Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2168
Peer-review started: December 15, 2023
First decision: December 22, 2023
Revised: January 11, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: May 15, 2024
Processing time: 145 Days and 23.7 Hours
Complement components could contribute to the tumor microenvironment and the systemic immune response. Nevertheless, their role in colorectal cancer (CRC) remains a contentious subject.
To elucidate the relationship between complement components and CRC risk and clinical characteristics.
Searches were conducted in PubMed, the Cochrane Library, and the China National Knowledge Infrastructure database until June 1, 2023. We included cohort studies encompassing participants aged ≥ 18 years, investigating the association between complement components and CRC. The studies were of moderate quality or above, as determined by the Agency for Healthcare Research and Quality. The meta-analysis employed fixed-effects or random-effects models based on the I² test, utilizing risk ratio (RR) and their corresponding 95% con
Data from 15 studies, comprising 1631 participants that met the inclusion criteria, were included in the meta-analysis. Our findings indicated that protein levels of cluster of differentiation 46 (CD46) (RR = 3.66, 95%CI: 1.75-7.64, P < 0.001), CD59 (RR = 2.86, 95%CI: 1.36-6.01, P = 0.005), and component 1 (C1) (RR = 5.88, 95%CI: 1.75-19.73, P = 0.004) and serum levels of C3 (standardized mean difference = 1.82, 95%CI: 0.06-3.58, P = 0.040) were significantly elevated in patients with CRC compared to healthy controls. Strong expression of CD55 or CD59 was associated with a higher incidence of lymph node metastasis, whereas strong CD46 expression correlated with a higher incidence of tumor differentiation compared to low CD46 expression (P < 0.05 for all). Although specific pooled results demonstrated notable heterogeneity, subgroup analyses pointed to regional differences as the primary source of inconsistency among the studies.
Our analysis underscores that increased levels of specific complement components are associated with a heightened risk of CRC, emphasizing the potential significance of monitoring elevated complement component levels.
Core Tip: This meta-analysis provides evidence emphasizing that increased levels of specific complement components are associated with a high risk of colorectal cancer (CRC). Compared with the healthy control group, protein levels of cluster of differentiation 46 (CD46), CD59, and component 1 (C1), and serum levels of C3 were significantly elevated in patients with CRC. Significantly, patients with pronounced expression of CD55 or CD59 have a higher incidence of lymph node metastasis. Nevertheless, these findings emphasize the need for methodologically rigorous studies with larger sample sizes to corroborate these preliminary conclusions.
- Citation: Zhu XL, Zhang L, Qi SX. Association of complement components with risk of colorectal cancer: A systematic review and meta-analysis. World J Gastrointest Oncol 2024; 16(5): 2168-2180
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2168.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2168
Colorectal cancer (CRC) is a prevalent and life-threatening malignancy that affects the colon and rectum[1]. It ranks as one of the most common cancers globally, contributing to significant morbidity and mortality rates[2]. The incidence of CRC varies across different regions, with higher rates observed in developed countries[3]. Lifestyle factors such as diet, sedentary behavior, obesity, and smoking correlate with an increased risk of developing CRC[4]. Despite advancements in screening and treatment options, CRC poses significant challenges. Many cases are diagnosed at advanced stages, limiting the effectiveness of curative interventions. Moreover, there is growing recognition of the heterogeneity within CRC, resulting in varying treatment responses and clinical outcomes. Therefore, identifying novel biomarkers and therapeutic targets for CRC is crucial to improve patient prognosis.
The complement system, an essential constituent of the innate immune system, is critical in defending the host against infections and maintaining tissue balance[5]. Activating the complement system initiates a series of events, including releasing various bioactive molecules and recruiting immune cells to inflammation sites[6]. Recent studies suggest that dysregulation of the complement system may contribute to tumorigenesis and cancer progression[7]. The involvement of the complement system has gained considerable attention in the context of CRC. Several complement components, including component 3 (C3), C4, and cluster of differentiation 55 (CD55) and CD59, are dysregulated in colorectal tumors[8]. Specific genetic variations in complement genes have also been linked to an increased susceptibility to CRC. Moreover, altered levels of complement proteins and their activation fragments have been observed in CRC patients, suggesting potential prognostic and diagnostic value. Complement components can promote tumor proliferation, angiogenesis, and invasion by interacting with specific receptors on cancer cells. CD55 serves as a marker of tumor aggression correlated with advanced stages and poor 7-year survival[9]. Additionally, the interaction between complement components and immune cells influences the tumor microenvironment and modulates the anti-tumor immune response[10]. Previous studies indicated that characterizing changes in complement levels and activity could aid in identifying individuals at high risk for CRC development[11]. Furthermore, targeting specific complement components or their associated pathways could provide novel therapeutic strategies for CRC treatment[12]. Therefore, comprehending the role of complement components in CRC pathogenesis holds promising implications for both diagnostic and therapeutic approaches.
Based on the existing scientific literature concerning the role of complement components in CRC, we conducted this systematic review and meta-analysis to explore the association between complement components and CRC risk. Moreover, we also aimed to determine whether specific complement components are associated with lymph node metastasis, differentiation, advanced stage, or distant metastasis. Through a scrupulous examination of published studies, this review contributes to an enriched understanding of the complement system’s role in colorectal carcinogenesis. This, in turn, may lay the groundwork for innovative advancements in CRC diagnosis, prognostic stratification, and therapeutic intervention.
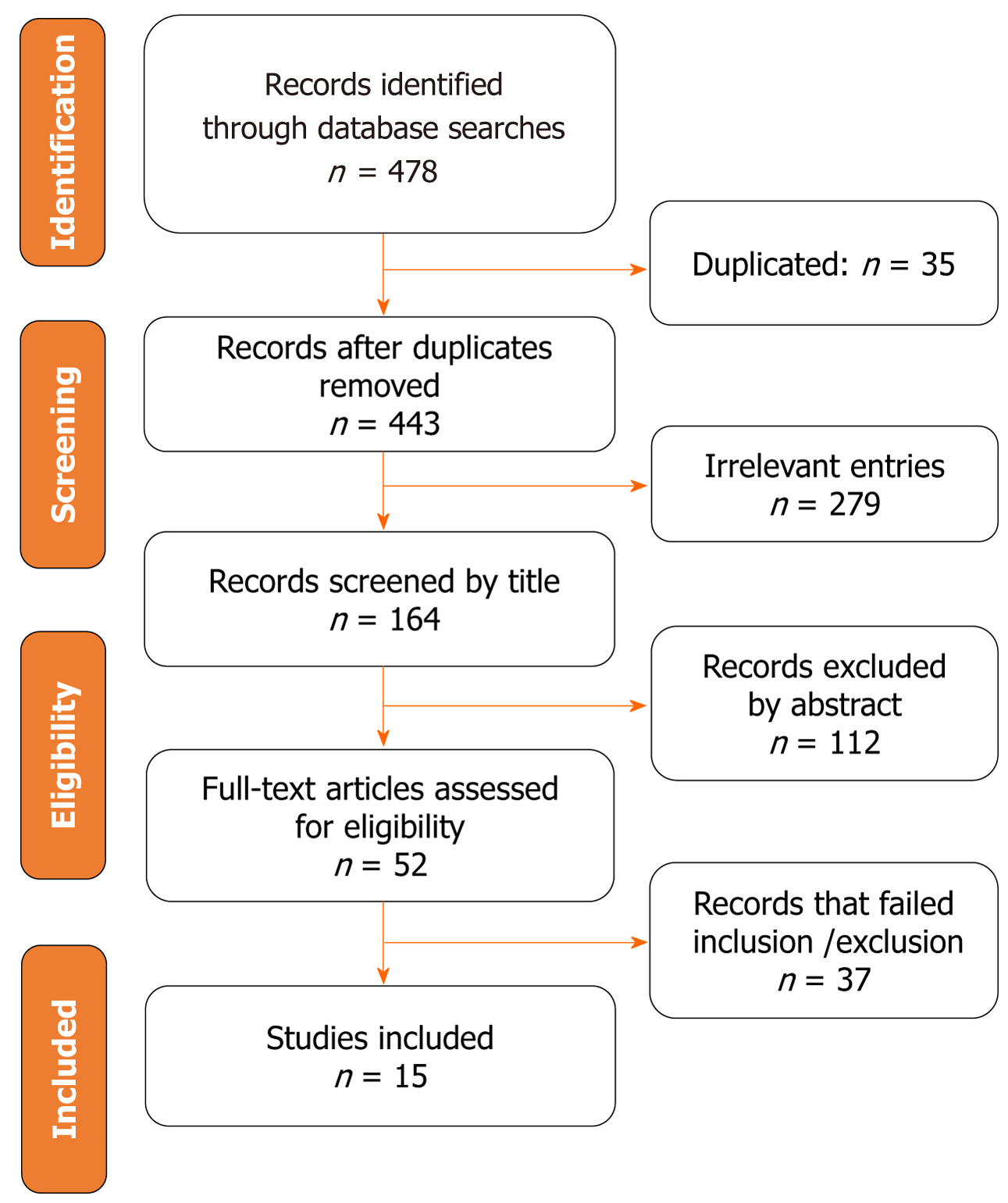
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[13]. The PRISMA statement comprises a 27-item checklist and a four-phase flow diagram (Figure 1). The meta-analysis was registered in PROSPERO (CRD42023444081), ensuring accurate and reliable results. PROSPERO encourages transparency by requiring researchers to provide detailed protocols, allowing others to assess the validity and reliability of the study methods. This transparency also helps find potential biases or conflicts of interest that may impact the findings.
Two investigators (Zhu XL and Qi SX) systematically searched PubMed (MEDLINE), the Cochrane Library, and the China National Knowledge Infrastructure database for relevant published studies until June 01, 2023. No language or country restrictions were applied. Two sets of search terms were used to retrieve potential studies related to complement components and CRC. The title or abstract of each study was screened using the following terms: (1) “Proteins, Complement System” or “Complement Protein” or “Proteins, complement” or “Complement” or “Complement, Hemolytic” or “Hemolytic complement” or “Complement Factors” or “CD59” or “CD55” or “CD46”; and (2) “colon cancer” or “rectal cancer” or “rectum cancer” or “colorectal cancer”. The detailed search strategy is shown in Supplementary Table 1. Additional relevant studies were identified through forward and backward citation tracking. Unpublished literature and conference papers were not incorporated into this study.
In order to conduct a comprehensive analysis, only studies that met the following predefined inclusion criteria were incorporated: (1) Patients with pathologically diagnosed CRC and healthy controls without CRC; (2) Original studies investigating the correlation between complement components and CRC; (3) Cohort studies, including prospective, retrospective, or case-control design; (4) Participants aged ≥ 18 years old; and (5) Studies reporting the measurement of serum or plasma concentrations of various complement proteins, or their expression assessed through immunohistochemistry (IHC) at the primary tumor site and in healthy control samples.
The following criteria were used to exclude studies: (1) Animal or basic studies; (2) Duplicate reports, reviews, editorials, conference abstracts, and case reports; (3) Retracted articles; and (4) Unavailable full-text articles or those with inadequate results or insufficient data.
Two researchers worked independently to extract pertinent data from the included studies, using a standardized form for data extraction. In cases where discrepancies arose, they were resolved through a meticulous re-evaluation, followed by a consultative discussion with a third author. The extracted information encompassed the following aspects: First author’s name, year of publication, country of origin, study design, number of cases and controls, time of the study, age and gender of participants, the positivity of complement components as determined by IHC, the cutoff value for categorizing high vs low expression of complement components through IHC, and the mean and SD of serum complement components. If a particular study did not include the SD value in the complete text, it was computed using the 95% confidence interval (CI) and the sample size per the guidelines delineated in the Cochrane Handbook[14]. Efforts were also made to contact the corresponding authors of the included studies to obtain any unclear or missing data.
To enhance the research credibility, we employed the Agency for Healthcare Research and Quality (AHRQ) to assess the included studies based on eleven criteria[15]. The articles underwent quality assessment based on the following scale: Studies scoring between 0 and 3 points were classified as having low quality. Studies with scores ranging from 4 to 7 points were categorized as having medium quality. Any study scoring 8 or more points was considered high quality (Supplementary Table 2). Our inclusion criteria only encompassed studies of medium quality or higher.
Statistical analyses were conducted per the recommendations of the Cochrane Collaboration. The risk ratio (RR) and its corresponding 95%CI were used to measure the difference in positive expression of complements between CRC patients and control samples, representing dichotomous data. For studies using different scales to measure the same outcome, the standardized mean difference (SMD) was used[14]. Heterogeneity among studies was assessed using the I² test, with values ≤ 25%, 25%-50%, 50%-75%, and > 75% indicating low, moderate, substantial, and considerable heterogeneity, respectively. A fixed-effects model was employed if no statistical heterogeneity was found (P > 0.1, I² < 50%); otherwise, a random-effects model was used. Sensitivity analysis involved systematically excluding one study at a time to identify the source of heterogeneity, assess the stability of the pooled results, and evaluate the impact of removing certain studies on the overall findings. Subgroup analysis was also performed to identify the source of heterogeneity based on region (Asian and non-Asian). Funnel plots and Egger’s test were conducted to evaluate the potential publication bias. Funnel plots provide a visual representation of study precision against effect size, while Egger’s test quantitatively evaluates asymmetry in the funnel plot. The Review Manager Version 5.3 software (The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen) was used to conduct all statistical analyses. Statistical significance was defined as a two-tailed P-value < 0.05.
Following an exhaustive search of the literature across three public databases, we identified 478 studies. Within this pool, 35 duplicates and 279 unrelated studies were found. After removing these duplicates and irrelevant articles, we meticulously reviewed the remaining articles by examining their titles and abstracts, excluding 149 more articles. This excluded set included 37 articles further assessed through a full-text review. Our meta-analysis incorporated 15 carefully selected studies. All studies have received ethical approvals. Figure 1 shows a detailed and comprehensive understanding of the selection procedure and its corresponding outcomes, providing an illustrative breakdown of the process.
The primary characteristics of the included studies are presented in Supplementary Table 3. Our meta-analysis comprised 15 identified studies[16-30] involving 1631 participants. These studies were published between 1993 and 2022 and were conducted in seven countries, namely, China, Denmark, England, Germany, the United States, Iran, and Ireland. The number of participants varied from 20 to 260 across the studies, with subjects’ mean age ranging from 47.5 to 71 years old. The quality scores of all the included studies ranged from 5 to 9 points, indicating above-average quality (Supplementary Table 2).
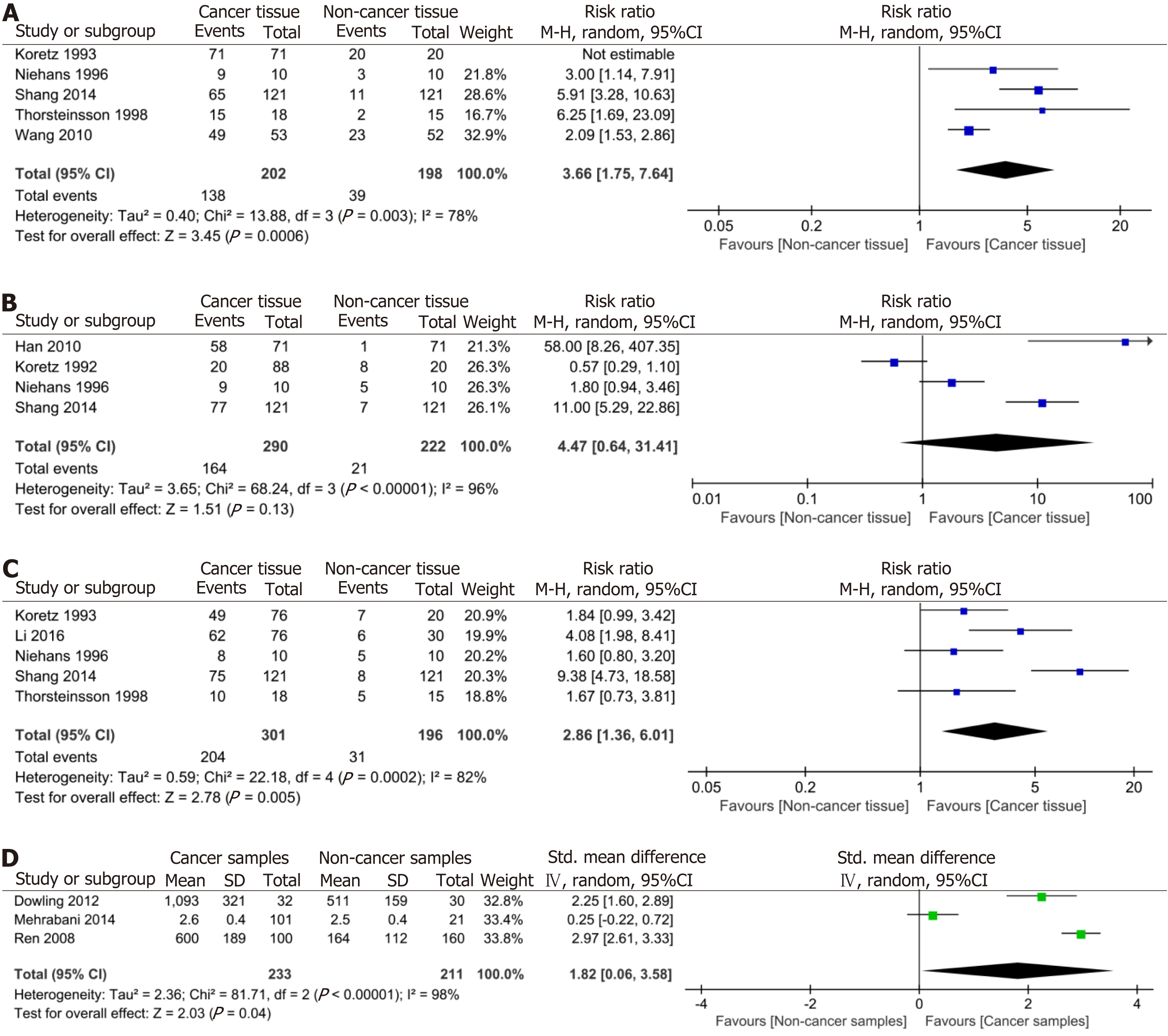
Our meta-analysis considered 11 studies with a sample size of 1167 that provided data on complement component proteins measured by IHC in CRC patients and control groups (Supplementary Table 3). Among these studies, five were suitable for quantitative assessment of the association between CD46 and CRC (Figure 2A). The results revealed that CD46 positivity in the CRC population did not exceed that in the control population (pooled RR = 2.93, 95%CI: 0.36-23.85, P = 0.310, I² = 99%, P for heterogeneity < 0.001).
Regarding CD55, four studies provided appropriate data for quantitative assessment of its association with CRC (Figure 2B). Our results demonstrated that CD55 positivity in CRC patients did not surpass that in the control group (pooled RR = 4.47, 95%CI: 0.64-31.41, P = 0.130, I² = 96%, P for heterogeneity < 0.001). For CD59, five studies provided relevant data to quantitatively assess its relationship with CRC (Figure 2C). The meta-analysis results indicated significantly higher CD59 positivity in patients with CRC compared to the control group (pooled RR = 2.86, 95%CI: 1.36-6.01, P = 0.005, I² = 82%, P for heterogeneity < 0.001). Moreover, two studies yielded relevant data for the quantitative evaluation of the association between C1 and CRC (Figure 2D). The result revealed that C1 positivity in patients with CRC exceeded that in the control group (pooled RR = 5.88, 95%CI: 1.75-19.73, P = 0.004, I² = 90%, P for heterogeneity < 0.001).
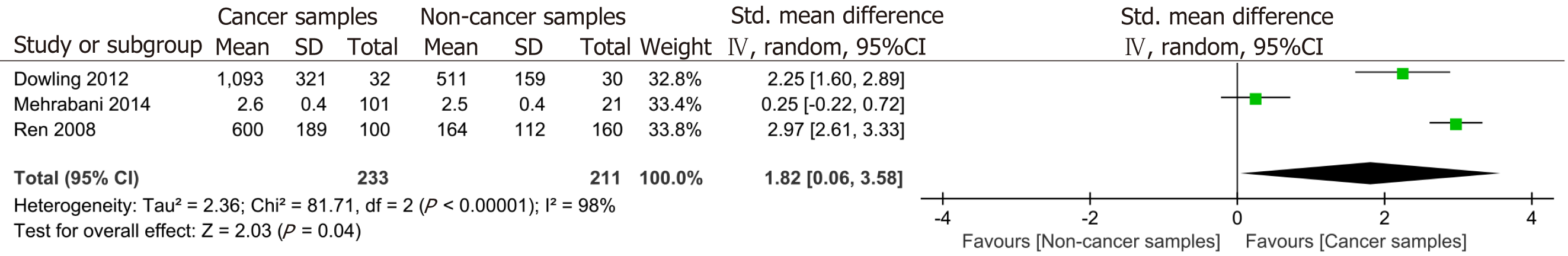
Three studies yielded relevant data for the quantitative evaluation of the association between serum C3 levels and CRC, exhibiting significant heterogeneity (I² = 98%, P < 0.001) (Figure 3). Considering the varying sensitivities of the three studies to detect serum levels of C3, we employed SMD and 95%CI for systematic analysis. The result indicated that serum C3 levels were significantly higher in the CRC population compared to the control population (pooled RR = 1.82, 95%CI: 0.06-3.58, P = 0.040, I² = 98%, P for heterogeneity < 0.001) (Figure 3). In addition, one study reported that the levels of alternative pathway and classical pathway-mediated C3 activation of the complement system in plasma of patients with CRC was comparable to those of healthy individuals[22].
In our analysis, we divided CRC patients into two groups based on the expression levels of complement proteins (high expression and low expression) according to the cutoff chosen by the original authors of the studies and examined the differences in clinical characteristics between these groups (Table 1). Two studies provided relevant data to assess the relationship between high and low expression of CD55 in CRC patients above 60 years old. The meta-analysis indicated no significant difference between the group with high CD55 expression and the group with low CD55 expression (pooled RR = 1.16, 95%CI: 0.92-1.45, P = 0.210, I² = 0%, P for heterogeneity = 0.580). Three studies yielded relevant data for the quantitative evaluation of the association between high and low expression of CD55 in CRC patients based on gender, lymph node metastasis, differentiation, T3 + T4 stage, or distant metastasis. The meta-analysis revealed that CRC patients with strong CD55 expression had a higher incidence of lymph node metastasis compared to those with low CD55 expression (pooled RR = 1.30, 95%CI: 1.03-1.63, P = 0.030, I² = 0%, P for heterogeneity = 0.700). However, no significant difference was found for other clinical characteristics (P > 0.05 for all).
| Clinicopathological feature | Reference | No. of studies | Model | Pooled RR (95%CI) | P value | Heterogeneity | |
| I2 (%) | P value | ||||||
| CD55 | |||||||
| Age (> 60 years old) | High vs low expression | 2 | Fixed | 1.16 (0.92-1.45) | 0.21 | 0 | 0.58 |
| Sex (male) | High vs low expression | 3 | Fixed | 1.09 (0.90-1.33) | 0.36 | 0 | 0.51 |
| Lymph node metastasis | High vs low expression | 3 | Fixed | 1.30 (1.03-1.63) | 0.03 | 0 | 0.70 |
| Differentiation | High vs low expression | 3 | Fixed | 1.09 (0.93-1.29) | 0.28 | 0 | 0.95 |
| T3 + T4 | High vs low expression | 3 | Fixed | 1.11 (0.99-1.24) | 0.08 | 0 | 0.95 |
| M1 | High vs low expression | 3 | Random | 1.52 (0.43-5.43) | 0.52 | 86 | < 0.001 |
| CD59 | |||||||
| Age (> 60 years old) | High vs low expression | 2 | Fixed | 0.88 (0.66-1.15) | 0.34 | 0 | 0.66 |
| Sex (male) | High vs low expression | 2 | Fixed | 0.85 (0.68-1.06) | 0.14 | 0 | 0.81 |
| Lymph node metastasis | High vs low expression | 2 | Fixed | 1.53 (1.12-2.08) | 0.007 | 53 | 0.14 |
| CD46 | |||||||
| Age (> 60 years old) | High vs low expression | 2 | Fixed | 1.15 (0.86-1.52) | 0.35 | 0 | 0.98 |
| Sex (male) | High vs low expression | 2 | Fixed | 0.87 (0.68-1.10) | 0.24 | 0 | 0.63 |
| Lymph node metastasis | High vs low expression | 2 | Random | 0.81 (0.39-1.66) | 0.56 | 83 | 0.01 |
| Differentiation | High vs low expression | 2 | Fixed | 1.30 (1.04-1.63) | 0.02 | 0 | 0.69 |
| T3 + T4 | High vs low expression | 2 | Random | 0.75 (0.35-1.60) | 0.46 | 80 | 0.03 |
Two studies provided relevant data to assess the relationship between high and low expression of CD59 in CRC patients above 60 years old, male patients, and those with lymph node metastasis (Table 1). The meta-analysis also indicated that CRC patients with strong CD59 expression had a higher incidence of lymph node metastasis than CRC patients with low CD59 expression (pooled RR = 1.53, 95%CI: 1.12-2.08, P = 0.007, I² = 53%, P for heterogeneity = 0.140). Three studies yielded relevant data for the quantitative evaluation of the association between high and low expression of CD46 in CRC patients above 60 years old, male patients, and those with lymph node metastasis, tumor differentiation, or T3 + T4 stage. The meta-analysis indicated that CRC patients with strong CD46 expression had a higher incidence of tumor differentiation than CRC patients with low CD46 expression (pooled RR = 1.30, 95%CI: 1.04-1.63, P = 0.020, I² = 0%, P for heterogeneity = 0.690). However, no significant difference was found for other clinical characteristics (P > 0.05 for all).
In our subgroup analysis, we aimed to identify the sources of heterogeneity in the association between the positive expression of complement component proteins and CRC based on region (Asian and non-Asian). Table 2 presents the summarized quantitative data for these subgroups. For CD46, a high level of heterogeneity was observed in both the Asian population (I² = 92%, P < 0.001) and the non-Asian population (I² = 88%, P = 0.003). Two studies from the Asian population and two from the non-Asian population were included in the analysis. These results suggested a higher proportion of CRC patients expressing CD46 in Asian (pooled RR = 3.43, 95%CI: 1.08-10.86, P = 0.040) and non-Asian populations (pooled RR = 4.37, 95%CI: 1.95-9.78, P < 0.001). Similar results were observed regarding CD59 (Asian population, pooled RR = 6.63, 95%CI: 4.06-10.82, P < 0.001; non-Asian population, pooled RR = 1.97, 95%CI: 1.22-3.18, P = 0.006). For CD55, two studies from the Asian population and two from the non-Asian population were included. The results indicated that more Asian CRC patients express CD55 (pooled RR = 16.88, 95%CI: 8.49-33.52, P < 0.001), while the association was not significant in the non-Asian population (pooled RR = 1.49, 95%CI: 0.19-11.97, P = 0.710).
| Subgroup | Study number | Risk ratio (95%CI) | I2 | P value |
| CD55 | ||||
| Asian | 2 | 16.88 (8.49-33.52) | 65% | < 0.001 |
| Non-Asian | 2 | 1.49 (0.19-11.97) | 88% | 0.71 |
| CD59 | ||||
| Asian | 2 | 6.63 (4.06-10.82) | 63% | < 0.001 |
| Non-Asian | 3 | 1.97 (1.22-3.18) | 0% | 0.006 |
| CD46 | ||||
| Asian | 2 | 3.66 (1.75-7.64) | 92% | 0.040 |
| Non-Asian | 2 | 4.37 (1.95-9.78) | 0% | < 0.001 |
After excluding individual studies one by one, we performed a sensitivity analysis to assess the stability and robustness of the pooled RR. After excluding the study by Koretz et al[17], which reported broadly positive expression of CD46 in both CRC tissues and controls, the results indicated a significant increase in CD46 positivity in the CRC population compared to the control population (pooled RR = 3.66, 95%CI: 1.75-7.64, P < 0.001). When the study by Koretz et al[19] was removed, the results showed that CD55 positivity in patients with CRC remained significantly higher than that in the control group (pooled RR = 9.21, 95%CI: 1.07-79.05, P = 0.040). However, high heterogeneity persisted with an I² value of 94% and a significant P-value for heterogeneity of < 0.001. Similarly, when the study by Shang et al[16] was excluded, the results indicated that CD59 positivity in patients with CRC remained significantly higher than that in the control group (pooled RR = 2.11, 95%CI: 1.35-3.32, P = 0.001). Notably, no significant heterogeneity was observed in this case, as indicated by an I² value of 38% and a non-significant P-value for heterogeneity of 0.180.
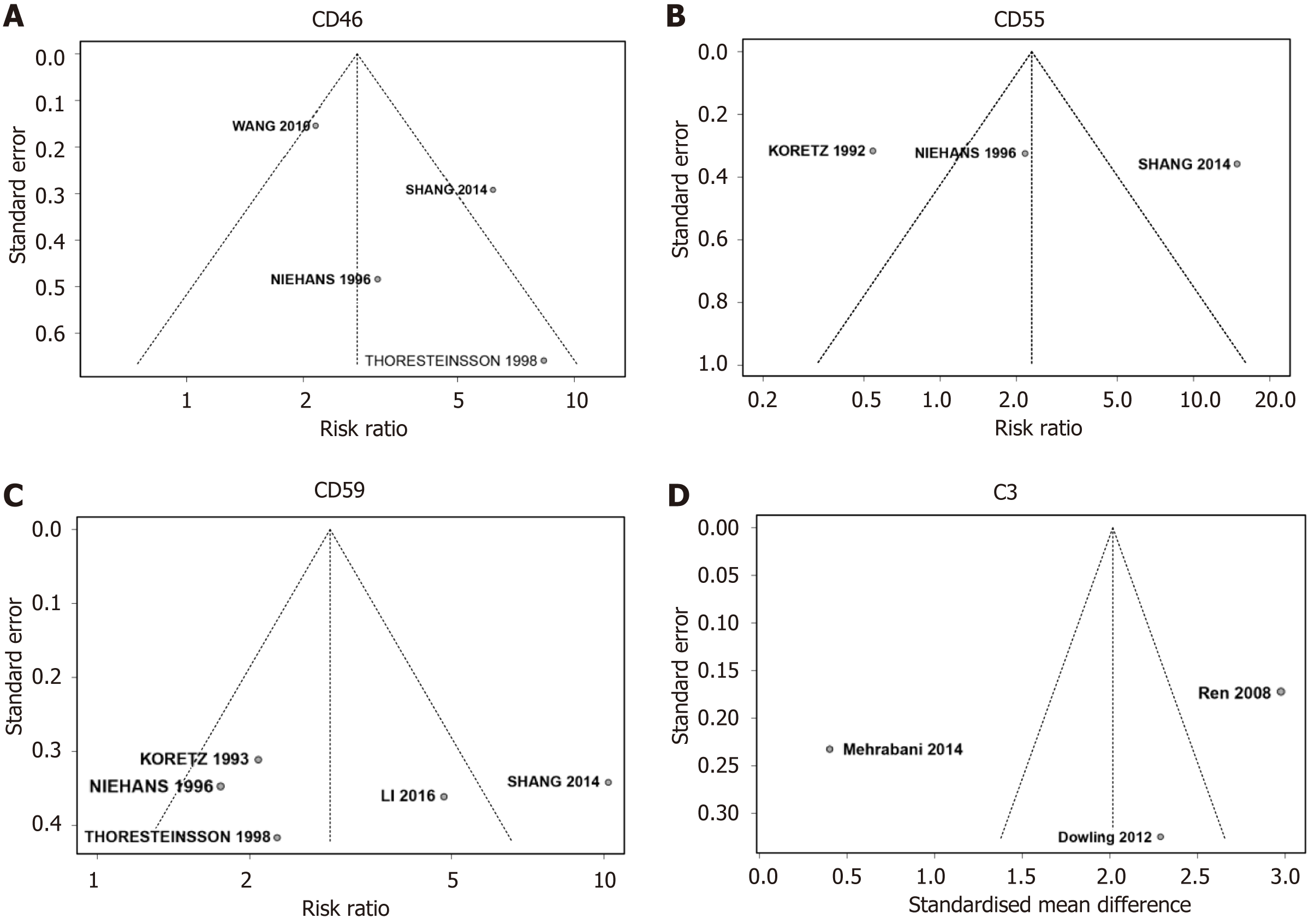
We assessed a select number of studies, uncovering considerable variability in the sample sizes across these studies. In response to this observation, we employed a funnel plot and Egger’s test to examine potential publication bias (Table 3). An asymmetrical funnel plot (Figure 4) hinted at publication bias in the relationship between specific complement components (CD46, CD55, CD59, and C3) and CRC risk. To substantiate this observation, we applied Egger’s test to four studies that evaluated CD46 and CD55 and to another five studies assessing CD59, as delineated in Table 3. Contrary to the implications of the funnel plot, the outcomes of Egger’s test did not yield evidence of statistically significant publication bias concerning the investigated complement components.
| Studies | Egger’s test | |||
| t | Bias | P value | ||
| CD46 | 4 | 1.34 | 2.34 | 0.311 |
| CD55 | 4 | 1.02 | 6.15 | 0.414 |
| CD59 | 5 | -0.09 | -1.15 | 0.931 |
| C3 | 3 | -0.44 | -9.27 | 0.734 |
CRC represents a complex disease characterized by intricate interplays among genetic, environmental, and immunological factors; approximately half of all patients may experience recurrence[31]. Within the diverse components of the immune system, the complement system has recently been recognized as a critical player, modulating the tumor microenvironment and consequently influencing CRC outcomes. Current evidence highlights that dysregulation of complement components plays a substantial role in both CRC initiation and progression[32]. Given the discordant findings in previous studies, we undertook a systematic review and meta-analysis to rigorously evaluate the association between complement components and the risk of CRC.
Upon analyzing the 12 included studies, it becomes clear that patients diagnosed with CRC consistently exhibit elevated levels of specific complement components (CD46, CD55, CD59, C3, and C1) compared to the healthy control group. Of particular significance is the observation that patients with pronounced expression of CD55 or CD59 manifest a higher incidence of lymph node metastasis. Likewise, those with robust CD46 expression tend to show a greater propensity for tumor differentiation than those with diminished CD46 expression. Although some pooled results reveal significant heterogeneity, further subgroup analyses propose that regional differences are likely the primary contributors to this variability among the studies. Notably, this meta-analysis represents the first study found within our limited search scope that explores the relationship between the complement system and the risk of CRC. This highlights the lack of knowledge regarding the role of the complement system in the progression of CRC and underscores the absence of a unified consensus.
The complement system comprises over 30 proteins, including soluble factors, cell surface receptors, and regulatory molecules[33]. It can be activated through three major pathways: Classical, lectin, and alternative pathways. Activation of the complement system leads to a series of proteolytic events, generating various bioactive fragments that mediate immune responses[34]. The complement system is a crucial link between innate and adaptive immunity, performing essential functions in the defense against pathogens, removing apoptotic cells, and preserving tissue homeostasis[35]. Several studies have reported alterations in complement component expression and activity in CRC. Upregulation of complement proteins, such as C3a, C5a, CD46, CD55, and CD59, has been observed in tumor tissues and correlated with disease stage and prognosis[11]. Dysregulated complement activation can promote tumor growth through various mechanisms, including enhancing angiogenesis, stimulating inflammation, and suppressing anti-tumor immune responses. Additionally, the complement system influences the ability of CRC cells to evade immune surveillance. Cancer cells can exploit complement regulatory proteins, such as CD46, CD55, and CD59, to protect themselves from complement-mediated lysis[32]. Moreover, complement activation can release immunosuppressive molecules, such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor, creating an immunosuppressive microenvironment that favors tumor progression[36]. These findings underscore the significance of the complement system in tumor immunoregulation, particularly in CRC.
The association between the complement system and tumor dissemination has been extensively studied at the pathological and serum levels. CD55 and CD59 are positively expressed in various cancers, including gallbladder carcinomas[37], pancreatic cancers[38], breast cancer[39], and CRC[40], and were significantly linked to lymph node metastasis, histological grade, vascular invasion, and clinical stage[21]. Mechanistically, the anaphylatoxins C3a and C5a, generated upon complement activation, play a crucial role in recruiting immune cells to the tumor microenvironment and lymph nodes, promoting inflammation and angiogenesis[36]. In the context of CRC, the signaling pathway involving the interaction between C5a and its receptor C5aR can potentially activate the nuclear factor-kappaB pathway and the transcription factor AP-1. This activation may facilitate the production of matrix metalloproteinases (MMP)-1 and MMP-9, which play a crucial role in degrading the extracellular matrix[41]. Furthermore, tumor cells can produce C5a, leading to an increased release of interleukin-10, TGF-β1, and monocyte chemoattractant protein-1. As a result, this process enhances the metastasis of CRC[36]. These processes are vital for establishing a pre-metastatic niche and facilitating tumor cell extravasation into lymph nodes. Complement components opsonize tumor cells, aiding their recognition and engulfment by phagocytes within lymph nodes[12]. This process is a critical defense mechanism against metastasis and eliminating circulating tumor cells. Complement activation products can regulate the balance between pro- and anti-inflammatory immune responses within lymph nodes[42]. Dysregulation of this delicate equilibrium may favor conditions that promote tumor cell survival, migration, and metastasis. In CRC, tumor cells generate the C3 component, which subsequently governs the reaction of macrophages and their anti-tumor immune response through the C3a-C3aR axis and PI3Kγ signaling pathway[12]. Furthermore, the DAF/CD55 has been recognized as a prospective biomarker for an unfavorable prognosis in colon cancer patients, as tumor cells expressing CD55 exhibit high resistance to complement-dependent cytotoxicity[11]. According to our study, CRC patients with strong CD55 or CD59 expression had a higher incidence of lymph node metastasis. The interplay between lymph node metastasis and complement activation represents a complex network of interactions with profound implications for cancer progression.
Recent research has indicated that the activation of complement plays a role in promoting cancer. Complement inhibitors, such as C5aR and C3aR blockers, could have therapeutic potential in treating various types of cancer[43]. Due to the known involvement of the C5a/C5aR1 signaling axis in immune infiltration within the CRC tumor microenvironment, several studies have explored the impact of complement C5 deficiency, particularly C5ar1, on CRC tumor development and found that it can ultimately prevent tumor occurrence[44,45]. Additionally, other C5aR antagonists, including PMX53, have effectively reduced tumor size in mice and enhanced the efficacy of anticancer chemotherapy[46]. Notably, modulation occurs when targeting the receptor C5aR rather than components C3 or C5, thereby protecting cancer patients from the risk of bacterial infections[44]. One exciting application of complement-targeted therapy in CRC treatment is its combination with immune checkpoint inhibitors without increasing myelosuppression, a well-known side effect of chemotherapy[32]. For instance, the combination of programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) antibodies with blockade of the C5a-C5aR signaling pathway significantly enhances the effectiveness of PD-1/PD-L1 antibodies in combating colon cancer and melanoma[47]. This paves the way for future clinical trials. To date, only one phase I clinical trial called STELLAR-001 (NCT03665129, https://clinicaltrials.gov/ct2/show/NCT03665129) has focused on investigating IPH5401 (anti-C5aR) in combination with durvalumab (anti-PD-1) for treating advanced solid tumors. Therefore, it further emphasizes the need for a comprehensive understanding of the relationship between the complement system and CRC to explore the risks and benefits of combining complement-targeted therapy with other anticancer drugs for treating CRC.
Subgroup analysis indicated that regional disparities may serve as heterogeneous sources of association between complement and CRC risk. One possible explanation is that different regions exhibit variations in lifestyle, genetics, environment, and dietary habits, contributing to differences in complement component levels and risk for CRC[4]. For instance, Asian regions may experience higher levels of economic stress and fewer opportunities for physical exercise. Notably, diet plays a significant role in the incidence of CRC[48]. Western diet, characterized by processed and red meats rather than fiber-rich grains, vegetables, and fruits, often represents a pro-inflammatory diet and is associated with an increased risk of CRC[49]. Previous studies have found that each additional point on the pro-inflammatory diet scale is associated with elevated C3 levels[50].
The strength of this study is that we determined the quality of the evidence for outcomes using the AHRQ approach and provided detailed results for all eligible studies. In addition, our research is the first meta-analysis and comprehensive investigation of the relationship between complement and CRC risk. Importantly, sensitivity analysis indicated that the pooled results were robust. These findings are vital and timely to help clinicians provide valuable insights into the role of complement in CRC patients.
Nevertheless, it is imperative to recognize certain limitations in this study. First, including solely retrospective cross-sectional or case-control studies may render the findings susceptible to selection bias. It may prevent us from establishing a temporal relationship between complement and CRC risk. Due to the lack of prospective studies, the identified associations between these complement components and the occurrence and development of CRC cannot be interpreted as causal relationships. Second, the constraints of a small sample size may increase the risk of non-representative data of the entire population, resulting in overestimating effects and amplifying systematic biases. Despite the absence of significant publication bias and the robustness of the sensitivity analysis, it must be noted that the association of complement components with CRC was examined in only two to five studies. Third, significant heterogeneity persisted across the analyses, a pattern that remained even after conducting subgroup analysis based on region. Lastly, the absence of a uniform cutoff value for delineating high or low complement density in CRC adds complexity. Collectively, these factors underscore the necessity for well-designed, higher-quality prospective studies in large sample sizes and more ethnic groups to validate and fortify the conclusions drawn from this research. For individuals at a higher risk of colorectal adenoma, a more comprehensive assessment of various complement components can be conducted, including IHC and serum testing. Subsequently, follow-up assessments can be performed to understand the ultimate progression of complement levels in CRC patients, exploring their association with clinical characteristics and prognosis.
The results from this systematic review and meta-analysis indicate that specific complement components, including CD46, CD55, CD59, C1, and C3, are associated with an elevated risk of CRC. Moreover, these components may hold promising potential as biomarkers for both clinical diagnosis and targeted treatment of CRC. These findings emphasize the need for methodologically rigorous studies with larger sample sizes to corroborate these preliminary conclusions. Such research efforts would validate these initial findings and contribute to an in-depth understanding of the multifaceted role of the complement system in the pathogenesis of CRC.
This meta-analysis focuses on the role of complement components in colorectal cancer (CRC). The contentious nature of this topic underscores the need for further investigation to elucidate the relationship between complement components and CRC risk and clinical characteristics.
Understanding the association between complement components and CRC is crucial for advancing our knowledge in this field. These findings will have significant implications for future research and potential therapeutic strategies.
This study aimed to determine the relationship between complement components and CRC risk and to analyze the clinical characteristics associated with these components. By achieving these objectives, the study aimed to contribute to future research by providing a comprehensive understanding of the role of complement components in CRC.
This meta-analysis utilized a systematic search approach in databases such as PubMed, the Cochrane Library, and the China National Knowledge Infrastructure database. Cohort studies meeting the inclusion criteria were analyzed using fixed-effects or random-effects models based on the I² test. Risk ratio and 95% confidence interval were calculated. Sensitivity and subgroup analyses were conducted to assess the robustness of the results and identify sources of heterogeneity.
The analysis included data from 15 studies comprising 1631 participants. Elevated protein levels of cluster of differentiation 46 (CD46), CD59, and component 1 (C1) and serum levels of C3 were significantly associated with CRC compared to healthy controls. Strong expression of CD55 or CD59 was linked to a higher incidence of lymph node metastasis, while strong CD46 expression correlated with a higher incidence of tumor differentiation. Notable, heterogeneity among the results was observed, primarily attributed to regional differences.
This study proposes that increased levels of specific complement components, such as CD46, CD59, C1, and C3, are associated with an elevated risk of CRC. The findings emphasize the potential significance of monitoring elevated complement component levels in CRC patients. The study does not propose new theories or methods but consolidates existing evidence to draw meaningful conclusions.
Future research in this field should focus on further investigating the regional differences observed in complement component levels and their association with CRC. Additional studies could explore the underlying mechanisms through which these components contribute to CRC development and progression. Moreover, it would be valuable to investigate the potential of targeting complement components as a therapeutic approach for CRC treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nooripour R, Iran S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1033] [Article Influence: 206.6] [Reference Citation Analysis (0)] |
| 3. | Dharwadkar P, Zaki TA, Murphy CC. Colorectal Cancer in Younger Adults. Hematol Oncol Clin North Am. 2022;36:449-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1586] [Article Influence: 264.3] [Reference Citation Analysis (2)] |
| 5. | Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2932] [Cited by in RCA: 2750] [Article Influence: 183.3] [Reference Citation Analysis (0)] |
| 6. | Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 1128] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 7. | O'Brien RM, Cannon A, Reynolds JV, Lysaght J, Lynam-Lennon N. Complement in Tumourigenesis and the Response to Cancer Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense. Immunol Rev. 2016;274:33-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 9. | Durrant LG, Chapman MA, Buckley DJ, Spendlove I, Robins RA, Armitage NC. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol Immunother. 2003;52:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32:433-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 11. | Bao D, Zhang C, Li L, Wang H, Li Q, Ni L, Lin Y, Huang R, Yang Z, Zhang Y, Hu Y. Integrative Analysis of Complement System to Prognosis and Immune Infiltrating in Colon Cancer and Gastric Cancer. Front Oncol. 2020;10:553297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Zha H, Wang X, Zhu Y, Chen D, Han X, Yang F, Gao J, Hu C, Shu C, Feng Y, Tan Y, Zhang J, Li Y, Wan YY, Guo B, Zhu B. Intracellular Activation of Complement C3 Leads to PD-L1 Antibody Treatment Resistance by Modulating Tumor-Associated Macrophages. Cancer Immunol Res. 2019;7:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15891] [Article Influence: 1589.1] [Reference Citation Analysis (1)] |
| 14. | Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions, Second Edition. United States: Wiley, 2019. |
| 15. | Guise JM, Chang C, Viswanathan M, Glick S, Treadwell J, Umscheid CA, Whitlock E, Fu R, Berliner E, Paynter R, Anderson J, Motu'apuaka P, Trikalinos T. Agency for Healthcare Research and Quality Evidence-based Practice Center methods for systematically reviewing complex multicomponent health care interventions. J Clin Epidemiol. 2014;67:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Shang Y, Chai N, Gu Y, Ding L, Yang Y, Zhou J, Ren G, Hao X, Fan D, Wu K, Nie Y. Systematic immunohistochemical analysis of the expression of CD46, CD55, and CD59 in colon cancer. Arch Pathol Lab Med. 2014;138:910-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Koretz K, Brüderlein S, Henne C, Möller P. Expression of CD59, a complement regulator protein and a second ligand of the CD2 molecule, and CD46 in normal and neoplastic colorectal epithelium. Br J Cancer. 1993;68:926-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Thorsteinsson L, O'Dowd GM, Harrington PM, Johnson PM. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Koretz K, Brüderlein S, Henne C, Möller P. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer. 1992;66:810-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996;149:129-142. [PubMed] |
| 21. | Han SL, Xu C, Wu XL, Li JL, Liu Z, Zeng QQ. The impact of expressions of CD97 and its ligand CD55 at the invasion front on prognosis of rectal adenocarcinoma. Int J Colorectal Dis. 2010;25:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zimmermann-Nielsen E, Baatrup G, Thorlacius-Ussing O, Agnholt J, Svehag SE. Complement activation mediated by mannan-binding lectin in plasma from healthy individuals and from patients with SLE, Crohn's disease and colorectal cancer. Suppressed activation by SLE plasma. Scand J Immunol. 2002;55:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Dowling P, Clarke C, Hennessy K, Torralbo-Lopez B, Ballot J, Crown J, Kiernan I, O'Byrne KJ, Kennedy MJ, Lynch V, Clynes M. Analysis of acute-phase proteins, AHSG, C3, CLI, HP and SAA, reveals distinctive expression patterns associated with breast, colorectal and lung cancer. Int J Cancer. 2012;131:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Mehrabani D, Shamsdin SA, Dehghan A, Safarpour A. Clinical significance of serum vascular endothelial growth factor and complement 3a levels in patients with colorectal cancer in southern Iran. Asian Pac J Cancer Prev. 2014;15:9713-9717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Deng H, Chen Y, Liu Y, Liu L, Xu R. Complement C1QC as a potential prognostic marker and therapeutic target in colon carcinoma based on single-cell RNA sequencing and immunohistochemical analysis. Bosn J Basic Med Sci. 2022;22:912-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Cui Y, Zhou Y, Zhang Y, Liang B, Shen Z, Jiang K, Yang X, Yin M, Ye Y. [Expression and clinical significance of CD55 in patients with stage Ⅲ~Ⅳ colon carcinoma]. Chinese Journal of Practical Surgery. 2022;42. [DOI] [Full Text] |
| 27. | Li Z, Jiang Z, Hao Z, An J, Gao M, Zhai J, Yang W. [Expression and clinical significance of CD59 in rectal carcinoma]. Chinese Journal of Current Advances in General Surgery. 2016;217-220. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Gou J, Jiang X, Qu H, Cui Y. [Complement C1 q/tumor necrosis factor-related protein 6 expression in colon cancer]. Chin J Gastroenterol Hepatol. 2019;28:313-316. [DOI] [Full Text] |
| 29. | Zhang J, Cai J, Cui Y, Jiang S, Wei J, Kim YC, Chan J, Thalakola A, Le T, Xu L, Wang L, Jiang K, Wang X, Wang H, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Corrigendum to Zhang J, Cai J, Cui Y, Jiang S, Wei J, Kim YC, Chan J, Thalakola A, Le T, Xu L, Wang L, Jiang K, Wang X, Wang H, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. "Role of the macula densa sodium glucose cotransporter type 1-neuronal nitric oxide synthase-tubuloglomerular feedback pathway in diabetic hyperfiltration." Kidney Int. 2022;101:541-550. Kidney Int. 2022;102:448. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Ren R, Gong C. [The value of serum complement C3a in the diagnosis of colon cancer]. Pract Oncol J. 2008;. [DOI] [Full Text] |
| 31. | Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M; Lateral Node Study Consortium. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 370] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 32. | Talaat IM, Elemam NM, Saber-Ayad M. Complement System: An Immunotherapy Target in Colorectal Cancer. Front Immunol. 2022;13:810993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34:2735-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 34. | Pouw RB, Ricklin D. Tipping the balance: intricate roles of the complement system in disease and therapy. Semin Immunopathol. 2021;43:757-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 35. | Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 929] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 36. | Piao C, Cai L, Qiu S, Jia L, Song W, Du J. Complement 5a Enhances Hepatic Metastases of Colon Cancer via Monocyte Chemoattractant Protein-1-mediated Inflammatory Cell Infiltration. J Biol Chem. 2015;290:10667-10676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Wu J, Lei L, Wang S, Gu D, Zhang J. Immunohistochemical expression and prognostic value of CD97 and its ligand CD55 in primary gallbladder carcinoma. J Biomed Biotechnol. 2012;2012:587672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | He Z, Wu H, Jiao Y, Zheng J. Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol Lett. 2015;9:793-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Yang YJ, Wang Z, Liao J, Liu M, Zhong XR, Zheng H, Wang YP. CD55 and CD59 expression protects HER2-overexpressing breast cancer cells from trastuzumab-induced complement-dependent cytotoxicity. Oncol Lett. 2017;14:2961-2969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Dash S, Wu CC, Chiang SF, Lu YT, Yeh CY, You JF, Chu LJ, Yeh TS, Yu JS. Extracellular Vesicle Membrane Protein Profiling and Targeted Mass Spectrometry Unveil CD59 and Tetraspanin 9 as Novel Plasma Biomarkers for Detection of Colorectal Cancer. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 41. | Kochanek DM, Ghouse SM, Karbowniczek MM, Markiewski MM. Complementing Cancer Metastasis. Front Immunol. 2018;9:1629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Read BJ, Won L, Kraft JC, Sappington I, Aung A, Wu S, Bals J, Chen C, Lee KK, Lingwood D, King NP, Irvine DJ. Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle immunogens. Cell Rep. 2022;38:110217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 44. | Ding P, Li L, Lv X, Zhou D, Wang Q, Chen J, Yang C, Xu E, Dai W, Zhang X, Wang N, Zhang W, Zhang L, Zhou Y, Gu H, Lei Q, Zhou X, Hu W. C5aR1 is a master regulator in Colorectal Tumorigenesis via Immune modulation. Theranostics. 2020;10:8619-8632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Downs-Canner S, Magge D, Ravindranathan R, O'Malley ME, Francis L, Liu Z, Sheng Guo Z, Obermajer N, Bartlett DL. Complement Inhibition: A Novel Form of Immunotherapy for Colon Cancer. Ann Surg Oncol. 2016;23:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, Werb Z, Coussens LM. Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell. 2018;34:561-578.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 47. | Zha H, Han X, Zhu Y, Yang F, Li Y, Li Q, Guo B, Zhu B. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology. 2017;6:e1349587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Deng Y, Wei B, Zhai Z, Zheng Y, Yao J, Wang S, Xiang D, Hu J, Ye X, Yang S, Wu Y, Li N, Xu P, Lyu J, Dai Z. Dietary Risk-Related Colorectal Cancer Burden: Estimates From 1990 to 2019. Front Nutr. 2021;8:690663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Shivappa N, Godos J, Hébert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 50. | Millar SR, Harrington JM, Perry IJ, Phillips CM. Associations between a protective lifestyle behaviour score and biomarkers of chronic low-grade inflammation: a cross-sectional analysis in middle-to-older aged adults. Int J Obes (Lond). 2022;46:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |