Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.859
Peer-review started: December 5, 2022
First decision: February 21, 2023
Revised: March 6, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 15, 2023
Processing time: 157 Days and 22.3 Hours
Hepatocellular carcinoma (HCC) is a common malignant tumor worldwide. Many regions across the world have issued various HCC diagnosis and treatment protocols to improve the diagnosis and targeted treatment of patients with HCC. However, real-world studies analysing the practice, application value, and existing problems of the China Liver Cancer (CNLC) staging system are scarce.
To analyze the current situation and problems associated with the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China.
We collected the medical records of all patients with HCC admitted to the First Affiliated Hospital of Zhengzhou University from January 1, 2011 to December 31, 2019, and recorded the hospitalization information of those patients until December 31, 2020. All information on the diagnosis and treatment of the target patients was recorded, and their demographic and sociological characteristics, CNLC stages, screening situations, and treatment methods and effects were analyzed. The survival status of the patients was obtained from follow-up data.
This study included the medical records of 3022 patients with HCC. Among these cases, 304 patients were screened before HCC diagnosis; their early-stage diagnosis rate was 69.08%, which was significantly higher than that of patients with HCC who were diagnosed without screening and early detection (33.74%). Herein, patients with no clinical outcome at discharge were followed up, and the survival information of 1128 patients was obtained. A Cox model was used to analyse independent risk factors affecting overall survival, which were revealed as age > 50 years, no screening, alpha-fetoprotein > 400 ng/mL, Child–Pugh grade B, and middle and late CNLC stages. Based on the Cox model survival analysis, in our study, patients with HCC identified via screening had significant advantages in overall and tumor-free survival after hepatectomy.
Early diagnosis and treatment can be achieved by screening groups at high risk for HCC based on the guidelines; however, real-world compliance is poor.
Core Tip: This retrospective study evaluated the current situation and problems associated with the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China. The findings revealed that 70% of hepatocellular carcinoma (HCC) treatments at the First Affiliated Hospital of Zhengzhou University were performed according to the guidelines. Patients who underwent liver resection in accordance with the guidelines had a significant survival advantage. Furthermore, screening for HCC high-risk groups according to the guidelines can achieve early diagnosis and treatment; however, real-world compliance is poor.
- Citation: Yan YW, Liu XK, Zhang SX, Tian QF. Real-world 10-year retrospective study of the guidelines for diagnosis and treatment of primary liver cancer in China. World J Gastrointest Oncol 2023; 15(5): 859-877
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/859.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.859
Hepatocellular carcinoma (HCC) is a common malignant tumor worldwide. According to 2020 data from the GLOBOCAN database, the global incidence of liver cancer ranked sixth among all malignant tumors, and its mortality ranked third[1]. In a recent study, the 1-, 3-, 5-, and 10-year survival rates of HCC in Asian countries were 34.8%, 19%, 18.1%, and 4.1%, respectively[2]. Many regions worldwide have issued HCC diagnosis and treatment protocols, such as the Barcelona Clinic Liver Cancer (BCLC) and Hong Kong Liver Cancer (HKLC) staging systems and guidelines from the European Association for the Study of the Liver (EASL), the Asian–Pacific Association for the Study of the Liver (APASL), and the Japan Society of Hepatology (JSH), to improve the diagnosis and targeted treatment of patients with HCC[3-7]. However, no unified, widely recognized staging scheme applies to all populations worldwide.
Liver cancer is the fourth most common malignancy and the second leading cause of cancer-related deaths in China[8]. Therefore, based on the current situation of HCC diagnosis, multidisciplinary comprehensive treatment, and research in China, the National Health Commission issued Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China in 2017[9] (referred to as ‘the guidelines’ hereafter) and updated them in 2019 and 2022[10]. Based on the liver function status, the tumor size, number, and invasion; and the general conditions of patients with HCC, a China Liver Cancer (CNLC) staging system was proposed for the Chinese population, including CNLC stages Ia, Ib, IIa, IIb, IIIa, IIIb, and IV[9,10]. The method of CNLC staging was first published in 2017[9]. Due to the short period since its publication, currently, there are no real-world studies on diagnosing and treating patients based on CNLC staging. Only a few studies have been performed on specific treatment methods in different CNLC stages[11-13]. The screening of high-risk HCC groups and the diagnosis and treatment of patients with HCC are also recommended by the guidelines. Some studies have analysed the situations of patients with HCC undergoing liver resection (LR) or interventional therapy based on CNLC staging[11-13]. In a comparative study, Vitale et al[14] found that CNLC staging was superior to BCLC staging as a prognostic staging system. However, these studies were traditional randomized controlled trials or comparative studies in literature reviews, and they only included patients who underwent LR or interventional therapy. There are some real-world studies on BCLC staging in clinical practice, but none exist on the practice of CNLC staging in real clinical settings in China[15-17]. Therefore, real-world studies analysing the practice, application value, and existing problems of CNLC staging in China’s real clinical environment are lacking[18,19]. Hence, we aimed to analyze the current situation and problems associated with Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China.
In this study, inpatients with liver cancer from the First Affiliated Hospital of Zhengzhou University, the largest hospital in China, were selected as the study group. The hospital is also home to the National Regional Liver Cancer Diagnosis and Treatment Centre and the National Liver Transplant Centre. We collected the complete medical records of all patients with HCC who were diagnosed and treated in the hospital from January 1, 2011, to December 31, 2019, and the hospitalization information of these patients was recorded until December 31, 2020. The data collected from the large sample of patients with HCC were classified into CNLC stages to evaluate the compliance between CNLC staging and real-life clinical diagnosis and treatment in China. These data were also used to systematically review and analyze the diagnosis and treatment of Chinese patients with HCC in the real world and identify the current situation and problems associated with the guidelines for HCC diagnosis and treatment in China to provide a reference to further optimize the screening, diagnosis, clinical staging, and treatment of HCC.
The statistical methods of this study were reviewed by Cheng Cheng from the Department of Epidemiology and Health Statistics at Zhengzhou University.
The main research results of this paper show that compliance in the clinical application of the Chinese guidelines for HCC diagnosis and treatment is good, screening compliance is poor,- and following the guidelines for screening and treatment can help patients to obtain certain survival benefits. This study also found that patients who underwent hepatectomy according to the guidelines had a significant advantage in tumor-free survival compared with those whose treatment did not follow the guidelines.
This was a real-world retrospective study based on the medical records system of the First Affiliated Hospital of Zhengzhou University.
The inclusion criteria involved patients: (1) With a final diagnosis of HCC; (2) aged ≥ 18 years at HCC diagnosis; (3) who had not received a related treatment in other hospitals before being diagnosed with HCC; (4) without complications with other serious immune system diseases, such as human immunodeficiency virus, syphilis, and leukemia; (5) without other malignant tumors; and (6) with complete clinical data.
The exclusion criteria included patients: (1) Without a final diagnosis of HCC; (2) with a diagnosis of intrahepatic cholangiocarcinoma (ICC) or HCC–ICC; (3) aged < 18 years; (4) with HCC diagnosed and treated in other hospitals; (5) with HCC diagnosed before January 1, 2011; (6) with other severe immune system diseases; (7) with other malignant tumors; and (8) with missing clinical data that could not be staged.
The guidelines define HCC as a malignant tumor of liver cells, while ICC refers to cancer of the intrahepatic bile duct branch lined with complex epithelial cells. The most common malignancies of ICC are adenocarcinomas. As this was a retrospective study, the patients included those who had been definitively clinically diagnosed with HCC and excluded those clinically diagnosed with ICC and HCC–ICC.
This study was approved (ZZUIRB2022-151) by Zhengzhou University. In accordance with national legislative and institutional requirements, participation in the study did not require written informed consent. The general guarantor of the study ensured the protection and confidentiality of the original data and examined the data analysis. This study was performed in accordance with the moral principles of the Declaration of Helsinki.
The main data collected included patients’ demographic characteristics, imaging and serological reports, treatment plans, and prognoses that could be traced back.
The following demographic and sociological characteristics were collected: (1) Hospitalization number; (2) sex; (3) age; (4) dates of admission and discharge; (5) clinical status at discharge; (6) presence of chronic diseases (e.g., hypertension, diabetes, and stroke); (7) presence of a family history of cancer; (8) presence of chronic viral hepatitis; (9) presence of cirrhosis; and (10) whether the patient was screened for HCC.
The patients’ imaging diagnostic reports included computed tomography and magnetic resonance imaging findings, liver biopsy records, and intraoperative images when diagnosed with HCC. The data collected included: (1) Number of tumors; (2) maximum diameter of a single tumor; (3) presence of extrahepatic or lymph node metastasis; (4) degree of tumor differentiation; (5) presence of ascites and its severity; and (6) presence of portal hypertension.
The serological reports of the patients included: (1) Alpha-fetoprotein (AFP); (2) total bilirubin; (3) albumin levels; and (4) prothrombin time. The AFP level in CNLC staging recommended by the guidelines is divided into three[9,10]: AFP ≤ 20, 20–400, and > 400 (ng/mL); according to the guidelines, an AFP level > 400 ng/mL is considered significantly increased.
The treatment information collected included: (1) Whether the patients accepted surgical treatment and the method, which included LR, radiofrequency ablation (RFA), liver transplantation (LT), and transcatheter arterial chemoembolization (TACE); and (2) whether the patients used oral anti-tumor drugs and the dosage regimen.
Patient survival: For patients with no clinical outcome in the hospital, we followed up with their families via telephone to determine the survival status of the patients. The main contents of the follow-up included: (1) Whether the patients survived until December 31, 2020; and (2) if the patient died, the cause and time of death were noted.
CNLC staging: We performed CNLC staging according to the guidelines, as follows: (1) CNLC Ia stage: performance status (PS): 0–2, Child–Pugh A/B liver function, single tumor, diameter ≤ 5 cm, with no vascular tumor thrombus and extrahepatic metastasis on imaging; (2) CNLC stage Ib: PS 0–2, Child–Pugh A/B liver function, single tumor, diameter > 5 cm, or two to three tumors, maximum diameter ≤ 3 cm, with no vascular tumor thrombus and extrahepatic metastasis; (3) CNLC stage IIa: PS 0–2, Child–Pugh A/B liver function, two to three tumors, maximum diameter > 3 cm, with no vascular tumor thrombus and extrahepatic metastasis; (4) CNLC stage IIb: PS 0–2, Child–Pugh A/B liver function, tumor number ≥ 4, status regardless of tumor diameter, with no vascular tumor thrombus and extrahepatic metastasis; (5) CNLC stage IIIa: PS 0–2, Child–Pugh A/B liver function, tumor status regardless of vascular tumor thrombus and no extrahepatic metastasis; (6) CNLC stage IIIb: PS 0–2, Child–Pugh A/B liver function, regardless of tumor status, whether there was vascular tumor thrombus on imaging, and extrahepatic metastasis; and (7) CNLC stage IV: PS 3–4 or Child–Pugh C liver function, regardless of tumor status, with presence or absence of vascular tumor thrombus on imaging and presence or absence of extrahepatic metastasis[9,10]. Patients with CNLC stages Ia, Ib, and IIa are generally considered to have early-stage HCC; those with CNLC stages IIb and IIIa are considered to have middle-stage HCC, and those with CNLC stages IIIb and IV are considered to have late-stage HCC.
The guidelines recommend that early screening for HCC includes liver ultrasound imaging and serum AFP level determination[9,10]. Furthermore, the guidelines identify high-risk groups for HCC as those with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection, excessive alcohol consumption, non-alcoholic steatohepatitis, cirrhosis caused by other causes, and a family history of liver cancer, especially in males older than 40 years[9,10].
In this study, patients with HCC who were diagnosed by screening were (1): Those who reported that they might have a high risk for HCC and those diagnosed with HCC via regular re-examination; and (2) those diagnosed with HCC during a routine physical examination without any symptoms.
The diagnosis and treatment of these patients in the real world and their compliance with the treatment recommended by the guidelines were analyzed. As this study was retrospective, information on the treatment methods received by the patients was collected from the hospital’s electronic medical records system and confirmed by attending clinicians.
The therapeutic effects of the patients in this study were reflected mainly in two aspects: (1) The survival analysis of 1128 patients traced back to the clinical outcomes based on different CNLC staging, whether it complied with the guidelines, and the main risk factors affecting the patients’ survival; and (2) the analysis of tumor-free survival and risk factors for postoperative tumor recurrence in patients with HCC who received LR.
The baseline characteristics and treatment modalities of the study population based on CNLC staging were summarized as frequencies (percentages), means (standard deviations), or medians (ranges). Categorical variables were summarized as numbers and percentages, and continuous variables were presented as medians, ranges, and 95%CI. Categorical data were compared using Chi-squared or Fisher’s exact tests, and continuous variables were compared using Mann–Whitney’s U test. The primary outcome was overall survival time and the secondary outcome was tumor-free survival time. The overall survival time since HCC diagnosis and the median tumor-free survival time after LR in each CNLC stage were determined using Kaplan–Meier estimations. Comparisons of overall survival probability rates between groups were illustrated using Kaplan–Meier survival curves, and the survival differences between groups were estimated using log–rank tests. Statistical significance was set at P < 0.05. Finally, we used Cox models to analyze independent factors affecting overall survival time and tumor-free survival in patients who received LR.
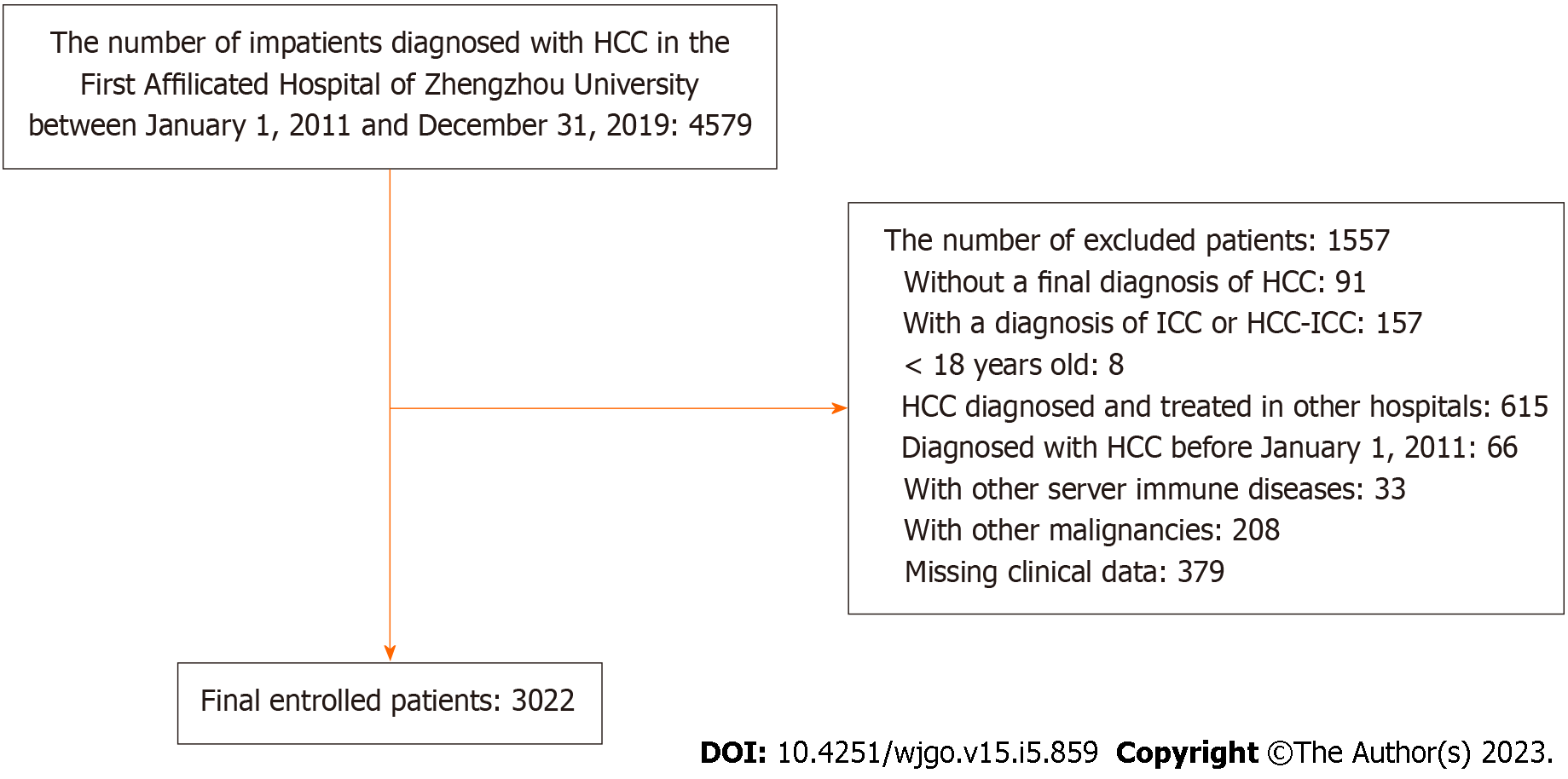
Between 2011 and 2019, 4579 patients were diagnosed with HCC and hospitalized in the First Affiliated Hospital of Zhengzhou University, with 3022 patients screened for inclusion in this study (Figure 1). Among the patients included, 2487 (82.3%) males and 535 (17.7%) females were diagnosed with HCC for the first time between the ages of 18 and 84 years, with an average age of 59.57 years. The proportions of patients with HCC with a history of smoking and drinking were 38.32% and 31.40%, respectively. Among the eligible patients, chronic liver disease was as follows: 2793 had HBV, 187 had HCV, 106 had non-alcoholic fatty liver disease (NAFLD), and 24 developed alcoholic liver disease (ALD). The following complications were noted: A total of 533 had hypertension, 360 had diabetes, and 95 had coronary heart disease. At initial diagnosis, 36.2% of the patients had normal AFP values (< 20 ng/mL), and 38.7% had significantly elevated AFP levels (> 400 ng/mL). The highest proportion of patients with significantly increased AFP was found in those with CNLC stage IV, reaching 59.5% (Table 1). The total number of patient hospitalizations was 14441, with an average hospitalization number and duration of 4.78 times and 9.59 d, respectively.
| Variables | CNLC staging | |||||||
| Total | Ia | Ιb | IIa | IIb | IIIa | IIIb | IV | |
| Sex | ||||||||
| Male | 2487 (82.3) | 492 (79.3) | 365 (81.7) | 47 (78.3) | 742 (83.7) | 468 (84.7) | 295 (81.4) | 78 (82.9) |
| Female | 535 (17.7) | 128 (20.7) | 82 (18.3) | 13 (21.7) | 145 (16.3) | 84 (16.3) | 67 (18.6) | 16 (17.1) |
| Age | ||||||||
| ≤ 50 | 884 (29.2) | 185 (29.8) | 116 (25.9) | 16 (26.7) | 245 (27.7) | 175 (31.7) | 112 (30.9) | 35 (37.3) |
| > 50 | 2138 (70.8) | 435 (70.2) | 331 (74.1) | 44 (73.3) | 642 (72.3) | 377 (68.3) | 250 (69.1) | 59 (62.7) |
| Chronic hepatitis | ||||||||
| HBV | 2793 (90.6) | 558 (90.0) | 398 (89.0) | 52 (86.6) | 808 (91.0) | 513 (92.9) | 323 (89.2) | 87 (92.5) |
| HCV | 187 (6.1) | 48 (7.7) | 22 (4.9) | 6 (10.0) | 54 (6.0) | 32 (5.7) | 19 (5.2) | 6 (6.3) |
| NAFLD | 106 (3.5) | 34 (32.0) | 27 (25.4) | 0 (0.0) | 23 (21.6) | 12 (11.3) | 8 (7.5) | 2 (1.8) |
| ALD | 24 (0.7) | 4 (16.6) | 3 (12.5) | 1 (4.1) | 7 (29.1) | 6 (25.0) | 2 (8.3) | 1 (4.1) |
| Complications | ||||||||
| Hypertension | 533 (17.6) | 125 (20.1) | 87 (19.4) | 9 (15.0) | 158 (17.8) | 82 (14.8) | 60 (16.5) | 12 (12.7) |
| Diabetes | 360 (11.9) | 75 (12.0) | 42 (9.3) | 12 (20.0) | 112 (12.6) | 68 (12.3) | 39 (10.7) | 12 (12.7) |
| Coronary heart disease | 95 (3.1) | 22 (3.5) | 15 (3.3) | 1 (1.6) | 30 (3.3) | 13 (2.3) | 12 (3.3) | 2 (2.1) |
| Smoking history | 1158 (38.32) | 215 (34.96) | 170 (39.63) | 25 (35.71) | 340 (38.16) | 223 (39.89) | 152 (41.76) | 33 (35.11) |
| Drinking history | 949 (31.40) | 182 (29.59) | 136 (31.70) | 18 (25.71) | 277 (31.09) | 176 (31.48) | 128 (35.16) | 32 (34.04) |
| AFP (ng/mL) | ||||||||
| ≤ 20 | 1094 (36.2) | 345 (55.7) | 187 (41.8) | 42 (70.0) | 318 (35.9) | 115 (20.8) | 90 (24.8) | 16 (17.0) |
| 20–400 | 757 (25.1) | 170 (27.4) | 101 (22.6) | 18 (30.0) | 223 (26.2) | 132 (23.9) | 80 (22.1) | 22 (23.5) |
| > 400 | 1171 (38.7) | 105 (16.9) | 159 (35.6) | 0 (0.0) | 336 (37.9) | 305 (55.2) | 192 (53.1) | 56 (59.5) |
Chinese Liver Cancer staging was performed for all patients with HCC, including 620 with CNLC stage Ia, 447 with CNLC stage Ib, 60 with CNLC stage IIa, 887 with CNLC stage IIb, 552 with CNLC stage IIIa, 362 with CNLC stage IIIb, and 94 with CNLC stage IV. The final diagnosis methods of patients with different CNLC stages also differed. The diagnosis methods of patients with CNLC stage Ia, Ib, and IIa were mainly pathology based, accounting for 70.3%, 60.4%, and 63.3%, respectively. The diagnosis methods of patients with CNLC stage IIb, IIIa, IIIb, and IV were mainly based on imaging examinations and clinical features (Table 1), accounting for 67.3%, 58.6%, 69.3%, and 81.9%, respectively. The liver function grades of the patients were as follows: There were 2191 patients with Child–Pugh grade A, 737 with Child–Pugh grade B, and 94 with Child–Pugh grade C (Table 2).
| CNLC staging | ||||||||
| Total (n = 3022) | Ia (n = 620) | Ιb (n = 447) | IIa (n = 60) | IIb (n = 887) | IIIa (n = 552) | IIIb (n = 362) | IV (n = 94) | |
| HCC diagnosis | ||||||||
| Pathology | 1390 (45.9) | 436 (70.3) | 270 (60.4) | 38 (63.3) | 290 (32.6) | 228 (41.3) | 111 (30.6) | 17 (18.1) |
| Imaging | 1632 (54.1) | 184 (29.6) | 117 (39.5) | 22 (36.6) | 597 (67.3) | 324 (58.6) | 251 (69.3) | 77 (81.9) |
| Child–Pugh grade | ||||||||
| A | 2191 (72.5) | 537 (86.6) | 378 (84.5) | 48 (80.0) | 625 (70.4) | 369 (66.8) | 234 (64.6) | 0 (0.0) |
| B | 737 (24.3) | 83 (13.4) | 69 (15.4) | 12 (20.0) | 262 (29.5) | 183 (33.1) | 128 (35.4) | 0 (0.0) |
| C | 94 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 94 (100.0) |
| Treatments | ||||||||
| LR | 803 (26.5) | 324 (52.2)1 | 188 (42.0)1 | 28 (46.6)1 | 102 (11.4)1 | 126 (22.8)1 | 35 (9.6) | 0 (0.0) |
| RFA | 203 (6.7) | 104 (16.7)1 | 27 (6.0)1 | 5 (8.3) | 48 (5.5) | 10 (1.8) | 6 (1.6) | 3 (3.1) |
| LT | 92 (3.0) | 10 (1.6)1 | 4 (0.8)1 | 0 (0.0) | 38 (4.2) | 25 (4.5) | 6 (1.6) | 9 (9.5)1 |
| TACE | 1052 (34.8) | 93 (15.0) | 145 (32.4)1 | 13 (21.6)1 | 422 (47.5)1 | 210 (38.0)1 | 141 (38.9)1 | 28 (29.7) |
| TACE + RFA | 185 (6.1) | 46 (7.4) | 33 (7.3)1 | 7 (11.6)1 | 67 (7.5) | 11 (1.9) | 15 (4.1)1 | 6 (6.3) |
| LR + RFA | 89 (2.9) | 23 (3.7)1 | 19 (4.2) | 4 (6.6)1 | 17 (1.8)1 | 19 (3.4)1 | 5 (1.3) | 2 (2.1) |
| Systemic anti-tumor therapy | 224 (7.4) | 4 (0.6) | 12 (2.6) | 1 (1.6) | 74 (8.3)1 | 58 (10.5)1 | 63 (17.4)1 | 13 (13.8)1 |
| BSC | 374 (12.3) | 16 (2.5) | 20 (4.4) | 2 (3.3) | 119 (13.4) | 93 (16.8) | 91 (25.1) | 33 (35.1)1 |
| Adherence1 | 2212 (73.2) | 461 (74.3) | 397 (88.8) | 52 (86.6) | 615 (69.3) | 413 (74.8) | 219 (60.4) | 55 (58.5) |
Of the 3022 patients with HCC, 304 (10.06%) were diagnosed during screening (Table 3), 69.08% were in the early stage, 28.29% were in the middle stage, and 2.63% were in the late stage. Among 2718 patients with HCC who were not diagnosed during screening, only 33.74% were in the early stage, which was a significantly lower level than in those who were screened (P < 0.01). Among the patients who were screened, 90.79% had Child–Pugh grade-A liver function, while only 70.46% of the patients who were not screened had Child–Pugh grade-A liver function, and the difference was statistically significant (P < 0.01) (Table 3). These results suggest that screening can detect early CNLC HCC stages and that these patients had better liver function. Additionally, patients with HCC who were screened had a greater probability of receiving curative treatment methods, such as LR and LT.
| Variable | Total (n = 3022) | Whether to undergo screening | χ2 | P value | |
| Yes, n = 304 (10.06%) | No, n = 2718 (89.94%) | ||||
| Sex | 0.254 | 0.614 | |||
| Male | 2487 (82.30) | 247 (81.25) | 2240 (82.41) | ||
| Female | 535 (17.70) | 57 (18.75) | 478 (17.59) | ||
| Age | 0.621 | 0.431 | |||
| < 50 | 884 (29.25) | 83 (27.30) | 801 (29.47) | ||
| ≥ 50 | 2138 (70.75) | 221 (72.70) | 1917 (70.53) | ||
| Chronic hepatitis | 1.891 | 0.595 | |||
| HBV | 2694 (89.15) | 266 (87.50) | 2428 (89.33) | ||
| HCV | 142 (4.70) | 19 (6.25) | 123 (4.53) | ||
| Mix | 45 (1.49) | 5 (1.64) | 40 (1.47) | ||
| None | 141 (4.66) | 14 (4.61) | 127 (4.67) | ||
| Smoking | 0.652 | 0.419 | |||
| Yes | 1158 (38.32) | 110 (36.18) | 1048 (38.56) | ||
| No | 1864 (61.68) | 194 (63.82) | 1670 (61.44) | ||
| Alcohol | 1.217 | 0.270 | |||
| Yes | 949 (31.40) | 87 (28.62) | 862 (31.71) | ||
| No | 2073 (68.60) | 217 (71.38) | 1856 (68.29) | ||
| Hypertension | 2.714 | 0.099 | |||
| Yes | 533 (17.64) | 64 (21.05) | 469 (17.26) | ||
| No | 2489 (82.36) | 240 (78.95) | 2249 (82.74) | ||
| Diabetes | 0.002 | 0.968 | |||
| Yes | 360 (11.91) | 36 (11.84) | 324 (11.92) | ||
| No | 2662 (88.09) | 268 (88.16) | 2394 (88.08) | ||
| Coronary heart disease | 3.559 | 0.059 | |||
| Yes | 95 (3.14) | 15 (4.93) | 80 (2.94) | ||
| No | 2927 (96.86) | 289 (95.07) | 2638 (97.06) | ||
| Family history of tumors | 0.009 | 0.923 | |||
| Yes | 473 (15.65) | 47 (15.46) | 426 (15.67) | ||
| No | 2549 (84.35) | 257 (84.54) | 2292 (84.33) | ||
| AFP (ng/mL) | 41.808 | 0.000a | |||
| ≤ 20 | 1094 (36.20) | 145 (47.70) | 949 (34.92) | ||
| 20-400 | 757 (25.05) | 93 (30.59) | 664 (24.43) | ||
| > 400 | 1171 (38.75) | 66 (21.71) | 1105 (40.65) | ||
| Child-Pugh Grade | 58.031 | 0.000a | |||
| A | 2191 (72.50) | 276 (90.79) | 1915 (70.46) | ||
| B | 737 (24.39) | 28 (9.21) | 709 (26.09) | ||
| C | 94 (3.11) | 0 (0.00) | 94 (3.45) | ||
| CNLC | 183.202 | 0.000a | |||
| Ia | 620 (20.52) | 141 (46.38) | 479 (17.62) | ||
| Ιb | 447 (14.79) | 57 (18.75) | 390 (14.35) | ||
| IIa | 60 (1.99) | 12 (3.95) | 48 (1.77) | ||
| IIb | 887 (29.35) | 59 (19.41) | 828 (30.46) | ||
| IIIa | 552 (18.27) | 27 (8.88) | 525 (19.32) | ||
| IIIb | 362 (11.98) | 8 (2.63) | 354 (13.02) | ||
| IV | 94 (3.10) | 0 (0.00) | 94 (3.46) | ||
Among 3022 patients, 2648 (87.62%) received active anti-tumor therapy, 803 received LR, 203 received RFA, 92 received LT, 1052 received TACE, 185 received TACE + RFA, 89 received TACE + LR, and 224 received systemic anti-tumor therapy in their first treatment. The remaining 374 received only palliative therapy or had their treatment discontinued, and the patient was discharged. Overall, 2212 patients received the treatment recommended by the guidelines, accounting for 73.20% (2212/3022) of all patients, and 83.76% (2212/2641) received active anti-tumor therapy. Among these patients, 74.3%, 88.8%, 86.6%, 69.3%, 74.8%, 60.4%, and 58.5% of those in stages Ia, Ib, IIa, IIb, IIIa, IIIb, and IV received the treatments recommended by the guidelines, respectively (Table 2). The results indicated that the treatment recommended by the guidelines was in good agreement with the clinical treatment of HCC in China during the last 10 years. Patients in the early and middle stages were more likely to accept the treatment recommended by the guidelines. Table 4 shows that 76.27% of the patients receiving guideline-recommended treatment had Child–Pugh grade-A liver function, while only 62.22% of the patients not receiving the treatment recommended by the guidelines had Child–Pugh grade-A liver function (P < 0.001). This suggests that the liver function status of the patients was an important factor affecting their treatment choice. Table 4 also shows the CNLC staging characteristics of 810 patients who did not receive the treatment recommended by the guidelines: Of these, 26.79% were in the early stage of HCC, 50.74% were in the middle stage, and 22.47% were in the late stage, indicating that most of the patients who did not receive the treatment recommended by the guidelines were in the middle stage of HCC.
| Variable | Total (n = 3022) | Compliance with guideline-recommended treatment | χ2 | P value | |
| Yes, n = 2212 (73.20%) | No, n = 810 (26.80%) | ||||
| Sex | 3.191 | 0.074 | |||
| Male | 2487 (82.30) | 1837 (83.05) | 650 (80.25) | ||
| Female | 535 (17.70) | 375 (16.95) | 160 (19.75) | ||
| Age | 0.652 | 0.419 | |||
| < 50 | 884 (29.25) | 656 (29.66) | 228 (28.15) | ||
| ≥ 50 | 2138 (70.75) | 1556 (70.34) | 582 (71.85) | ||
| Chronic hepatitis | 2.046 | 0.309 | |||
| HBV | 2694 (89.15) | 1979 (89.47) | 715 (88.27) | ||
| HCV | 142 (4.70) | 96 (4.34) | 46 (5.68) | ||
| Mixed | 45 (1.49) | 36 (1.63) | 9 (1.11) | ||
| None | 141 (4.66) | 101 (4.56) | 40 (4.94) | ||
| AFP (ng/mL) | 1.859 | 0.395 | |||
| ≤ 20 | 1094 (36.20) | 786 (35.53) | 308 (38.02) | ||
| 20–400 | 757 (25.05) | 555 (25.09) | 202 (24.94) | ||
| > 400 | 1171 (38.75) | 871 (39.38) | 300 (37.04) | ||
| Child–Pugh grade | 59.828 | 0.000a | |||
| A | 2191 (72.50) | 1687 (76.27) | 504 (62.22) | ||
| B | 737 (24.39) | 470 (21.25) | 267 (32.96) | ||
| C | 94 (3.11) | 55 (2.49) | 39 (4.81) | ||
| CNLC | 74.941 | 0.000a | |||
| Early stage (Ia, Ib, and IIa) | 1127 (37.29) | 910 (41.14) | 217 (26.79) | ||
| Middle stage (IIb and IIIa) | 1439 (47.62) | 1028 (46.47) | 411 (50.74) | ||
| Late stage (IIIb and IV) | 456 (15.09) | 274 (12.39) | 182 (22.47) | ||
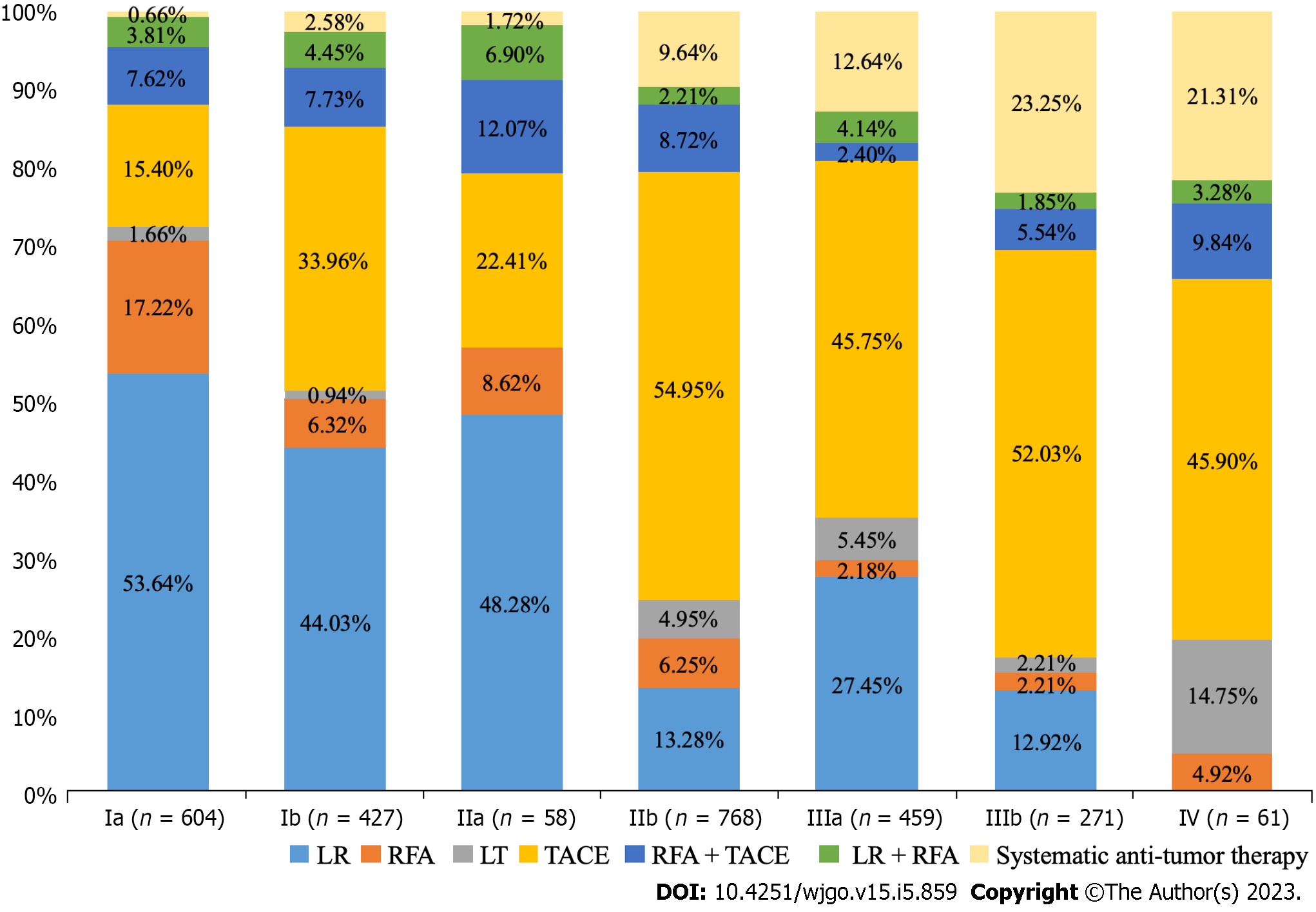
Real-world data showed that among the 2648 patients receiving active anti-tumor therapy, the treatment choice was not the same in each CNLC stage (Figure 2). LR and RFA accounted for 53.64% and 17.22%, respectively, inpatients with CNLC stage Ia HCC, while in patients with CNLC stage Ib HCC, LR (44.03%) and TACE (33.96%) were mainly selected. In patients with CNLC stage IIa, treatment was mainly with LR (48.28%) and TACE (22.41%), while those with CNLC stage IIb were treated mainly with TACE (54.95%) and LR (13.28%). In CNLC stage IIIa, TACE (45.75%) and LR (27.45%) were mostly selected, while patients in CNLC stage IIIb received mainly TACE (52.03%) and systemic anti-tumor therapy (23.25%). Among 94 patients with CNLC stage IV HCC, 61 received anti-tumor therapy, with most receiving TACE (45.90%) and LT (21.31%). These results suggest that patients in the early stage of HCC were more likely to receive LR, while those in the middle and late stages were more likely to receive TACE.
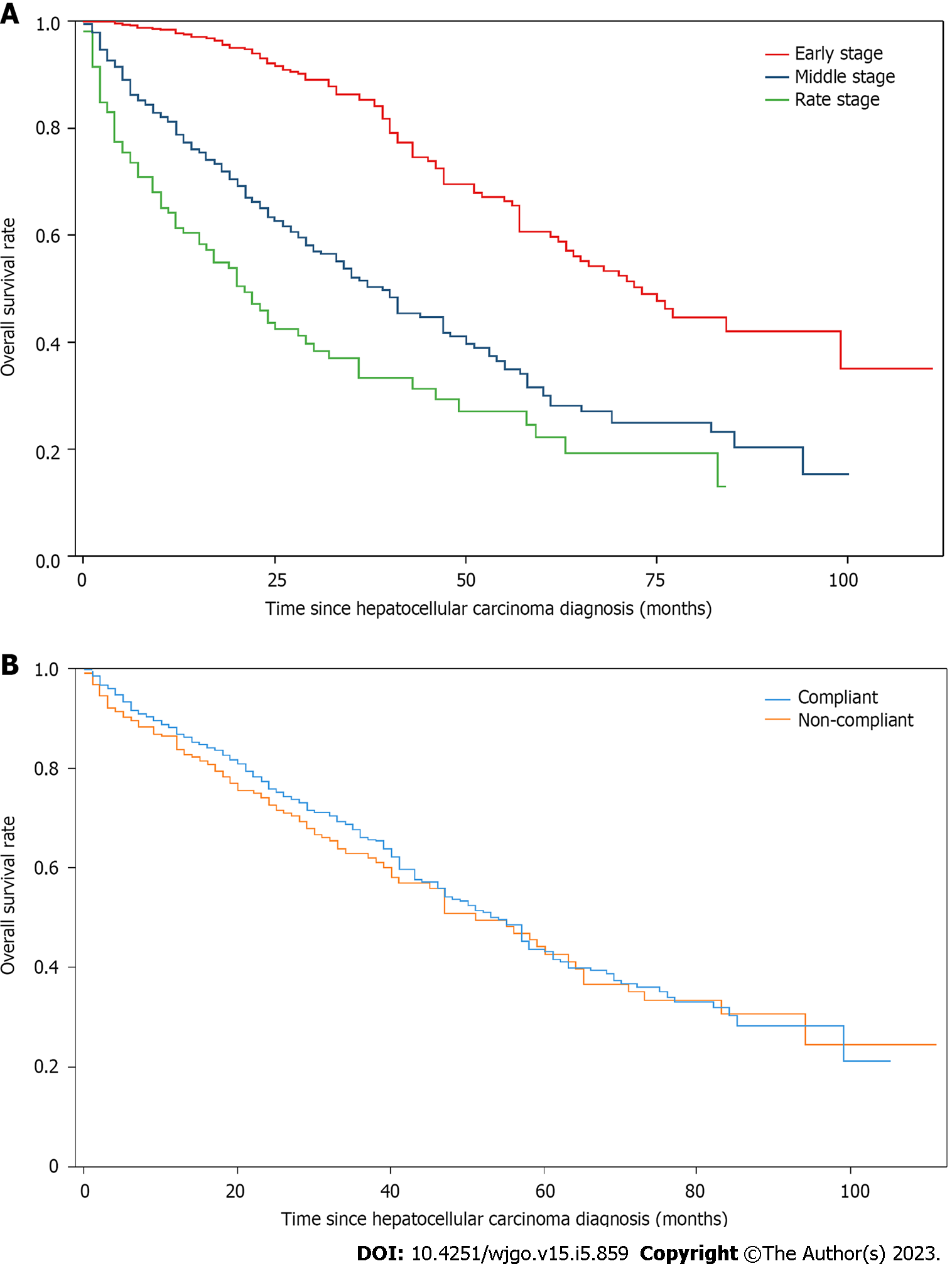
Overall survival analysis: In this study, patients who did not have a clinical outcome during hospitalization were followed up to December 31, 2020. We collected the clinical outcomes of 1128 patients. Among the general clinical characteristics of these patients, 922 (81.74%) were male, and 798 (70.74%) were over the age of 50. There were 992 (87.94%) patients with HBV, 56 (4.96%) with HCV, 62 (5.50%) with NAFLD, and 18 (1.60%) with ALD. There were 226 (20.04%) patients with hypertension, 159 (14.10%) with diabetes, and 33 (2.93%) with coronary heart disease. Of the patient population, 408 (36.17%) and 337 (29.88%) had a history of smoking and drinking, respectively. There were 870 (77.13%) cases of Child–Pugh grade A, 228 (20.21%) cases of Child–Pugh Grade B, and 30 (2.66%) cases of Child–Pugh grade C (Table 5). In terms of overall survival, 407 deaths and 721 survivals were observed, with a median survival of 54.0 mo (95%CI: 50.0–61.0). Statistically significant differences were observed in overall survival among patients with different CNLC staging (P < 0.001), with a median survival of 73 mo (95%CI: 63.0–99.0) in the early stage, 39 mo (95%CI: 33.0–47.0) in the middle stage, and 21 mo (95%CI: 15.0–29.0) in the late stage (Figure 3A). No statistically significant differences were observed in terms of overall survival between patients who complied with the treatment recommended by the guidelines and those who did not (P = 0.344). The median survival was 54 mo (95%CI: 47.0–58.0) and 51 mo (95%CI: 41.0–64.0), respectively (Figure 3B).
| Variables | CNLC staging | |||||||
| Total | Ia | Ιb | IIa | IIb | IIIa | IIIb | IV | |
| Sex | ||||||||
| Male | 922 (81.74) | 261 (80.56) | 152 (79.58) | 21 (75.00) | 252 (84.00) | 151 (84.36) | 60 (78.95) | 25 (83.33) |
| Female | 206 (18.26) | 63 (19.44) | 39 (20.42) | 7 (25.00) | 48 (16.00) | 28 (15.64) | 16 (21.05) | 5 (16.67) |
| Age | ||||||||
| ≤ 50 | 330 (29.26) | 105 (32.41) | 51 (26.70) | 8 (28.57) | 74 (24.67) | 54 (30.17) | 23 (30.26) | 15 (50.00) |
| > 50 | 798 (70.74) | 219 (67.59) | 140 (73.30) | 20 (71.43) | 226 (75.33) | 125 (69.83) | 53 (69.74) | 15 (50.00) |
| Chronic hepatitis | ||||||||
| HBV | 992 (87.94) | 284 (87.65) | 167 (87.43) | 21 (75.00) | 266 (88.67) | 161 (89.94) | 68 (89.47) | 25 (83.33) |
| HCV | 56 (4.96) | 21 (6.48) | 8 (4.19) | 3 (10.71) | 12 (4.00) | 8 (4.47) | 2 (2.63) | 2 (6.67) |
| NAFLD | 62 (5.50) | 14 (4.32) | 14 (7.33) | 3 (10.71) | 17 (5.67) | 6 (3.35) | 6 (7.89) | 2 (6.67) |
| ALD | 18 (1.60) | 5 (1.54) | 2 (1.05) | 1 (3.57) | 5 (1.67) | 4 (2.23) | 0 (0.00) | 1 (3.33) |
| Complications | ||||||||
| Hypertension | 226 (20.04) | 70 (21.60) | 38 (19.90) | 5 (17.86) | 65 (21.67) | 28 (15.64) | 17 (22.37) | 3 (10.00) |
| Diabetes | 159 (14.10) | 2 (12.96) | 21 (10.99) | 6 (21.43) | 49 (16.33) | 24 (13.41) | 11 (14.47) | 6 (20.00) |
| Coronary heart disease | 33 (2.93) | 7 (2.16) | 8 (4.19) | 1 (3.57) | 10 (3.33) | 2 (1.12) | 4 (5.26) | 1 (3.33) |
| Smoking | 408 (36.17) | 121 (37.35) | 68 (35.60) | 8 (28.57) | 101 (33.67) | 67 (37.43) | 30 (39.47) | 13 (43.33) |
| Drinking | 337 (29.88) | 98 (30.25) | 53 (27.75) | 7 (25.00) | 88 (29.33) | 50 (27.93) | 29 (38.16) | 12 (40.00) |
| AFP (ng/mL) | ||||||||
| ≤ 20 | 491 (43.53) | 184 (56.79) | 90 (47.12) | 11 (39.29) | 129 (43.00) | 46 (25.70) | 24 (31.58) | 7 (23.33) |
| 20–400 | 289 (25.62) | 92 (28.40) | 43 (22.51) | 8 (28.57) | 75 (25.00) | 47 (26.26) | 16 (21.05) | 8 (26.67) |
| > 400 | 348 (30.85) | 48 (14.81) | 58 (30.37) | 9 (32.14) | 96 (32.00) | 86 (48.04) | 36 (47.37) | 15 (50.00) |
| Child–Pugh grade | ||||||||
| A | 870 (77.13) | 284 (87.65) | 168 (87.96) | 23 (82.14) | 213 (71.00) | 132 (73.74) | 50 (65.79) | 0 (0.00) |
| B | 228 (20.21) | 40 (12.35) | 23 (12.04) | 5 (17.86) | 87 (29.00) | 47 (26.26) | 26 (34.21) | 0 (0.00) |
| C | 30 (2.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 30 (100.00) |
Subsequently, we used the Cox model to analyse the risk factors affecting patients’ survival. The univariate results showed that age, screening, Child–Pugh grade, AFP level, and CNLC staging were risk factors affecting the survival time of patients with HCC (P < 0.05). A multivariate analysis used to verify these results revealed that the following independent risk factors affected overall survival: Age > 50 years [hazard ratio (HR): 1.359, 95%CI: 1.081–1.709], no screening (HR: 2.181, 95%CI: 1.435–3.313), AFP > 400 ng/mL (HR: 1.576, 95%CI: 1.256–1.977), Child–Pugh grade B (HR: 1.813, 95%CI: 1.480–2.252), middle-stage HCC (HR: 2.610, 95%CI: 2.056–3.312), and late-stage HCC (HR: 3.967, 95%CI: 2.827–5.591) (Table 6).
| Variables | Univariate analysis | Multivariate analysis | |
| P value | P value | Hazard ratio (95%CI) | |
| Sex | 0.5736 | — | |
| Age (> 50) | 0.0074a | 0.0087b | 1.359 (1.081–1.709) |
| Screening | < 0.001b | 0.0003b | 2.181 (1.435–3.313) |
| HBV | 0.0703 | — | |
| HCV | 0.0354 | — | |
| Child-Pugh grade | < 0.001b | ||
| A | 1 reference | ||
| B | < 0.001b | 1.813 (1.480–2.252) | |
| C | 0.3798 | 1.274 (0.742–2.190) | |
| CNLC staging | < 0.001b | ||
| Early | 1 reference | ||
| Middle | < 0.001b | 2.610 (2.056–3.312) | |
| Late | < 0.001b | 3.967 (2.827–5.591) | |
| AFP (ng/mL) | < 0.001b | ||
| ≤ 20 | 1 reference | ||
| 20–400 | 0.615 | 1.070 (0.822–1.392) | |
| > 400 | < 0.001b | 1.576 (1.256–1.977) | |
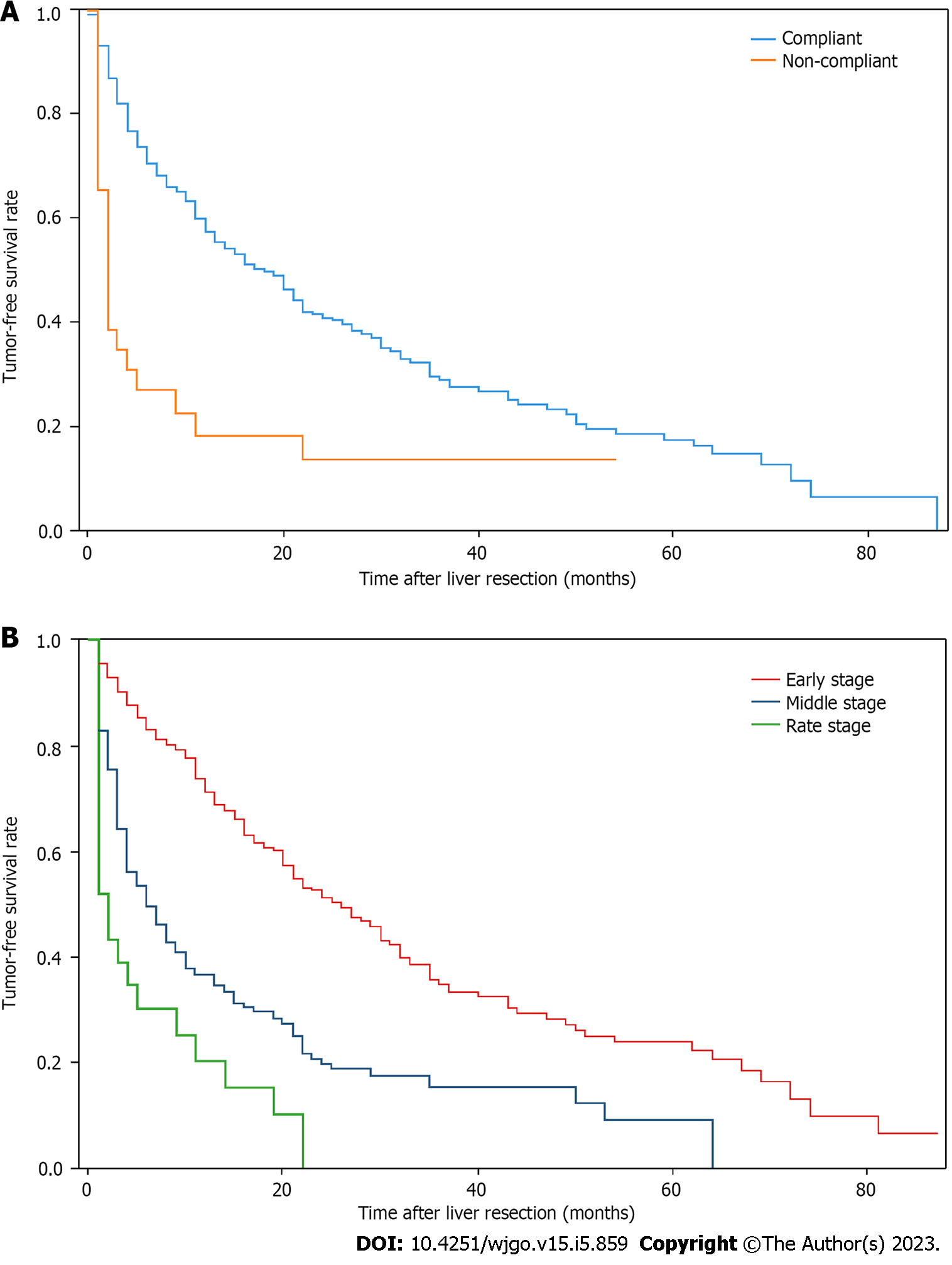
Tumor-free survival in patients with HCC treated with LR: Overall, 803 patients underwent LR, including 768 who received LR as recommended by the guidelines and 35 who did not receive LR as recommended by the guidelines. Statistically significant differences were observed in terms of tumor-free survival between patients who received guideline-recommended LR treatment and those who received non-guideline-recommended LR treatment (P < 0.001), with median tumor-free survival of 18 mo (95%CI: 14.0–21.0) and 2 mo (95%CI: 1.0-4.0), respectively (Figure 4A). Among the 803 patients receiving LR at different CNLC stages, 540 patients had early-stage HCC; of these, 200 experienced a recurrence during the study period, with a maximum of 87 mo and a median of 24 mo of tumor-free survival (95%CI: 20.0–30.0). A total of 228 patients had middle-stage HCC, 130 of whom experienced a recurrence during the study, with a maximum tumor-free survival of 59 mo and a median of 5 mo (95%CI: 4.0–7.0). Finally, 35 patients had late-stage HCC, 22 of whom experienced a recurrence during the study, with a maximum tumor-free survival of 54 mo and a median of 2 mo (95%CI: 1.0–4.0). The results showed a statistically significant difference in tumor-free survival between patients who received LR in the early stage of HCC and those who received LR in the middle and late stages of the disease (P < 0.001). No statistically significant differences were observed in tumor-free survival between patients who received LR in the middle and late stages of HCC (P = 0.099) (Figure 4B).
The Cox model was used to analyze the risk factors for postoperative recurrence in patients undergoing LR. The univariate results showed that sex, Child–Pugh grade, AFP level, and CNLC staging were independent factors for tumor recurrence (P < 0.05). These results were verified using a multivariate analysis, revealing that AFP > 400 ng/mL (HR: 1.612, 95%CI: 1.256–2.070), Child–Pugh grade B (HR: 1.771, 95%CI: 1.243–2.524), middle-stage HCC (HR: 2.556, 95%CI: 2.032–3.215), and late-stage HCC (HR: 3.312, 95%CI: 2.113–5.192) were independent factors affecting the postoperative recurrence of HCC (Table 7).
| Variables | Univariate analysis | Multivariate analysis | |
| P value | P value | Hazard ratio (95%CI) | |
| Sex | 0.0455a | 0.0164a | 0.694 (0.515–0.935) |
| Age (> 50) | 0.9711 | — | |
| Screening | 0.0678 | — | |
| HBV | 0.7773 | — | |
| HCV | 0.3531 | — | |
| Child-Pugh grade | 0.0012b | 0.0016b | 1.771 (1.243–2.524) |
| CNLC staging | < 0.001b | ||
| Early | 1 reference | ||
| Middle | < 0.001b | 2.556 (2.032–3.215) | |
| Late | < 0.001b | 3.312 (2.113-5.192) | |
| AFP (ng/mL) | < 0.001b | ||
| ≤ 20 | 1 reference | ||
| 20–400 | 0.338 | 1.139 (0.873–1.487) | |
| > 400 | < 0.001b | 1.612 (1.256–2.070) | |
Liver cancer staging is important for selecting treatment options and evaluating prognosis. Many staging systems exist, such as the BCLC, EASL, APASL, JSH, and HKLC systems[3-7]. Among them, the most widely used is the BCLC staging system. The CNLC staging system was established specifically for Chinese people by the National Health Commission in combination with China’s specific national conditions and practice accumulation[9,10]. As China has the highest number of liver cancer cases of all countries[1], evaluating the use of China’s guidelines for HCC diagnosis and treatment in a real-world setting can provide a broad clinical reference for global liver cancer prevention and treatment.
The strength of this study is that we collected real-world data from a large sample of patients with HCC and conducted CNLC staging for those patients to evaluate the compliance between CNLC staging and real clinical diagnosis and treatment in China. We also performed a systematic review and an analysis of the diagnosis and treatment choices of Chinese patients with HCC in the real world.
The guidelines recommend that imaging and pathological diagnosis are used in HCC diagnosis. In this study, the diagnosis methods of patients in CNLC stages Ia, Ib, and IIa were mainly pathology based, with frequencies of 70.3%, 60.4%, and 63.3%, respectively. The diagnosis methods of patients in CNLC stages IIb, IIIa, IIIb, and IV were based mainly on imaging examinations and clinical features, with frequencies of 67.3%, 58.6%, 69.3%, and 81.9%, respectively.
In this study, 304 patients were screened before HCC diagnosis. No statistical differences were observed in demographic characteristics between screened and unscreened patients; however, significant differences were observed in terms of liver function grade, AFP levels, and CNLC staging. Among those patients diagnosed via screening, 90.79% had Child–Pugh grade-A liver function, while only 70.46% of unscreened patients had the same. The AFP levels of screened patients were low when they were diagnosed with HCC, and only 21.71% of the patients had significantly increased AFP levels (> 400 ng/mL). The proportion of patients who were not screened with significantly increased AFP levels was 40.56%.
Regarding different HCC stages, 69.08%, 28.29%, and 2.63% of patients with early-, middle-, and late-stage HCC were detected via screening, while 33.74%, 49.78%, and 16.48% of patients were detected without screening, respectively. The guidelines recommend the regular screening of groups at high risk for HCC to detect more patients with early-stage HCC. Thus, screening can detect more patients with early-stage HCC and encourage patients to undergo curative treatments, thus extending their survival time.
The guidelines recommend screening for groups at high risk for HCC; however, cluster sampling screening is only available in pilot cities, and a planned and regular national screening program is lacking[20]. Currently, some Asian countries and regions, for example, Korea and Japan, have unified national screening programs, and stratified and phased screening programs have been implemented in Taiwan for groups at high risk for HCC. Because of these programs, screening has detected more patients with early-stage HCC, leading to more effective treatment, longer survival, and better quality of life[21-24]. In Europe and the United States, numerous studies have demonstrated the efficacy and cost-effectiveness of HCC screening[25-27]. For example, in a prospective study conducted in France, HCC screening was cost-effective while prolonging patients’ survival, even after adjusting for lead-time bias[28]. In a study by Da Fonseca et al[29], improved compliance and targeting were key factors in HCC screening. The present study discovered that only 10.06% of patients in the real medical environment received screening or regular physical examinations before HCC diagnosis, indicating that in the real world, Chinese patients with HCC have poor screening compliance.
Studies on compliance with the guidelines in clinical practice in China are limited, with only a few studies on the effectiveness of specific treatment methods based on CNLC staging[11-13] and a systematic review comparing the advantages and disadvantages of CNLC staging with those of other staging methods[14]. In China and other regions, several related studies have been conducted on compliance with BCLC guidelines in clinical practice. For example, in a retrospective study conducted by Zhong et al[30] in China, treatment in BCLC staging was compared with that in CNLC staging in clinical practice. However, that study did not analyse the screening, diagnosis, and prognosis of patients, which are limitations. In a prospective study based on 160 patients in Korea, Kim et al[15] found that only 66% of patients adhered to the treatment regimen recommended by the BCLC guidelines. The main reasons for not using the recommended treatment included refusing surgery, uncertain malignant nodules, lack of a suitable donor, and financial problems. In a study conducted at a general hospital in Italy, Borzio et al[16] found that overall adherence to the BCLC guidelines reached 70%, with better adherence by patients in the early and late stages than by those in the middle stage, possibly due to greater heterogeneity in patients in the middle stage. In a multi-center study in Argentina, overall adherence to the BCLC guidelines among patients with HCC was only 50%, which may have been related to the lack of flexibility in BCLC staging and to clinical decision-making by physicians[17].
In this study, we discovered that 73.20% of the treatment methods used in the clinical diagnosis and treatment of patients with HCC in China were consistent with those recommended by the guidelines. Among patients in different HCC stages, 80.75%, 71.39%, and 60.01% of those with early-, middle-, and late-stage HCC received treatments compliant with the guidelines, respectively, which may have been related to the fact that patients in the early stage have more treatment options and are more likely to accept radical treatments[31]. Due to the large individual heterogeneity among patients with middle-stage HCC, selecting appropriate clinical treatment methods is challenging for physicians. Therefore, patients may choose treatment methods over or under those recommended in the clinical diagnosis and treatment guidelines, that are closely related to their liver function and general activity status[32]. In all patients receiving treatment, more than 50% of those with early-stage HCC received LR therapy for the first time, while more than 50% of patients with middle- and late-stage HCC received TACE.
Since most patients were hospitalized in the study hospital after HCC diagnosis, we were able to analyze tumor-free survival in those who underwent LR. This study’s results showed a significant difference in tumor-free survival between patients with early-stage HCC and those with middle- and late-stage HCC who received LR, while no statistical differences were observed between patients in the middle and late stages. Statistically significant (P < 0.001) differences were observed in tumor-free survival between patients who complied with the guidelines while receiving LR and those who did not. In the analysis of influencing factors for recurrence after hepatectomy, patients with Child–Pugh grade-A liver function were considered as a reference because no patient with Child–Pugh grade-C liver function received hepatectomy. Patients with Child–Pugh grade-B liver function experienced more recurrence after hepatectomy (P < 0.01), indicating that the status of patients’ liver function was an important risk factor for recurrence after LR[33].
In a cohort study by Liao et al[11], two nomograms were constructed to compare the reliability of tumor node metastasis (TNM), BCLC, and CNLC staging in predicting the prognosis of Chinese patients with HCC undergoing LR therapy. The results showed that AFP levels and CNLC staging were the main factors affecting the tumor-free survival of patients. Similarly, CNLC staging predicted the prognosis of patients better than TNM and BCLC staging. However, a cohort study by Li et al[13] that examined the prognosis of patients who underwent LR at different stages showed that age, AFP level, tumor size, and tumor number were the main factors affecting early postoperative recurrence.
LR remains the best treatment for HCC. Patients with different stages have shown survival advantages after LR, especially those with early HCC[13]. Similarly, the guidelines recommend that LR be used for eligible patients with late-stage HCC as it has advantages over other treatment methods[13]. In a meta-analysis, survival rates were significantly higher in an LR group than in a TACE group among patients with HCC at BCLC stages B and C[34]. Our study’s results also revealed a significant difference in tumor-free survival between patients with early-stage HCC and those with middle- and late-stage HCC after LR, with those at the early stage demonstrating a significant survival advantage after LR. The guidelines recommend LR for eligible patients with middle-stage HCC after screening; however, compared with those in the late stage, LR-treated patients with middle-stage HCC have an advantage in terms of tumor-free survival, but it is limited. More studies are required to demonstrate the survival benefits and cost-effectiveness of LR compared with those of other treatments for patients with middle- and late-stage HCC.
This study’s results showed that patients who were treated in accordance with the guidelines had a survival advantage over those who were not, but the advantage was limited (54 vs 51 mo), with no statistical difference. This result may be related to the fact that the treatment recommended by the guidelines is relatively fixed. Depending on the actual situation, patients may receive more positive or negative treatment since various factors affect patients’ treatment plans. This result is also reflected in a study by Yen et al[31] that showed that in patients with stage-B and -C BCLC, the choice of treatment over the guidelines had survival advantages for these patients. In contrast, in patients with BCLC at stages 0 and C, treatment options under the recommended treatment guidelines did not affect overall survival. Moreover, a study conducted in Italy by Guarino et al[35] revealed that receiving BCLC-recommended treatment had no significant impact on patients’ overall survival.
However, some studies on patients with specific treatment modalities and specific HCC stages have revealed that receiving the treatment methods recommended in the guidelines provides survival benefits for patients[36-39]. Our study’s results also revealed that patients who received LR treatment based on the guidelines had a significant advantage in terms of tumor-free survival compared with those who did not. We discovered significant differences in the survival time of patients with different stages of HCC. We discovered that the main risk factors affecting the survival of patients with HCC were age > 50 years, no screening, AFP > 400 ng/mL, Child–Pugh grade B, and middle and late CNLC stages. A retrospective study conducted in China by Wang and Li[40] showed that hepatitis B surface antigen, AFP levels, Child–Pugh grade, BCLC stage, antiviral therapy, and treatment methods were important prognostic factors for HCC, and BCLC stage and tumor size were independent prognostic factors, which were similar results to those obtained in our study. Hence, Child–Pugh grade, AFP level, and clinical HCC stage are risk factors affecting the survival of patients with HCC.
This study has some limitations. The data used in this study were obtained from the inpatient information of a single regional medical center, and they should be verified by future multi-center studies. Some patients who were diagnosed with HCC in our study hospital were discharged and treated in other medical institutions. Consequently, the treatment information of these patients could not be obtained, which might have led to the underestimation of the treatment level received. Some patients with ICC may have been included in the study while some with HCC may have been excluded because pathological examinations were not performed in all cases. The attending physician followed the guidelines used to distinguish HCC and ICC; however, it is impossible to rule out ICC or HCC-ICC absolutely because the guidelines are not 100% accurate. The studied patients were followed up; however, only 1128 patients were traced in this retrospective study due to the long study period and the loss of samples, which might have affected the HCC survival rate analysis to some extent. Prospective studies are required to further understand patient survival.
This study’s results suggest that screening enables the early diagnosis of HCC. However, due to the retrospective nature of the study, data on patients’ willingness to undergo screening as well as influencing factors, such as wealth or area of residence, could not be collected. Finally, large-sample multi-center studies are required to provide high-quality evidence for the screening, diagnosis, and treatment guidelines of HCC in China.
The findings revealed that 70% of HCC treatments at the First Affiliated Hospital of Zhengzhou University were performed according to the guidelines. The most frequently used treatment for patients at all stages of HCC was that recommended in the guidelines. No benefit was demonstrated in patients with HCC as a whole who received guideline-recommended treatment; however, those who underwent LR in accordance with the guidelines had a significant survival advantage. We also demonstrated that although screening groups at high risk for HCC according to the guidelines can achieve early diagnosis and treatment, real-world compliance is poor.
Hepatocellular carcinoma (HCC) is a common malignant tumor worldwide; however, no staging scheme that would apply across populations is currently described in the extant literature. Although a China Liver Cancer (CNLC) staging system was proposed for the Chinese population, there is no study regarding the practice of CNLC staging in real clinical settings in China, which the current study addresses.
Although HCC patients detected through screening in the current study had a significant survival advantage compared with those who were not screened, the screening compliance in patients remained was poor. Therefore, improving patients’ screening compliance would be the key to achieving early HCC diagnosis and treatment, which we have identified as the direction of future research.
Most importantly, the study highlighted that although no benefit was demonstrated in HCC patients for receiving the treatment recommended by the guidelines, the patients who underwent liver resection in accordance with the guidelines had a significant survival advantage.
The results of the current study demonstrated that patients who were treated according to the CNLC guidelines had a survival advantage over those who were not treated per the guidelines. However, this advantage was limited i.e., 54 vs 51 mo, and no statistical difference was observed. This result also corroborated with a study, which demonstrated survival advantage for Barcelona Clinic Liver Cancer stages B and C with the choice of treatment over the guidelines. Prospective studies are required to further understand the survival of patients.
The hospitalisation information of patients with HCC admitted to the First Affiliated Hospital of Zhengzhou University was obtained, which included demographic characteristics, imaging and serological reports, treatment, and patients’ prognosis. Thereafter, the CNLC staging was done according to the guidelines. Each stage was characterised by the performance status, tumor number and diameter, liver function status using Child-Pugh A/B, and infringements such as vascular tumor thrombus and extrahepatic metastasis on imaging.
The study aimed to investigate the present situation and problems of HCC diagnosis and treatment guidelines in China, since real-world studies for the existing problems of CNLC staging in China’s clinical practice are lacking.
The method of CNLC staging includes CNLC stages Ia, Ib, IIa, IIb, IIIa, IIIb, and IV, and it is based on the liver function status, tumor size, number, and invasion, and the general conditions of patients with HCC. However, given that the first method of CNLC staging was published as recently as 2017, only a few studies have addressed specific treatment methods in different CNLC staging. Since this study highlights significant difference in the tumor-free survival time between patients undergoing hepatectomy according to the guideline and those undergoing hepatectomy without the guideline, these findings will aid in future research to improve clinical decision making for HCC treatment.
We thank the hepatobiliary surgeons of the First Affiliated Hospital of Zhengzhou University who provided clinical advice for analysing the case data. We are also grateful to our colleagues for their assistance in checking the data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Raffele E, Italy; El-Shishtawy MM, Egypt; Masuda S, Japan S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Hassanipour S, Vali M, Gaffari-Fam S, Nikbakht HA, Abdzadeh E, Joukar F, Pourshams A, Shafaghi A, Malakoutikhah M, Arab-Zozani M, Salehiniya H, Mansour-Ghanaei F. The survival rate of hepatocellular carcinoma in Asian countries: a systematic review and meta-analysis. EXCLI J. 2020;19:108-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2871] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 5. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1637] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 7. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 8. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2386] [Article Influence: 397.7] [Reference Citation Analysis (1)] |
| 9. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 10. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (1)] |
| 11. | Liao R, Wei XF, Che P, Yin KL, Liu L. Nomograms Incorporating the CNLC Staging System Predict the Outcome of Hepatocellular Carcinoma After Curative Resection. Front Oncol. 2021;11:755920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Qin J, Huang Y, Zhou H, Yi S. Efficacy of Sorafenib Combined With Immunotherapy Following Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma: A Propensity Score Analysis. Front Oncol. 2022;12:807102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 13. | Li C, Wang H, Chen R, Zhang H, Xu Y, Zhang B, Li Y, Zhang C, Yang Y, Wang X, Li X. Outcomes and recurrence patterns following curative hepatectomy for hepatocellular carcinoma patients with different China liver cancer staging. Am J Cancer Res. 2022;12:907-921. [PubMed] |
| 14. | Vitale A, Farinati F, Finotti M, Di Renzo C, Brancaccio G, Piscaglia F, Cabibbo G, Caturelli E, Missale G, Marra F, Sacco R, Giannini EG, Trevisani F, Cillo U; Associazione Italiana Per Lo Studio Del Fegato Aisf Hcc Special Interest Group; Italian Liver Cancer Ita Li Ca Study Group. Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Kim SE, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Applicability of the BCLC staging system to patients with hepatocellular carcinoma in Korea: analysis at a single center with a liver transplant center. Korean J Hepatol. 2011;17:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Borzio M, Fornari F, De Sio I, Andriulli A, Terracciano F, Parisi G, Francica G, Salvagnini M, Marignani M, Salmi A, Farinati F, Carella A, Pedicino C, Dionigi E, Fanigliulo L, Cazzaniga M, Ginanni B, Sacco R; EpaHCC Group. Adherence to American Association for the Study of Liver Diseases guidelines for the management of hepatocellular carcinoma: results of an Italian field practice multicenter study. Future Oncol. 2013;9:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Piñero F, Marciano S, Fernández N, Silva J, Zambelo Y, Cobos M, Zerega A, Ridruejo E, Miguez C, Ameigeiras B, D'Amico C, Gaite L, Coronel M, Bermúdez C, Rosales C, Romero G, McCormack L, Reggiardo V, Colombato L, Gadano A, Rubinstein F, Silva M; Argentinean Association for the Study of Liver Diseases (A. A.E.E.H). Adherence to Barcelona Clinic Liver Cancer therapeutic algorithm for hepatocellular carcinoma in the daily practice: a multicenter cohort study from Argentina. Eur J Gastroenterol Hepatol. 2018;30:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sun X, Tan J, Tang L, Guo JJ, Li X. Real world evidence: experience and lessons from China. BMJ. 2018;360:j5262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Mospan AR, Morris HL, Fried MW. Real-world evidence in hepatocellular carcinoma. Liver Int. 2021;41 Suppl 1:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Dai M, Mao AY, Shi JF. [Sustainability of cancer screening program in urban China: a multicenter assessment from service supplier's and demander's perspectives]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:139-141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Kuo MJ, Chen HH, Chen CL, Fann JC, Chen SL, Chiu SY, Lin YM, Liao CS, Chang HC, Lin YS, Yen AM. Cost-effectiveness analysis of population-based screening of hepatocellular carcinoma: Comparing ultrasonography with two-stage screening. World J Gastroenterol. 2016;22:3460-3470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kim HL, An J, Park JA, Park SH, Lim YS, Lee EK. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology. 2019;69:1599-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Kwon JW, Tchoe HJ, Lee J, Suh JK, Lee JH, Shin S. The Impact of National Surveillance for Liver Cancer: Results from Real-World Setting in Korea. Gut Liver. 2020;14:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, Iwata Y, Enomoto H, Saito M, Imanishi H, Nishiguchi S. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res. 2012;42:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Lima PH, Fan B, Bérubé J, Cerny M, Olivié D, Giard JM, Beauchemin C, Tang A. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR Am J Roentgenol. 2019;213:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, Rapaccini G, Nolfo MAD, Benvegnù L, Zoli M, Borzio F, Giannini EG, Caturelli E, Chiaramonte M, Pinna AD; Italian Liver Cancer (ITA. LI.CA) Group. Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012;56:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Eltabbakh M, Zaghla H, Abdel-Razek W, Elshinnawy H, Ezzat S, Gomaa A, Waked I. Utility and cost-effectiveness of screening for hepatocellular carcinoma in a resource-limited setting. Med Oncol. 2015;32:432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Letouzé E, Imbeaud S, Zucman-Rossi J, Audureau E, Roudot-Thoraval F, Nahon P; ANRS CO12 CirVir Group. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology. 2018;155:431-442.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Da Fonseca LG, Forner A. Adherence and quality are key for a beneficial HCC surveillance. Liver Int. 2020;40:751-753. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Zhong JH, Peng NF, You XM, Ma L, Xiang X, Wang YY, Gong WF, Wu FX, Xiang BD, Li LQ. Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: A real-world study. Oncotarget. 2017;8:18296-18302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Yen YH, Cheng YF, Wang JH, Lin CC, Chen CH, Wang CC. Adherence to the modified Barcelona Clinic Liver Cancer guidelines: Results from a high-volume liver surgery center in East Asias. PLoS One. 2021;16:e0249194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Li JW, Goh BG, Chang PE, Tan CK. Barcelona Clinic Liver Cancer outperforms Hong Kong Liver Cancer staging of hepatocellular carcinoma in multiethnic Asians: Real-world perspective. World J Gastroenterol. 2017;23:4054-4063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, Ma L, Qi LN, Ou BN, Li LQ. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 34. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Guarino M, Tortora R, de Stefano G, Coppola C, Morisco F, Salomone Megna A, Izzo F, Nardone G, Piai G, Adinolfi LE, D'Adamo G, Gaeta GB, Messina V, Francica G, De Girolamo V, Coppola N, Persico M, Di Costanzo GG; Progetto Epatocarcinoma Campania Group. Adherence to Barcelona Clinic Liver Cancer guidelines in field practice: Results of Progetto Epatocarcinoma Campania. J Gastroenterol Hepatol. 2018;33:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Farinati F, Vanin V, Giacomin A, Pozzan C, Cillo U, Vitale A, Di Nolfo AM, Del Poggio P, Benvegnu' L, Rapaccini G, Zoli M, Borzio F, Giannini EG, Caturelli E, Trevisani F; Italian Liver Cancer (ITA. LI.CA) group. BCLC stage B hepatocellular carcinoma and transcatheter arterial chemoembolization: a 20-year survey by the Italian Liver Cancer group. Liver Int. 2015;35:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Pecorelli A, Lenzi B, Gramenzi A, Garuti F, Farinati F, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Cabibbo G, Felder M, Morisco F, Gasbarrini A, Baroni GS, Foschi FG, Biasini E, Masotto A, Virdone R, Bernardi M, Trevisani F; Italian LiverCancer (ITA. LI.CA) group. Curative therapies are superior to standard of care (transarterial chemoembolization) for intermediate stage hepatocellular carcinoma. Liver Int. 2017;37:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 39. | Radu P, Groza I, Iancu C, Al Hajjar N, Andreica V, Sparchez Z. Treatment of hepatocellular carcinoma in a tertiary Romanian center. Deviations from BCLC recommendations and influence on survival rate. J Gastrointestin Liver Dis. 2013;22:291-297. [PubMed] |
| 40. | Wang CY, Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from China. Medicine (Baltimore). 2019;98:e14070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |