Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104194
Revised: March 30, 2025
Accepted: April 15, 2025
Published online: May 15, 2025
Processing time: 71 Days and 3.1 Hours
Gastrointestinal dual-contrast ultrasonography (DCUS) is characterized by its high resolution, sensitivity, and specificity.
To determine the accuracy of DCUS in predicting lymph node metastasis in middle-aged and elderly patients with gastric cancer (GC).
A total of 100 middle-aged and elderly patients with GC admitted to the Fourth Affiliated Hospital of Soochow University (Dushu Lake Hospital, Suzhou, China) between April 2022 and April 2024 were selected. The baseline data and lymph node metastasis status were collected. DCUS combined with intravenous contrast technology was used to calculate the enhancement time (ET), time to peak (TTP), and slope of the ascending branch wash-in rate (WIR). These indicators were used in assessing lymph node metastasis in patients with GC.
Among 100 middle-aged and elderly patients with GC, 35 (35.00%) had lymph node metastases. GC patients with lymph node metastasis had a higher propor
DCUS-mediated assessment of ET, TTP, and WIR can effectively predict and evaluate lymph node metastasis status in patients with GC, with higher sensitivity when used in combination.
Core Tip: Accurate assessment of lymph node metastasis is crucial for middle-aged and elderly patients with gastric cancer (GC), and dual-contrast ultrasonography can effectively predict and evaluate lymph node metastasis status in GC patients.
- Citation: Jiang Y, Xu SH, Han L, Lu N, Huang S, Wang L. Accuracy of dual-contrast gastrointestinal ultrasonography in predicting lymph node metastasis in older adults with gastric cancer. World J Gastrointest Oncol 2025; 17(5): 104194
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104194.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104194
Gastric cancer (GC) is a prevalent malignant tumor associated with various factors, including genetics, environment, dietary habits, and lifestyle. It has a relatively high incidence rate in middle-aged and elderly populations. With the increasing aging of the global population, the diagnosis and treatment of GC have emerged as timely topics in clinical medicine[1,2]. Over the past several decades, research on GC has made remarkable progress; however, many questions remain unanswered. While various diagnostic methods are currently available including endoscopy, the accurate predic
A total of 100 middle-aged and elderly patients with GC admitted to the Fourth Affiliated Hospital of Soochow Univer
Detailed baseline data for each patient, including sex, age, body mass index, disease course, tumor stage, tumor location, and tumor size, were recorded through a medical record review, patient inquiry, and utilization of imaging materials.
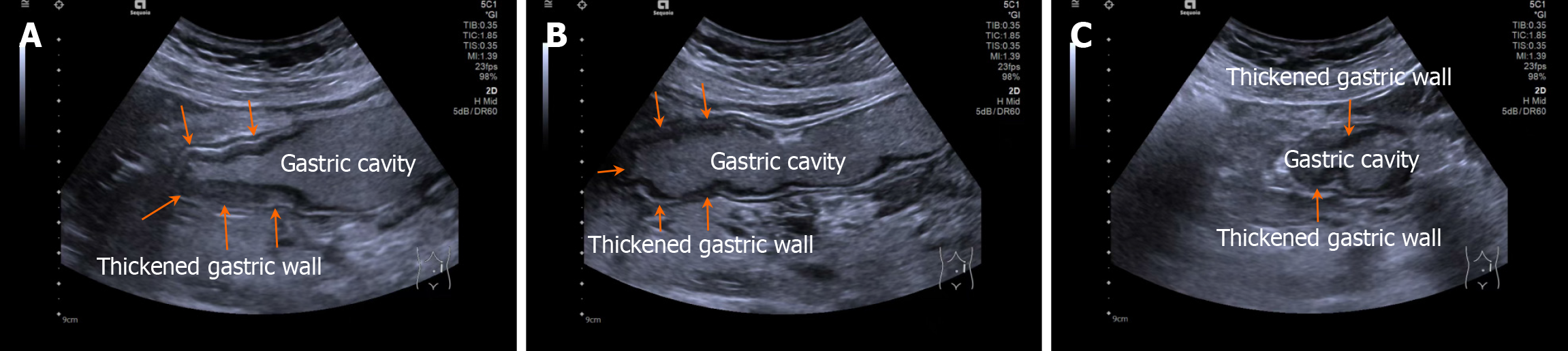
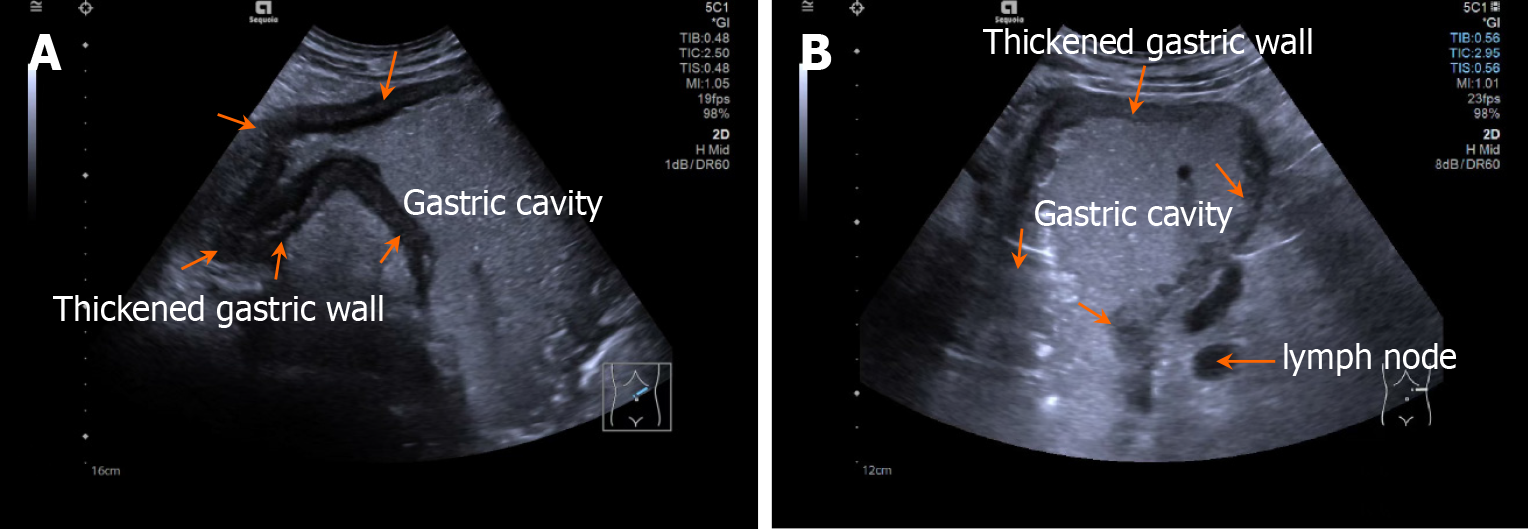
Patients fasted for > 8 hours and orally ingested 500 mL of prepared gastrointestinal contrast ultrasonography agent (Huzhou Dongya Medical Supplies Co., Ltd., Zhejiang; medical device registration number 20212060179) 5 minutes before the examination using a Siemens-SEQ1 color ultrasonography diagnostic device (registration number: National medical device registration import 20192060012) for gastrointestinal contrast ultrasonography. During the examination, the patient was adjusted to different positions, including supine, left and right lateral decubitus, and semi-sitting, with an appropriate amount of ultrasonography coupling agent applied. A probe was used to perform multi-plane scanning of the digestive tract from the cervical esophagus to the duodenum, with frequency and mechanical index set at 2-4 MHz and 0.07, respectively. After the basic contour structure of the stomach was clearly displayed, the thickness of the gastric wall, peristalsis, and the distribution of contrast agent in the gastric cavity were observed in detail. Representative images are shown in Figures 1 and 2.
A channel was established in the patient's elbow vein, and sulfur hexafluoride microbubbles (Bracco Suisse SA, Switzer
After DCUS examination, all patients underwent radical surgery for GC within 2 weeks, and lymph node metastasis status was recorded.
All data collected in this study were processed using the SPSS version 26.0 statistical software. Categorical data are expressed as percentages (%) and were subjected to χ2 testing. Normal distribution of continuous data was determined by the Shapiro-Wilk test; they are presented as mean ± SD and were analyzed using t-tests. Receiver operating characteristic curves were constructed for the ET, TTP, and WIR, and their combined diagnostic performance in predicting GC lymph node metastasis. The area under the curve (AUC) was calculated; an AUC > 0.90 indicated high diagnostic value, 0.7 < AUC ≤ 0.9 indicated moderate diagnostic value, and 0.5 < AUC ≤ 0.7 indicated poor diagnostic value. Statistical signifi
Histopathological examination of the tissue collected during radical gastrectomy of the 100 middle-aged and elderly patients with GC demonstrated that 35 (35.00%) exhibited lymph node metastasis, whereas 65 (65.00%) did not.
There were no statistically significant differences in sex, age, body mass index, disease duration, or tumor diameter between GC patients with and without lymph node metastasis (P > 0.05). The proportion of patients with lymph node metastasis at TNM stage II was 28.57%, which was significantly higher than the 6.15% of those without lymph node metastasis (P < 0.05) (Table 1).
| Data class | Lymph node metastasis (n = 35) | No metastasis (n = 65) | t/χ2 | P value |
| Sex | ||||
| Male | 18 (51.43) | 35 (53.85) | 0.053 | 0.817 |
| Female | 17 (48.57) | 30 (46.15) | ||
| Age (years) | 56.24 ± 5.71 | 55.38 ± 5.43 | 0.742 | 0.461 |
| Body mass index (kg/m2) | 24.17 ± 1.15 | 24.09 ± 1.03 | 0.356 | 0.723 |
| Duration of disease (years) | 2.31 ± 0.46 | 2.24 ± 0.51 | 0.677 | 0.501 |
| Tumor diameter (mm) | 4.08 ± 0.75 | 3.97 ± 0.72 | 0.718 | 0.475 |
| TNM staging | ||||
| Phase I | 25 (71.43) | 61 (93.85) | 9.496 | 0.002 |
| Phase II | 10 (28.57) | 4 (6.15) |
The ET and TTP values of GC patients with lymph node metastasis were lower than those without lymph node metastasis, whereas the WIR value was higher, with all differences being statistically significant (P < 0.05; Table 2).
| Group | n | ET (s) | TTP (s) | WIR |
| Lymph node metastasis | 35 | 15.45 ± 4.37 | 8.16 ± 2.32 | 7.36 ± 1.97 |
| No metastasis | 65 | 22.08 ± 6.74 | 11.87 ± 4.28 | 6.17 ± 1.25 |
| t | - | 5.249 | 4.758 | 3.689 |
| P value | - | < 0.001 | < 0.001 | < 0.001 |
The AUC values for ET, TTP, WIR, and combined diagnosis of GC lymph node metastasis using DCUS were all > 0.7, with the optimal assessment achieved when the cutoff values for ET, TTP, and WIR were set at 16.32 seconds, 10.67 seconds, and 7.02, respectively (Table 3).
| Index | Sensitivity | Specificity | Youden index | AUC value | 95%CI | Cut-off value |
| ET | 0.615 | 0.807 | 0.421 | 0.792 | 0.708-0.891 | 16.32 |
| TTP | 0.930 | 0.569 | 0.495 | 0.776 | 0.684-0.867 | 10.67 |
| WIR | 0.725 | 0.961 | 0.676 | 0.751 | 0.604-0.904 | 7.02 |
| Combined diagnosis | 0.782 | 0.748 | 0.535 | 0.836 | 0.769-0.932 | - |
GC is a highly malignant gastrointestinal tumor with an increasing global incidence. It is particularly prevalent among middle-aged and elderly populations. Postoperative survival time in patients with GC is associated with various factors, including general physical condition, age, sex, lifestyle habits, psychological state, and postoperative treatment, and is closely related to the occurrence of lymph node metastasis. Studies have indicated that the 5-year survival rate for patients with GC without lymph node metastasis is approximately 40%-50%, whereas those with lymph node metastasis, it is only 0%-15%[6-8]. Therefore, employing safe and effective methods to accurately predict lymph node metastasis in middle-aged and elderly patients with GC is important for treatment planning and prognostic assessment.
Under normal physiological conditions, blood perfusion into the lymph node is typically at a relatively low level. This implies that in the absence of external interference, the internal blood flow within the lymph nodes is limited to meet their basic physiological needs. When tumor cells begin to invade and metastasize, there is a significant change in the hemodynamics within the lymph nodes, which is usually characterized by an increase in blood perfusion or a change in distribution patterns. Specifically, tumor cell invasion leads to the blood vessel dilation within lymph nodes, thereby in
Additionally, the study results showed that the AUC values for ET, TTP, WIR, and the combined diagnosis of GC lymph node metastasis using DCUS were all > 0.7, with the optimal assessment achieved when the cutoff values for ET, TTP, and WIR were set at 16.32 seconds, 10.67 seconds, and 7.02, respectively. The AUC value is an important indicator of diagnostic efficacy[19,20]. In this study, the AUC values were all > 0.7, indicating that DCUS has a high diagnostic efficacy in predicting lymph node metastasis in patients with GC. In particular, the combined diagnostic AUC value reached 0.836, indicating that combining the three indicators of ET, TTP, and WIR for diagnosis can more comprehensively assess lymph node metastasis, providing a more accurate basis for clinical decision-making.
To further elucidate the significance of our findings, we conducted a detailed comparison of our results with those reported in literature. In recent years, numerous studies have explored the application of various imaging techniques to predict lymph node metastasis in GC. For instance, Wen et al[20] demonstrated that lymph node metastasis in GC patients is closely associated with TNM staging, which is consistent with our findings. Additionally, Riely et al[21] reported that the assessment of lymph node metastasis using multidetector computed tomography in patients with GC can achieve an AUC value of 0.854, which is comparable to the AUC value obtained from DCUS in our study. However, the unique strength of our study lies in the combined use of ET, TTP, and WIR for diagnosis, with a combined diagnostic AUC value of 0.836, which was higher than that of any single indicator. This indicates that the combined diagnostic approach has a significant advantage for evaluating lymph node metastasis in GC.
Moreover, a previous study demonstrated that quantitative dynamic contrast-enhanced MRI has high diagnostic value in assessing lymph node metastasis in patients with GC, with an AUC value close to our results[22,23]. This further corroborates the scientific validity and effectiveness of the proposed method. Nonetheless, the combined diagnostic approach in our study offers greater feasibility and operability in clinical applications, as DCUS is a non-invasive and convenient imaging technique that is well suited for widespread use in clinical practice.
In this study, DCUS revealed that patients with GC and lymph node metastasis exhibited higher TNM stage propor
DCUS can effectively predict and evaluate the lymph node metastasis status in patients with GC through assessment of ET, TTP, and WIR. The combination of all three values has higher diagnostic value, suggesting the potential for the application of this technique in clinical practice.
| 1. | Wang Q. Refining Radiomics by Integrating Vascular Information from Color Doppler Ultrasound for Assessing Lymph Node Metastasis in Endometrial Cancer. Acad Radiol. 2024;31:4730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Eggermont C, Nené LEH, Koekelkoren FHJ, van der Toorn YR, Snetselaar LD, Kroah-Hartman M, Genders RE, Kelleners-Smeets NWJ, Hollestein LM, van Kester MS, Wakkee M. The impact of routine ultrasonography on nodal metastasis in head and neck cutaneous squamous cell carcinoma: A retrospective multicentre cohort study. J Eur Acad Dermatol Venereol. 2023;37:e1136-e1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Matsumoto C, Enomoto N, Yamada K, Kato D, Yagi S, Nohara K, Kokudo N, Misumi K, Igari T. Gastric cancer with a giant lymph node metastasis: a case report and review of the literature. Clin J Gastroenterol. 2023;16:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Imtiaz S, Berger Y, Gleeson E, Williams HS, Durham DM, Mahajan D, Buseck A, Tharakan S, Zheng S, Macfie R, Labow D, Cohen NA, Golas BJ, Sarpel U, Hiotis SP. T1 Gastric Cancer Is Associated With a High Incidence of Regional Lymph Node Metastases. J Surg Res. 2023;287:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Onal C, Guler OC, Erpolat P, Hurmuz P, Sutera P, Deek MP, Elmali A, Yilmaz MT, Koken UH, Yavuz M, Ozyigit G, Tran PT. Evaluation of Treatment Outcomes of Prostate Cancer Patients With Lymph Node Metastasis Treated With Definitive Radiotherapy: Comparative Analysis of PSMA PET/CT and Conventional Imaging. Clin Nucl Med. 2024;49:e383-e389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 6. | van der Fels CAM, Leliveld A, Buikema H, van den Heuvel MC, de Jong IJ. VEGF, EGFR and PSMA as possible imaging targets of lymph node metastases of urothelial carcinoma of the bladder. BMC Urol. 2022;22:213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Liro M, Śniadecki M, Wycinka E, Wojtylak S, Brzeziński M, Jastrzębska J, Wydra D. Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Issa PP, Mueller L, Hussein M, Albuck A, Shama M, Toraih E, Kandil E. Radiologist versus Non-Radiologist Detection of Lymph Node Metastasis in Papillary Thyroid Carcinoma by Ultrasound: A Meta-Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Ginat DT, Juloori A, Vivar OI, Farber LA, Gooi Z, Rosenberg AJ. Imaging Features of Intratumoral Injection of NBTXR3 for Head and Neck Squamous Cell Carcinoma Lymph Node Metastases. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Pampena R, Raucci M, Mirra M, Lombardi M, Piana S, Kyrgidis A, Peccerillo F, Paganelli A, Garbarino F, Pellacani G, Longo C. The role of ultrasound examination for early identification of lymph-node metastasis of cutaneous squamous cell carcinoma: results from a single institutional center. Ital J Dermatol Venerol. 2021;156:479-483. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Pierantoni C, Lisotti A, Fusaroli P. Prediction of the Risk of Lymph Node Metastases in Early Gastric Cancer: Contrast-Enhanced Harmonic Endoscopic Ultrasonography May Help. Gut Liver. 2021;15:940-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Arjmandi F, Mootz A, Farr D, Reddy S, Dogan B. New horizons in imaging and surgical assessment of breast cancer lymph node metastasis. Breast Cancer Res Treat. 2021;187:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Wang X, Kou H, He H, Lu M, Zhou L, Wang L. Difference in Perfusion Parameters Between Gastric Cancer and Gastric Stromal Tumors: Evaluation With Oral Contrast Plus Contrast-Enhanced Ultrasonography. Front Oncol. 2020;10:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Wang K, Jiang X, Ren Y, Ma Z, Cheng X, Li F, Xiao J, Yu Z, Jiao Z. The significance of preoperative serum carcinoembryonic antigen levels in the prediction of lymph node metastasis and prognosis in locally advanced gastric cancer: a retrospective analysis. BMC Gastroenterol. 2020;20:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Yu Y, Shi LL, Zhang HW, Wang Q. Performance of contrast-enhanced ultrasound for lymph node metastasis in papillary thyroid carcinoma: a meta-analysis. Endocr Connect. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Geng M, Geng M, Wei R, Chen M. Artificial intelligence neural network analysis and application of CT imaging features to predict lymph node metastasis in non-small cell lung cancer. J Thorac Dis. 2022;14:4384-4394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Lin WW, Zhong Q, Guo J, Yu S, Li K, Shen Q, Zhuo M, Xue E, Lin P, Chen Z. A Preoperative Prediction Model for Lymph Node Metastasis in Patients with Gastric Cancer Using a Machine Learning-based Ultrasomics Approach. Curr Med Imaging. 2024;20:e15734056291074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Zhong Y, Xiao YY, Ye JY, Jian GL, Huang WJ. Diagnostic efficacy of contrast-enhanced gastric ultrasonography in staging gastric cancer: a meta-analysis. BMC Cancer. 2024;24:422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Zhang S, Liu R, Wang Y, Zhang Y, Li M, Wang Y, Wang S, Ma N, Ren J. Ultrasound-Base Radiomics for Discerning Lymph Node Metastasis in Thyroid Cancer: A Systematic Review and Meta-analysis. Acad Radiol. 2024;31:3118-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Wen DY, Chen JM, Tang ZP, Pang JS, Qin Q, Zhang L, He Y, Yang H. Noninvasive prediction of lymph node metastasis in pancreatic cancer using an ultrasound-based clinicoradiomics machine learning model. Biomed Eng Online. 2024;23:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M, Desai AP, Dilling TJ, Dowell J, Durm GA, Gettinger S, Grotz TE, Gubens MA, Juloori A, Lackner RP, Lanuti M, Lin J, Loo BW, Lovly CM, Maldonado F, Massarelli E, Morgensztern D, Mullikin TC, Ng T, Owen D, Owen DH, Patel SP, Patil T, Polanco PM, Riess J, Shapiro TA, Singh AP, Stevenson J, Tam A, Tanvetyanon T, Yanagawa J, Yang SC, Yau E, Gregory KM, Hang L. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22:249-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 207] [Article Influence: 207.0] [Reference Citation Analysis (0)] |
| 22. | Wei J, Zhang Y, Wang Z, Wu X, Zhang J, Bu Z, Ji J. Identification of lymph node metastasis by computed tomography in early gastric cancer. Chin J Cancer Res. 2021;33:671-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Chen Z, Hu D, Ye G, Xu D. Quantitative Evaluation of Extramural Vascular Invasion of Rectal Cancer by Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Contrast Media Mol Imaging. 2022;2022:3038308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |