45-59 years, while in the other age groups it remained stable.
Bertuccio et al[9] analyzed the mortality rates for intrahepatic CCA using the official death certification data from the World Health Organization Database from 1990 to 2010 in 12 major selected European countries. The authors found that mortality for intrahepatic CCA increased by around 9% in both males and females from 1990 to 2008 reaching rates of 1.1/100000 for males and 0.75/100000 for females. The highest rates were seen in the United Kingdom, Germany, and France[9]. Only a few recent studies have analyzed epidemiological data related to the hospitalization rates and outcome of CCAs in Italy[10,11].
Gallbladder cancer is the most common cancer of the biliary tract and is a highly fatal condition. Globally, the incidence of this tumor shows remarkable geographical variability, with the highest incidence rates in South America, South Asia, and Southeast Asia[12]. In particular, according to the 2022 Global Cancer Observatory data, Bolivia, Chile, Bangladesh, and Nepal have the highest rates, ranging from 4.4 to 7.6 per 100000[13]. The incidence of this tumor steadily increases with age, reaching its maximum in the seventh decade of life. Females are more affected than males[14].
Therefore, the purpose of our study was to investigate the most recent epidemiological trend of CCA and gallbladder cancer in Northeast Italy, using regional data collected from hospital discharge records (HDRs) over a 17-year period.
We performed a 17-year (2007-2023) retrospective analysis of HDRs of the Veneto Region. The administrative database contains information on all patients discharged from public or accredited private hospitals. Diagnoses are based on the International Classification of Diseases, 9th rev., clinical modification (ICD-9-CM, 2007). HDRs concerning subjects not residing in the Veneto Region were excluded. In 2022, the Veneto Region had an age index of 195.1 and an average population of 4.8 million inhabitants, 51% of whom were females, and the mean age was 46.6 years.
Patients with biliary tract cancer were identified by selecting all HDRs containing one of the following ICD-9-CM codes: Intrahepatic CCA (155.1); primary gallbladder cancer (156.0); primary extrahepatic biliary tract cancer (156.1); malignant neoplasm of other specified sites of gallbladder and extrahepatic bile ducts (156.8); and malignant neoplasm of biliary tract, part unspecified site (156.9). The primary diagnosis and up to five secondary diagnoses were recorded.
Selected hospitalizations were classified into three groups: Intrahepatic CCA (ICD-9-CM: 155.1); extrahepatic CCA (ICD-9-CM: 156.1, 156.8); and gallbladder cancer and unspecified biliary tract (ICD-9-CM: 156.0, 156.9). If the same patient was readmitted to a hospital for the same condition, only the first admission was considered. Through HDRs we collected demographic data (date of birth, gender, and place of residence) and clinical information (clinical manifestation and discharge or death). We conducted the analysis using five age groups (0-44 years; 45-54 years; 55-64 years; 65-74 years; and 75 + years).
The number of hospital admissions and hospitalization rates by year, by type of diagnosis, and by age group were evaluated. The annual hospitalization rates were estimated by dividing the number of hospitalizations by the population of Veneto Region according to data from the Statistics Office of the Veneto Region.
Data were analyzed by the χ2 test for categorical data and the Student’s t-test for continuous data to assess differences in percentages and averages, respectively. Hospitalization rates were standardized to the 2024 population of the Veneto Region. Specifically, age-standardized hospitalization rates were computed through direct standardization, utilizing 5-year age groups, with the population of Veneto Region as of January 1, 2024 as the reference. Joinpoint regression was conducted to evaluate the significance of trends over time, expressed as annual percentage change (APC). A P value < 0.05 was considered significant. The analyses were performed using the Statistical Package for the Social Sciences (SPSS 28.0; SPSS Inc., Chicago, IL, United States) and Microsoft Excel software.
HDRs were obtained from the administrative databases of the Veneto Region, and the disclosure and utilization of such records for educational and scientific purposes do not necessitate approval from ethical committees. On January 24, 2023, the Veneto Region implemented the code of conduct for the use of health data for educational and scientific publication purposes (Official Bulletin of the Region, No. 10), as established by the European Committee (European Regulation 2016/679). This implementation received approval from the Italian Personal Data Protection Authority on January 14, 2021. Adhering to the current Italian privacy legislation, the publication and utilization of HDR data, along with the processing methods, must occur exclusively in aggregate form, without any reference to patients’ personal information. Prior to providing access to the authors, all personal data that could potentially lead to identification was substituted with anonymous codes in accordance with current privacy regulations (Legislative Decree No. 196 of June 30, 2003).
In the period of 2007-2023 in the Veneto Region, 10778 first hospital admissions for biliary tract cancers in the main or secondary diagnosis were recorded, yielding 29.4% (n = 3170) for intrahepatic CCA, 33.2% (n = 3583) for extrahepatic CCA, and 37.3% (n = 4025) for gallbladder cancer and unspecified biliary tract (Table 1). Overall, the absolute number of hospitalizations for biliary tract cancers remained stable during the period considered, with an average of 634 hospitalizations per year (186 hospitalizations/year for intrahepatic CCA, 211 for extrahepatic CCA, and 237 for gallbladder cancer/unspecified biliary tract).
| Year | Total | Intrahepatic CCA | Extrahepatic CCA | Gallbladder cancer and unspecified biliary tract |
| 2007 | 692 | 172 (24.9) | 239 (34.5) | 281 (40.6) |
| 2008 | 681 | 191 (28.0) | 259 (38.0) | 231 (33.9) |
| 2009 | 651 | 198 (30.4) | 225 (34.6) | 228 (35.0) |
| 2010 | 544 | 156 (28.7) | 197 (36.2) | 191 (35.1) |
| 2011 | 603 | 169 (28.0) | 232 (38.5) | 202 (33.5) |
| 2012 | 577 | 194 (33.6) | 192 (33.3) | 191 (33.1) |
| 2013 | 794 | 234 (29.5) | 268 (33.8) | 292 (36.8) |
| 2014 | 609 | 197 (32.3) | 202 (33.2) | 210 (34.5) |
| 2015 | 695 | 214 (30.8) | 222 (31.9) | 259 (37.3) |
| 2016 | 579 | 188 (32.5) | 182 (31.4) | 209 (36.1) |
| 2017 | 667 | 226 (33.9) | 213 (31.9) | 228 (34.2) |
| 2018 | 621 | 184 (29.6) | 194 (31.2) | 243 (39.1) |
| 2019 | 672 | 202 (30.1) | 217 (32.3) | 253 (37.6) |
| 2020 | 574 | 152 (26.5) | 183 (31.9) | 239 (41.6) |
| 2021 | 580 | 162 (27.9) | 180 (31.0) | 238 (41.0) |
| 2022 | 628 | 168 (26.8) | 180 (28.7) | 280 (44.6) |
| 2023 | 611 | 163 (26.7) | 198 (32.4) | 250 (40.9) |
| Total | 10778 | 3170 (29.4) | 3583 (33.2) | 4025 (37.3) |
Table 2 summarizes the main characteristics of the cohort. Among the 10778 subjects hospitalized for biliary tract cancers, 51.3% (n = 5527) were males. Overall, the mean age was 72.9 ± 11.3 years, with significant differences between gender (males 71.8 ± 10.8 vs females 74.2 ± 11.7; P < 0.001) and between type of cancer (intrahepatic CCA: 71.3 ± 11.5, extrahepatic CCA: 74.1 ± 11.1, gallbladder cancer/unspecified site: 73.2 ± 11.2; P < 0.001). Specifically, 48.9% were over 75 years old, 29.5% were between 65-74 years old and 21.5% were under 65. The frequency of biliary tract cancers increased with age and was greater in the 65-74 and 75 + age groups for all cancer types. In particular the frequency of subjects over 75 was higher among hospitalizations with extrahepatic CCA (53.9% vs 42.3% intrahepatic CCA vs 49.6% gallbladder cancer; P < 0.001) and the frequency of subjects 65-74 was higher among hospitalizations with intrahepatic CCA (32.4% vs 27.9% extrahepatic CCA and 28.7% gallbladder cancer; P < 0.001).
| Characteristic | Total | Intrahepatic CCA | Extrahepatic CCA | Gallbladder cancer and unspecified biliary tract | P value |
| Gender | |||||
| Males | 5527 (51.3) | 1793 (56.6) | 1959 (54.7) | 1775 (44.1) | < 0.001 |
| Females | 5251 (48.7) | 1377 (43.4) | 1624 (45.3) | 2250 (55.9) | |
| Age group (years) | |||||
| 0-44 | 160 (1.5) | 68 (2.1) | 44 (1.2) | 48 (1.2) | < 0.001 |
| 45-54 | 547 (5.1) | 189 (6.0) | 165 (4.6) | 193 (4.8) | |
| 55-64 | 1615 (15.0) | 545 (17.2) | 441 (12.3) | 629 (15.6) | |
| 65-74 | 3184 (29.5) | 1027 (32.4) | 1000 (27.9) | 1157 (28.7) | |
| 75 + | 5272 (48.9) | 1341 (42.3) | 1933 (53.9) | 1998 (49.6) | |
| Age | 72.9 ± 11.3 | 71.3 ± 11.5 | 74.1 ± 11.1 | 73.2 ± 11.2 | < 0.001 |
| Hospital stay (days) | 16.9 ± 16.5 | 15.9 ± 15.5 | 18.1 ± 18.1 | 16.6 ± 16.5 | < 0.001 |
| Hospitalization regimen | |||||
| Ordinary | 9893 (91.8) | 2834 (89.4) | 3401 (94.9) | 3658 (90.9) | < 0.001 |
| Day surgery | 790 (7.3) | 326 (10.3) | 164 (4.6) | 300 (7.5) | |
| Week surgery | 95 (0.9) | 10 (0.3) | 18 (0.5) | 67 (1.7) | |
| Biliary tract cancer in main diagnosis | 8094 (75.1) | 2458 (77.5) | 2754 (76.9) | 2882 (71.6) | < 0.001 |
| Surgery during hospital stay | 9246 (85.8) | 2730 (86.1) | 3143 (87.7) | 3373 (83.8) | < 0.001 |
| In-hospital mortality | 1903 (17.7) | 574 (18.1) | 609 (17.0) | 720 (17.9) | NS |
| Total | 10778 (100.0) | 3170 (100.0) | 3583 (100.0) | 4025 (100.0) |
Among hospitalizations for gallbladder cancer/unspecified site there was a higher frequency of females (55.9% vs 45.3% in extrahepatic CCA and 43.4% in intrahepatic CCA, P < 0.001). The mean hospital stay was 16.9 days, which was longer than for extrahepatic CCA (18.1 days). Hospitalizations for biliary tract cancers in the main diagnosis were 75.1% (8094) of the total. The death of the subject during hospitalization occurred in 17.7% of cases (n = 1903), without significant differences between cancer types. Overall, 91.8% of hospitalizations occurred in ordinary regimen, and 85.8% of hospitalized subjects (9246/10778) underwent a procedure (surgical or other interventions) during the hospital stay.
Of these patients, 69.9% (6465/9246) underwent a procedure on the digestive system, 27.5% (2540/9246) underwent a diagnostic-therapeutic procedure, and 2.6% (241/9246) underwent other interventions. The majority (81.5%) of the digestive system interventions were classified as the main intervention (5266), and 18.5% (1199) were classified as secondary intervention. Over 90% of the digestive system interventions consisted of interventions on the gallbladder and bile ducts (49.6%), interventions on the liver (24.6%), incision, removal, and anastomosis of the intestine (7.7%), and other interventions on the abdominal region (11.8%). As shown in Table 3, the frequency of digestive system procedures was higher in the 75 + age group for all procedures except those involving the pancreas and liver where the frequencies were higher among patients aged 65 to 74 years. Among those who underwent a procedure, in-hospital mortality occurred most frequently among those who underwent esophagus procedures (25%), other stomach procedures (18.2%), other procedures in the abdominal region (15%), and incision, removal, and anastomosis of the intestine (14.9%). Overall, admissions involving digestive procedures had a lower in-hospital mortality rate (9.7%) compared to those involving only diagnostic procedures (26.9%).
| Total | 0-44 | 45-54 | 55-64 | 65-74 | 75 + | In-hospital mortality | Total (n) |
| 150 (1.6) | 476 (5.1) | 1447 (15.7) | 2807 (30.4) | 4366 (47.2) | 1335 (14.4) | 9246 | |
| Digestive system interventions | 110 (1.7) | 341 (5.3) | 1014 (15.7) | 2000 (30.9) | 3000 (46.4) | 627 (9.7) | 6465 |
| Gallbladder and biliary tract procedures | 37 (1.2) | 121 (3.8) | 394 (12.3) | 829 (25.8) | 1826 (56.9) | 305 (9.5) | 3207 |
| Liver procedures | 43 (2.7) | 114 (7.2) | 326 (20.5) | 624 (39.3) | 481 (30.3) | 92 (5.8) | 1588 |
| Other abdominal region procedures | 15 (2.0) | 54 (7.1) | 150 (19.7) | 272 (35.7) | 271 (35.6) | 114 (15.0) | 762 |
| Incision, removal, and anastomosis of the intestine | 5 (1.0) | 26 (5.3) | 68 (13.7) | 133 (26.9) | 263 (53.1) | 74 (14.9) | 495 |
| Pancreas procedures | 6 (2.3) | 19 (7.3) | 50 (19.1) | 100 (38.2) | 87 (33.2) | 17 (6.5) | 262 |
| Other stomach procedures | 1 (1.0) | 2 (2.0) | 20 (20.2) | 28 (28.3) | 48 (48.5) | 18 (18.2) | 99 |
| Stomach incision and excision | 1 (5.9) | 1 (5.9) | 2 (11.8) | 5 (29.4) | 8 (47.1) | 2 (11.8) | 17 |
| Other bowel procedures | 1 (7.7) | 0 (0) | 1 (7.7) | 5 (38.5) | 6 (46.2) | 1 (7.7) | 13 |
| Procedures on the esophagus | 0 (0.0) | 2 (16.7) | 2 (16.7) | 2 (16.7) | 6 (50.0) | 3 (25.0) | 12 |
| Procedures on the rectum, rectosigmoid, and perirectal tissues | 0 (0.0) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 0 (0.0) | 6 |
| Hernia repair | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 |
| Procedures on the anus | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100) | 1 (100) | 1 |
| Appendix procedures | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Diagnostic-therapeutic procedures | 31 (1.2) | 113 (4.4) | 384 (15.1) | 723 (28.5) | 1289 (50.7) | 682 (26.9) | 2540 |
| Other interventions | 9 (3.7) | 22 (9.1) | 49 (20.3) | 84 (34.9) | 77 (32.0) | 26 (10.8) | 241 |
The standardized hospitalization rates for biliary tract cancers are shown in Figure 1A. Overall, the average annual hospitalization rate was 14.8 cases (per 100000 inhabitants), with specific rates of 4.3 for intrahepatic CCA, 5.0 for extrahepatic CCA and 5.5 for gallbladder cancer and unspecified biliary tract. Overall, hospitalization rates for biliary tract cancers exhibited a significant downward trend from 2007 to 2023 with rates decreasing from 18.2 to 12.8 per 100000 inhabitants [APC = -2.1, 95% confidence interval (CI): -2.9 to -1.0]. In particular hospitalization rates for intrahepatic and extrahepatic CCA decreased while hospitalizations for gallbladder cancer showed no significant changes in the period with an average rate of 5.5 (per 100000 inhabitants). Intrahepatic CCA displayed a significant decreasing trend from 2007 to 2023 (APC = -2.0, 95%CI: -3.2 to -0.7), going from 4.9 cases per 100000 inhabitants in 2009 to 3.4 in 2023. Extrahepatic CCA also decreased significantly from 6.7 cases per 100000 inhabitants in 2008 to 3.8 in 2022 (APC = -3.2, 95%CI: -4.2 to
Hospitalization rates for intrahepatic and extrahepatic CCA were significantly higher in males throughout the observed period (Figure 1B). The average rate of intrahepatic CCA was 5.6 per 100000 inhabitants among males and 3.3 per 100000 inhabitants among females. Hospitalization of males did not have significant changes until 2015 (average 6.1) but decreased thereafter from 6.6 in 2015 to 4.1 in 2023 (APC = -5.4, 95%CI: -8.7 to -2.0). Among females, hospitalization rates decreased from 4.0 in 2008 to 2.8 in 2023 (APC = -2.1, 95%CI: -3.6 to -0.5).
The average rate of extrahepatic CCA was 6.4 per 100000 inhabitants among males and 3.9 per 100000 inhabitants among females. Over the observed period, the hospitalization rates decreased significantly from 7.8 to 5.3 (APC = -3.1, 95%CI: -4.2 to -2.0) for males and from 5.3 to 3.3 (APC = -3.5, 95%CI: -4.8 to -2.1) for females. Conversely, hospitalization rates for gallbladder cancer/unspecified biliary tract among males and females had a generally overlapping trend (average 5.5) with slightly higher rates among females from 2007 to 2009 (6.8 vs 6.0) and among males from 2017 to 2023 (5.7 vs 5.0) without any significant difference.
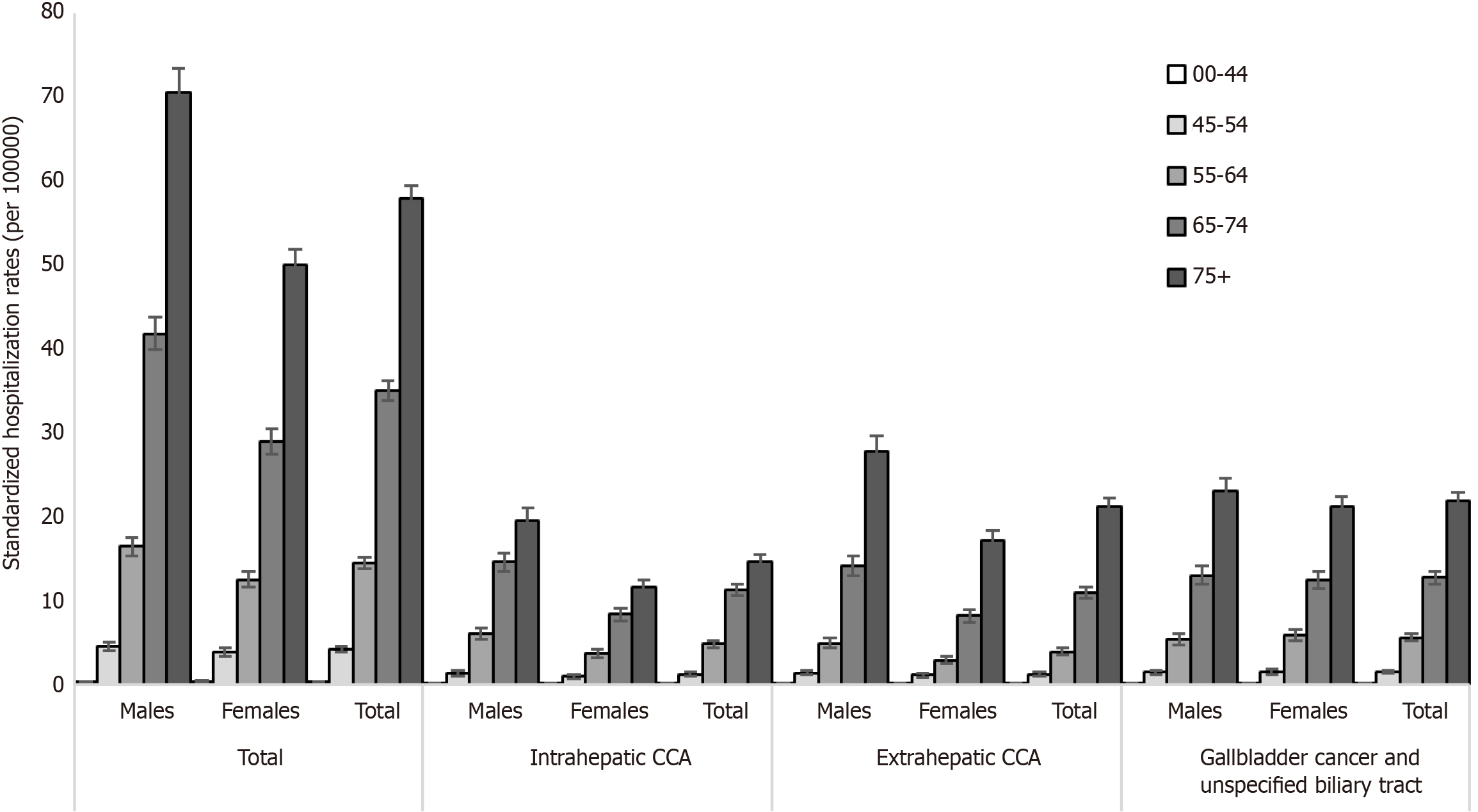
The hospitalization rates for biliary tract cancers (per 100000 inhabitants) stratified by gender and age group are shown in Figure 2. Overall, hospitalization rates increased with age and were highest among males compared to females. The highest rates occurred among subjects over 75 years of age with 57.9 (95%CI: 56.4-59.5) cases per 100000 inhabitants (males: 70.5, 95%CI: 67.7-73.4 vs females: 50.0, 95%CI: 48.2-51.9) and among subjects aged 65-74 with 35.1 (95%CI: 33.9 to 36.3) cases (per 100000 inhabitants) (males: 41.8, 95%CI: 39.9-43.7 vs females 29.0, 95%CI: 27.5-30.6). Among subjects aged 55-64, the hospitalization rates of males and females were 16.4 (95%CI: 15.4-17.5) and 12.5 (95%CI: 11.6-13.5), respectively. No significant differences according to gender were observed among people aged 0-44 and 45-54, with a rate of 0.4 (95%CI: 0.3-0.4) and 4.2 (95%CI: 3.8-4.5), respectively.
Stratifying the study population by cancer type and age group, the highest hospitalization rates occurred among subjects both over 75 years and in the class of age of 65-74 for all types of cancer. Hospitalization rates for intrahepatic CCA among subject aged 55-64, 65-74, and over 75 were 4.9 (95%CI: 4.5-5.3), 11.3 (95%CI: 10.6-12.0), and 14.7 (95%CI: 13.9-15.5), respectively, with higher rates among males than females in all three age groups (6.1, 95%CI: 5.4-6.7 vs 3.7, 95%CI: 3.2-4.2 in the 55-64 group; 14.6, 95%CI: 13.5-15.7 vs 8.3, 95%CI: 7.5-9.2 in the 65-74 group; and 19.6, 95%CI: 18.1-21.1 vs 11.6, 95%CI: 10.7-12.5 in the over 75 group).
Hospitalization rates for extrahepatic CCA among subject aged 55-64, 65-74, and over 75 were 3.9 (95%CI: 3.6-4.3), 11.0 (95%CI: 10.3-11.7), and 21.3 (95%CI: 20.3-22.2), respectively, with higher rates among males than females in all three age groups (5.0, 95%CI: 4.4-5.6 vs 3.0, 95%CI: 2.5-3.4 in the 55-64 group; 14.2, 95%CI: 13.0-15.3 vs 8.2, 95%CI: 7.4-9.0 in the 65-74 group; and 27.9, 95%CI: 26.1-29.7 vs 17.2, 95%CI: 16.1-18.3 in the over 75 group).
Finally, hospitalization rates for gallbladder cancer increased from 5.6 (95%CI: 5.2-6.1) in the 55-64 group to 12.7 (95%CI: 12.0-13.5) in the 65-74 group and to 22.0 (95%CI: 21.0-22.9) in the over 75 group without any significant difference by gender.
The main findings of our retrospective analysis indicate the following: (1) The total number of hospitalizations for biliary tract cancers remained stable over the past 17 years; (2) Standardized hospitalization rates for intrahepatic and extrahepatic CCA decreased while hospitalizations for gallbladder cancer showed no significant changes in the period; and (3) Diagnosis of CCA and gallbladder cancer is increasing with advancing age in both genders.
In the period 2007-2023, we observed that the hospitalization rates for intrahepatic CCA had a decreasing temporal trend. This declining trend observed in our study differs from the main national and international studies in the literature that report a progressive increase over the previous decades in the incidence and mortality rates of intrahepatic CCA both in the United States[15,16] and in Europe[17]. In Europe in a previous study using the hospitalization data during the 2000-2009 period, we documented an increasing rate of intrahepatic CCA, accounting more males than females[10]. The trend towards an increasing incidence of intrahepatic CCA was also reported by Alvaro et al[11] on the basis of epidemiological data collected from the Italian cancer registries. In the United States the study by Saha et al[18], analyzing Surveillance, Epidemiology, and End Results Program datasets, reported a 128% increase in the estimated incidence of intrahepatic CCA over the past 40 years, from 0.44 cases per 100000 person-years in 1973 to 1.18 cases per 100000 person-years in 2012 (APC of 2.30%). Similarly, Patel[19] reported an increase of estimated incidence rates for intrahepatic CCA in the United States from 0.13 per 100000 in 1973 to 0.67 per 100000 in 1997, with an estimated APC incidence over this period of 9.11%. Furthermore, Storandt et al[20] postulated that there will be a doubling of the incidence of intrahepatic CCA in White females and males from 2001 to 2029.
The reasons for the increased incidence of intrahepatic CCA observed worldwide in the previous studies are not clear yet. It remains an open question whether this increase in incidence is authentic or an apparent increase due to improved diagnosis or misclassification of intrahepatic CCA[21,22]. A possible explanation can be found in the complexity of classifications used for coding CCAs and in the different revisions of the ICD coding system for liver and biliary tracts cancers over the past three decades[23]. Given that perihilar CCA is the most common subtype of CCA in the United States[1] and has probably been misclassified as intrahepatic CCA under the second edition of the ICD for Oncology, CCA rates in Surveillance, Epidemiology, and End Results (SEER) between 1992 and 2000 may have been misreported. This has resulted in overreporting intrahepatic CCA by 13% and underreporting extrahepatic CCA by 15%[24]. The hypothesis of diagnostic misclassification and the variations in classification systems between different countries over the years could therefore explain the differences in the results regarding intrahepatic CCA obtained in our study compared to previous studies in the literature.
Another hypothesis to be considered is the possible role of risk factors in determining the increase in intrahepatic CCA frequency. A recent study conducted in Northeast Thailand reported that in addition to Opistorchis viverrine infection diabetes mellitus plays an important role as a risk factor for CCA. Specifically, regions characterized by a high distribution of CCA often coincide with elevated occurrences of both Opistorchis viverrini infection and diabetes mellitus[25].
It is important to point out that there are some exceptions to this increasing trend observed over the previous decades. For example, in Denmark an analysis of the Danish Cancer Registry data regarding a 25-year period (1978-2002) revealed a decrease in the standardized incidence rate of intrahepatic CCA (from 1.27 to 0.46 per 100000 people). More specifically, intrahepatic CCA rates were three times higher in Denmark than in the United States until the mid-1980s when the rate in the United States started to rise and the Danish rate started to decrease[26]. A French study conducted in Burgundy also suggested that the incidence of intrahepatic CCA remained relatively stable from 1976 to 2005[27]. In the United States Javle et al[28] reported that the increase in the incidence of intrahepatic CCA may be a real increase not attributable to reclassification issues.
We found that the hospitalization rates of extrahepatic CCA had a decreasing trend from 2007 to 2023. This data is consistent with other international epidemiological studies, which point out that over the previous three decades the incidence rate of extrahepatic CCA had a decreasing trend[26] or remained stable[7]. In particular, a French population-based study conducted in an area of one million people over a 30-year period (1976-2005) showed that the age-standardized incidence rates for extrahepatic CCA remained stable over the past 30 years[27].
Our study showed a practically constant trend in the hospitalization rates for gallbladder cancer and unspecified biliary tract cancer over a period of 17 years. However, there are conflicting results between the different studies in the literature regarding the trend of incidence of gallbladder cancer. For example, in the United States the incidence rate decreased from 1973 to 2009 for males, while the incidence rate for females decreased from 1973 to the mid-1990s but has then remained stable ever since[29]. In Southern Finland and Sweden the incidence appeared to decrease[30,31], while it increased in Asia, especially in India[32].
Another aspect that we examined concerns the hospitalization rates stratified by age groups, gender, and type of diagnosis in the period of 2007-2023. Our analysis showed that hospitalization rates for both intrahepatic and extrahepatic CCA increased with age and were highest among males. This result is consistent with the fact that the peak of CCA incidence is between the fifth and the seventh decade of life and the average age at diagnosis is over 50 years[33]. It rarely occurs before the age of 40 except in patients with PSC[34]. The disease affects both genders, but males have a higher incidence of CCA than females with ratios of 1:1.2-1.5[35,36]. Moreover, in a previous study using the hospitalization data during the 2000-2009 period, we documented a trend toward higher intrahepatic and extrahepatic CCA hospitalization rates with increasing age with males involved more often than females[10].
Recently, molecular profiling has revealed distinct genomic alterations in CCA subtypes with up to half of patients with CCA harboring actionable mutations. Next-generation sequencing plays a crucial role in identifying targetable gene variants that can influence disease prognosis and treatment response[37]. Interestingly, Tsilimigras et al[38] comprehensively assessed the genomic profile of intrahepatic CCA using a large dataset of patients who underwent next-generation sequencing of their tumors. Their evidence suggests ethnicity and sex differences in genomic profiling. For instance, IDH1 mutations are more frequent in White patients, while FGFR2 alterations are more common among Black patients. Males are more likely to harbor TP53 mutations, whereas females more frequently exhibit IDH1, FGFR2, and BAP1 mutations. The varying prevalence of these mutations across demographic groups may contribute to disparities in incidence and outcomes, potentially explaining some of the differences observed in estimates from different countries, as previously discussed[38].
Our study showed that hospitalization rates for gallbladder cancer/unspecified biliary tract increased with age, especially in subjects over 75 years, without any significant difference by gender. These findings align with previous literature studies that showed higher rates of gallbladder cancer in the seventh decade of life[14] and an incidence that increased consistently with age[39]. However, gallbladder cancer is noted to affect females more than males. Previous studies revealed that females have a three to six times higher incidence than males, and this is thought to be due to a higher prevalence of gallstone disease in females[39,40].
Our HDR data estimated an in-hospital mortality rate of 17.7% over the entire study period in hospitalizations with a diagnosis of biliary tract cancer. Unfortunately, the data source does not allow for the analysis of long-term survival. Studies on the topic have reported that surgery for CCA has improved, leading to a significant increase in long-term survival after resection (median overall survival increased from 16 months to 30 months), with a decreased operative risk despite a more aggressive surgical approach[41]. Pathological factors, such as tumor differentiation, lymphovascular invasion, perineural invasion, and lymph node involvement, have been confirmed as independent predictors of both overall and disease-free survival in multivariate analysis[41].
In our study, we observed that admissions involving digestive procedures had a lower risk of in-hospital mortality compared to those involving only diagnostic procedures. This is likely because surgical procedures are typically performed on tumors considered resectable. Among digestive system surgeries, the highest in-hospital mortality rates were observed in cases involving intestinal removal and other abdominal operations. The frequency of interventions increased with age and can be attributed to the age bias. Unfortunately, in the absence of detailed anatomical-pathological data (tumor grading, staging, resection margins, the presence of vascular or perineural invasion) as well as clinical history (e.g., medical treatments such as radiotherapy or chemotherapy), it is not possible to assess the impact of procedure severity on fatal outcomes while adjusting for potential covariates.
Another limitation of the present study was the inability to identify potential risk factors associated with the development of CCA, such as PSC. This is due to the nature of the available data source, which is primarily administrative. As reported in other studies using the same data source, our classification relies on the diagnosis codes recorded in the HDRs, which are still based on the ICD-9-CM system in the Veneto Region[42]. This introduces quantitative distortions due to coding practices and reverse reporting bias, resulting in limited sensitivity despite high specificity in identifying cases. It is also important to acknowledge issues related to the consistency of HDR data entry, particularly when different criteria are used to assign specific diagnostic codes. While HDRs allow for the reconstruction of a patient’s entire hospital care pathway and provide readily available data, this source has certain limitations in terms of accuracy. As a result, this tool does not allow for the identification of PSC due to the lack of a specific diagnostic code. The condition is classified under ICD-9-CM code No. 576.1, which encompasses a broader category of cholangitis types, including PSC, stenosing cholangitis, suppurative cholangitis, chronic cholangitis, and acute cholangitis. Consequently, the correlation between PSC and CCA could not be accurately investigated. Nevertheless, the strength of our study was in its representative sample size and the long-term study period.
Our study, conducted over a 17-year period, reported a decreasing hospitalization rate for intrahepatic and extrahepatic CCA and a stable trend for gallbladder cancer. These trends differ from the results of a previous study conducted in the same geographical area over the 2000-2009 period, suggesting a change in the epidemiology of CCA during the previous decades. Further research is required to fully understand the causes of this phenomenon and identify potential risk factors related to this new epidemiological trend.
The authors sincerely thank Laino P for the professional review of the English language in this manuscript.
| 1. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1549] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 2. | Gopal P, Robert ME, Zhang X. Cholangiocarcinoma: Pathologic and Molecular Classification in the Era of Precision Medicine. Arch Pathol Lab Med. 2024;148:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 4. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 5. | Aune D, Sen A, Norat T, Riboli E, Folseraas T. Primary sclerosing cholangitis and the risk of cancer, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11:10646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 7. | Pascale A, Rosmorduc O, Duclos-Vallée JC. New epidemiologic trends in cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2023;47:102223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Witjes CD, Karim-Kos HE, Visser O, de Vries E, IJzermans JN, de Man RA, Coebergh JW, Verhoef C. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB (Oxford). 2012;14:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Floreani A, Lisiero M, Baldovin T, Baldo V. Epidemiological aspects of biliary tree tumors in a region of northern Italy: emerging trends and sex-based differences. Eur J Gastroenterol Hepatol. 2013;25:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R; AISF Cholangiocarcinoma committee. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 187] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 13. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8160] [Article Influence: 8160.0] [Reference Citation Analysis (2)] |
| 14. | Halaseh SA, Halaseh S, Shakman R. A Review of the Etiology and Epidemiology of Gallbladder Cancer: What You Need to Know. Cureus. 2022;14:e28260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Gad MM, Saad AM, Faisaluddin M, Gaman MA, Ruhban IA, Jazieh KA, Al-Husseini MJ, Simons-Linares CR, Sonbol MB, Estfan BN. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin Res Hepatol Gastroenterol. 2020;44:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Jiang Y, Jiang L, Li F, Li Q, Yuan S, Huang S, Fu Y, Yan X, Chen J, Li H, Li S, Liu J. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022;22:546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 17. | Tavolari S, Brandi G. Mutational Landscape of Cholangiocarcinoma According to Different Etiologies: A Review. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 19. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 797] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 20. | Storandt MH, Tella SH, Wieczorek MA, Hodge D, Elrod JK, Rosenberg PS, Jin Z, Mahipal A. Projected Incidence of Hepatobiliary Cancers and Trends Based on Age, Race, and Gender in the United States. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Raoof M, Singh G. Rising trends in intrahepatic cholangiocarcinoma incidence and mortality: getting at the root cause. Hepatobiliary Surg Nutr. 2019;8:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Blendis L, Halpern Z. An increasing incidence of cholangiocarcinoma: why? Gastroenterology. 2004;127:1008-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 24. | Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Thinkhamrop K, Suwannatrai K, Kelly M, Suwannatrai AT. Spatial analysis of cholangiocarcinoma in relation to diabetes mellitus and Opisthorchis viverrini infection in Northeast Thailand. Sci Rep. 2024;14:10510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Jepsen P, Vilstrup H, Tarone RE, Friis S, Sørensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Lepage C, Cottet V, Chauvenet M, Phelip JM, Bedenne L, Faivre J, Bouvier AM. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Javle M, Lee S, Azad NS, Borad MJ, Kate Kelley R, Sivaraman S, Teschemaker A, Chopra I, Janjan N, Parasuraman S, Bekaii-Saab TS. Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017. Oncologist. 2022;27:874-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 29. | Rahman R, Simoes EJ, Schmaltz C, Jackson CS, Ibdah JA. Trend analysis and survival of primary gallbladder cancer in the United States: a 1973-2009 population-based study. Cancer Med. 2017;6:874-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Koppatz H, Takala S, Peltola K, But A, Mäkisalo H, Nordin A, Sallinen V. Gallbladder cancer epidemiology, treatment and survival in Southern Finland - a population-based study. Scand J Gastroenterol. 2021;56:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Kilander C, Lagergren J, Ljung R, Sadr-Azodi O. The population-based incidence and mortality of biliary tract cancer in Sweden. Cancer Epidemiol. 2018;56:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Malhotra RK, Manoharan N, Shukla NK, Rath GK. Gallbladder cancer incidence in Delhi urban: A 25-year trend analysis. Indian J Cancer. 2017;54:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F, Cacciatore M, Guido M. Cholangiocarcinoma. Pathologica. 2021;113:158-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 34. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 35. | Goral V. Cholangiocarcinoma: New Insights. Asian Pac J Cancer Prev. 2017;18:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 37. | Stenzinger A, Vogel A, Lehmann U, Lamarca A, Hofman P, Terracciano L, Normanno N. Molecular profiling in cholangiocarcinoma: A practical guide to next-generation sequencing. Cancer Treat Rev. 2024;122:102649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 38. | Tsilimigras DI, Stecko H, Ntanasis-Stathopoulos I, Pawlik TM. Racial and Sex Differences in Genomic Profiling of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2024;31:9071-9078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder cancer: epidemiology and genetic risk associations. Chin Clin Oncol. 2019;8:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 40. | Alkhayyat M, Abou Saleh M, Qapaja T, Abureesh M, Almomani A, Mansoor E, Chahal P. Epidemiology of gallbladder cancer in the Unites States: a population-based study. Chin Clin Oncol. 2021;10:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, Bresadola F, Calise F, Dalla Valle R, D'Amico DF, Gennari L, Giulini SM, Guglielmi A, Jovine E, Pellicci R, Pernthaler H, Pinna AD, Puleo S, Torzilli G, Capussotti L; Italian Chapter of the International Hepato-Pancreato-Biliary Association, Cillo U, Ercolani G, Ferrucci M, Mastrangelo L, Portolani N, Pulitanò C, Ribero D, Ruzzenente A, Scuderi V, Federico B. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 42. | Cocchio S, Cozzolino C, Furlan P, Cozza A, Tonon M, Russo F, Saia M, Baldo V. Pneumonia-Related Hospitalizations among the Elderly: A Retrospective Study in Northeast Italy. Diseases. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |