Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1218
Peer-review started: March 3, 2022
First decision: April 17, 2022
Revised: May 10, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: July 15, 2022
Processing time: 131 Days and 20.4 Hours
Nearly 80% of patients with pancreatic ductal adenocarcinoma (PDAC) develop cachexia along their disease course. Cachexia is characterized by progressive weight loss, muscle wasting, and systemic inflammation and has been linked to poorer outcomes and impairments in quality of life. Management of PDAC cachexia has historically involved a multidisciplinary effort comprised of nutri
Core Tip: Cachexia is a hallmark of pancreatic cancer and is characterized by muscle wasting, weight loss, and systemic inflammation. Despite advancements in nutritional support, pancreatic enzyme replacement therapy, and pharmacologic interventions for treating pancreatic cancer cachexia, it continues to have a significant negative impact on patient outcomes. We detail the results of a recent prospective clinical trial wherein cachectic patients with advanced pancreatic cancer achieved weight stability with 12 wk of enteral feeding. Notably, gut microbiome changes and an increased abundance of a specific microbe associated with enteral feeding highlight a potentially novel approach to mitigate cachexia through microbial modulation.
- Citation: Hendifar A, Akinsola R, Muranaka H, Osipov A, Thomassian S, Moshayedi N, Yang J, Jacobs J, Devkota S, Bhowmick N, Gong J. Gut microbiome and pancreatic cancer cachexia: An evolving relationship. World J Gastrointest Oncol 2022; 14(7): 1218-1226
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1218.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1218
Pancreatic cancer is an aggressive malignancy characterized by progressive therapeutic resistance and a multifactorial syndrome of weight loss, muscle wasting, and systemic inflammation known as cachexia[1,2]. Cachexia is pervasive and an unfortunate hallmark of pancreatic cancer as nearly 85% of patients with pancreatic ductal adenocarcinoma (PDAC) will meet the definition of cancer cachexia along their disease course[3,4]. Cancer cachexia is generally defined as a multifactorial syndrome characterized by progressive loss of skeletal muscle mass (with or without loss of fat mass) that is not fully reversible through conventional means of nutritional support and leads to ongoing impairment in patient function[5]. Diagnostic criteria for cancer cachexia have been defined by international consensus guidelines as well (Table 1).
| Definition of cancer cachexia is met with one of the following |
| Weight loss > 5% over past 6 mo (in absence of simple starvation) |
| Body mass index < 20 and weight loss > 2% |
| Evidence of sarcopenia with weight loss > 2%1 |
The management of PDAC cachexia is multidisciplinary and has historically been comprised of the following: Nutritional support with oral nutrition supplements and involvement of a registered dietitian, pancreatic enzyme replacement therapy, exercise, pharmacologic interventions, and in select cases, specialized nutrition support through the use of enteral or parenteral nutrition[4,6]. The importance of systemic therapy for the underlying PDAC cannot be underscored as well given that the negative impact of cancer cachexia on patient outcomes can be offset, to a degree, with systemic chemotherapy[7]. Despite the mechanisms of PDAC cachexia having been increasingly described, the cachexia syndrome in pancreatic cancer patients remains difficult to treat with a profoundly negative impact on outcomes including overall survival (OS), response to chemotherapy, and quality-of-life[6,7]. As such, novel interventions for PDAC cachexia are of high unmet need.
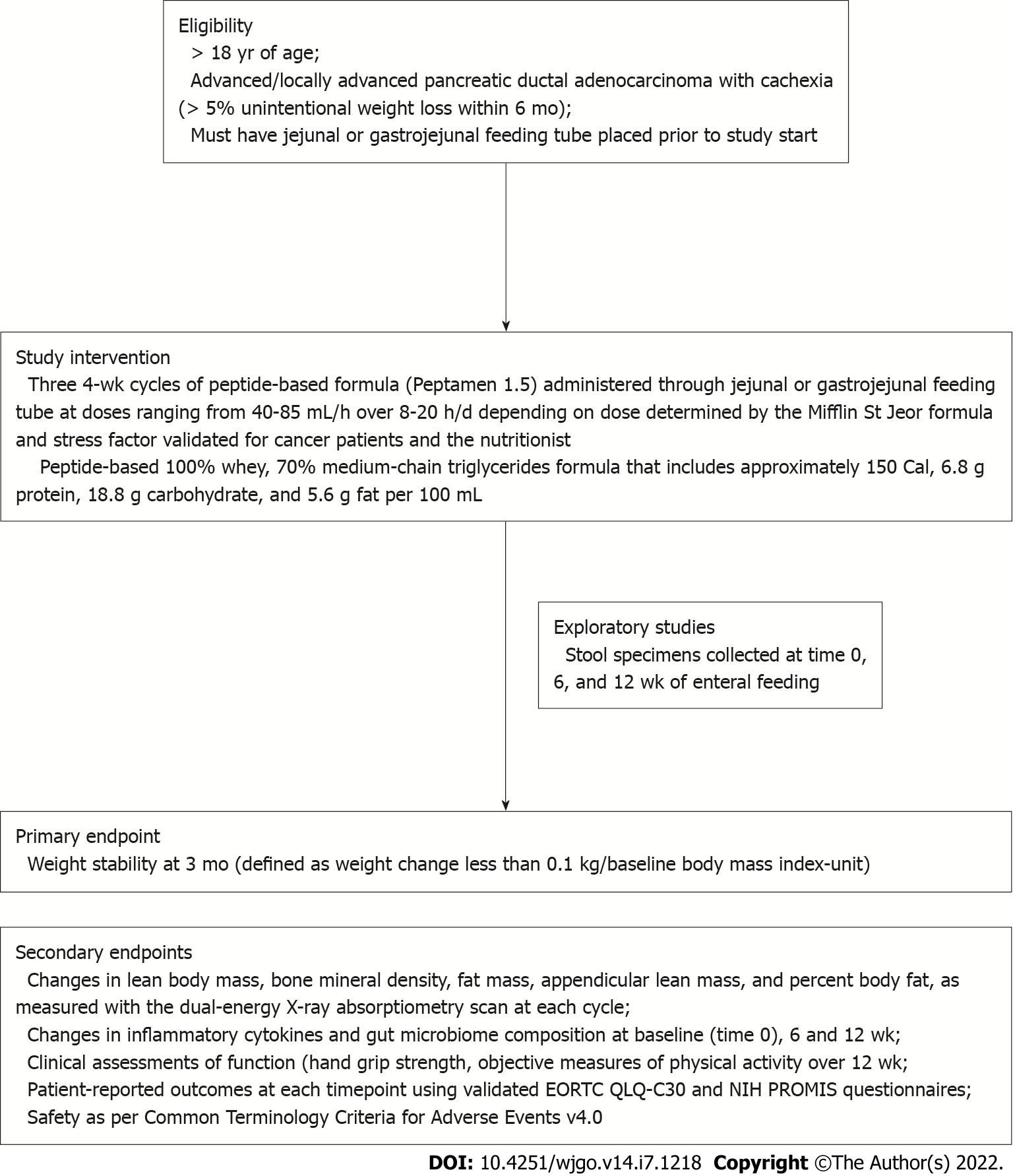
Our group has historically focused on the development of biomarkers and therapeutic strategies for PDAC cachexia across interventional and observational trials[1,8]. We conducted PANCAX-1 (NCT02400398), which was a single-institution, single-arm prospective clinical trial, to evaluate the feasibility and efficacy of enteral feeding on weight stability in cachectic patients with advanced pancreatic cancer[9,10]. Eligible patients included those aged > 18 years having been diagnosed with advanced or locally advanced pancreatic cancer and cachexia. Candidates were required to have a jejunal or gastrojejunal feeding tube placed prior to study intervention. Cachexia was defined using consensus criteria (Table 1). Anticancer therapy or previous surgical resection for pancreatic cancer was permitted. Patients were enrolled to receive the study intervention of a peptide-based formula (Peptamen 1.5) over three 4-week cycles (total of 12 wk) of enteral feeding as per protocol (Figure 1).
The primary endpoint was weight stability at 3 mo, defined as weight loss < 0.1 kg/baseline body mass index (BMI)-unit. Secondary endpoints included changes in body composition measurements, clinical metrics of function and activity, safety, and patient-reported outcomes (PROs).
From April 2015-March 2019, a total of 31 patients were consented onto the study. From this, 16 patients were able to complete all 12 wk of enteral tube feeding and were deemed evaluable for the primary endpoint. The study achieved its primary endpoint whereby weight stability was achieved in 10/16 patients (62.5%). Additionally, enteral feeding was associated with improvement in key secondary outcomes including decreases in body fat mass and inflammatory markers (CRP) but increases in lean body mass (Table 2). Improvements were seen in PROs using both NIH PROMIS and EORTC QLQ-C30 scores from baseline to 12 wk of enteral feeding in this cohort[9].
| Outcome (n = 16) | Change (SD) |
| Average weight (kg) | +1.29 (5.8) |
| Body mass index (kg/m2) | +0.6 (1.7) |
| % Body fat | -1.6 (5) |
| Bone mineral density (T-score) | -0.01 (0.02) |
| Body fat mass (g) | -602 (2794) |
| Lean body mass (g) | +1273.1 (4078) |
| Appendicular lean mass (kg/m2) | +0.45 (0.62) |
| C-reactive protein (mg/mL) | -9.77 (SE 11.6) |
| Patient-reported outcomes | |
| NIH PROMIS (mean difference in score) | |
| Pain interference | -7.5 (P = 0.05) |
| Fatigue | -7.1 (P = 0.06) |
| Depression | -10.4 (P = 0.006) |
| EORTC QLQ-C30 (mean difference in score) | |
| Global health | +13.3 (P = 0.05) |
The PANCAX-1 trial successfully demonstrated the feasibility and efficacy of enteral feeding alongside systemic chemotherapy for the treatment of cachexia in patients with advanced PDAC. Despite a drop in consented subjects who were unable to complete 12-wk of enteral feeding due to advanced disease, deteriorating performance status, and/or rapid changes in symptom burden as expected from a high-risk population, enteral feeding resulted in weight stability and improved PROs in cachectic patients with advanced pancreatic cancer. We next sought to identify predictive biomarkers associated with weight stability in this prospective cohort for insight into the possible mechanisms by which enteral feeding served an effective intervention for cachexia. In preplanned exploratory studies of the PANCAX-1 prospective cohort, blood and stool samples were collected longitudinally for profiling of inflammatory cytokines and the gut microbiome.
Our group characterized for the first time the gut microbiome composition in patients with advanced pancreatic cancer treated with enteral feeding for cachexia[11]. DNA extraction and sequencing of the 16S ribosomal RNA gene was performed on fecal samples, as previously described[12], with several unique findings (Table 3). Firstly, in stool samples collected over 12 wk of enteral feeding, differential abundance testing identified an increased relative abundance of the Gram-negative genus Veillonella (P = 0.0150) and the Gram-positive genus Actinomyces (P = 0.0390). As Veillonella represented a bacterial genus that increased in abundance over time with enteral feeding for PDAC cachexia, it was interesting to discover that a significantly increased abundance of Veillonella was also identified in baseline stool samples of cachectic patients who achieved weight stability with enteral feeding.
| Stool microbiome assessment1 | Outcome |
| Relative abundance from baseline (time 0) to 12 wk (n = 6) | Increased abundance: Veillonella genera (P = 0.015); Actinomyces genera (P = 0.039). Decreased abundance: Bacteroides genera (P = 0.015); Butyricicoccus genera (P = 0.039) |
| Relative abundance in baseline stool samples from subjects achieving weight stability (n = 8) | Increased abundance: Veillonella genera (P = 0.0006). Decreased abundance: Bifidobacterium genera (P = 2.35 × 10-5)2 |
| Diversity indices (n = 8) | Weight stability associated with reduced diversity by Chao1 index of richness (P = 0.0208) but not reduced species richness and evenness by Shannon index (P = 0.187) |
Veillonella are Gram-negative, anaerobic bacteria known for its lactate fermenting abilities and are nonpathogenic colonizers of the intestines and oral mucosa in humans whereby Veillonella atypica and its active metabolite propionate has been shown to enhance physical performance in mouse models[13]. Interestingly, in a separate cohort of cachectic patients comprised predominantly of subjects with pancreatic cancer, Veillonella was among the most abundant bacterial genera among cachectic cancer patients[14]. In a comparison of the oral microbiome collected from PDAC patients and healthy controls, Veillonella were among the genera in significantly greater abundance in salivary samples from healthy controls than those with PDAC[15]. Furthermore, when comparing subjects with resectable vs unresectable PDAC, Veillonella was found to be the most abundant bacterial genera in those with less advanced, resectable disease when compared to more advanced, unresectable disease. When compared to healthy controls, Veillonella had the lowest odds ratio (OR) for risk of PDAC development across all sampled oral bacteria (OR 0.187, 95% confidence interval 0.055-0.631, P = 0.007). The relative abundance of Veillonella in saliva samples was observed to show a gradual decline from healthy controls to those with resectable PDAC and unresectable PDAC. The lowest abundance of Veillonella was observed in saliva samples from subjects with unresectable PDAC, whereas the highest abundance of Veillonella was observed in the saliva from healthy subjects.
The relationship between the gut microbiome and cachexia has long been implicated in earlier investigations wherein alterations in gut microbiome composition were associated with anorexia nervosa and low BMI states, body weight loss, low muscle mass, low appetite, and systemic inflammation[16]. However, the role that the gut microbiome plays in the cachexia process has been better established with studies on its impact with systemic inflammation and muscle wasting, which are hallmarks of cachexia[16-18].
Inflammation has classically served in host defense against pathogens but has increasingly been shown to be equally important in tissue repair, regeneration, and remodeling with programmed cell death including apoptosis, necroptosis, and pyroptosis representing means to clear dying cells and promote tissue homeostasis[19,20]. In this sense, localized transient inflammation is generally protective, helping the host to remove harmful stimuli including physical, chemical, carcinogenic, and infectious and facilitate degradation of dying cells as a nutritional source to facilitate tissue regeneration. However, the inflammatory response underlying cachexia often is characterized by impairment in the correct utilization of nutrients such that meeting energy and protein requirements in patients with cachexia without addressing inflammation can result in improper restoration of body composition as most proteins and energy are diverted to production of acute-phase proteins and adi
Animal studies have illustrated several key findings of the gut microbiome-cachexia relationship: (1) Gut microbes can lead to muscle wasting through decreasing amino acid availability for the host or synthesis of noxious bacterial metabolites (e.g., indoxyl sulfate and lipopolysaccharide or LPS) that activate PI3K/AKT, NF-κB, and MAPK (p38, JNK, ERK) signaling to upregulate Atrogin-1/MAFbx and MuRF1 genes encoding E3 ubiquitin ligases; (2) Pathogen-associated molecular patterns (PAMPs) from microbes (e.g., circulating peptidoglycans, LPS, bacterial nucleic acids, short-chain fatty acids or SCFA, branched-chain amino acids or BCAAs, or flagellin) can induce muscle atrophy by stimulating the Toll-like receptor/NF-kB pathway; (3) Increased gut permeability in cachectic disorders and subsequent translocation of PAMPs from the gut lumen can stimulate pro-inflammatory cytokine cascades; and (4) Depletion of certain bacterial conditions can induce muscle wasting by activating the AMPK-FoxO3-Atrogin-1/MuRF1 cascade and BCAA catabolism, reducing expression of growth factors and muscle growth-related genes (IGF-1, myogenin, SIK1, and MyoD), increasing myostatin, and impairing neuromuscular junction function and mitochondrial function[16-18].
Based on this preclinical rationale, it is not surprising that early efforts for gut microbiota-targeted nutritional interventions of cachexia have already begun exploration. For example, administration of a mixture of Lactobacillus reuteri and Lactobacillus gasseri to cachectic mice with leukemia restored the levels of these bacteria in the gut while reducing inflammation and partially counteracting the induction of muscle atrophy markers[24]. Administration of bacterial metabolites have also shown applicability in the treatment of cachexia where pectic oligosaccharides given to leukemic mice with cachexia was able to delay the cachectic phenotype and spare fat mass while increasing abundance of Bacteroides dorei[25].
There is growing evidence to suggest that Veillonella represents a genus of gut bacteria that is protective against PDAC and performance enhancing in human subjects[13,15]. In our preplanned analyses of the gut microbiome in stool samples serially collected from a prospective cohort of enteral fed patients with PDAC cachexia, we identified compositional changes in the gut microbiome and an increase in abundance of the bacterial genus Veillonella over time with enteral feeding associated with weight stability. We are therefore the first to posit another beneficial role of Veillonella as a microbe associated with weight stability in the treatment of cachexia. However, before positioning Veillonella as a potential future and novel intervention to mitigate cachexia, there are several lessons that can be learned from microbial manipulation strategies thus far.
Interventions to target the gut microbiome in cancer cachexia can largely be classified into: (1) Prebiotics, which are nondigestible substrates that can induce growth or activity of microorganisms in the host; (2) Probiotics that contain live microorganisms to be introduced to the host; and (3) Synbiotics, which are mixtures of live microorganisms and substrates utilized by the host (combination of prebiotics and probiotics)[17].
Recently, the double-blind, randomized phase II TRANSIT trial enrolled patients with unresectable or metastatic gastroesophageal junction adenocarcinoma who were planned to receive standard first-line chemotherapy and met criteria for cachexia to receive allogenic fecal microbiota transplantation (FMT) from obese donors or autologous FMT (control)[26]. Donor and recipients delivered fresh fecal samples within 6 h before use on day of fecal infusions wherein the feces were mixed until fully homogenized and the fecal solution filtered to remove food derived debris. The filtrate was then transferred to a 1000-mL sterile bottle and stored at room temperature. Enrolled subjects underwent bowel lavage with polyethylene glycol solution through a nasoduodenal tube to remove endogenous fecal contamination. This was followed by infusion of the gut microbiota solution over 30 minutes approximately. The primary outcome of this study was effect of allogenic FMT on satiety after 4 wk with secondary outcomes on cachexia domains including nutritional and appetite assessments and conventional cancer efficacy outcomes.
Between August 2016 to January 2019, 24 patients were randomized to receive allogenic FMT (n = 12) and autologous FMT (n = 12). Donors for allogenic FMT were all healthy overweight or obese subjects by BMI criteria. There was no significant difference in satiety levels, caloric intake, or change in any other measure related to cachexia between allogenic and autologous FMT groups. There was no difference in completion rates or adverse events associated with chemotherapy across groups either. However, those receiving allogenic FMT had higher disease-control rates at 12 wk, longer median OS (365 d vs 227 d), hazard ratio 0.38 (95% confidence interval: 0.14-1.05, P = 0.057) and longer progression-free survival (204 d vs 93 d) than those receiving autologous FMT. The microbiome composition from the allogenic recipients resembled the donor microbiome more closely after the FMT compared to baseline, suggestive of proper engraftment of donor microbiota.
The phase II TRANSIT trial, although negative, should be praised for testing the feasibility of such an approach in human subjects with cancer cachexia. There are multiple take-away points from this important study that need to be considered in future applications of microbial interventions in human subjects with cachexia. Firstly, although microbiome analyses revealed a significant shift in microbiome composition following allogenic FMT, a specific microbe or group of microbes mediating the beneficial oncological outcomes in the allogenic group were not identified. Is a healthy obese subject the ideal donor for FMT to treat cancer cachexia? An alternative mechanism could entail the administration of microbes isolated from stool of successfully treated patients for cachexia. This could arguably reflect the compositional changes in the gut microbiome indicative of a responding host to anti-cachexia therapy. The microbiome in an obese individual could also differ significantly from those of non-obese individuals and can induce weight gain or weight loss dependent on a variety of environmental and host biologic factors[26]. The uniqueness of the PANCAX-1 cohort lies in the fact that all subjects received enteral feeding as their primary source of nutrition thereby representing a homogeneous and internally controlled population for microbiome and metabolomics analyses. The finding that Veillonella was a microbe of interest with increased abundance over time with enteral feeding and was associated with weight stability in cachectic patients with advanced PDAC receiving enteral feeding provides an innovative opportunity to explore microbial interventional strategies for cachexia with this organism.
However, individual microbes may not be sufficient to elect pro- or anti-cachexia effects alone. Animal models have demonstrated that a series of functional and structural changes occur in the gut bacterial population during the development of cachexia[27]. Microbial dysbiosis has shown to play a role in shaping the gut microbiome and pancreatic tumorigenesis as well[28]. We showed that weight stabilizing, cachexia therapy through enteral feeding was associated with multiple taxonomic shifts including increased abundance of the Veillonella genus (P = 0.015) and Actinomyces genus (P = 0.039) and decreased abundance of the Bacteroides genus (P = 0.015) and Butyricicoccus genus (P = 0.039) (Table 3). In this sense, it would be prudent for future studies to evaluate the impact of microbial dysbiosis on cachexia, with emphasis on community microbes that altogether contribute to anti- or pro-cachexia effects in microbial interventional strategies for cachexia.
Lastly, it would be important to consider that microorganisms themselves may not be the key constituent for developing therapies against cachexia. Instead, the active metabolites of the gut microbiota may be just as (if not more) important in contributing to anti- or pro-cachexia effects. Here, studies have shown that branched-chain amino acids, LPS, polyamines, and metabolites of other biosynthetic pathways have correlated with altered microbial flora, tumorigenesis, and development of cachexia across animal models[28,29]. Therefore, the logical next step in addition to exploring the potential of Veillonella as a microbial intervention in the treatment of cachexia would be for metabolomics to profile the active metabolite(s) of this microorganism and microbial communities associated with weight stabilization on cachexia therapy. The impact of these metabolites as a mechanism to address the cachexia syndrome could then be formally evaluated in preclinical models[30].
Cachexia represents a multifactorial syndrome of weight loss, muscle wasting, and systemic inflammation that is pervasive across multiple advanced disease states. Using PDAC as a model, we identified a unique relationship between the gut microbiome and treatment of cachexia in a prospective cohort of advanced PDAC subjects treated with enteral feeding. Specifically, an increased abundance in the bacterial genus Veillonella was observed over time in stool samples of cachectic subjects effectively treated with weight-stabilizing intervention through 12 wk of enteral feeding. Our findings are hypothesis-generating and add to an exciting body of evidence suggesting a potential role for microbial-based interventions for cachexia. Future clinical translation of microbial modulation to mitigate cachexia will need to consider the role of microbial dysbiosis and microbial-derived metabolites in cachexia as well.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manojlovic N, Serbia; Yu L, Singapore S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Gong J, Guan M, Forsmark CE, Petzel MQ, Placencio-Hickok V, Hendifar A. Fecal elastase, an assay for exocrine pancreatic insufficiency, has clinical utility in patients with pancreatic ductal adenocarcinoma. Therap Adv Gastroenterol. 2020;13:1756284820964319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Gong J, Tuli R, Shinde A, Hendifar AE. Meta-analyses of treatment standards for pancreatic cancer. Mol Clin Oncol. 2016;4:315-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Fearon KC, Voss AC, Hustead DS; Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 513] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Hendifar AE, Petzel MQB, Zimmers TA, Denlinger CS, Matrisian LM, Picozzi VJ, Rahib L; Precision Promise Consortium. Pancreas Cancer-Associated Weight Loss. Oncologist. 2019;24:691-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3840] [Article Influence: 274.3] [Reference Citation Analysis (0)] |
| 6. | Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N, Pandol SJ, Tuli R, Hendifar AE. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol. 2014;5:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Hendifar AE, Chang JI, Huang BZ, Tuli R, Wu BU. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J Gastrointest Oncol. 2018;9:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Hendifar AE, Kim S, Tighiouart M, Hautamaki E, Kim H, Ng C, Liu J-Y, Scher KS, Klempner SJ, Placencio-Hickok V, Gresham G, Gong J. A phase I study of nanoliposomal irinotecan and 5-fluorouracil/folinic acid in combination with interleukin-1-alpha antagonist for advanced pancreatic cancer patients with cachexia (OnFX). J Clin Oncol. 2020;38 4634 PMID:Not applicable.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Gresham G, Placencio-Hickok VR, Lauzon M, Nguyen T, Kim H, Mehta S, Paski S, Pandol SJ, Osipov A, Gong J, Jamil LH, Nissen N, Lo SK, Hendifar AE. Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient-reported outcomes in pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Hendifar AE, Gresham G, Kim H, Guan M, Liu J-Y, Minton B, Bhuiyan D, Langeslay R, Rogatko A, Gong J, Placencio-Hickok V. A prospective trial of elemental enteral feeding in patients with pancreatic cancer cachexia (PANCAX-1). J Clin Oncol. 2020;38:726. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Moshayedi N, Yang J, Lagishetty V, Jacobs J, Placencio-Hickok V, Osipov A, Hendifar AE, Gong J. Fecal microbiome composition in pancreatic cancer cachexia and response to nutrition support. J Clin Oncol. 2021;39:4129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Jacobs JP, Lin L, Goudarzi M, Ruegger P, McGovern DP, Fornace AJ Jr, Borneman J, Xia L, Braun J. Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency. Gut Microbes. 2017;8:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, Yang Z, Hattab MW, Avila-Pacheco J, Clish CB, Lessard S, Church GM, Kostic AD. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 14. | Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J, van Helvoort A, Smidt ML, Kruitwagen RFPM, Baade-Corpelijn L, Olde Damink SWM, Rensen SS. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle. 2021;12:2007-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 15. | Wei AL, Li M, Li GQ, Wang X, Hu WM, Li ZL, Yuan J, Liu HY, Zhou LL, Li K, Li A, Fu MR. Oral microbiome and pancreatic cancer. World J Gastroenterol. 2020;26:7679-7692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 16. | Genton L, Mareschal J, Charretier Y, Lazarevic V, Bindels LB, Schrenzel J. Targeting the Gut Microbiota to Treat Cachexia. Front Cell Infect Microbiol. 2019;9:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ziemons J, Smidt ML, Damink SO, Rensen SS. Gut microbiota and metabolic aspects of cancer cachexia. Best Pract Res Clin Endocrinol Metab. 2021;35:101508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Liu C, Cheung WH, Li J, Chow SK, Yu J, Wong SH, Ip M, Sung JJY, Wong RMY. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1393-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 19. | Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 2431] [Article Influence: 405.2] [Reference Citation Analysis (0)] |
| 20. | Yang Y, Jiang G, Zhang P, Fan J. Programmed cell death and its role in inflammation. Mil Med Res. 2015;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | Laviano A, Koverech A, Mari A. Cachexia: clinical features when inflammation drives malnutrition. Proc Nutr Soc. 2015;74:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Arabi YM, Reintam Blaser A, Preiser JC. Less is more in nutrition: critically ill patients are starving but not hungry. Intensive Care Med. 2019;45:1629-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 427] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 24. | Bindels LB, Beck R, Schakman O, Martin JC, De Backer F, Sohet FM, Dewulf EM, Pachikian BD, Neyrinck AM, Thissen JP, Verrax J, Calderon PB, Pot B, Grangette C, Cani PD, Scott KP, Delzenne NM. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7:e37971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Bindels LB, Neyrinck AM, Salazar N, Taminiau B, Druart C, Muccioli GG, François E, Blecker C, Richel A, Daube G, Mahillon J, de los Reyes-Gavilán CG, Cani PD, Delzenne NM. Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PLoS One. 2015;10:e0131009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | de Clercq NC, van den Ende T, Prodan A, Hemke R, Davids M, Pedersen HK, Nielsen HB, Groen AK, de Vos WM, van Laarhoven HWM, Nieuwdorp M. Fecal Microbiota Transplantation from Overweight or Obese Donors in Cachectic Patients with Advanced Gastroesophageal Cancer: A Randomized, Double-blind, Placebo-Controlled, Phase II Study. Clin Cancer Res. 2021;27:3784-3792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | de Maria YNLF, Aciole Barbosa D, Menegidio FB, Santos KBNH, Humberto AC, Alencar VC, Silva JFS, Costa de Oliveira R, Batista ML Jr, Nunes LR, Jabes DL. Analysis of mouse faecal dysbiosis, during the development of cachexia, induced by transplantation with Lewis lung carcinoma cells. Microbiology (Reading). 2021;167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Mendez R, Kesh K, Arora N, Di Martino L, McAllister F, Merchant N, Banerjee S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis. 2020;41:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 29. | Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E, Galffy G, Berta J, Weiss GJ, Sommer MOA, Panagiotou G. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 2021;15:3207-3220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Tsvetikova SA, Koshel EI. Microbiota and cancer: host cellular mechanisms activated by gut microbial metabolites. Int J Med Microbiol. 2020;310:151425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |