Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.1
Peer-review started: September 24, 2022
First decision: November 15, 2022
Revised: December 6, 2022
Accepted: December 27, 2022
Article in press: December 27, 2022
Published online: January 15, 2023
Processing time: 107 Days and 17.9 Hours
Improvements in early screening, new diagnostic techniques, and surgical treatment have led to continuous downward trends in hepatocellular carcinoma (HCC) morbidity and mortality rates. However, high recurrence and refractory cancer after hepatectomy remain important factors affecting the long-term prognosis of HCC. The clinical characteristics and prognosis of recurrent HCC are heterogeneous, and guidelines on treatment strategies for recurrent HCC are lacking. Therapies such as surgical resection, radiofrequency ablation, and transhepatic arterial chemoembolization are effective for tumors confined to the liver, and targeted therapy is a very important treatment for unresectable recurrent HCC with systemic metastasis. With the deepening of the under
Core Tip: Hepatocellular carcinoma (HCC) is a highly recurrent malignancy; at present, there is a lack of definite treatment strategies for recurrent HCC. Recurrent HCC has particularities in recurrence mode and tumor microenvironment, so its treatment strategy should be different from that of primary HCC. Systemic therapy based on the immune microenvironment has emerged as a potential alternative treatment option for advanced HCC. Combining local therapy and systemic therapy for recurrent HCC may achieve better effects and is worthy of further exploration.
- Citation: Liang J, Bai Y, Ha FS, Luo Y, Deng HT, Gao YT. Combining local regional therapy and systemic therapy: Expected changes in the treatment landscape of recurrent hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(1): 1-18
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/1.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.1
Primary liver cancer is a common digestive system tumor with a high degree of malignancy. According to global epidemiological statistics in 2020, the number of new cases of liver cancer ranks 7th among malignant tumors, and liver cancer-related death ranks 2nd among tumor-related mortality[1]. Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy. Surgical resection is the most effective radical treatment method, but the 5-year recurrence rate after surgery is as high as 40%-70%[2-4]. An appropriate treatment plan for patients with recurrent HCC is key to increasing the survival of patients after surgery. Although different regions and societies have developed many guidelines for the treatment of HCC, guidance on treatment strategies for recurrent HCC is lacking due to the heterogeneity of recurrent cancer and the presence of impaired liver function, portal hypertension, and vascular invasion[5-7]. Developing a suitable treatment plan for recurrent HCC and prolonging the survival time of patients are issues that remain to be studied.
Local regional therapies for intrahepatic tumors, including traditional surgical resection, liver transplantation, ablation therapy, and transhepatic arterial chemoembolization (TACE), remain the main approaches by which patients can obtain long-term benefits. Systemic therapy refers to antitumor therapies such as molecular targeted drugs, immunotherapy, and chemotherapy and has become an important component of antitumor therapies. Many retrospective studies advocate repeat resection (RR) as the preferred method for the treatment of recurrent HCC. Researchers believe that in the case of limited liver lesions and sufficient reserve function, the safety and effectiveness of RR are similar to those of initial hepatectomy. The 5-year overall survival (OS) rate is 25%-87%[8-10]. However, due to limited liver reserves after liver resection and the heterogeneity of recurrence patterns, the number of patients who meet the criteria RR in actual clinical practice is limited. Salvage liver transplantation (SLT) is also an effective radical treatment option. Compared with RR, SLT can simultaneously eliminate tumors and radically treat primary liver disease and has a longer survival time[11,12]. However, due to the shortage of donor organs in some areas and the limited capacity for liver transplantation, SLT implementation is low. Currently, nonsurgical local regional therapies, such as radiofrequency ablation (RFA) and TACE, are common treatments for unresectable recurrent HCC[13,14]. Due to the success of immune-targeted drugs such as atezolizumab and bevacizumab, combination therapies have been used in the systemic treatment of HCC. These treatments are likely to be a new paradigm for improving the long-term prognosis of recurrent HCC.
This paper aims to review the recurrence pattern of HCC and the changes in the tumor microenvironment; provide updated data on liver-targeted therapy for recurrent HCC, including the application value and status of systemic therapy and combined local therapy in recurrent HCC; and explore treatment strategies for improving the prognosis of recurrent HCC patients.
Recurrence of HCC after radical treatment is very common, and the recurrence rate after hepatectomy is approximately 70%. Many scholars have studied the risk factors for and recurrence patterns of HCC. Male sex, a high degree of fibrosis, HBV load, tumor diameter, and vascular invasion are positively correlated with HCC recurrence[15-18]. The recurrence pattern of HCC is often divided into early recurrence and late recurrence[19,20]. Early recurrence is considered to be caused by occult intrahepatic metastasis of the primary tumor and has a worse prognosis[21], as it is more likely to be accompanied by extrahepatic metastasis[22]. In contrast, late recurrence is considered to be HCC of polyclonal origin unrelated to the primary tumor or neoplastic foci, mostly with intrahepatic metastasis. Early recurrence is usually associated with the invasive pathological characteristics of the tumor, such as large tumor size, multiple tumors, poor differentiation and vascular infiltration[23-26]. The main manifestation of late recurrence is intrahepatic recurrence, and the cause of recurrence is usually related to underlying liver diseases, such as uncontrolled hepatitis, the extent of cirrhosis and HBV replication[27,28]. Differentiating recurrence patterns is important for monitoring and preventing recurrent HCC and selecting different treatment strategies[29,30].
Regarding the time limits for early relapse and late recurrence, most studies have considered the time limit for early recurrence to be 2 years after surgery[2,16,19]. A recent multicenter study used the survival period as the evaluation standard; early recurrence was limited to 8 mo or 6 mo after surgery, which allowed the survival period after recurrence to be better distinguished[22,31]. However, determining recurrence patterns based on the time of recurrence alone may lead to inaccurate results. Recently, molecular genetic techniques such as microsatellite instability, loss of heterozygosity, and short tandem repeat and next-generation sequencing have been used to analyze the origin of tumor clones to more accurately identify foci origins, recurrence patterns and mechanisms[30,32]. Intrahepatic metastatic hepatocellular carcinoma (IM-HCC) refers to recurrent tumors derived from the same monoclonal cells as the primary tumor. Multicenter origin hepatocellular carcinoma (MO-HCC) is caused by polyclonal cancer[33]. Early and late recurrence suggests IM-HCC and MO-HCC, respectively. In addition, early recurrence is usually diffuse and spreads through the portal venous system, while the prognosis of late recurrence is better. Distinguishing the source of tumor heterogeneity by cloning techniques explains the low survival associated with early recurrence. Recurrence patterns suggest that the time of HCC recurrence and invasiveness should be considered when choosing a treatment. Although clarifying the clonal origin of recurrent HCC has certain application prospects, its application in clinical practice requires more standardized diagnostic procedures and further technical capabilities[34].
Liquid biopsy based on biomarkers is being explored as a new approach for monitoring HCC recurrence. The detection of circulating tumor DNA (ctDNA) by gene sequencing is used to detect minimal residual disease and predict the recurrence of HCC earlier and more accurately[35]. Moreover, comparing the mutations of the driver gene in ctDNA in the blood and in the primary tumor tissue in patients with recurrent HCC may help reveal the heterogeneity of recurrent HCC[36].
The liver is rich in a variety of immunocompetent cells, including liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, NK cells, lymphocytes, and dendritic cells[37]. These immune cells interact with surrounding stromal cells to form a complex immune dynamic network and play important roles in the proliferation, migration, invasion and angiogenesis of HCC[38]. Immune cell infiltration, fibroblast proliferation, and the induction of angiogenesis in the tumor microenvironment (TME) are closely related to the progression and metastasis of HCC[39].
The inhibitory environment of immune cells and tumor blood vessels with abnormal structures and functions together constitute the HCC microenvironment, which plays a key role in determining the outcome of HCC, including the risk of recurrence after HCC resection or ablation[40]. Tumor-infiltrating lymphocytes (TILs) are a representative component of the host antitumor immune response[41]. CD3-, CD4-, CD8-, and Foxp3-positive T lymphocytes are the most common subpopulations of TILs[42]. An increase in the number of TILs, especially activated cytotoxic T lymphocytes, is associated with the prognosis and survival of patients with HCC[43,44]. Meta-analysis of multiple studies has shown that tumor-infiltrating CD8- positive T cells are a strong prognostic factor for recurrence-free survival in HCC patients after hepatectomy[45,46]. Nakagawa et al[47] reported that TILs were significantly associated with a high recurrence rate and poor OS in all study patients, including HBV-associated and non-B non-C HCC patients. Most studies have shown that CD3+ and CD4+ TILs play a protective role in HCC[46,48,49]. Another study of HCC patients after surgical resection found that high CD3+ TIL density in the center and margin of a tumor predicted low recurrence and long-term disease-free survival (DFS)[50]. Fu et al[51] found that CD4 (+) T-cell dysfunction caused by FoxP3 (+) regulatory T cells was associated with a high recurrence rate in HCC patients.
Additionally, the proportion of TILs in multiple subpopulations is related to tumor activity and has predictive value for HCC recurrence. Mathai et al[52] found that a high postoperative Foxp3+/CD8+ TIL ratio was associated with poor tumor differentiation, high recurrence, and low OS and DFS in HCC patients.
The infiltration of immunosuppressive cell types [such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs)] is also associated with the high recurrence rate of HCC. Tregs facilitate the immune escape of tumor cells by inhibiting the function of antigen-presenting cells and hindering the proliferation or activation of NK cells and effector T cells[53]. High Treg expression is associated with poor DFS in HCC patients[54,55]. The proportion of Tregs and cytotoxic T cells is an independent predictor of postoperative recurrence and survival[56]. MDSCs mediate tumor angiogenesis and exert immunosuppressive effects by inhibiting dendritic cells and NK cells[57]. A systematic retrospective study showed that the proportion of MDSCs was associated with poor clinical outcomes after HCC resection or local ablation[58] and was associated with early postoperative recurrence of HCC[59]. Additionally, some studies have suggested that the inhibitory effect of MDSCs and highly programmed death ligand 1 (PD-L1) inhibitors have a synergistic effect[60]. The high expression of PD-L1 in tumor cells or immune cells is associated with highly invasive tumors and is a predictor of recurrence[61,62].
Vascular endothelial growth factor (VEGF) is an important cytokine involved in angiogenesis during tumor proliferation and plays a key role in HCC invasion and recurrence[63-65]. VEGF accelerates the early recurrence of HCC after radiofrequency by promoting the proliferation of CD133+ cancer stem cells[66]. Additionally, VEGF regulates immunosuppressive cells such as MDSCs and Tregs to promote tumor immune escape[67,68]. Therefore, we speculate that VEGF-induced immunosuppression plays a key role in the TME of HCC and is also an important driving factor in HCC recurrence.
The complex interaction between immune cells and effector molecules in the TME of HCC alters immune status and promotes or inhibits the growth of HCC. The recurrence of HCC has been shown to be related to immune characteristics. An in-depth understanding of the TME provides a basis for the exploration of targeted multimodal immunity and targeted therapy.
Traditionally, local regional therapies for HCC are divided into curative and palliative treatments. The former group includes surgery, liver transplantation, RFA, microwave ablation (MWA), and percutaneous ethanol injection, while the latter group includes conventional TACE, drug-eluting bead TACE (DEB-TACE), chemotherapy, and radiotherapy. Intrahepatic recurrence is the main type of HCC recurrence; therefore, local treatment for intrahepatic tumors clearly dominates recurrent HCC treatment. RR of recurrent HCC is considered the best treatment option for patients with resectable tumors[8,69], and high survival rates have been extensively reported in the literature[70,71]. A recent meta-analysis of RR for recurrent HCC showed that the 5-year OS was 42%-55%, the 5-year DFS was 19%-39%, and the 2-year and 5-year OS and DFS were superior to those of RFA[72]. A randomized controlled study of early recurrent HCC showed that there was no significant difference in 5-year OS for patients who received RR or RFA (43.6% vs 38.5%). However, for tumors with a diameter larger than 3 cm, RR may be associated with a better local disease control rate and longer survival[73]. For patients with isolated recurrent lesions and adequate liver function, resection remains one of the preferred treatment decisions, and the occurrence of serious complications does not differ from RFA treatment[74]. When the number of lesions is large or there is vascular invasion, the use of RR is restricted[75]; additionally, declines in liver function and portal hypertension progression may be related to poor postoperative outcomes, complications, and a high decompensation rate[76,77]. In clinical practice, only approximately 15%-30% of HCC patients who relapse after initial resection are eligible for RR[75,78]. The implementation of laparoscopic hepatectomy has reduced the incidence of postoperative complications and the length of the hospital stay[79], but the survival time remains similar to that of open hepatectomy, with no significant improvement in the overall prognosis of recurrent HCC patients[80,81].
SLT is considered an effective and curative treatment approach that can simultaneously resolve liver tumors, potential liver dysfunction and the original underlying diseases. For recurrent HCC, as long as patients meet the criteria, the prognosis is relatively high[11,82,83]; the 5-year survival rate is 42%-73%[84]. A systematic retrospective analysis of the prognosis of SLT and RR in the treatment of recurrent HCC showed that patients in the SLT group generally had larger liver lesions but better relapse-free survival (RFS) than those in the RR group and that the OS of the two groups was similar[85]. The main limitation of this treatment technique is the shortage of liver donors and the high level of technical requirements[86].
Compared with RR and SLT, ablation therapy (including RFA and MWA) is minimally invasive, has a good local control rate and low rate of postoperative liver dysfunction and is a reproducible treatment for recurrent HCC[70,78]. For early recurrent HCC with small lesions, the 5-year survival rate is 26.7%-64.5%[8,14,70,87,88]. The 5-year long-term survival of patients with BCLC stage 0/A receiving RFA is comparable to that of patients receiving RR, but the length of the hospital stay is shorter, and there are fewer complications[88,89]. There is no significant difference in long-term survival between RFA and TACE treatments in patients with early recurrent HCC (BCLC stage 0/A, diameter < 3 cm)[90,91]. However, a meta-analysis that included patients with early and mid-stage recurrent HCC showed that the 1-, 3-, and 5-year survival rates were higher for the RFA treatment group than for the TACE treatment group[92]. The indications for local ablation are limited in terms of the size and number of lesions, and there are also certain requirements for HCC location. If HCC recurs after local ablation, TACE is the most commonly used rescue treatment.
Due to the heterogeneity of recurrent HCC, the options for radical treatment are limited for patients with larger lesions and multicentric lesions. TACE is the most widely used treatment for recurrent HCC, accounting for approximately 60% of patients with recurrent HCC[93-95]. The indications for TACE include impaired liver function, a large number of tumors, complex location, combined portal vein invasion and contraindications for RR. TACE has always been the main strategy for treating unresectable intrahepatic multiple recurrence with the goal of preserving liver function[95]. TACE can be used for patients with early recurrent HCC and later-stage HCC. Consequently, the prognosis after treatment varies greatly, but overall, TACE can improve the OS of multifocal tumor patients. A number of studies have shown that the 5-year survival rate after TACE treatment for recurrent HCC is 12-56%[70,83,84,87,96]. A prospective study showed that for patients with early HCC recurrence, the 5-year survival rate after TACE treatment was 27.7%, which is lower than those for RR and RFA. However, TACE treatment is more suitable for multifocal tumors and early-stage (≤ 1 year) recurrent HCC[87]. Because TACE embolizes the blood supply artery, it may be more effective for patients with early recurrence due to microvascular invasion (MVI) and tumor remnants[78,97]. For early recurrent HCC with MVI, TACE combined with RFA treatment results in better survival than surgical resection or RFA alone[98]. In recent years, DEB-TACE and transarterial radioembolization with yttrium-90-labeled microspheres have been applied to unresectable HCC and have shown a better objective remission rate, disease control rate and survival[99-102].
As a palliative treatment, TACE can be combined with other treatments and has advantages. A long-term follow-up observation of recurrent HCC with a diameter less than 5 cm showed that the 1-, 3-, and 5-year DFS rates after TACE combined with RFA treatment were 55.1%, 22.5%, and 9.7%, respectively, significantly higher than those after TACE treatment alone (41.1%, 9.9%, and 4.9%)[103]. A study of DEB-TACE combined with RFA for the treatment of recurrent HCC showed that the 1-, 2-, and 3-year OS rates were 90%, 82.3%, and 66.4%, respectively, percentages that were superior to those after surgical resection and RFA alone[104]. Compared with using RFA or TACE alone, combining the two methods has demonstrated advantages in the control of early and mid-stage recurrent HCC. In the case of insufficient residual liver reserves after surgical resection, the combination of the two local treatments is effective in the treatment of recurrent HCC[105,106].
For unresectable HCC, other local liver-directed therapies, including yttrium-90, stereotactic body radiation therapy (SBRT), proton beam therapy (PBT), and hepatic artery infusion chemotherapy (HAIC), have been applied in clinical practice, but there is still a lack of strong evidence to replace traditional treatment methods. Studies have reported on the efficacy of yttrium-90 radiotherapy, SBRT, PBT, and HAIC in recurrent HCC and confirmed that these treatments inhibit the progression of recurrent HCC with few side effects. These methods can be used as new adjuvant treatments[107-110]. However, more data on the treatment of recurrent intrahepatic HCC are needed.
Before the advent of sorafenib, systemic treatment for HCC was lacking. Sorafenib is the standard first-line drug for the systemic treatment of HCC. Although sorafenib prolongs the survival time of patients with advanced HCC, its objective response rate is low, and side effects lead to poor patient tolerance. In 2017, lenvatinib was approved by the Food and Drug Administration as a first-line treatment for advanced HCC, and regorafenib, cabotinib, and ramucirumab were successively approved as second-line treatments for advanced HCC. With the deepening of the understanding of the immune microenvironment of HCC, immunotherapy that modulates the immune function of the body to enhance the tumor immune response and block tumor immunosuppression has become a new direction for the treatment of HCC. Among immunotherapy drugs, immune checkpoint inhibitors (ICIs) are the most widely used. However, the efficacy rates of single-agent ICIs in the treatment of HCC are still very low, indicating that it is necessary to use ICIs in combination with other drugs to improve efficacy[111]. As a tumor with abundant blood vessels, HCC is driven by growth factors such as VEGF. Abnormal angiogenesis contributes to tumor growth and metastasis. Therefore, combining antiangiogenic drugs and ICI therapy may be an ideal strategy to further overcome tumor immunosuppression and to optimize the efficacy of ICI therapy by inducing tumor vascular normalization. High efficacy rates of dual immunotherapy with ICIs plus tyrosine kinase inhibitors (TKIs), ICIs plus VEGF inhibitors, and ICIs combined with anti-cytotoxic T lymphocyte-associated protein 4 have been reported in recent studies of unresectable HCC[112-114]. The latest NCCN guidelines have been updated[115], and the IMbrave150 trial has established atezolizumab combined with bevacizumab as a new first-line treatment standard for patients with advanced HCC. This combination has also become the preferred first-line treatment for HCC[116,117]. Systemic treatment has been shown to be beneficial for the survival of patients with advanced HCC[114].
The high recurrence rate of early-stage HCC after radical treatment has spurred studies of adjuvant therapy for patients with a high risk of postoperative recurrence[5,118,119]. Phase III clinical trials of sorafenib as an adjuvant therapy after hepatectomy or ablation have not shown a positive effect on RFS and OS[120]. Although there is no definitive evidence to confirm the effectiveness of this treatment and it is not recommended in current mainstream treatment guidelines, given the highly invasive characteristics of recurrent HCC, systemic treatment should have value in the early prevention of recurrence[121,122]. Therefore, many clinical studies are exploring this topic[26,123].
Early studies found that compared with primary HCC, cell lines derived from recurrent HCC have stem cell-like characteristics and are more susceptible to TIL-mediated recognition and cytotoxicity; therefore, it is beneficial for immunotherapy to target recurrent HCCs with stem cell-like characteristics[124]. Studies have found that high PD-L1 expression in tumor cells or immune cells is associated with more aggressive tumors and is a predictor of HCC recurrence[61,62]. Given these immune characteristics, immunotherapy for recurrent HCC can improve the tumor immune environment and shrink tumors by reversing the immunosuppression induced by HCC. Because most clinical studies of systemic treatment exclude HCC patients with a history of treatment, there are few clinical studies on systemic treatment for recurrent HCC, and some of the available clinical evidence is for recurrent HCC after liver transplantation[125-135]. In these studies, TKIs were mainly used (Table 1); the 1-year survival rate of patients with recurrence after transplantation treated with sorafenib was 63%, and the average time to progression was 5.6 mo. However, increased adverse events should be a concern for the combination of sorafenib with immunosuppressants[136]. For patients with a history of liver transplantation, ICI treatment should be considered very carefully due to the possibility of transplant rejection[137]. However, patients with HCC recurrence after hepatectomy are not subject to this limitation.
| Ref. | Medication regimen | Study type | Number of patients | OS (mo) | PFS (mo) | 1-yr OS |
| Iavarone et al[125] | Regorafenib | Retrospective | 28 | 12.9 | ||
| Li et al[126] | Sorafenib 79%; lenvatinib 2.3%; chemotherapy 4.7%; chemotherapy plus bevacizumab 7.0%; pembrolizumab 2.3% | Retrospective | 41 | 17.0 | ||
| Li et al[127] | Sorafenib | Retrospective | 10 | 10.0 | ||
| de'Angelis et al[128] | Sorafenib | Retrospective | 15 | 60% | ||
| Gomez-Martin et al[129] | Sorafenib | Retrospective | 31 | 19.3 | 6.77 | |
| Zavaglia et al[130] | Sorafenib | Retrospective | 11 | 5.0 | ||
| Staufer et al[131] | Sorafenib | Retrospective | 13 | 7.0 | ||
| López Ortega et al[132] | Sorafenib | Retrospective | 17 | 62% | ||
| Weinmann et al[133] | Sorafenib | Retrospective | 11 | 20.1 | ||
| Piñero et al[134] | Lenvatinib | Case | 1 | 84.0 | ||
| Sotiropoulos et al[135] | Sorafenib | Retrospective | 14 | 25.0 |
A retrospective study by Li et al[138] examined 58 patients with early recurrence after hepatectomy and found that the average survival time of patients receiving TKIs combined with programmed cell death protein 1 (PD-1) treatment was 35.8 mo, a significant increase of 17.8 mo compared with the average survival time of patients receiving TKIs alone. He et al[139] reported a patient with residual liver recurrence, multiple pulmonary metastases, and suspected splenic metastasis. After the successive application of sorafenib and regorafenib followed by lenvatinib combined with pembrolizumab treatment, partial remission (PR) was achieved, along with 24 mo of progression-free survival (PFS) and 60 mo of postoperative OS.
Systemic therapy has become the standard treatment regimen for advanced HCC. The risk of tumor recurrence is higher for recurrent HCC than for the original tumor[95]. Therefore, there is a more urgent need for safe and effective drugs to reduce the effect of tumor recurrence on patient survival. The role of systemic therapy can be expanded from an adjuvant therapy to an active treatment strategy.
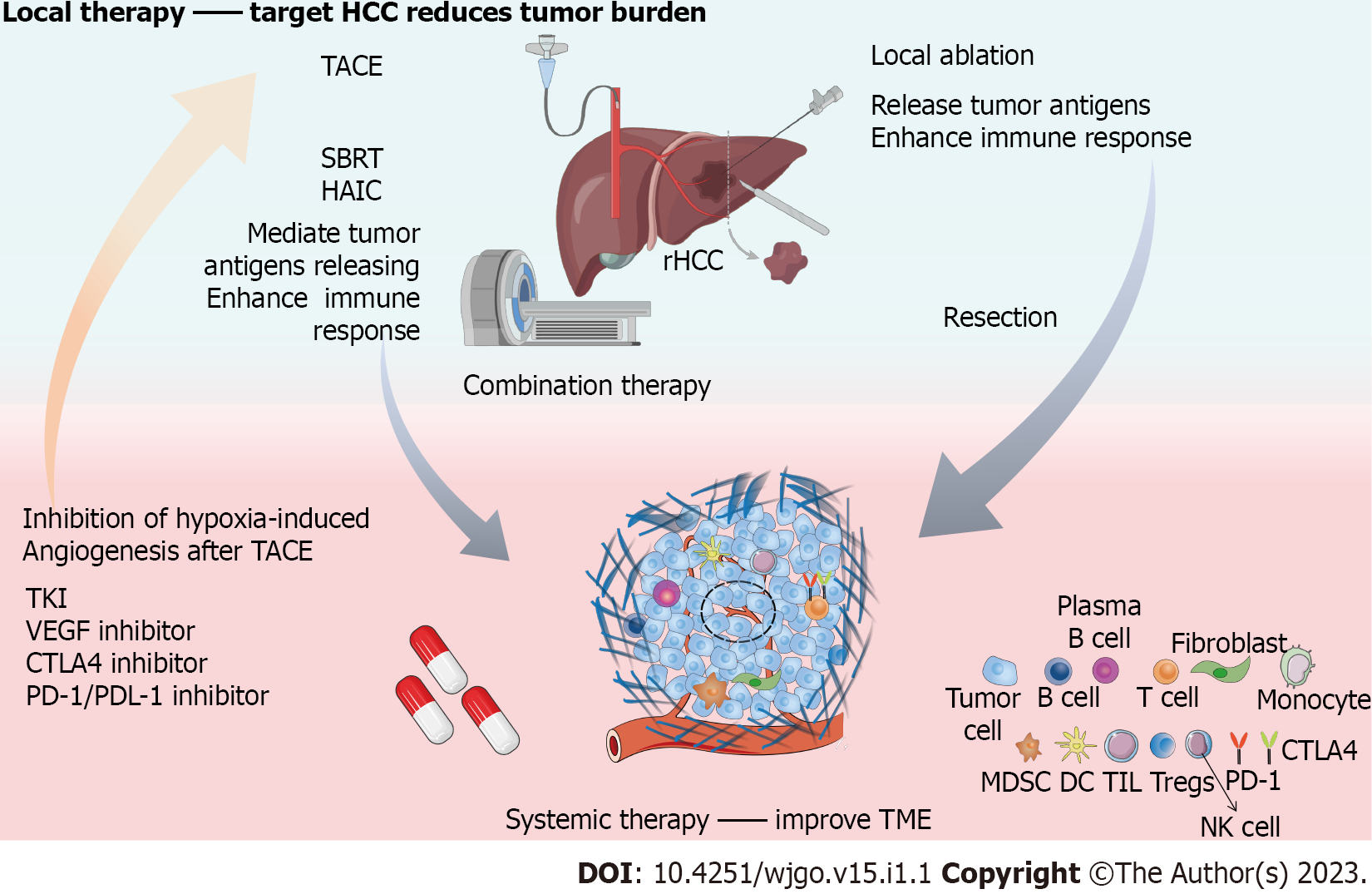
Continuous progress in immunizations and targeted drugs have brought breakthroughs in treatment patterns in recent years, including new treatment methods and guidelines and expansion from single choices to multiple choices. The published data for combination therapy show that the combination of local therapy and systemic therapy has a theoretical basis and is technically feasible[106,140]. Although ICIs show good therapeutic effects, dysfunctional T cells that infiltrate the tumour microenvironment (TME) are one of the reasons for ICI failure[141], and the ratio of T-cell activity to tumor burden is a key predictor of the clinical efficacy of ICIs[142,143]. Therefore, reducing the tumor burden by local regional therapy in combination with ICI therapy will better improve patient prognosis, and improvements in the HCC microenvironment due to combination therapy may also improve patient outcomes (Figure 1).
After multiple percutaneous ablation or TACE treatments, many patients experience disease progression and liver function deterioration[144,145]. Regardless of the treatment strategy, physical performance and the maintenance of residual liver function are prerequisites for cancer treatment[146]. Combining local regional therapy with systemic therapy at an appropriate time may prolong the survival of patients with recurrent HCC and improve their prognosis. Combined systemic therapy can reduce tumor progression, thereby reducing recurrence and prolonging the interval between local regional therapies to ensure residual liver function. Patients with recurrent HCC who may benefit from combination therapy include: (1) Patients with a high risk of recurrence after the remission of RR and ablation; (2) patients with progression under TACE; and (3) patients with distant metastasis.
The effect of local treatment on the body may create conditions for improving the efficacy of systemic treatment. TACE causes local hypoxia in tumors, and the expression of hypoxia-responsive genes regulated by hypoxia-inducible factor-1α triggers the expression of VEGF, leading to angiogenesis, which can promote tumor recurrence and metastasis[147-149]. Thus, combining VEGF inhibitors with TACE may improve local tumor control by inhibiting hypoxia-induced angiogenesis after TACE. Ablation combined with immunotherapy is also considered a potentially beneficial method. Ablation therapy itself may help activate antigen-specific CD4+ and CD8+ T cells in HCC patients[150,151]. Heat-induced tissue necrosis releases tumor antigens and induces the accumulation of inflammatory cytokines [interleukin (IL)-1β, IL-6, IL-8, and tumour necrosis factor alpha (TNF)] around the necrotic area. Therefore, RFA can increase the sensitivity of HCC cells to immunotherapy[152-154]. Compared with RFA, cryoablation induces a stronger immune response, including increasing the levels of IL-1, IL-6, NF-κB, and TNF-α and upregulating the expression of PD-L1/PD-1 in the circulation[155].
Different local therapies can be combined with systemic therapy successively or simultaneously to obtain strong immune stimulation, slow tumor vascular reconstitution, and increase antitumor efficacy[156]. In primary HCC, objective response rates (ORRs) and disease control rates (DCRs) of 50.0% and 78.6% were observed in microwave ablation combined with apatinib and carrelizumab in the treatment of advanced hepatocellular carcinoma, respectively[157]. In a randomized, multicenter prospective study, TACE plus sorafenib in patients with unresectable HCC resulted in longer PFS (25.2 mo vs 13.5 mo) than TACE alone[158]. Real-world evidence showed a higher tumor response with the combination of HAIC, anti-PD-1 antibodies and TKIs in patients with advanced HCC[159]. These encouraging data contributed to the application of combination therapy in recurrent HCC.
Due to the relatively recent clinical application of immunotherapy, current follow-up studies on combination therapy for recurrent HCC mainly focus on ablation therapy or interventional therapy combined with TKI drugs, with preliminary results for the effect of combination therapy on relapse and OS[160-170] (Table 2). A multicenter retrospective study by Wei et al[168] included 211 patients with early postoperative recurrence of HCC with MVI, and the survival rate after RFA combined with sorafenib treatment was significantly better than that after RFA treatment alone. Feng et al[169] evaluated the efficacy of sorafenib combined with RFA in 128 patients with BCLC stage 0-B1 HCC. Among them, 75% of patients had recurrent HCC; the 1-, 2-, and 3-year OS rates after combined treatment were 64.0%, 58.7%, and 50.3%, respectively, which were significantly higher than those after RFA treatment. Mahn et al[170] provided a case report of a 36-year-old patient with HCC recurrence after transplantation. The patient underwent surgery, radiofrequency ablation, and second-line treatment with sorafenib and cabozantinib and achieved a survival time longer than 10 years after tumor recurrence. Although current data on recurrent HCC are limited, many clinical studies of the combination of systemic treatment and local treatment are ongoing (Table 3).
| Ref. | Interventions | Study type | Disease | Outcome |
| Wang et al[160] | Anti-PD-1 + RFA (n = 40) vs RFA (n = 40) | Retrospective | Recurrent HCC after resection | RFS 39.1 wk vs 19.3 wk, P = 0.002 |
| Gu et al[161] | Apatinib + TACE (n = 40) vs TACE (n = 40) | Prospective | Intrahepatic recurrence after resection | RFS 17.2 mo vs 12.5 mo, P = 0.041 |
| Peng et al[162] | Sorafenib + TACE (n = 128) vs TACE (132) | Retrospective | Intermediate-stage recurrent HCC | 5-yr OS: 38.9% vs 20.5%, P = 0.01; 5-yr PFS, 37.5% vs 18.7%, P = 0.003 |
| Wan et al[163] | Sorafenib + TACE (n = 127) vs TACE (n = 127) | Retrospective | Unresectable recurrent HCC | OS 30.7 mo vs 18.2 mo, P = 0.003 |
| Zhou et al[164] | Sorafenib + RFA (n = 174) vs RFA (n = 174) | Retrospective (multicenter) | Recurrent HCC after curative resection | 1-, 3-, 5-yr OS: 97.7%, 83.7%, 54.7% vs 93.1%, 61.3%, 30.9%, P < 0.001 |
| Guo et al[165] | Camrelizumab + TACE (n = 20) vs TACE (n = 59) | Retrospective | Recurrent HCC after curative resection | ORR 40.0% vs 56.9% NS; PFS 6 mo vs 9 mo NS |
| Iwata et al[166] | Sorafenib + HAIC | Case | Recurrent HCC after resection | OS 24 mo |
| Chen et al[167] | Toripalimab + TKI + Radiation (n = 17) | Prospective | Advanced recurrent HCC | 1-, 2-yr OS: 60%, 24% |
| Wei et al[168] | Sorafenib + RFA (n = 103) vs RFA (n = 108) | Retrospective (multicenter) | Early-stage recurrent HCC with MVI | RFS: 17.7 mo vs 13.1 mo, P < 0.001; OS: 32.0 mo vs 25.0 mo, P = 0.002 |
| Feng et al[169] | Sorafenib + RFA (n = 48) vs RFA (n = 40) | Retrospective (multicenter) | BCLC Stage 0-B1 recurrent HCC | 1-, 2-, 3-, and 4-yr OS: 85.6%, 64.0%, 58.7%, 50.3% vs 80.7%, 47.2%, 30.9%, 30.9%, P = 0.036 |
| Mahn et al[170] | Resection, RFA; Sorafenib, Cabozantinib | Case | Recurrent HCC after LT | OS longer than 10 yr |
| Clinical trials number | Study type | Agent(s) | Local regional therapy | Estimated enrollment | Primary endpoint |
| chiCTR2100044057 | Phase II | Camrelizumab + Bevacizumab | Microwave Ablation | 30 | PFS |
| chiCTR2100046533 | Prospective | Apatinib + Camrelizumab | TACE | 37 | PFS |
| NCT05162898 | Prospective | Toripalimab + Lenvatinib | Radiofrequency Ablation | 90 | PFS |
| NCT05010434 | Phase II | Sintilimab + Bevacizumab | Radiofrequency Ablation | ORR | |
| NCT05444478 | Prospective | Lenavatinib | Microwave Ablation | 274 | 3-yr PFS% |
| NCT05277675 | Prospective | Tislelizumab/Sintilimab + Lenvatinib/Bevacizumab | Radiofrequency Ablation | 160 | 1-yr PFS% |
| NCT05162898 | Prospective | Toripalimab + Lenvatinib | Radiofrequency ablation | 90 | RFS |
| NCT04663035 | Phase II | Tislelizumab | Ablation | 120 | 1-yr PFS% |
| NCT05355155 | Phase II | Bevacizumab + Biosimilar IBI305 | - | 15 | ORR |
| NCT05103904 | Phase II | Lenvatinib | - | 19 | ORR |
| NCT04615143 | Prospective | Tislelizumab/Tislelizumab + Levatinib | - | 80 | PFS |
| NCT04564313 | Phase I | Camrelizumab | - | 20 | ORR |
| NCT04237740 | Phase III | Relenvatinib | - | 40 | 3-yr PFS% |
| NCT04204850 | Phase II | Cabozantinib | 20 | DCR |
In animal model studies, radiotherapy (including conventional radiotherapy and SBRT) has been shown to enhance the immune response to ICIs and treatment efficacy by upregulating the expression of major histocompatibility complex class I, mediating the release of tumor antigens, and increasing the number of TILs[171,172]. Oxaliplatin enhanced the efficacy of ICIs in a mouse model of lung cancer by inducing immunogenic cell death and depleting macrophages[173]. Although these mechanisms have been validated in animal models and HCC models, clinical research data are lacking. However, the combination of the two treatments holds promise for HCC[174].
In summary, there is a lack of guidance for the treatment of recurrent HCC based on the recurrence patterns of recurrent HCC and the characteristics of the TME. The treatment mode and prognosis of recurrent HCC differ from those for the initial diagnosis of HCC. In the era of targeted immunotherapy, surgery and local regional therapy continue to be irreplaceable. However, due to the bimodal distribution of recurrence, early combined systemic therapy may be the most ideal choice for patients with early recurrence to eliminate or control residual tumor cells. For patients with late recurrence, local regional therapy can be performed first, followed by observation and follow-up. Timely combined systemic treatment should be performed considering the staging and progression risk of HCC. Additionally, an inherent advantage of recurrent HCC is that the tumor pathology obtained in the previous stage can be used to evaluate the accuracy of treatment and predict the effectiveness of systemic treatment. However, many problems with the systemic treatment of recurrent HCC must still be resolved, and not all patients can benefit from it. Systemic treatment has limitations, such as a low response rate and a lack of effective clinical biomarkers. The identification of populations that will benefit from these treatments and development of reliable predictive markers and models are areas that need to be explored. Although combination therapy with local regional therapy and systemic therapy may have good application prospects, further improvements in the safety and effectiveness of combined therapy are needed. The correct timing, dose, and reasonable combinations to maintain residual liver function and achieve better survival need to be established. The above clinical problems need to be further explored through a large number of clinical trials.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: World Journal of Hepatology, No. 02937551; International Society for Extracellular Vesicles, No. 57890631.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lang SA, Germany; Mohamed SY, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 2. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 3. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 659] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 4. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 5. | European Association for the Study of the Liver. ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 6. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 7. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 8. | Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, Chen MS, Zhang YF, Guo RP. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection versus radiofrequency ablation. Cancer Med. 2020;9:2997-3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J, Liu Z. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol. 2010;8:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Lacaze L, Scotté M. Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J Hepatol. 2015;7:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 11. | Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Yamashita Y, Yoshida Y, Kurihara T, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, Uchiyama H, Shirabe K, Maehara Y. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: Repeat hepatectomy versus salvage living donor liver transplantation. Liver Transpl. 2015;21:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ji J, Gu J, Wu JZ, Yang W, Shi HB, Liu S, Zhou WZ. The "Six-and-Twelve" Score for Recurrent HCC Patients Receiving TACE: Does it Still Work? Cardiovasc Intervent Radiol. 2021;44:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg. 2015;39:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Kim J, Kang W, Sinn DH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Substantial risk of recurrence even after 5 recurrence-free years in early-stage hepatocellular carcinoma patients. Clin Mol Hepatol. 2020;26:516-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 17. | Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, Kim SY, Sinn DH, Kim JM, Kim K, Ha SY. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann Surg. 2021;273:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 18. | Jin B, Du S, Yang H. HBsAg seroclearance reduces the risk of late recurrence in HBV-related HCC. J Hepatol. 2022;77:1469-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 20. | Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, Ueno H, Okusaka T, Arai Y, Takayasu K. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Wang MD, Li C, Liang L, Xing H, Sun LY, Quan B, Wu H, Xu XF, Wu MC, Pawlik TM, Lau WY, Shen F, Yang T. Early and Late Recurrence of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Oncologist. 2020;25:e1541-e1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Wei T, Zhang XF, Bagante F, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Grigorie R, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Lv Y, Aldrighetti L, Pawlik TM. Early Versus Late Recurrence of Hepatocellular Carcinoma After Surgical Resection Based on Post-recurrence Survival: an International Multi-institutional Analysis. J Gastrointest Surg. 2021;25:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Huang L, Li J, Yan J, Cao J, Liu C, Zhang X, Wu M, Yan Y. Early recurrence after curative resection in oligonodular hepatocellular carcinoma. Hepatogastroenterology. 2013;60:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, Xia Y, Li J, Wang K, Yan Z, Wu D, Zhang B, Hüser N, Shen F. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Shimoda M, Tago K, Shiraki T, Mori S, Kato M, Aoki T, Kubota K. Risk Factors for Early Recurrence of Single Lesion Hepatocellular Carcinoma After Curative Resection. World J Surg. 2016;40:2466-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Nahon P, Vibert E, Nault JC, Ganne-Carrié N, Ziol M, Seror O. Optimizing curative management of hepatocellular carcinoma. Liver Int. 2020;40 Suppl 1:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16:792-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Zhang X, Li C, Wen T, Yan L, Li B, Yang J, Wang W, Xu M, Lu W, Jiang L. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Wang B, Xia CY, Lau WY, Lu XY, Dong H, Yu WL, Jin GZ, Cong WM, Wu MC. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg. 2013;217:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Liu YW, Yong CC, Lin CC, Wang CC, Chen CL, Cheng YF, Wang JH, Yen YH. Six months as a cutoff time point to define early recurrence after liver resection of hepatocellular carcinoma based on post-recurrence survival. Updates Surg. 2021;73:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 32. | Li Q, Wang J, Juzi JT, Sun Y, Zheng H, Cui Y, Li H, Hao X. Clonality analysis for multicentric origin and intrahepatic metastasis in recurrent and primary hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Gupta S, Khan S, Kawka M, Gujjuri R, Chau I, Starling N, Cunningham D, Jiao LR, Gall T. Clinical utility of clonal origin determination in managing recurrent hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Xie DY, Fan HK, Ren ZG, Fan J, Gao Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin Transl Gastroenterol. 2019;10:e00006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Cai Z, Chen G, Zeng Y, Dong X, Li Z, Huang Y, Xin F, Qiu L, Xu H, Zhang W, Su X, Liu X, Liu J. Comprehensive Liquid Profiling of Circulating Tumor DNA and Protein Biomarkers in Long-Term Follow-Up Patients with Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:5284-5294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 36. | Zhao L, Jiang L, Liu Y, Wang X, Song J, Sun Y, Bai Y, Dong X, Sun L, Wu J, Jiao Y, Zhao X. Integrated analysis of circulating tumour cells and circulating tumour DNA to detect minimal residual disease in hepatocellular carcinoma. Clin Transl Med. 2022;12:e793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Chen Y, Tian Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol Immunol. 2021;18:57-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 39. | Oura K, Morishita A, Tani J, Masaki T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 40. | Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. 2020;14:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Zheng X, Jin W, Wang S, Ding H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front Immunol. 2021;12:729705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Itoh S, Yoshizumi T, Yugawa K, Imai D, Yoshiya S, Takeishi K, Toshima T, Harada N, Ikegami T, Soejima Y, Kohashi K, Oda Y, Mori M. Impact of Immune Response on Outcomes in Hepatocellular Carcinoma: Association With Vascular Formation. Hepatology. 2020;72:1987-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 44. | Li XS, Li JW, Li H, Jiang T. Prognostic value of programmed cell death ligand 1 (PD-L1) for hepatocellular carcinoma: a meta-analysis. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, Jin L, Ding W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e13923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Yao W, He JC, Yang Y, Wang JM, Qian YW, Yang T, Ji L. The Prognostic Value of Tumor-infiltrating Lymphocytes in Hepatocellular Carcinoma: a Systematic Review and Meta-analysis. Sci Rep. 2017;7:7525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 47. | Nakagawa S, Umezaki N, Yamao T, Kaida T, Okabe H, Mima K, Imai K, Hashimoto D, Yamashita YI, Ishiko T, Chikamoto A, Baba H. Survival impact of lymphocyte infiltration into the tumor of hepatocellular carcinoma in hepatitis B virus-positive or non-B non-C patients who underwent curative resection. Hepatol Res. 2018;48:E126-E132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Schoenberg MB, Hao J, Bucher JN, Miksch RC, Anger HJW, Mayer B, Mayerle J, Neumann J, Guba MO, Werner J, Bazhin AV. Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, Heinrich B, Yu SJ, Zhang Q, Wilson A, Shi ZD, Swenson R, Greten TF. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 50. | Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, Island E, Satoskar R, Banovac F, Jha R, Kachhela J, Feng P, Zhang T, Tesfaye A, Prins P, Loffredo C, Marshall J, Weiner L, Atkins M, He AR. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol Res. 2016;4:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 51. | Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, Shi J, Fu B, Liu Z, Zhang JY, Jin L, Zhao Y, Lau GK, Zhao J, Wang FS. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 52. | Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155-5163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 54. | Sun L, Xu G, Liao W, Yang H, Xu H, Du S, Zhao H, Lu X, Sang X, Mao Y. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: a meta-analysis. Oncotarget. 2017;8:39658-39672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Zhao HQ, Li WM, Lu ZQ, Yao YM. Roles of Tregs in development of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20:7971-7978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 892] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 57. | Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 58. | Zhang X, Fu X, Li T, Yan H. The prognostic value of myeloid derived suppressor cell level in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0225327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14(+) HLA-DR(-/low) myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 61. | Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship With clinical and pathological features. Hepatology. 2016;64:2038-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 62. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 658] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 63. | Villani R, Vendemiale G, Serviddio G. Molecular Mechanisms Involved in HCC Recurrence after Direct-Acting Antiviral Therapy. Int J Mol Sci. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 65. | Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 66. | Liu K, Hao M, Ouyang Y, Zheng J, Chen D. CD133(+) cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci Rep. 2017;7:41499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 68. | Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012;2012:492920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Meniconi RL, Komatsu S, Perdigao F, Boëlle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg. 2021;273:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 72. | Yang Y, Yu H, Tan X, You Y, Liu F, Zhao T, Qi J, Li J, Feng Y, Zhu Q. Liver resection versus radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2021;38:875-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 74. | Wei F, Huang Q, Zhou Y, Luo L, Zeng Y. Radiofrequency ablation versus repeat hepatectomy in the treatment of recurrent hepatocellular carcinoma in subcapsular location: a retrospective cohort study. World J Surg Oncol. 2021;19:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther. 2016;16:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017;18:e101-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 77. | Qin L, Li C, Xie F, Wang Z, Wen T. Are inflammation-based markers useful in patients with hepatocellular carcinoma and clinically significant portal hypertension after liver resection? Biosci Trends. 2020;14:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 79. | Machairas N, Papaconstantinou D, Stamopoulos P, Prodromidou A, Garoufalia Z, Spartalis E, Kostakis ID, Sotiropoulos GC. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res. 2018;38:3181-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Liang Y, Lin C, Zhang B, Cao J, Chen M, Shen J, Feng X, Xiao G, Pan L, Chen K, Maher H, Cai X. Perioperative outcomes comparing laparoscopic with open repeat liver resection for post-hepatectomy recurrent liver cancer: A systematic review and meta-analysis. Int J Surg. 2020;79:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 81. | Di Sandro S, Danieli M, Ferla F, Lauterio A, De Carlis R, Benuzzi L, Buscemi V, Pezzoli I, De Carlis L. The current role of laparoscopic resection for HCC: a systematic review of past ten years. Transl Gastroenterol Hepatol. 2018;3:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Fang JZ, Xiang L, Hu YK, Yang Y, Zhu HD, Lu CD. Options for the treatment of intrahepatic recurrent hepatocellular carcinoma: Salvage liver transplantation or rehepatectomy? Clin Transplant. 2020;34:e13831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Zheng J, Cai J, Tao L, Kirih MA, Shen Z, Xu J, Liang X. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 84. | Aquina CT, Eskander MF, Pawlik TM. Liver-Directed Treatment Options Following Liver Tumor Recurrence: A Review of the Literature. Front Oncol. 2022;12:832405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Kostakis ID, Machairas N, Prodromidou A, Stamopoulos P, Garoufalia Z, Fouzas I, Sotiropoulos GC. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Transplant Proc. 2019;51:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Ma KW, Chok KSH, She WH, Chan ACY, Cheung TT, Dai WC, Fung JYY, Lo CM. Defining Optimal Surgical Treatment for Recurrent Hepatocellular Carcinoma: A Propensity Score Matched Analysis. Liver Transpl. 2018;24:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571-104581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Yin X, Hua T, Liang C, Chen Z. Efficacy of re-resection versus radiofrequency ablation for recurrent Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma (HCC) after resection for primary HCC. Transl Cancer Res. 2019;8:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Chen R, Gan Y, Ge N, Chen Y, Wang Y, Zhang B, Ye S, Ren Z. Transarterial Chemoembolization versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Resection within Barcelona Clinic Liver Cancer Stage 0/A: A Retrospective Comparative Study. J Vasc Interv Radiol. 2016;27:1829-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Koh PS, Chan AC, Cheung TT, Chok KS, Dai WC, Poon RT, Lo CM. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford). 2016;18:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Gou H, Liu S, Zhu G, Peng Y, Li X, Yang X, He K. Effectiveness of radiofrequency ablation versus transarterial chemoembolization for recurrent hepatocellular carcinoma: A meta-analysis. Acta Radiol Open. 2022;11:20584601221085514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Zu QQ, Liu S, Zhou CG, Yang ZQ, Xia JG, Zhao LB, Shi HB. Chemoembolization of recurrent hepatoma after curative resection: prognostic factors. AJR Am J Roentgenol. 2015;204:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017;52:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Kim KM. Nonsurgical multidisciplinary approach for recurrent hepatocellular carcinoma after surgical resection. Hepat Oncol. 2015;2:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 96. | Midorikawa Y, Takayama T, Moriguchi M, Yagi R, Yamagishi S, Nakayama H, Aramaki O, Yamazaki S, Tsuji S, Higaki T. Liver Resection Versus Embolization for Recurrent Hepatocellular Carcinoma. World J Surg. 2020;44:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, Shin WY. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Sun X, Yang Z, Mei J, Lyu N, Lai J, Chen M, Zhao M. The guiding value of microvascular invasion for treating early recurrent small hepatocellular carcinoma. Int J Hyperthermia. 2021;38:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Bzeizi KI, Arabi M, Jamshidi N, Albenmousa A, Sanai FM, Al-Hamoudi W, Alghamdi S, Broering D, Alqahtani SA. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 100. | Han T, Yang X, Zhang Y, Li G, Liu L, Chen T, Zheng Z. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A meta-analysis. Biosci Trends. 2019;13:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Yang B, Liang J, Qu Z, Yang F, Liao Z, Gou H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS One. 2020;15:e0227475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Kirchner T, Marquardt S, Werncke T, Kirstein MM, Brunkhorst T, Wacker F, Vogel A, Rodt T. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol (NY). 2019;44:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Song Q, Ren W, Fan L, Zhao M, Mao L, Jiang S, Zhao C, Cui Y. Long-Term Outcomes of Transarterial Chemoembolization Combined with Radiofrequency Ablation Versus Transarterial Chemoembolization Alone for Recurrent Hepatocellular Carcinoma After Surgical Resection. Dig Dis Sci. 2020;65:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, Lv SY. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. 2020;12:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Chu HH, Kim JH, Yoon HK, Ko HK, Gwon DI, Kim PN, Sung KB, Ko GY, Kim SY, Park SH. Chemoembolization Combined with Radiofrequency Ablation for Medium-Sized Hepatocellular Carcinoma: A Propensity-Score Analysis. J Vasc Interv Radiol. 2019;30:1533-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Sparchez Z, Radu P, Bartos A, Nenu I, Craciun R, Mocan T, Horhat A, Spârchez M, Dufour JF. Combined treatments in hepatocellular carcinoma: Time to put them in the guidelines? World J Gastrointest Oncol. 2021;13:1896-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 107. | Ali R, Riaz A, Gabr A, Abouchaleh N, Mora R, Al Asadi A, Caicedo JC, Abecassis M, Katariya N, Maddur H, Kulik L, Lewandowski RJ, Salem R. Clinical outcomes of Y90 radioembolization for recurrent hepatocellular carcinoma following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:2195-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Walburn T, Moon AM, Hayashi PH, Gerber D, Sanoff HK, McGinty KA, Mauro D, Tepper J, Wang K. Stereotactic Body Radiation Therapy for Recurrent, Isolated Hepatocellular Carcinoma Lymph Node Metastasis With or Without Prior Liver Transplantation. Cureus. 2020;12:e9988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Lee BH, Lee DS, Cho CW, Yun SS. Role and limitation of neoadjuvant hepatic arterial infusion chemotherapy in advanced hepatocelluar carcinoma patients with Child-Pugh class A. World J Surg Oncol. 2019;17:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, Park JW. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol. 2021;74:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 111. | Longo V, Brunetti O, Azzariti A, Galetta D, Nardulli P, Leonetti F, Silvestris N. Strategies to Improve Cancer Immune Checkpoint Inhibitors Efficacy, Other Than Abscopal Effect: A Systematic Review. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 112. | Burton EM, Tawbi HA. Bispecific Antibodies to PD-1 and CTLA4: Doubling Down on T Cells to Decouple Efficacy from Toxicity. Cancer Discov. 2021;11:1008-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39:2991-3001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 114. | Vogel A, Bathon M, Saborowski A. Advances in systemic therapy for the first-line treatment of unresectable HCC. Expert Rev Anticancer Ther. 2021;21:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 576] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 116. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 962] [Article Influence: 320.7] [Reference Citation Analysis (0)] |
| 117. | Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, Bai Y, Gu S, Li L, Hernandez S, Xu DZ, Mulla S, Wang Y, Shao H, Cheng AL. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer. 2021;10:296-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 118. | Lu LC, Cheng AL, Poon RT. Recent advances in the prevention of hepatocellular carcinoma recurrence. Semin Liver Dis. 2014;34:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Zhong JH, Zhong QL, Li LQ, Li H. Adjuvant and chemopreventive therapies for resectable hepatocellular carcinoma: a literature review. Tumour Biol. 2014;35:9459-9468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 121. | Chen DS, Hurwitz H. Combinations of Bevacizumab With Cancer Immunotherapy. Cancer J. 2018;24:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 122. | Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 123. | Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, Lee HC, Yopp A, Chow P, Qin S. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 124. | Xu X, Xing B, Hu M, Xu Z, Xie Y, Dai G, Gu J, Wang Y, Zhang Z. Recurrent hepatocellular carcinoma cells with stem cell-like properties: possible targets for immunotherapy. Cytotherapy. 2010;12:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, Manini MA, López MF, Anders M, Pinter M, Rodríguez MJB, Cristóbal MR, Soteras GA, Piñero F, Villadsen GE, Weinmann A, Crespo G, Mazzaferro V, Regnault H, Giorgio M, González-Diéguez ML, Donato MF, Varela M, Wörns MA, Bruix J, Lampertico P, Reig M. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 126. | Li BCW, Chiu J, Shing K, Kwok GGW, Tang V, Leung R, Ma KW, She WH, Tsang J, Chan A, Cheung TT, Lo CM, Yau T. The Outcomes of Systemic Treatment in Recurrent Hepatocellular Carcinomas Following Liver Transplants. Adv Ther. 2021;38:3900-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Li XH, Zhong KB, Liu Y, Yang DH, Zhou J. [Safety and efficacy of Sorafenib in treatment of tumor recurrence in liver transplantation recipients]. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1608-1610. [PubMed] |
| 128. | de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, Compagnon P, Lim C, Costentin C, Calderaro J, Luciani A, Feray C, Azoulay D. Role of Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation. Prog Transplant. 2016;26:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 130. | Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 131. | Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | López Ortega S, González Grande R, Santaella Leiva I, De la Cruz Lombardo J, Jiménez Pérez M. Efficacy and Safety of Sorafenib After Liver Transplantation: Experience in Our Center. Transplant Proc. 2020;52:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 133. | Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 134. | Piñero F, Thompson M, Marín JI, Silva M. Lenvatinib as first-line therapy for recurrent hepatocellular carcinoma after liver transplantation: Is the current evidence applicable to these patients? World J Transplant. 2020;10:297-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Sotiropoulos GC, Nowak KW, Fouzas I, Vernadakis S, Kykalos S, Klein CG, Paul A. Sorafenib treatment for recurrent hepatocellular carcinoma after liver transplantation. Transplant Proc. 2012;44:2754-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 137. | Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Li Z, Han N, Ren X, Zhang Y, Chu X. Effectiveness of TKI Inhibitors Combined With PD-1 in Patients With Postoperative Early Recurrence of HCC: A Real-World Study. Front Oncol. 2022;12:833884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 139. | He X, Peng Y, Zhou Z, Li W. Immune Checkpoint Inhibitor-Based Systemic Therapy Shows Remarkable Curative Effect in a Hepatocellular Carcinoma Patient With Intractable Postoperative Recurrence and Metastases: A Case Report and Literature Review. Front Oncol. 2022;12:784224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 140. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 595] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 141. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3297] [Cited by in RCA: 4772] [Article Influence: 397.7] [Reference Citation Analysis (1)] |
| 142. | Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D'Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1253] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 143. | Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, Ribas A, Hodi FS, Hamid O, Robert C, Daud A, Dronca R, Hersey P, Weber JS, Patnaik A, de Alwis DP, Perrone A, Zhang J, Kang SP, Ebbinghaus S, Anderson KM, Gangadhar TC. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res. 2018;24:4960-4967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 144. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 145. | Hiraoka A, Kumada T, Kudo M, Hirooka M, Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, Joko K, Shimada N, Deguchi A, Ishikawa T, Imai M, Tsuji K, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics). Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig Dis. 2017;35:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 146. | Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 147. | Jia ZZ, Huang YQ, Feng YL, Jiang GM. [Correlations between serum hypoxia inducible factor-1α, vascular endothelial growth factor and computed tomography perfusion imaging at pre-and post-TACE in patients with primary hepatic carcinoma]. Zhonghua Yi Xue Za Zhi. 2013;93:1472-1475. [PubMed] |
| 148. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 149. | Jia ZZ, Jiang GM, Feng YL. Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J. 2011;26:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 150. | Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 151. | Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 152. | Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, Guha C, Hodge JW. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8:e70417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 153. | Fagnoni FF, Zerbini A, Pelosi G, Missale G. Combination of radiofrequency ablation and immunotherapy. Front Biosci. 2008;13:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 154. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 155. | Jansen MC, van Hillegersberg R, Schoots IG, Levi M, Beek JF, Crezee H, van Gulik TM. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery. 2010;147:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 156. | Huang JT, Zhang S, Yang YH, Zhang ZC, Jiang N, Li WC, Shen J, Zhong BY, Zhu XL. Recent Update on Immunotherapy and Its Combination With Interventional Therapies for Hepatocellular Carcinoma. Clin Med Insights Oncol. 2022;16:11795549221134832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 157. | Li X, Zhang Q, Lu Q, Cheng Z, Liu F, Han Z, Yu X, Yu J, Liang P. Microwave ablation combined with apatinib and camrelizumab in patients with advanced hepatocellular carcinoma: A single-arm, preliminary study. Front Immunol. 2022;13:1023983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 158. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 159. | Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, Zhang X, Wang XD, Cao G, Chen H, Liu P, Xu HF, Gao QZ, Yang RJ. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 160. | Wang X, Liu G, Chen S, Bi H, Xia F, Feng K, Ma K, Ni B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia. 2021;38:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 161. | Gu H, Li J, You N, Wu K, Wang Z, Wang L, Zhu Y, Liu Q, Peng X, Zheng L. Efficacy and safety of apatinib combined with transarterial chemoembolization (TACE) in treating patients with recurrent hepatocellular carcinoma. Ann Transl Med. 2020;8:1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 162. | Peng Z, Chen S, Xiao H, Wang Y, Li J, Mei J, Chen Z, Zhou Q, Feng S, Chen M, Qian G, Peng S, Kuang M. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology. 2019;292:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 163. | Wan X, Zhai X, Yan Z, Yang P, Li J, Wu D, Wang K, Xia Y, Shen F. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7:83806-83816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 164. | Zhou Q, Wang X, Li R, Wang C, Wang J, Xie X, Li Y, Li S, Mao X, Liang P. Sorafenib as adjuvant therapy following radiofrequency ablation for recurrent hepatocellular carcinoma within Milan criteria: a multicenter analysis. J Gastroenterol. 2022;57:684-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Guo Y, Ren Y, Chen L, Sun T, Zhang W, Sun B, Zhu L, Xiong F, Zheng C. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 166. | Iwata N, Ishii Y, Yoshida Y, Kikuchi T, Mita H, Adachi Y, Minami T, Ikeda K, Makiguchi Y, Hata F, Kato Y, Endo T. [A Case in Which Multiple Post-Operatively Recurring Hepatocellular Carcinomas Showed a Positive Response to Sorafenib-Hepatic Arterial Infusion Chemotherapy (HAIC)Sequential Therapy]. Gan To Kagaku Ryoho. 2021;48:215-218. [PubMed] |
| 167. | Chen B, Zhai YR, Li YX, Wang SL, Yu T, Tang Y, Fang H, Yang Z, Zhao P, Liu Y, Song YW, Lu NN, Jing H, Qi SN, Yang Y, Wang L, Wu J, Wu F, Rong W, Niu L, Zeng H, Song Y, Sun Y, Li N. Previous/Concurrent Radiation Enhanced the Response of Toripalimab in Advanced and Recurrent Liver Cancer: A Pilot Study. Int J Radiat Oncol Biol Phys. 2021;111 Suppl:e34. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 168. | Wei MC, Zhang YJ, Chen MS, Chen Y, Lau WY, Peng ZW. Adjuvant Sorafenib Following Radiofrequency Ablation for Early-Stage Recurrent Hepatocellular Carcinoma With Microvascular Invasion at the Initial Hepatectomy. Front Oncol. 2022;12:868429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 169. | Feng X, Xu R, Du X, Dou K, Qin X, Xu J, Jia W, Wang Z, Zhao H, Yang S, Guo C, Liu T, Ma K. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol. 2014;109:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 170. | Mahn R, Sadeghlar F, Bartels A, Zhou T, Weismüller T, Kupczyk P, Meyer C, Gaertner FC, Toma M, Vilz T, Knipper P, Glowka T, Manekeller S, Kalff J, Strassburg CP, Gonzalez-Carmona MA. Multimodal and systemic therapy with cabozantinib for treatment of recurrent hepatocellular carcinoma after liver transplantation: A case report with long term follow-up outcomes. Medicine (Baltimore). 2021;100:e27082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 171. | Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 536] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 172. | Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 173. | Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Recept Signal Transduct Res. 2019;39:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 174. | Lee BM, Seong J. Radiotherapy as an immune checkpoint blockade combination strategy for hepatocellular carcinoma. World J Gastroenterol. 2021;27:919-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |