Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.687
Peer-review started: November 27, 2023
First decision: December 18, 2023
Revised: December 27, 2023
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 15, 2024
Processing time: 99 Days and 20.3 Hours
The Alcian blue (AB) and periodic acid Schiff (PAS) stains are representative mucus markers in gastric signet ring cell carcinoma (SRCC). They are low-cost special staining methods used to detect acidic mucus and neutral mucus, respectively. However, the clinical importance of the special combined AB and PAS stain is unclear.
To investigate AB expression, PAS expression and the AB-to-PAS (A/P) ratio in gastric SRCC patients and to assess patient prognosis.
Paraffin-embedded sections from 83 patients with gastric SRCC were stained with AB and PAS, and signet ring cell positivity was assessed quantitatively. Immunohistochemical staining for Ki67, protein 53 (P53) and human epidermal growth factor receptor 2 (HER2) was performed simultaneously. The cancer-specific survival (CSS) rate was estimated via Kaplan-Meier analysis. Cox proportional hazards models were used for univariate and multivariate survival analyses.
Kaplan-Meier survival analysis revealed that the 3-year CSS rate was significantly greater in the high-PAS-expression subgroup than in the low-PAS-expression subgroup (P < 0.001). The 3-year CSS rate in the A/P ≤ 0.5 group was significantly greater than that in the A/P > 0.5 group (P = 0.042). Univariate Cox regression analysis revealed that the factors affecting prognosis included tumor diameter, lymph node metastasis, vessel carcinoma embolus, tumor stage, the A/P ratio and the expression of Ki67, P53 and the PAS. Cox multivariate regression analysis confirmed that low PAS expression [hazard ratio (HR) = 3.809, 95% confidence interval (CI): 1.563-9.283, P = 0.003] and large tumor diameter (HR = 2.761, 95%CI: 1.086-7.020, P = 0.033) were independent risk factors for poor prognosis.
A/P > 0.5 is potentially a risk factor for prognosis, and low PAS expression is an independent risk factor in the prognosis of gastric SRCC. PAS expression and the A/P ratio could help in predicting the clinical prognosis of patients with SRCC.
Core Tip: Through retrospective analysis of 83 patients with gastric signet ring cell carcinoma (SRCC) and paraffin-embedded sections from 83 patients were stained with Alcian blue (AB), periodic acid Schiff (PAS), Ki67, protein 53, and human epidermal growth factor receptor 2, we confirmed that AB to PAS (A/P) ratio > 0.5 is a potential risk factor for the prognosis and low PAS expression is an independent risk factor for the prognosis of gastric SRCC. PAS expression and A/P ratio could help in predicting the clinical prognosis of SRCC patients. However, our study had a limited sample size this and further follow-up studies are still needed.
- Citation: Lin J, Chen ZF, Guo GD, Chen X. Impact of Alcian blue and periodic acid Schiff expression on the prognosis of gastric signet ring cell carcinoma. World J Gastrointest Oncol 2024; 16(3): 687-698
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/687.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.687
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death globally[1]. According to epidemiological statistics, there were approximately 1.033 million new cases of signet ring cell carcinoma (SRCC) and 783000 deaths globally in 2018[2]. The incidence rate of SRCC was 14.4% (192/1330) of the gastric adenocarcinoma cases in our hospital from 2015 to 2022. According to the 3rd edition of the World Health Organization (WHO), more than 50% of gastric SRCC tumors are composed of signet ring cells. The tumor cells were categorized into five types based on their cellular features: Classical (showing a typical signet ring appearance with abundant intracytoplasmic mucin that pushed the nucleus to the periphery), histiocytoid (cells with central nuclei resembling histiocytes), eosinophilic (cells with deeply eosinophilic cytoplasm containing abundant minute granules positive for neutral mucin), small cell (cells with little or no mucin) and anaplastic (cells with little or no mucin) types[3]. Since 2010, the 4th edition of the WHO has included the term “poorly cohesive carcinoma”, which refers to classical SRCC and four other subtypes[4]. However, the term poorly cohesive carcinoma has not been widely used[5]; therefore, in our research, the definition of gastric SRCC was based on the 3rd edition of the WHO.
SRCC is usually regarded as having a poor prognosis and being more aggressive. It has been reported that the SRCC subtype is an independent risk factor for poor prognosis in gastric cancer patients, and the 5-year and 10-year survival rates of gastric SRCC patients are significantly lower than those of patients with other types of gastric adenocarcinoma (5-year survival rate: 19.2% vs 25.8%; 10-year survival rate: 16.0% vs 22.1%)[6]. However, there are also reports with varying opinions regarding the survival outcomes of patients with different stages of gastric SRCC[7,8].
It is well known that signet ring cells can be stained blue-green by Alcian blue (AB) combined with acidic mucus and stained red by periodic acid-Schiff (PAS) combined with neutral mucus[3]. PAS is a staining method used to detect polysaccharides, such as glycogen, glycoproteins, glycolipids and mucin. Gomori[9] demonstrated that the carbon chain of polysaccharides is actually broken between C2 and C3, leading to the formation of aldehydes, which can be demonstrated by reaction with Schiff’s and Fuchsin sulfurous acid. This reaction results in the formation of intense red compounds. The AB dyes dissolved in 3% acetic acid to make a pH = 2.5 solution are copper phthalocyanines with a variety of cationic side chains, and they are useful for staining carbohydrate polyanions[10]. The cationic groups of AB are easily removed under very mild conditions, producing insoluble blue/bluish-green pigments[11]. This reaction enhances the sensitivity of detecting anions in acidic mucus. Previous researchers have explored the value of the AB and PAS staining reactions in tumor diagnosis and prognosis[12,13]. However, the clinical importance of special AB combined with PAS staining in treating gastric SRCC is unclear and controversial. To determine the possible mechanisms underlying the tumorigenesis and heterogeneity of gastric SRCC at the cellular level, we examined the expression of neutral mucin and acid mucin in tumor cells from each patient. Our study was designed to predict the prognosis of gastric SRCC by mucus expression and to preliminarily explore the possible impact of different pondus hydrogenii strains of cell mucus on tumor development.
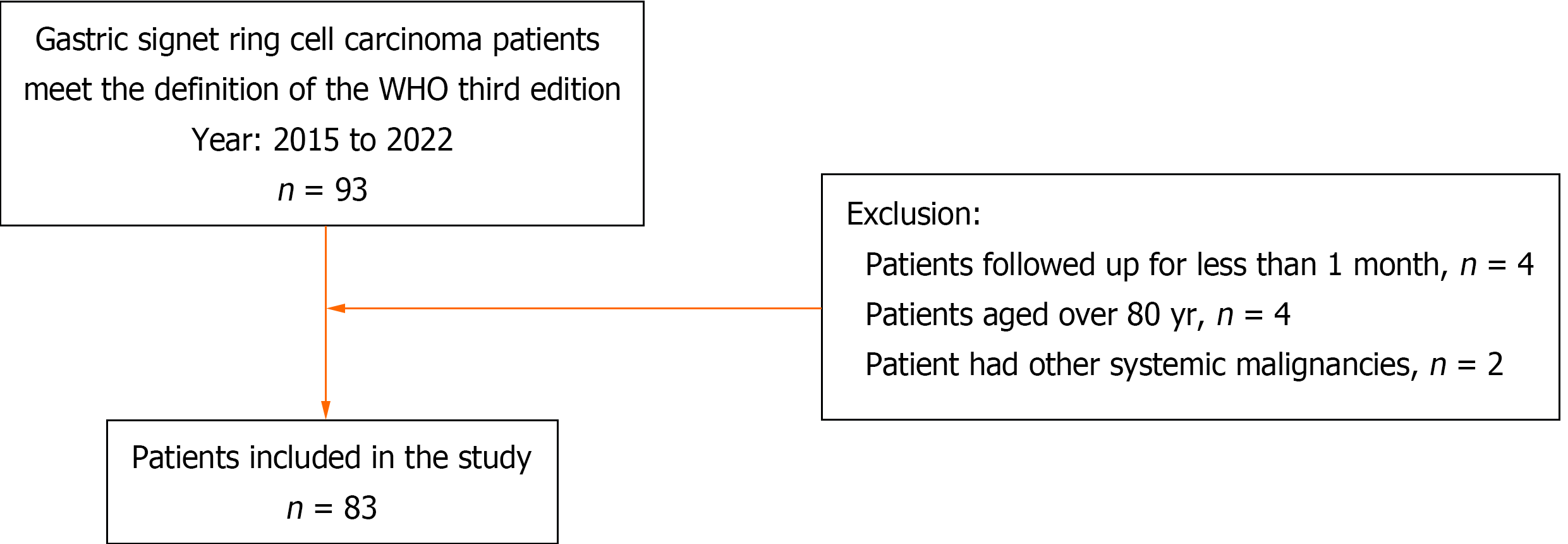
The clinical records of 93 patients diagnosed with gastric SRCC (according to the third edition of the WHO) in our hospital from January 2015 to February 2020 were reviewed. Four patients lost to follow-up after one month were excluded; 4 patients aged older than eighty years and 2 patients with other systemic malignancies were excluded. Finally, 83 patients were enrolled in our study. The detailed patient selection flowchart is shown in Figure 1. Fifty-four of these patients underwent distal gastrectomy, 25 underwent total gastrectomy, and 4 underwent endoscopic submucosal dissection. The follow-up time or survival time for each patient was calculated from the time of pathological diagnosis. The endpoint event was defined as death from cancer. None of the patients had received any preoperative radiotherapy or chemotherapy. The clinicopathological parameters of the patients are provided in the Supplementary material.
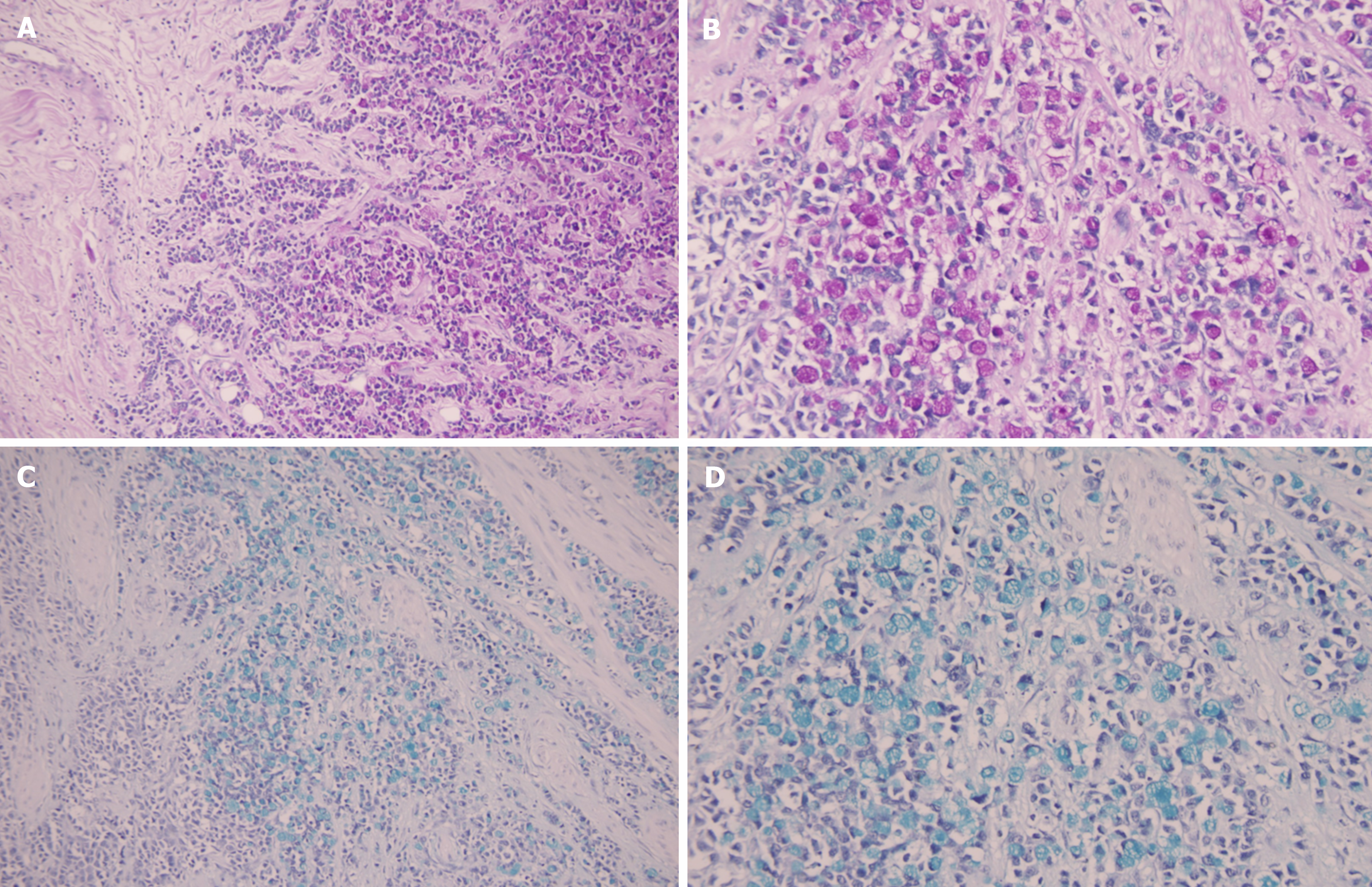
After routine deparaffinization and dehydration, the slides were rinsed in distilled water: (1) For PAS staining, sections were incubated in 1% periodic acid solution for 10 min and then subjected to Schiff’s reagent for 20 min; and (2) For AB staining, sections were immersed in a 3% acetic acid solution for 3 min followed by incubation in AB solution (pH = 2.5) for 30 min at room temperature. Nuclear staining with hematoxylin was performed for 30 s. After dehydration and transparentization, the samples were mounted with neutral gum. The neutral mucus material was red after PAS staining. Red- and purplish red-stained cells were considered positive. In AB-positive stained signet ring cells, the mucus had a light bluish-green to deep bluish-green color. The nuclei of the cells were blue. The positive controls used for PAS and AB were antral epithelium and colonic epithelium tissues, respectively.
Two qualified pathologists independently assessed the AB and PAS reaction products in all patients by counting one thousand tumor cells on each slide. The results were recorded as the percentage of tumor cells showing AB or PAS positivity. We took the average of the two percentages for each patient. The high AB or PAS expression groups comprised those patients with proportion of AB- or PAS-positive signet ring cells > 50%, and the low AB or PAS expression groups comprised those patients with the corresponding proportions ≤ 50%.
When calculating the AB to PAS (A/P) ratio, we replaced negative expression with AB and PAS staining results, with a positive expression result of 1%. The difference in the expression ratio between the AB and PAS could be calculated without affecting the grouping of the positive expression data.
The sections were subjected to immunohistochemical staining using Ki67 (dilution 1:800), protein 53 (P53) (dilution 1:500), and human epidermal growth factor receptor 2 (HER2) (dilution 1:1000) antibodies. Antigen retrieval was achieved by incubating the sections in citrate (pH = 6.0) with microwave heating for 10 min. The sections were then placed in 0.3% H2O2 in ethanol for 20 min to block endogenous peroxidase activity. The slides were incubated overnight at 4 °C and then incubated with horseradish peroxidase-conjugated secondary antibody for 30 min. The sections were developed in diaminobenzidine solution and counterstained with hematoxylin. The slices were washed with phosphate buffered saline during each step. Materials were purchased from Fuzhou Maixin Biotech Co. For positive controls for Ki67 and P53, chronic tonsillitis and colonic carcinoma tissue were used. HER2-negative and -positive control tissues were obtained from breast cancer tissues (negative, positive 1+, positive 3+) via the tissue microarray technique.
For Ki67 assessment, tiny yellowish, tan or brown particles indicated a positive signal. For P53 assessment, the intensity (color) of P53 staining was graded as 0 (colorless), 1 (yellow), 2 (tan), or 3 (brown), and the percentage of positive cells was scored as 0 (0%), 1 (1%-10%), 2 (11%-50%), 3 (51%-75%), or 4 (> 75%), respectively, for each core. A product of staining intensity and percentage of positive cells > 3 was considered to indicate positive staining, and a product ≤ 3 was considered to indicate negative staining. For HER2 assessment, patients were scored in accordance with the HER2 scoring system for gastric cancer[14]. Two qualified pathologists independently assessed the immunoreactivity of Ki67, P53, and HER2.
All the statistical analyses were conducted using SPSS software (version 23.0; SPSS, Chicago). The patients’ clinicopathological characteristics were analyzed using the χ2 test or Fisher’s exact test for categorical variables. Univariate Cox regression analysis was used to screen potential prognostic risk factors. Subsequently, independent risk factors were further screened by multivariate Cox regression analysis (Method: Forward: LR). The hazard ratio (HR) and 95% confidence interval (CI) were also reported for each risk factor. For survival analysis, the survival rate was calculated by the Kaplan-Meier method, and the log-rank method was used to compare the survival rates between the different groups. P < 0.05 was considered to indicate statistical significance.
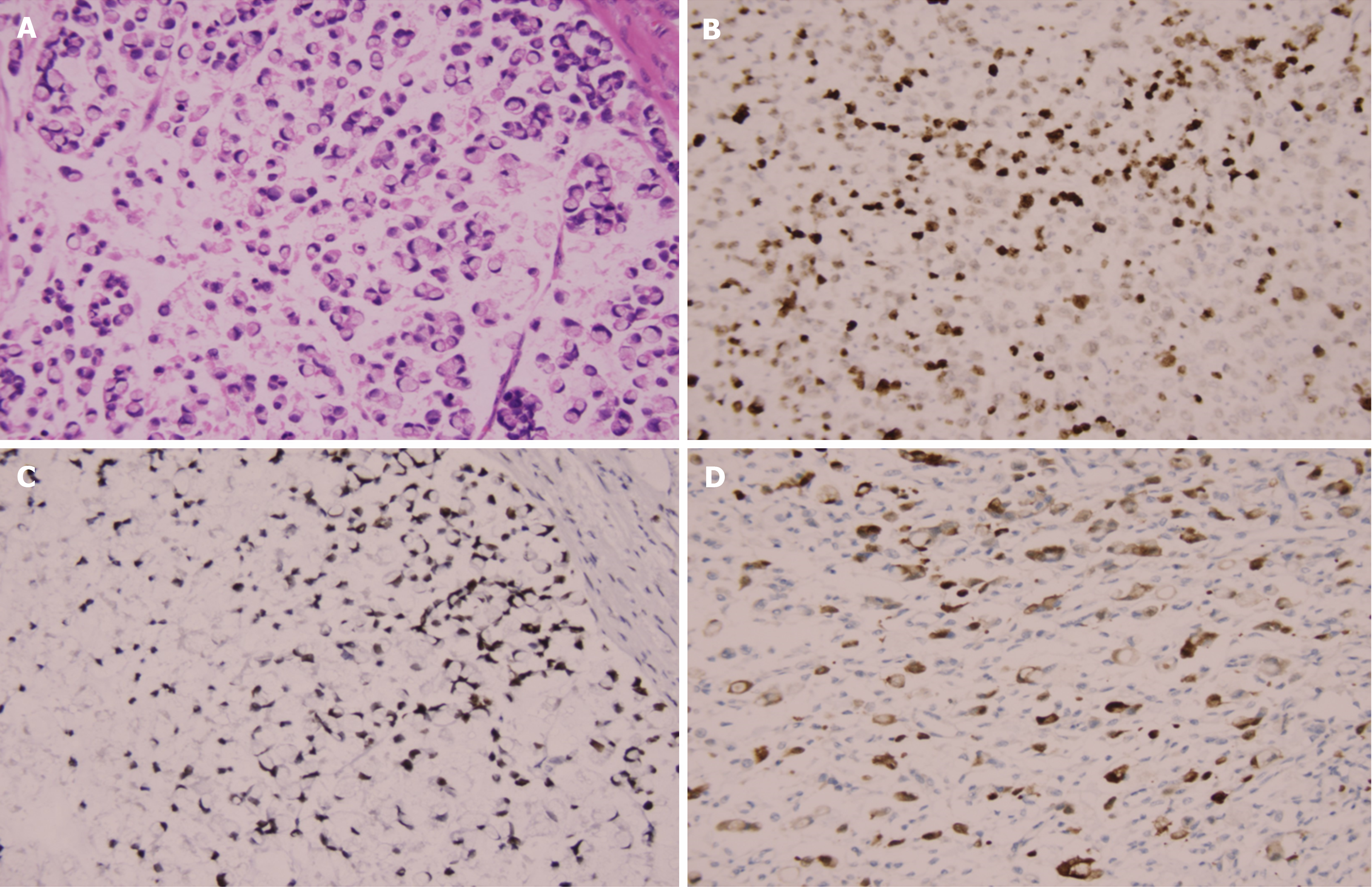
The 83 patients at diagnosis ranged from 24 to 76 years old, with a median age of 39.7 years. Among them, 32 (38.55%) were males and 51 (61.45%) were females. The median ages for males and females were 56.1 and 48.6 years, respectively. The mean postoperative follow-up was 34 months (range: 21-61). There were 21 (25.3%) patients at the early stage and 62 (74.7%) at the advanced stage. By tumor diameter, 43 (51.8%) patients were ≤ 3 cm, 40 (48.2%) were > 3 cm, 33 (39.8%) patients had no vessel carcinoma embolus, 50 (60.2%) had vessel carcinoma embolus, 33 (39.8%) patients had no lymph node metastasis, and 50 (60.2%) patients had lymph node metastasis. K67 and P53 were stained in the nucleus, and HER2 was stained in the cytomembrane (Figure 2). The percentages of P53- and HER2-positive patients were 62.7% and 24.10%, respectively. The percentage of Ki67-positive cells > 50% was 25.3%. PAS was used to stain the signet ring cells as prominent purplish red/red, and AB was used to stain the tumor cells blueish-green (Figure 3). PAS staining revealed that 33 (39.8%) patients in the low-grade group had ≤ 50% PAS-positive cells, 50 (60.2%) had > 50% PAS-positive cells, and only 4 patients in the low-grade group had no PAS-positive material. With regard to the response of signet ring cells to AB staining, 48 patients had > 50% AB-positive cells, 20 patients had ≤ 50% AB-positive cells, and 15 patients were negative. The patients’ clinicopathological parameters at the time of diagnosis are shown in Table 1.
| Factors | Total | PAS | P value | |
| n = 83 | ≤ 50% positive | > 50% positive | ||
| Age (yr) | 0.583 | |||
| ≤ 60 | 63 (75.9) | 24 (72.7) | 39 (78.0) | |
| > 60 | 20 (24.1) | 9 (27.3) | 11 (22.0) | |
| Gender | 0.898 | |||
| Male | 32 (38.6) | 13 (39.4) | 19 (38.0) | |
| Female | 51 (61.4) | 20 (60.6) | 31 (62.0) | |
| Tumor diameter | 0.347 | |||
| ≤ 3 | 43 (51.8) | 15 (45.5) | 28 (56.0) | |
| > 3 | 40 (48.2) | 18 (54.5) | 22 (44.0) | |
| Vessel carcinoma embolus | 0.608 | |||
| No | 33 (39.8) | 12 (36.4) | 21 (42.0) | |
| Yes | 50 (60.2) | 21 (63.6) | 29 (58.0) | |
| Lymph node metastasis | 0.331 | |||
| Negative | 33 (39.8) | 11 (33.3) | 22 (44.0) | |
| Positive | 50 (60.2) | 22 (66.7) | 28 (56.0) | |
| Tumor stage | 0.006 | |||
| Early | 21 (25.3) | 3 (9.1) | 18 (36.0) | |
| Advanced | 62 (74.7) | 30 (90.9) | 32 (64.0) | |
| Ki67 | 0.394 | |||
| ≤ 50% | 62 (74.7) | 23 (69.7) | 39 (78.0) | |
| > 50% | 21 (25.3) | 10 (30.3) | 11 (22.0) | |
| P53 | 0.281 | |||
| Negative | 31 (37.3) | 10 (30.3) | 21 (42.0) | |
| Positive | 52 (62.7) | 23 (69.7) | 29 (58.0) | |
| HER2 | 0.283 | |||
| Negative | 63 (75.9) | 23 (69.7) | 40 (80.0) | |
| Positive | 20 (24.1) | 10 (30.3) | 10 (20.0) | |
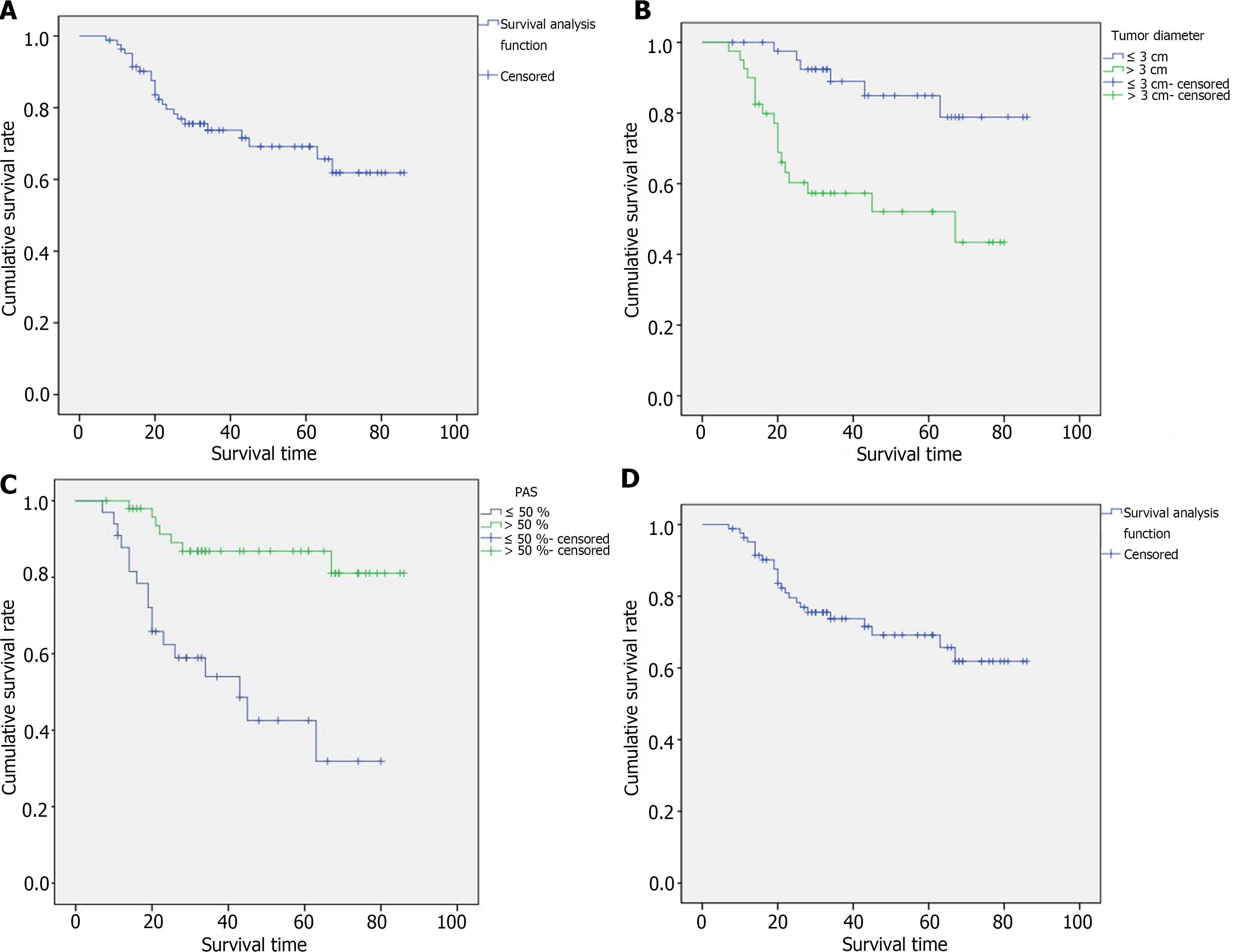
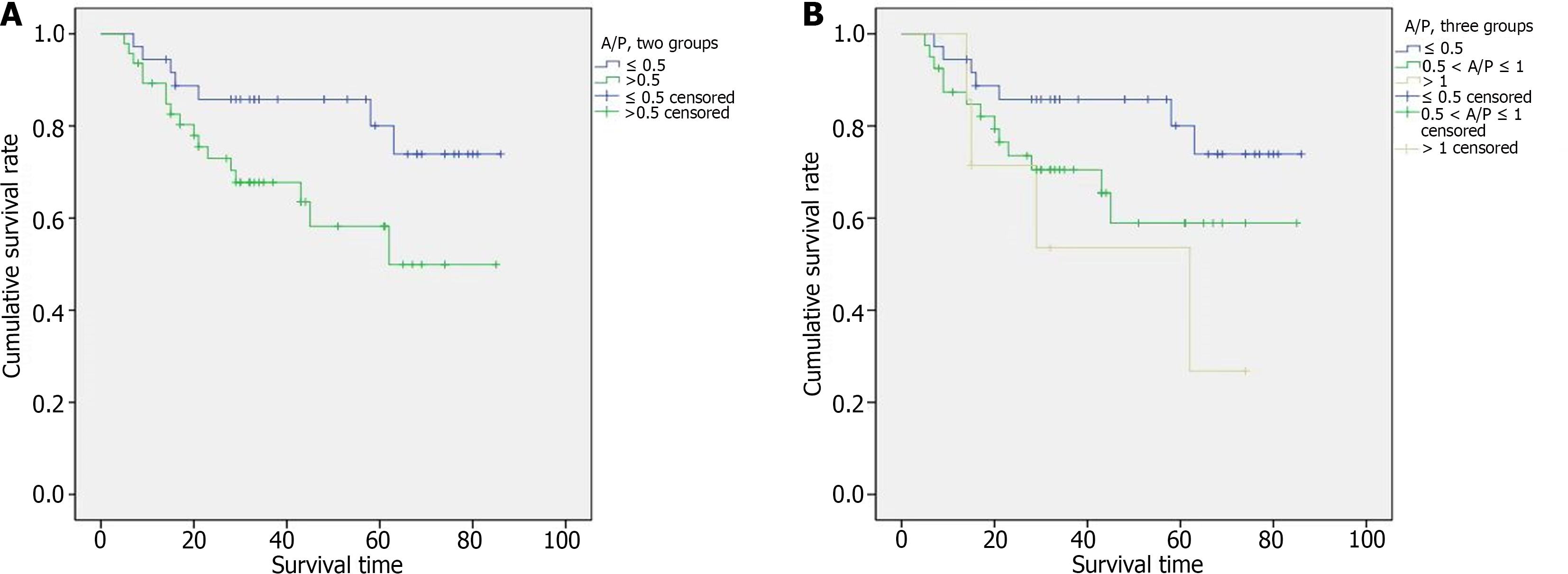
The 3-year cancer-specific survival (CSS) rate of all gastric SRCC patients was 73.7% (Figure 4A). Among the 21 patients with early-stage disease, the 3-year CSS rate was 100%. Among the 62 patients with advanced-stage disease, the 3-year CSS rate was 63.7%. Kaplan-Meier survival curves revealed that patients with a tumor diameter > 3 cm had significantly poorer CSS than did those with a tumor diameter ≤ 3 cm (Figure 4B, P = 0.001). The degree of PAS expression tended to correlate with prognosis, and the 3-year CSS rates in the high PAS expression group and low PAS expression group were 86.8% and 54.0%, respectively (Figure 4C, P < 0.001). Even when considering only advanced cancers, there was still a significant difference in prognosis between the two groups, and the 3-year CSS rates were 78.2% and 49.1%, respectively (Figure 4D, P = 0.003). AB expression was not significantly associated with patient survival. Among the 83 patients with gastric SRCC, the A/P ratio ranged from 0.11 to 5.00. After all patients were assigned to two groups according to the A/P ratio, the median survival durations were 48 and 30 months for patients with A/P ≤ 0.5 and > 0.5, respectively. For the 36 patients with A/P ≤ 0.5, the 3-year CSS rate was 85.8%. For the 47 patients with A/P > 0.5, the 3-year CSS rate was 67.8%. Kaplan-Meier survival curves revealed that an A/P ≤ 0.5 was strongly correlated with a better prognosis (Figure 5A, P = 0.042). When the patients categorized into three groups, the median survival times of the 36, 40 and 7 patients with A/P ≤ 0.5, 0.5 < A/P ≤ 1 and A/P > 1 were 48, 31 and 29 months, respectively. For patients with A/P ≤ 0.5, 0.5 < A/P ≤ 1, and A/P > 1, the 3-year CSS rates were 85.8%, 60.5%, and 53.6%, respectively. Among the patients in the three groups, the A/P ratio tended to correlate with prognosis: The lower the A/P ratio, the better the survival. However, statistical significance was not apparent (Figure 5B, P = 0.067).
Kaplan-Meier survival curves revealed that high PAS expression and an A/P ≤ 0.5 were significantly correlated with a better prognosis. Therefore, we analyzed the relationships between these two indicators and mucus and clinicopathologic features to further explore their prognostic value in gastric SRCC. Fifty patients exhibited metastasis to the lymph nodes; 22 (66.7%) had low PAS expression, and 28 (56.0%) had high PAS expression. Among the other 33 patients with no evidence of metastasis, 11 (33.3%) had low PAS expression, and 22 (44.0%) had high PAS expression. However, in the group with low PAS expression, the number of patients with lymph node metastasis was twice that of patients without metastasis, and no significant difference was observed between lymph node metastasis and PAS expression (P = 0.331). Three (9.1%) of the 21 patients at the early stage and 30 (90.9%) of the 62 patients at the advanced stage had significantly lower PAS expression (P = 0.006). The relative expression of Ki67 and p53 was lower in the high PAS expression group than in the low PAS expression group. However, the difference was not significant (P > 0.05). Moreover, there were no significant differences in any clinicopathologic features or A/P ratios. The patient clinicopathological parameters and the degree of PAS expression at the time of diagnosis are shown in Table 1.
Univariate Cox regression analysis revealed that large tumor diameter (HR = 4.428, 95%CI: 1.750-11.199, P = 0.002), vessel carcinoma embolus (HR = 3.634, 95%CI: 1.351-9.775, P = 0.011), lymph node metastasis (HR = 3.006, 95%CI: 1.120-8.066, P = 0.029), advanced tumor stage (HR = 42.232, 95%CI: 1.201-1485.160, P = 0.039), > 50% positive expression of Ki67 (HR = 2.254, 95%CI: 0.984-5.165, P = 0.055), positive expression of P53 (HR = 2.532, 95%CI: 0.995-6.443, P = 0.051), low PAS expression (HR = 5.284, 95%CI: 2.164-12.905, P < 0.001) and A/P > 0.5 group (HR = 1.402, 95%CI: 1.028-1.910, P = 0.033) were potential independent risk factors. After multivariate Cox regression analysis, low PAS expression (HR = 3.809, 95%CI: 1.563-9.283, P = 0.003) and large tumor diameter (HR = 2.761, 95%CI: 1.086-7.020, P = 0.033) emerged as independent risk factors for prognosis. Univariate and multivariate Cox regression analyses based on the clinicopathologic parameters of the gastric SRCC patients are shown in Table 2.
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||
| ≤ 60 | 1 | |||||
| > 60 | 1.461 | 0.624-3.418 | 0.382 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.865 | 0.384-1.949 | 0.727 | |||
| Tumor diameter | ||||||
| ≤ 3 | 1 | 1 | ||||
| > 3 | 4.428 | 1.750-11.199 | 0.002 | 2.761 | 1.086-7.020 | 0.033 |
| Venous tumor emboli | ||||||
| No | 1 | |||||
| Yes | 3.634 | 1.351-9.775 | 0.011 | |||
| Lymph node metastasis | ||||||
| No | 1 | |||||
| Yes | 3.006 | 1.120-8.066 | 0.029 | |||
| Tumor stage | ||||||
| Early | 1 | 1 | ||||
| Advanced | 42.232 | 1.201-1485.160 | 0.039 | 159858 | 0.000-2.26E+153 | 0.945 |
| Ki67 | ||||||
| ≤ 50% | 1 | |||||
| > 50% | 2.254 | 0.984-5.165 | 0.055 | |||
| P53 | ||||||
| Negative | 1 | |||||
| Positive | 2.532 | 0.995-6.443 | 0.051 | |||
| HER2 | ||||||
| Negative | 1 | |||||
| Positive | 1.232 | 0.487-3.114 | 0.659 | |||
| PAS | ||||||
| > 50% | 1 | 1 | ||||
| ≤ 50% | 5.284 | 2.164-12.905 | 0 | 3.809 | 0.108-0.640 | 0.003 |
| AB | ||||||
| ≤ 50% | 1 | |||||
| > 50% | 0.985 | 0.970-3.114 | 1.002 | |||
| A/P | ||||||
| ≤ 50% | 1 | |||||
| > 0.5 | 1.402 | 1.028-1.910 | 0.033 | |||
According to our study, gastric SRCC exhibits different clinicopathological features compared with other types of gastric adenocarcinoma; for example, it occurs more frequently in females and develops at a relatively younger age. This finding was similar to related reports from previous studies[15,16], although the exact cause was not yet clear. It has been proposed that the cancerization of SRCC might be influenced by sex hormones, especially estrogen receptor (ER). However, there was no evidence that ER played a key role[17]. Due to the differences in epidemiology, the risk factors leading to the cancerization of SRCC are still controversial. Previous studies suggested that E-cadherin, which is encoded by the CDH1 gene and leads to loss of cell contact by disruption of adherent junctions, may be involved in SRCC initiation, and CDH1 mutations seem to be the most frequent abnormality leading to SRCC[18,19]. Its specific pathogenic mechanisms are unclear because of poor understanding of its etiology; however, one of the prominent processes at the cellular level involves the accumulation of different amounts of mucin within the cytoplasm. We analyzed the expression and ratio of acidic mucus to neutral mucus in tumor cells by performing AB and PAS special staining. We attempted to clarify the correlation between mucus in signet ring cells and patient prognosis by AB and PAS special staining methods.
Several studies have suggested that gastric SRCC has a better prognosis than other types of gastric adenocarcinoma at an early stage, but the survival outcome of patients with advanced-stage disease is still controversial[16,20-23]. According to our present analysis of the prognosis of 83 SRCC patients, the 3-year CSS rate was 73.7%, and that of patients with advanced-stage disease was 63.7%. These results are not widely divergent from those of previous studies on the prognosis of gastric SRCC[24]. Lymph node metastasis has been reported to be an independent prognostic risk factor for gastric SRCC[25]. Our study showed that lymph node metastasis significantly affects patient prognosis but was not an independent risk factor according to multivariate Cox analysis. This may be related to our insufficient sample size. Tumor diameter was an independent prognostic risk factor for gastric SRCC in our study. However, several studies have reported that tumor diameter is not associated with poor outcome[22,26]. These controversial results may be related to the depth of invasion and require further confirmation.
Ki67 (encoded by the MKI67 gene) is a proliferation marker protein correlated with poor differentiation and worse biological behaviors[27,28]. P53 gene alterations are missense mutations, most of which lead to the synthesis of a mutant protein and thus massive overexpression of the protein product[29]. Both of these factors were confirmed to be potential biomarkers for predicting the prognosis of gastric cancer[30-32]. In our study, Kaplan-Meier analysis revealed that Ki67 and P53 expression significantly affected patient prognosis (χ2 = 3.932, P = 0.047; χ2 = 4.093, P = 0.043). The higher the expression of Ki67 and P53, the poorer the prognosis. HER2 belongs to the human epidermal receptor family[33]. Upon HER2 dimerization among the receptors of the family, downstream tyrosine kinase signaling cascades are activated, thus triggering cell proliferation, migration and invasion[34]. It has also been proven to be associated with the prognosis of gastric SRCC[35], but our research did not support this view.
Gastric SRCC with pure classical signet ring cells is relatively rare and is usually present in the early stage and limited to the intramucosal layer. Its morphology is often lost when tumors develop and transform into the other 4 types of tumors, especially in invasive areas[36]. We observed cell morphology and found that the loss of cell morphology was mainly manifested by a reduction in the amount of mucus in the cytoplasm, and the number of PAS- or AB-positive cells decreased correspondingly. The intracytoplasmic mucus content differs among the five different subtypes of signet ring cells, and the accumulation of mucins results in either large, small, or even absent vacuoles. In other words, the loss of morphological differentiation of typical signet ring cells decreases or even abolishes PAS or AB expression. This result was consistent with previous research[12,25]. We analyzed the correlation between mucus content and prognosis by detecting PAS and AB expression in different types of signet ring cells and confirmed that low PAS expression was an independent risk factor for poor prognosis and that AB expression was not significantly associated with patient survival. However, there is also a different opinion that PAS and AB staining of signet ring cells reflects the character and degree of maturity of mucous granules, and PAS-positive tumor cells are more active and more immature than AB-positive cells are[37]. Takenoshita et al[13] indicated that alterations in the properties of mucin occur during the progression of signet ring cells based on reactions to PAS and AB staining. Our research, which demonstrated that the A/P ratio is related to the survival of SRCC patients (a lower A/P ratio is associated with a worse prognosis), was consistent with previous findings. Therefore, mucin in the cytoplasm could play an important role in cancer progression. The amount of AB- and PAS-positive materials in mucus varies between different subtypes of signet ring cells[13,38,39]. We found that the expression of neutral mucus is closely related to patient prognosis. The lower the neutral mucus concentration is, the worse the prognosis. The presence of acidic mucus is not directly related to prognosis. However, our study suggested that as the ratio of acidic mucus to neutral mucus in signet ring cells increases, the survival rate of patients significantly decreases. We speculated that when tumor cells differentiate in a more malignant direction, the intracytoplasmic mucin ionizes more anions, leading to acidification. Then, the isoelectric point of the cytoplasm changes, and the material moves to the acid side to bind to additional AB dye, which is basic. Therefore, these patients had a greater A/P ratio. We preliminarily confirmed that the different concentrations and pH values of intracytoplasmic mucus could play a role in tumor cell differentiation and progression and affect patient prognosis.
There are several limitations to our study. First, our study was a single-center, retrospective study with a limited sample size. Second, we had a shorter follow-up period. In addition, other unknown physiological and path
This study demonstrated that low PAS expression was an independent risk factor for poor prognosis and that A/P > 0.5 was potentially a risk factor for poor prognosis. The PAS and A/P ratio can be used to evaluate the prognosis of patients with gastric SRCC. PAS and AB staining is helpful for determining the prognosis of gastric SRCC patients, and the cost of these methods is low.
There were few studies on the prognosis of patients with gastric signet ring cell carcinoma (SRCC) and the clinical significance in gastric SRCC of the combined Alcian blue (AB) and periodic acid Schiff (PAS) is unclear and controversial.
To explore the prognostic predictors in patients with gastric SRCC.
This study aimed to investigate the AB expression, PAS expression and AB to PAS ratio (A/P) in gastric SRCC and assess the prognosis.
A total of 83 patients with gastric SRCC were selected for retrospective analysis and their paraffin-embedded sections were stained by AB and PAS, Ki67, protein 53 (P53) and human epidermal growth factor receptor 2. Kaplan-Meier analysis and Cox proportional-hazard models were used for statistical analyses.
The 3-year cancer-specific survival rate showed that: (1) High PAS expression group was significantly higher than that of low PAS expression group (P < 0.001); and (2) A/P ≤ 0.5 group was significantly higher than A/P > 0.5 group (P = 0.042). Univariate Cox regression analysis showed that the factors affecting prognosis included tumor diameter, lymph node metastasis, vessel carcinoma embolus, tumor stage, A/P ratio and the expression of Ki67, P53 and PAS. Multivariate Cox regression analysis conformed that low PAS expression and large tumor diameter were independent risk factors for prognosis.
A/P > 0.5 is a potential risk factor for the prognosis and low PAS expression is an independent risk factor for the prognosis of gastric SRCC. PAS expression and A/P ratio could help in predicting the clinical prognosis of SRCC patients.
AB and PAS stains can be routinely used for SRCC diagnosis to help determine prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2820] [Article Influence: 564.0] [Reference Citation Analysis (5)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55742] [Article Influence: 7963.1] [Reference Citation Analysis (132)] |
| 3. | Fenoglio-Preiser C, Carneiro F, Correa P. Gastric carcinoma. In: Hamilton SR, Aaltonen LA. Pathology and genetics: tumors of the digestive system (WHO classification of tumor pathology). Lyon: IAP IRAC Press, 2000: 39-52. |
| 4. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Correction to: Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Karnaukhov NS, Khomeriki SG, Derizhanova IS, Mantsov AA, Izrailov RE, Tsvirkun VV, Khatkov IE. [Poorly cohesive gastric carcinoma. Validity of using the term; translation variants]. Arkh Patol. 2021;83:69-72. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Benesch MGK, Mathieson A. Epidemiology of Signet Ring Cell Adenocarcinomas. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Kim YH, Park JH, Park CK, Kim JH, Lee SK, Lee YC, Noh SH, Kim H. Histologic purity of signet ring cell carcinoma is a favorable risk factor for lymph node metastasis in poorly cohesive, submucosa-invasive early gastric carcinoma. Gastric Cancer. 2017;20:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Gomori G. The periodic-acid Schiff stain. Am J Clin Pathol. 1952;22:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Fagan C, Dapson RW, Horobin RW, Kiernan JA. Revised tests and standards for Biological Stain Commission certification of alcian blue dyes. Biotech Histochem. 2020;95:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Scott JE. Alcian blue. Now you see it, now you don't. Eur J Oral Sci. 1996;104:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Kerckhoffs KGP, Liu DHW, Saragoni L, van der Post RS, Langer R, Bencivenga M, Iglesias M, Gallo G, Hewitt LC, Fazzi GE, Vos AM, Renaud F, Yoshikawa T, Oshima T, Tomezzoli A, de Manzoni G, Arai T, Kushima R, Carneiro F, Grabsch HI. Mucin expression in gastric- and gastro-oesophageal signet-ring cell cancer: results from a comprehensive literature review and a large cohort study of Caucasian and Asian gastric cancer. Gastric Cancer. 2020;23:765-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Takenoshita S, Hashizume T, Katoh R, Koitabashi H, Nagamachi Y. Mucin production and subsequent interstitial fibrosis in gastric-cancer with signet-ring cells. Oncol Rep. 1994;1:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5300] [Article Influence: 353.3] [Reference Citation Analysis (3)] |
| 15. | Postlewait LM, Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Swords D, Jin LX, Cho CS, Winslow ER, Cardona K, Staley CA, Maithel SK. The Prognostic Value of Signet-Ring Cell Histology in Resected Gastric Adenocarcinoma. Ann Surg Oncol. 2015;22 Suppl 3:S832-S839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Franko J, Le VH, Tee MC, Lin M, Sedinkin J, Raman S, Frankova D. Signet ring cell carcinoma of the gastrointestinal tract: National trends on treatment effects and prognostic outcomes. Cancer Treat Res Commun. 2021;29:100475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Humar B, Blair V, Charlton A, More H, Martin I, Guilford P. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res. 2009;69:2050-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho-Simöes M. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, Wang Y. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zaafouri H, Jouini R, Khedhiri N, Khanchel F, Cherif M, Mesbahi M, Daghmouri A, Mahmoudi W, Akremi S, Sabbah M, Benzarti Y, Hadded D, Gargouri D, Bader MB, Maamer AB. Comparison between signet-ring cell carcinoma and non-signet-ring cell carcinoma of the stomach: clinicopathological parameters, epidemiological data, outcome, and prognosis-a cohort study of 123 patients from a non-endemic country. World J Surg Oncol. 2022;20:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Fujiyoshi Y, Eimoto T. Chromogranin A expression correlates with tumour cell type and prognosis in signet ring cell carcinoma of the stomach. Histopathology. 2008;52:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, Yamauchi H. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Zeng M, Zhou J, Wen L, Zhu Y, Luo Y, Wang W. The relationship between the expression of Ki-67 and the prognosis of osteosarcoma. BMC Cancer. 2021;21:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Wei L, Shi C, Zhang Y. Expression of miR-34a and Ki67 in nasopharyngeal carcinoma and the relationship with clinicopathological features and prognosis. Oncol Lett. 2020;19:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Davidoff AM, Humphrey PA, Iglehart JD, Marks JR. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci U S A. 1991;88:5006-5010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 211] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Go SI, Ko GH, Lee WS, Lee JH, Jeong SH, Lee YJ, Hong SC, Ha WS. The Use of CD44 Variant 9 and Ki-67 Combination Can Predicts Prognosis Better Than Their Single Use in Early Gastric Cancer. Cancer Res Treat. 2019;51:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Wu CW, Lin YY, Chen GD, Chi CW, Carbone DP, Chen JY. Serum anti-p53 antibodies in gastric adenocarcinoma patients are associated with poor prognosis, lymph node metastasis and poorly differentiated nuclear grade. Br J Cancer. 1999;80:483-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res. 2006;12:5112-5117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Li F, Meng G, Tan B, Chen Z, Ji Q, Wang X, Liu C, Niu S, Li Y, Liu Y. Relationship between HER2 expression and tumor interstitial angiogenesis in primary gastric cancer and its effect on prognosis. Pathol Res Pract. 2021;217:153280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med. 2019;381:1284-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 35. | Qiu MZ, Li Q, Wang ZQ, Liu TS, Liu Q, Wei XL, Jin Y, Wang DS, Ren C, Bai L, Zhang DS, Wang FH, Li YH, Xu RH. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer. 2014;134:2468-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Arai T. Where does signet-ring cell carcinoma come from and where does it go? Gastric Cancer. 2019;22:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Yamashiro K, Suzuki H, Nagayo T. Electron microscopic study of signet-ring cells in diffuse carcinoma of the human stomach. Virchows Arch A Pathol Anat Histol. 1977;374:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 38. | Bakkelund K, Fossmark R, Nordrum I, Waldum H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J Histochem Cytochem. 2006;54:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Santini D, Bazzocchi F, Mazzoleni G, Ricci M, Viti G, Marrano D, Martinelli G. Signet-ring cells in advanced gastric cancer. A clinical, pathological and histochemical study. Acta Pathol Microbiol Immunol Scand A. 1987;95:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |