Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.1968
- This article has been corrected.
- See: World J Gastrointest Oncol. Sep 15, 2023; 15(9): 1673-1674
Peer-review started: May 30, 2022
First decision: June 21, 2022
Revised: July 4, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: October 15, 2022
Processing time: 136 Days and 22 Hours
Interleukin (IL)-34 is a pro-inflammatory cytokine involved in tumor development. The role of IL-34 in the proliferation and epithelial-mesenchymal transition (EMT) of gastric cancer (GC) remains to be investigated.
To investigate whether and how IL-34 affects the proliferation of GC cells and EMT.
Using immunohistochemical staining, the expression of IL-34 protein was detected in 60 paired GC and normal paracancerous tissues and the relationship between IL-34 and clinicopathological factors was analyzed. The expression of IL-34 mRNA and protein in normal gastric epithelial cell lines and GC was detected using quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting, respectively. Stable IL-34 knockdown and overexpression in AGS cell lines were established by lentiviral infection and validated by qRT-PCR and western blotting. The cholecystokinin-8 assay, clone formation assay, cell scratch assay, and transwell system were used to detect GC cell proliferation, clone formation, migration, and invasion capacity, respectively. The effects of IL-34 on the growth of GC transplant tumors were assessed using a subcutaneous transplant tumor assay in nude mice. The effects of IL-34 on the expression level of EMT-associated proteins in AGS cells were examined by western blotting.
Expression of IL-34 protein and mRNA was higher in GC cell lines than in GES-1 cells. Compared to matched normal paraneoplastic tissues, the expression of IL-34 protein was higher in 60 GC tissues, which was correlated with tumor size, T-stage, N-stage, tumor, node and metastasis stage, and degree of differentiation. Knockdown of IL-34 expression inhibited the proliferation, clone formation, migration, and invasion of AGS cells, while overexpression of IL-34 promoted cell proliferation, clone formation, migration, and invasion. Furthermore, the reduction of IL-34 promoted the expression of E-cadherin in AGS cells but inhibited the expression of vimentin and N-cadherin. Overexpression of IL-34 inhibited E-cadherin expression but promoted expression of vimentin and N-cadherin in AGS cells. Overexpression of IL-34 promoted the growth of subcutaneous transplanted tumors in nude mice.
IL-34 expression is increased in GC tissues and cell lines compared to normal gastric tissues or cell lines. In GC cells, IL-34 promoted proliferation, clone formation, migration, and invasion by regulating EMT-related protein expression cells. Interference with IL-34 may represent a novel strategy for diagnosis and targeted therapy of GC.
Core Tip: Our study provides novel evidence that interleukin (IL)-34 contribute the growth and metastasis of gastric cancer (GC). IL-34 is up-regulated in GC cell lines and tissues, which is correlated with tumor size, grade of differentiation and tumor, node and metastasis stage. IL-34 enhances the ability of proliferation, clone formation, migration and invasion of GC cells and regulates the expression levels of epithelial-mesenchymal transition-associated proteins, suggesting that IL-34 may be an effective target for the therapy of GC.
- Citation: Li CH, Chen ZM, Chen PF, Meng L, Sui WN, Ying SC, Xu AM, Han WX. Interleukin-34 promotes the proliferation and epithelial-mesenchymal transition of gastric cancer cells. World J Gastrointest Oncol 2022; 14(10): 1968-1980
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/1968.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.1968
Gastric cancer (GC) is one of the most aggressive cancers, with approximately 1 million new cases diagnosed worldwide in 2020 and an estimated 769000 deaths, ranking fifth in incidence and fourth in mortality globally[1]. Rates are two-fold higher in men than in women[1]. East Asia, including China, Japan, and Korea, is the hotspot of incidence and mortality of GC[2]. In Japan and Korea, screening programs have led to substantial reductions in GC-associated mortality[2]. However, in China, due to the lack of extensive initial screening for early GC, most patients present with advanced GC[3]. Radial surgery is the main modality for the treatment of locally progressive GC, and the combination of chemotherapy and targeted therapy can improve the prognosis. During 2007-2021, the 5-year relative survival rate of patients with GC continued to increase[4]. The overall 5-year age-standardized relative survival rates in 2007-2011, 2012-2016, and 2017-2021 were 38.3%, 40.6%, and 42.9%, respectively[4]. However, the overall relative survival of patients with GC remains low[4]. In particular, the survival rate of patients with distant-stage GC remains very low (10%)[4]. Targeted therapy alone or in combination with chemotherapy has shown potential advantages in the treatment of advanced GC[5]. Trastuzumab is a targeted therapy that effectively improves the prognosis of HER2-positive patients with GC; however, less than 20% of patients with GC are HER2-positive, which means that the remaining 80% of patients with GC do not benefit from the drug[1]. Therefore, identifying more effective targets will provide new ideas for targeted therapy for GC.
Epithelial-mesenchymal transition (EMT) refers to the biological process in which epithelial cells are transformed into cells with mesenchymal properties through a complex series of mechanisms in response to relevant factors[6-8]. In cancer, EMT is associated with tumorigenesis, invasion, metastasis, and resistance to therapy. Different states of EMT exhibit different functional characteristics in cancer and are associated with tumor proliferation, propagation, plasticity, invasion, and metastasis[9]. Exosomes have been proposed as a therapeutic tool to control the development of EMT and influence the progression of cancer[10]. In addition, Pinin induces EMT and malignant progression in hepatocellular carcinoma[11]. Furthermore, Wu et al[12] found that interleukin (IL)-6 secreted by cancer-associated fibroblasts promotes EMT and GC metastasis through the JAK2/STAT3 signaling pathway, Li et al[13] reported that tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in GC cells, and Tian et al[14] reported that SERPINH1 regulates EMT and GC metastasis through the Wnt/β-catenin signaling pathway. Clarifying the regulatory mechanism of EMT in the development of GC is of great practical importance in attempting to understand the occurrence and metastasis of GC.
IL-34 is a new cytokine discovered in 2008 that binds to its functional receptor and plays a role in the regulation of cell differentiation, proliferation, angiogenesis, inflammation, and the immune response[15-17]. IL-34 has been found to play a pro-cancer role in a variety of tumors, including thyroid, colorectal, and liver cancers[18-20]. In particular, Zhang et al[18] found that IL-34 promotes tumor proliferation and activates the ERK signaling pathway in papillary thyroid cancer cells. However, it remains unclear whether IL-34 regulates GC cell migration and invasion. Furthermore, it is also unknown whether IL-34 can regulate GC proliferation in vitro and in vivo. This study aimed to elucidate the relationship between IL-34 and the clinicopathological characteristics of patients with GC and the role of IL-34 in the proliferation and the EMT of GC cells.
A total of 60 patients diagnosed with GC (age >18 years) were collected from patients who underwent surgical resection at The First Affiliated Hospital of Anhui Medical University from November 2019 to June 2020. Patients with a history of preoperative radiotherapy, long-term drug treatment, inflammatory diseases, rheumatic immune diseases, and genetic-related diseases were excluded. Information collected included sex, age, tumor diameter, T-stage, N-stage, M-stage, tumor, node and metastasis (TNM) stage, and grade (well, moderate, and poor). All tumors were staged according to the Eighth Edition of American Joint Committee on Cancer TNM classification. All procedures performed in studies with human participants were reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Anhui Medical University and were carried out in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study. Approval for the ethical use of clinical samples was obtained from the Institutional Review Board (No: Quick-PJ2019-09-11).
Tumor tissues collected from patients with GC were fixed in 4.0% paraformaldehyde, embedded in paraffin, and sliced into 4-μm sections. The sections were incubated with 3.0% hydrogen peroxide to inactivate endogenous peroxidase and then blocked in 5.0% bovine serum albumin after antigen retrieval. After blocking, sections were incubated with anti-IL-34 monoclonal antibody (863800; ZEN-BIOSCIENCE; 1:150) overnight at 4°C, followed by secondary antibody (ab150077; Abcam; 1:500). Sections were visualized with a diaminobenzidine solution and hematoxylin counterstain. Images were acquired with a microscope (Nikon Eclipse E200). Two pathologists blinded to clinical data independently scored tissue staining. The percentage of staining was evaluated and scored as follows: 0, < 1% staining; 1, 1%-25% staining; 2, 26%-50% staining; 3, 51%-75% staining; and 4, > 75% staining. The intensity of staining was defined as follows: 0, no signal; 1, weak; 2, moderate; and 3, strong. Scoring formula = percentage of positive tumor cell score × positive cell staining score. A total score of < 5 was considered negative and > 6 was considered positive.
Total RNA was isolated from tissues and cells with the TRIzol reagent (Thermo Fisher). Reverse transcription was achieved using Vazyme Biotech kits. The cDNA amplification was then performed using SYBR Premix Ex Taq (Vazyme Biotech) using a Roche 480 light cycler. PCR was carried out for 40 cycles according to the following procedure: 95°C for 60 s, and 60°C for 30 s. Relative mRNA expression of target genes were calculated using the 2−ΔΔCt method after normalization with GAPDH expression. Primer sequences were as follows: IL-34, 5’-TTGACGCAGAATGAGGAGTG-3’(forward); 5’-CCCTCGTAAGGCACACTG AT-3’(reverse); GAPDH,5’-CAGGAGGCATTGCTGATGAT-3’(forward); 5’-GAAGG CTGGGGCTCATTT-3’(reverse).
Protein was obtained by lysis of tissues or cells using RIPA buffer containing protease inhibitors (Thermo Fisher), and protein concentration was quantified using the BCA Protein Assay kit (Beyotime Biotechnology). Protein was separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane according to standard protocols and then blocked with 5% skim milk in TBST for 1 h. The membranes were incubated overnight at 4°C with the following antibodies: anti-E-cadherin (1:5000, Abcam), anti-N-cadherin (1:5000, Abcam), anti-Vimentin (1:5000, Abcam), anti-GAPDH (1:5000, Abcam), anti-Vimentin (1:5000, Abcam), anti-GAPDH (1:5000, Abcam), anti-IL-34 (1:1000, ZEN-BIOSCIENCE) and then incubated with horseradish peroxidase conjugated secondary antibodies (1:2000, Proteintech Group) at room temperature for 1 h after washing 3 times using TBST. The relative band density was determined with the ECL Western Blotting Substrate Kit (EMD Millipore) using Tanon 5200 Multifunctional Imaging System (Shanghai, China). GAPDH was used as an internal control.
The human normal gastric mucosal epithelial cell line (GES-1) and human GC cell lines (AGS, MKN-45 and HGC-27) were provided by Prof. Aman Xu (The First Affiliated Hospital of Anhui Medical University). Cells were grown in RPMI 1640 medium supplemented with 10% FBS (BI), 100 U/mL penicillin (Gibco). The cells were kept in a humidified incubator at 37°C with a mixture of 95% air and 5% CO2.
The short hairpin RNA (shRNA) and IL-34 overexpression plasmid sequences were synthesized by the Public Protein/Plasmid Library. The shRNA sequences were as follows: shRNA-IL-34, 5’-CAGAGCCCTCATTGCAGTATG-3’ and Negative control, 5’-GTTCTCCGAACGTGTCACGTT-3’. The IL-34 overexpression plasmid is a lentiviral expression vector for human IL-34 with an inserted sequence size of 729 bp and no fused CopGFP protein at its C-terminus. Target plasmids with IL-34, shRNA-IL-34, or shRNA control were transfected into AGS cells using lentivirus and selected by adding puromycin after 48 h, then viable cells were diluted to 50 cells/mL and inoculated into 96-well plates, respectively, and continued to be screened with drugs until satisfactory monoclonal cell lines were selected.
For the cholecystokinin (CCK) assay, cells were seeded in 96-well culture plates at about 3000 cells per well. At 0, 24, 48, 72, and 96 h, CCK-8 agent was added to each well and cells were treated for 2 h. Absorbance values at 450 nm were measured using an enzyme labeler (USCN KIT INC, China). For the clone formation assay, cells were seeded in six-well plates at 400 cells per well and then cultured for 2 wk. Cells were fixed and stained for 30 min in a 35% methanol solution with 1% crystal violet, and then the number of stained cells was counted.
The cells were seeded in six-well plates (2 × 104/well). A scratch was made through the cell layer along the central axis using a sterile plastic tip after complete cell attachment. After scratching, the cells were washed 3 times with PBS to remove floating cells and to make the scratch visible, and then the medium was replaced with fresh medium to continue the culture. The scratch was photographed at 0 h and 24 h. The photos were imported into Image J software and the cell closure was calculated according to the software instructions.
The cell invasion assay was performed using Transwell chambers with a pore size of 8 µm. A 200 μL cell suspension (2 × 103/L) was added to the upper chamber with Matrigel and placed in a 24-well plate containing 700 μL medium with 10% FBS. After 24 h of incubation at 37°C and 5% CO2, cells that invaded toward the lower surface of the membrane were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and counted.
All applicable national and institutional guidelines for the care and use of animals were followed. The ethics approval for the animal protocols for this study was obtained by the Animal Research Committee of Anhui Medical University (No. SC20200513). Ten 4-wk-old female nude mice purchased from Shanghai SLAC Laboratory Animal Co., Ltd were randomly divided into two groups (experimental and control groups), five in each group. Pre-cultured AGS cells (overexpression group and control group) in good growth condition were digested separately using trypsin, centrifuged, and a cell suspension (1 × 106 cells) was injected into the inguinal region of mice. The mice in both groups were maintained in the same feeding environment and nutritional conditions. The animals were sacrificed at the end of the experiments for an observation period of 28 d. Tumor weights were recorded per mouse and tumor volumes were calculated using the modified formula of (length × width2)/2.
SPSS statistical software (version 22.0) statistical software was used for statistical analysis and GraphPad Prism (version 8.0.2) was used for graphing. Measurement data were first examined using the Kolmogorov-Smirnov test to check whether the measurement data of each group had a normal distribution. Results are expressed as the mean ± SE for measurement data with a normal distribution. Comparisons between two groups were performed using the two-tailed Student’s t test. One-way analysis of variance was used for multiple group comparisons, and the LSD-t test was used for pairwise comparisons. All results are from three independent replicate experiments. P values < 0.05 were used to indicate statistically significant differences.
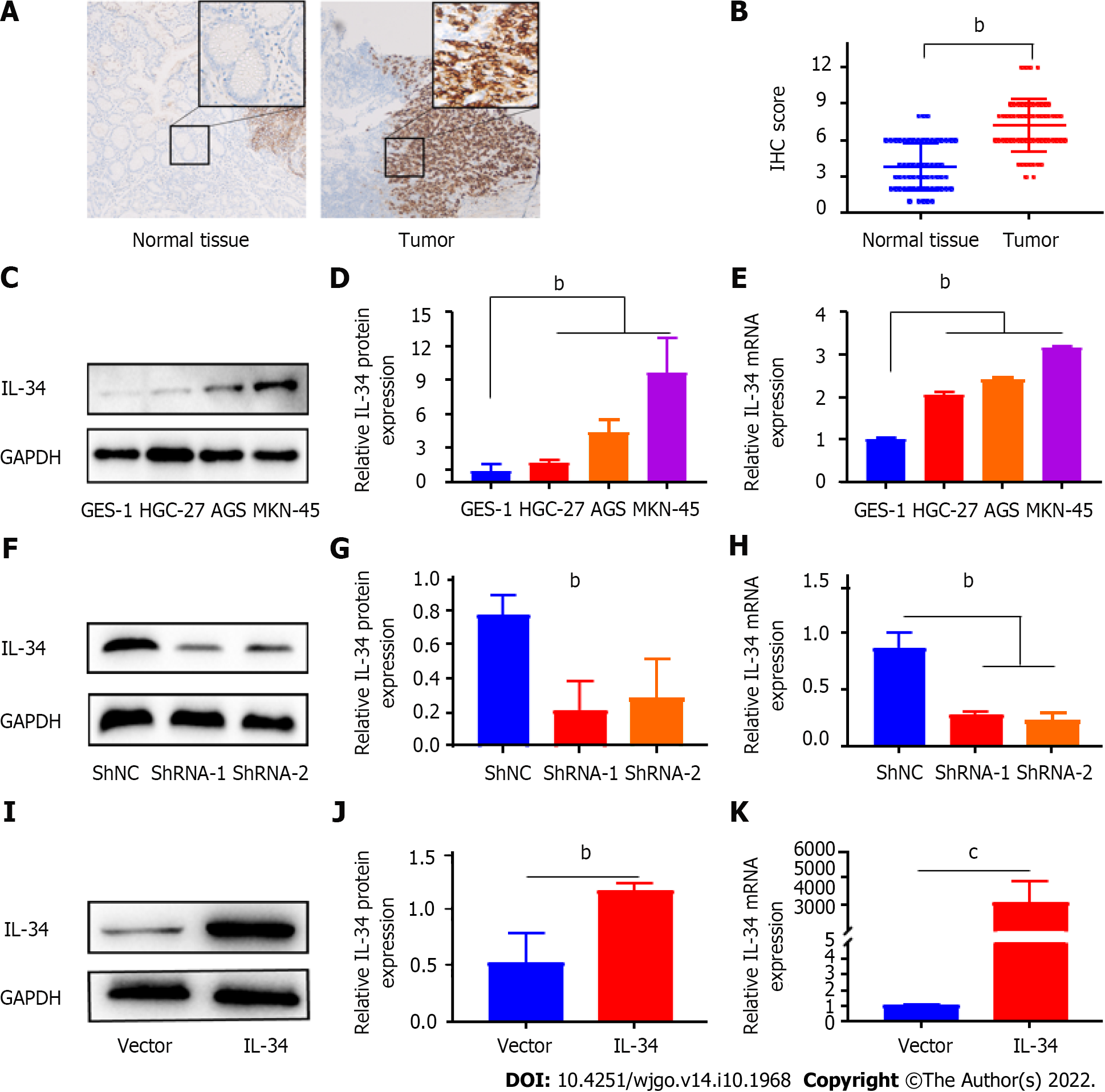
The results of immunohistochemical staining showed that IL-34 expression increased in GC tissues compared to paired normal gastric tissues (Figure 1A and B, P < 0.01). Meanwhile, as shown in Figure 1C-E, the expression of IL-34 mRNA and protein in GC cell lines (AGS, HGC-27, and MKN-45) were higher than in normal gastric mucosal cells GES-1 (P < 0.01). Furthermore, the relationship between IL-34 and clinicopathological characteristics was also analyzed, and the results showed that tumor size in GC patients with higher expression of IL-34 was greater than with lower expression of IL-34 (P < 0.05, Table 1). Furthermore, IL-34 expression correlated with the depth of invasion, the degree of differentiation, lymph node metastasis, and the TNM stage of GC patients, but not with age, sex, or distant metastasis (P > 0.05, Table 1). Altogether, these findings suggested that IL-34 might function as an oncogene in the development of GC.
| Characteristics | Expression of IL-34 | χ2/t | P value | |
| Lower (n = 29) | Higher (n = 31) | |||
| Age (yr) | 62.14 ± 11.23 | 63.87 ± 10.642 | 0.976 | 0.945 |
| Tumor size (cm) | 4.69 ± 1.74 | 6.52 ± 3.06 | 0.003 | 0.006 |
| Sex | ||||
| Male | 16 | 21 | 1.001 | 0.317 |
| Female | 13 | 10 | ||
| T-stage | ||||
| T1 + T2 | 13 | 4 | 7.520 | 0.006 |
| T3 + T4 | 16 | 27 | ||
| N-stage | ||||
| N0 | 17 | 8 | 6.638 | 0.010 |
| N1 + N2 + N3 | 12 | 23 | ||
| M0 stage | ||||
| Yes | 1 | 2 | 0.000 | 1.000 |
| No | 28 | 29 | ||
| TNM stages | ||||
| I + II | 17 | 10 | 4.207 | 0.040 |
| III + IV | 12 | 21 | ||
| Grade | ||||
| Well + moderate | 11 | 4 | 5.006 | 0.025 |
| Poor | 18 | 27 | ||
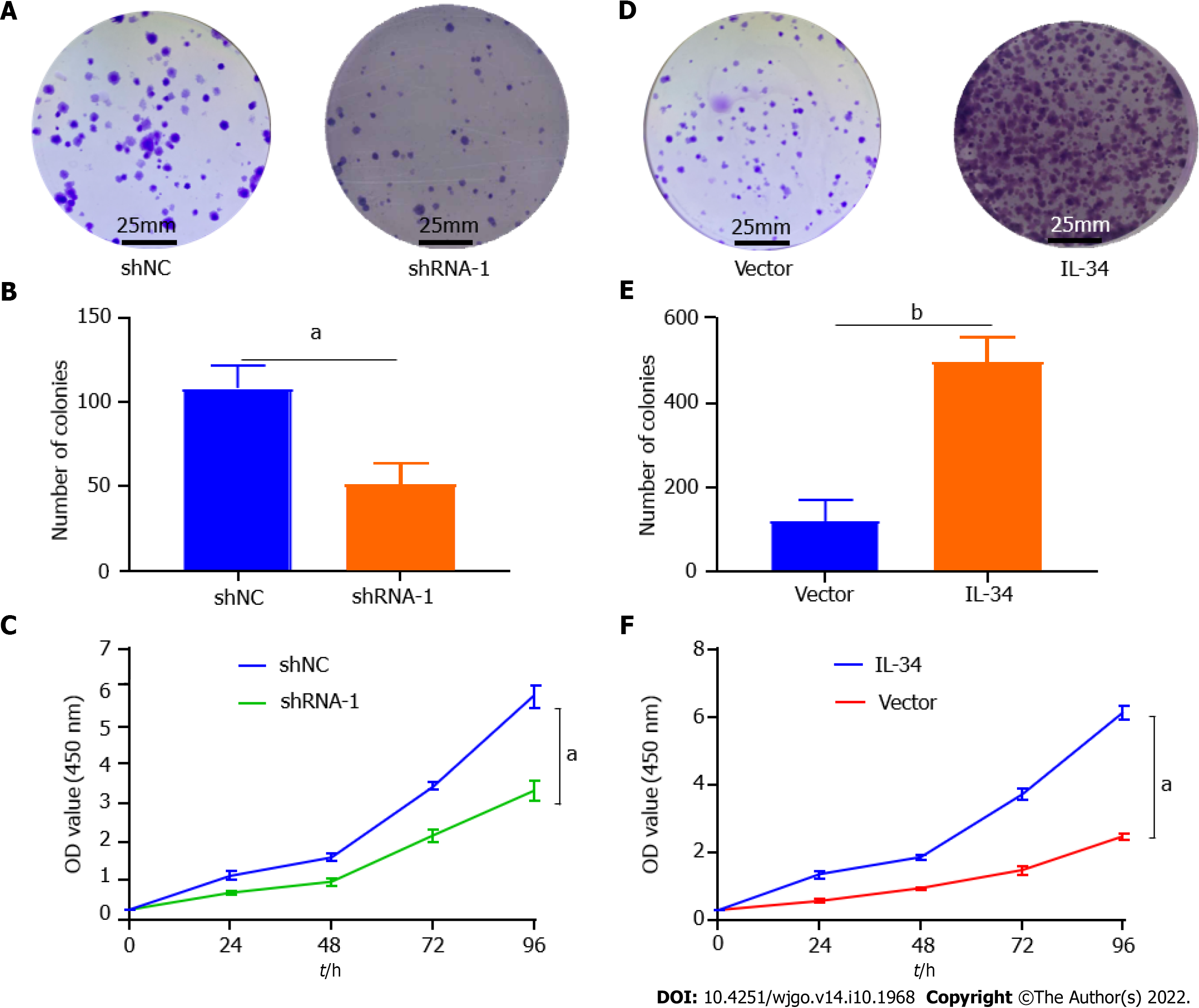
AGS cell lines with stable knockdown or overexpression of IL-34 were successfully obtained by introducing plasmids containing shRNA-IL-34 or the cds fragment (coding sequence) of the IL-34 gene into AGS cells by lentiviral infection, followed by puromycin pressurization screening. As shown in Figure 1F-J. To identify the effects of IL-34 on GC cell proliferation, we evaluated the formation of CCK-8 and plate clones in AGS cells with different expression of IL-34. The results showed that the number of AGS cell clones in the stable knockdown group (shRNA-1) was less than in the control group (shNC) (Figure 2A and B, P < 0.01). Meanwhile, the proliferation rate of AGS cells in the shRNA-1 group was also slowed compared to the shNC group (Figure 2C, P < 0.05). Furthermore, as shown in Figure 2C and D, the clone formation was significantly increased following the stable overexpression of the IL-34 compared to cells transfected with Vector alone (Figure 2E, P < 0.05). In addition, the CCK-8 assay also showed that the proliferation ability of AGS cells in the stable IL-34 overexpression group was significantly enhanced compared to the Vector-transfected cells, and the difference was statistically significant (Figure 2F, P < 0.05). Therefore, IL-34 accelerated the proliferation and clone formation of AGS cells.
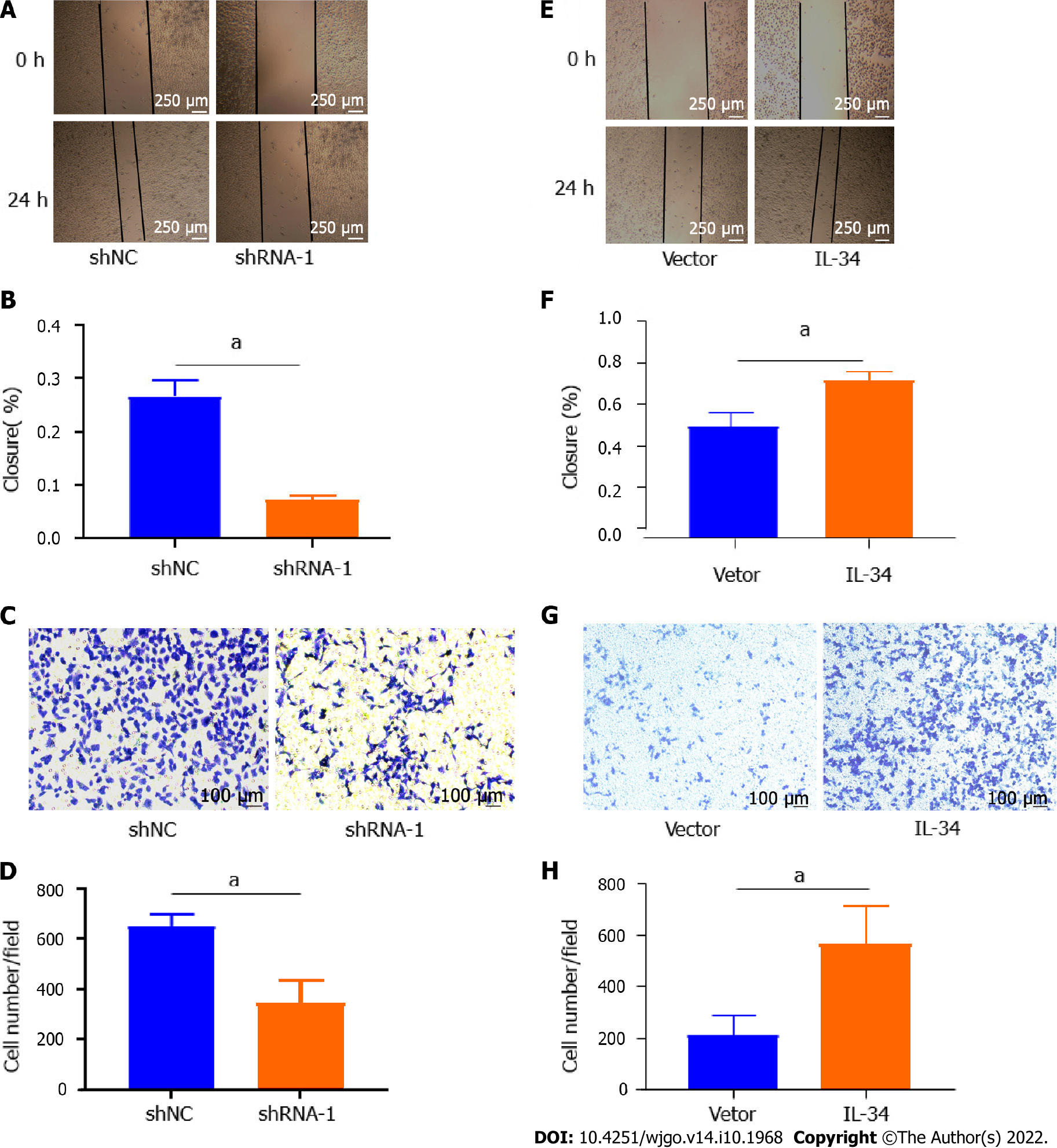
To verify the effect of IL-34 on GC migration and invasion ability, we examined the migration and invasion capacity of AGS cell lines in the shNC group and in the shRNA-1 group. As shown in Figure 3A and B, the migration capacity of AGS cells in the shRNA-1 group was reduced compared to the shNC group (P < 0.05). Meanwhile, the transwell assay showed that the invasion of AGS cells in the shRNA-1 group was weaker than that of the shNC group (Figure 3C and D, P < 0.05). As shown in Figure 3E-H, the migration and invasion of AGS cell lines was enhanced following the stable overexpression of IL-34 compared to cells transfected with Vector alone (P < 0.05). Taken together, IL-34 overexpression promoted migration and invasiveness of AGS cells.
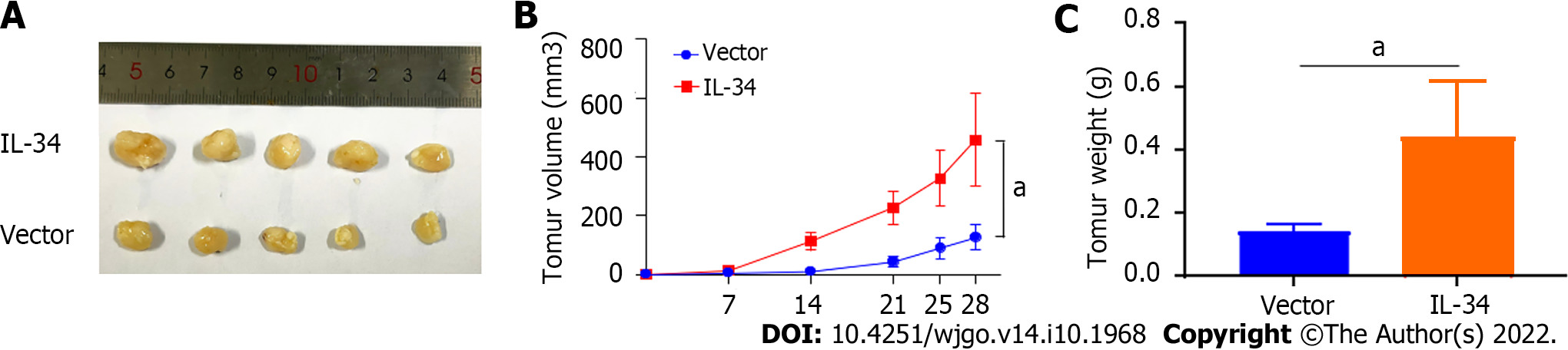
To further verify the effects of IL-34 on GC transplant tumors in vivo, we constructed a subcutaneous transplant tumor model in nude mice. As shown in Figure 4A and B, the tumors of the nude mice in the IL-34 group grew faster compared to the Vector implanted group (P < 0.05). Furthermore, tumor weight in the IL-34 group was higher than in the Vector group (Figure 4C, P < 0.05). Therefore, IL-34 overexpression promoted the growth of subcutaneous transplantation tumors in nude mice.
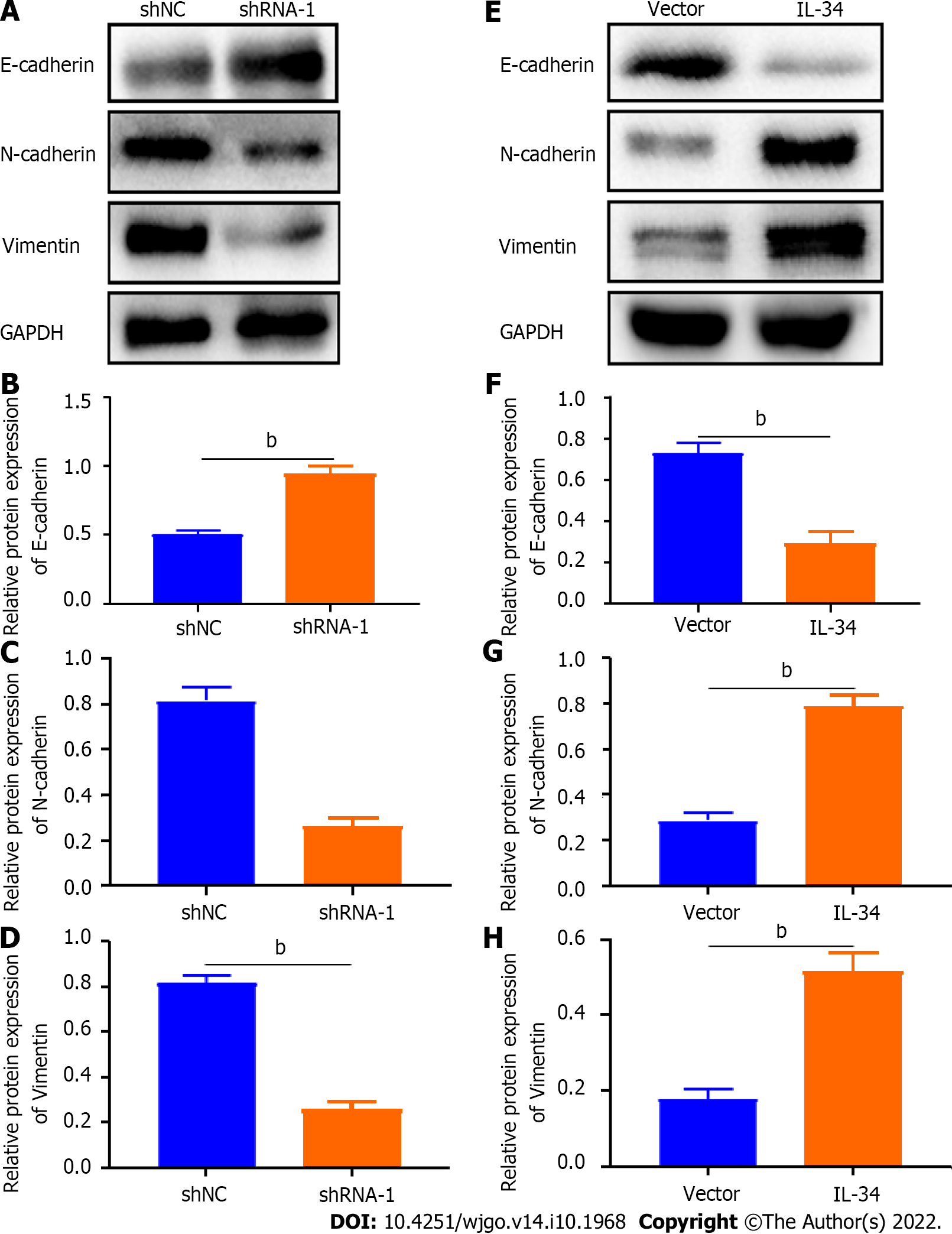
The EMT process is characterized by a decreased expression of the epithelial cell marker (E-cadherin) and an elevated expression of the mesenchymal cell marker (vimentin) and the tumor invasion promoter marker (N-cadherin)[24]. As shown in Figure 5A-D, knockdown increased the expression of the E-cadherin protein expression but decreased the expression of vimentin and N-cadherin in AGS cells (P < 0.01). In contrast, overexpression of IL-34 suppressed E-cadherin expression and increased vimentin and N-cadherin protein expression in AGS cells (Figure 5E-H, P < 0.01). Overall, IL-34 promoted the migration and invasion of AGS cells by regulating the expression of EMT-associated proteins.
In recent years, IL-34, a newly discovered cytokine, has been reported to influence tumorigenesis and progression by binding to receptors triggering multiple intracellular pathways, which mediate cellular processes such as cell proliferation, the cell cycle, and protein phosphorylation[16]. Zhou et al[17] found that IL-34 binds to the colony stimulating factor 1 receptor (CSF-1R) to control the survival, proliferation, and differentiation of tumor-associated macrophages, and patients with higher expression of IL-34 and higher density of tumor-associated macrophages had a poorer prognosis and shorter overall survival and recurrence-free period. Additionally, Zhang et al[18] found that IL-34 Levels were significantly up-regulated in serum and tissue samples from patients with thyroid cancer, which were strongly correlated with tumor size, TNM stage, and lymph node metastasis. Kobayashi et al[19] found that IL-34 is a prognostic factor in colorectal cancer. Irie et al[20] found that IL-34 promotes hepatoblastoma cells progression via autocrine and paracrine mechanisms. Kajihara et al[21] found that IL-34 expression was upregulated in triple-negative breast cancer and that overall survival was worse in patients with triple-negative breast cancer with high expression of IL-34. Consistent with the above findings, we found that IL-34 expression levels were correlated with the clinicopathological characteristics of the patients, including tumor size, T-stage, N-stage, stage TNM, and tumor grade of differentiation. More specifically, patients with GC with lower expression of IL-34 had smaller tumors, earlier tumor stages (T-stage and N-stage, and TNM stage) and well-differentiated tumors. Additionally, CCK-8 assays and plate clone formation assays showed that the capacity for proliferation and clone formation of GC cells was enhanced after IL-34 overexpression, while the knockdown of IL-34 suppressed the proliferation and clone formation of GC cells. Similarly, Franzè et al[22] also found that IL-34 promoted colorectal cancer cell proliferation by regulating the growth of tumor-associated macrophages. In this study, we also attempted to further validate the effects of IL-34 on GC growth in vivo by constructing subcutaneous tumor nude mice models. The results showed that the overexpression of IL-34 accelerated the growth rate of GC tumors compared to cell xenograft containing only Vector. Regrettably, due to the limitations of nude mouse subcutaneous tumor models, no supporting evidence was observed with regard to macrophage and lymphocyte infiltration into tumors (data was not shown), making it difficult to investigate the role of tumor-associated macrophages in IL-34 mediating GC progression.
It is well known that EMT is involved in GC metastasis, which is characterized by changes in migration, invasion, and expression of proteins associated with EMT, including E-cadherin, vimentin, and N-cadherin[6,23-25]. Previous studies have found that EMT is strongly associated with the proliferation and metastasis of GC[12-14]. For example, cancer-associated fibroblasts promote EMT and GC metastasis via the JAK2/STAT3 signaling pathway[12], while tumor neutrophils induce EMT to promote migration and invasion in GC cells[13]. SERPINH1 regulates the progression of EMT and GC through the Wnt/β-catenin pathway[14]. Furthermore, ZMYM1 promotes EMT and metastasis of GC cells by recruiting the CtBP/LSD1/CoREST complex to bind to the E-cadherin promoter and mediating its repression[26]. IL-34 has been reported to promote EMT and activate the ERK signaling pathway in papillary thyroid cancer cells[15]. However, it remains unclear whether IL-34 regulates the EMT process of GC. In the present study, overexpression of IL-34 enhanced migration and invasion ability, while knockdown of IL-34 impaired metastasis in GC cells. Furthermore, we also found that the N-cadherin and vimentin proteins were up-regulated in GC cells after overexpression of IL-34, while the knockdown of IL-34 decreased the expression of N-cadherin and vimentin. In human papillary thyroid cancer, it has also been reported that IL-34 regulates the expression of E-cadherin, vimentin, and N-cadherin, which is consistent with our results.
Previous studies have shown that interactions between IL-34 and its functional receptors trigger several intracellular pathways that ultimately control the growth and progression of many types of cancers. For example, multiple studies have found that IL-34 promotes tumor cell growth and invasion through the activation of the ERK signaling pathway[27-29], while inhibition of the ERK signaling pathway inhibits the tumor-promoting effects of IL-34[30]. Currently, the factors and molecular mechanisms that regulate IL-34 in cancer cells are still unknown. We speculate that IL-34 induction is associated with oncogenic mutations that trigger signal that sustain carcinogenesis, and that production of cytokines and chemokines sustains carcinogenesis[31]. Furthermore, it is possible that the altered synthesis of IL-34 depends on changes in miRNA expression, a class of small noncoding RNAs that regulate a wide range of biological processes by altering the expression and translation of their target messenger RNA genes[32]. Finally, immune and stromal cells present in the tumor microenvironment secrete many inflammatory mediators, growth factors such as TNF- a, IL-1b, and IL-6, and these small molecules stimulate tumor cells to secrete IL-34[33,34].
A limitation of the present study is that we did not further investigate the mechanism of action of IL-34 in GC. During the novel coronavirus epidemic, we were temporarily unable to conduct further mechanistic studies due to lack of time and funding constraints. However, we have identified nuclear factor-kappa B as a potential mechanism that may regulate IL-34 expression in GC by single cell sequencing. In the next step, we will continue to validate the mechanism of IL-34 regulation of proliferation, migration and EMT expression in GC.
Our study provides novel evidence that IL-34 contributes to GC growth and metastasis. IL-34 is up-regulated in GC cell lines and tissues, and is correlated with tumor size, grade of differentiation, and TNM stage. IL-34 enhances the capacity for cancer cell proliferation, clone formation, migration, and invasion, and regulates the expression of EMT-associated proteins, suggesting that IL-34 may be an effective target for the therapy of GC.
Interleukin (IL)-34 is an inflammatory cytokine that is also involved in the development of several tumors. However, the role of IL-34 in the proliferation and epithelial-mesenchymal transition (EMT) of gastric cancer (GC) remains to be investigated.
To investigate the effect of IL-34 on the proliferation of GC cells and to find new therapeutic targets for the treatment of GC.
To clarify the effect of IL-34 on the prognosis of GC patients and the effect of IL-34 on the proliferation and EMT of GC cells.
The expression of IL-34 protein in GC tissues and cells was detected using immunohistochemical staining and Western blotting. In vitro, stable IL-34 knockdown and overexpressed GC cell lines were cultured, and the proliferation, clone formation, migration and invasion ability of GC cells were examined using cholecystokinin-8 assay, clone formation assay, cell scratch assay and transwell assay, respectively. In vivo, the effect of IL-34 on GC transplantation tumor growth was assessed using a subcutaneous tumor transplantation assay in nude mice. Western blotting was used to detect the association of IL-34 protein with EMT-related protein expression levels.
IL-34 expression is elevated in GC cells and tissues, and IL-34 expression levels correlated with tumor size, T stage, N stage, tumor, node and metastasis stage, and degree of differentiation. In vitro, endogenous upregulation of IL-34 promoted GC cell proliferation and EMT. In vivo, IL-34 overexpression promoted subcutaneous graft tumor growth in nude mice.
IL-34 expression is increased in GC tissues and cell lines. IL-34 promotes the proliferation and epithelial-mesenchymal transition of GC cells.
IL-34 may represent a new strategy for the diagnosis and targeted treatment of GC. Further search for potential cancer-promoting mechanisms of IL-34 is needed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan; Nath L, India; Qin Y; Tanabe S, Japan; Zhang Z, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64129] [Article Influence: 16032.3] [Reference Citation Analysis (174)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2809] [Article Influence: 561.8] [Reference Citation Analysis (5)] |
| 3. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1120] [Article Influence: 186.7] [Reference Citation Analysis (1)] |
| 4. | Li Y, Feng A, Zheng S, Chen C, Lyu J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control. 2022;29:10732748221099227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J; RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 vs adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 254] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 6. | Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed Pharmacother. 2021;133:110909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 7. | Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 814] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 8. | Zhang J, Tian XJ, Zhang H, Teng Y, Li R, Bai F, Elankumaran S, Xing J. TGF-β-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal. 2014;7:ra91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 9. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1874] [Article Influence: 267.7] [Reference Citation Analysis (0)] |
| 10. | Jiang J, Li J, Zhou X, Zhao X, Huang B, Qin Y. Exosomes Regulate the Epithelial-Mesenchymal Transition in Cancer. Front Oncol. 2022;12:864980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Qiao K, Chen C, Liu H, Qin Y. Pinin Induces Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma by Regulating m6A Modification. J Oncol. 2021;2021:7529164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li C, Yan M, Zhu Z, Liu B, Su L. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741-20750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Li S, Cong X, Gao H, Lan X, Li Z, Wang W, Song S, Wang Y, Li C, Zhang H, Zhao Y, Xue Y. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 14. | Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging (Albany NY). 2020;12:3574-3593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 15. | Garceau V, Smith J, Paton IR, Davey M, Fares MA, Sester DP, Burt DW, Hume DA. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J Leukoc Biol. 2010;87:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Ségaliny AI, Brion R, Brulin B, Maillasson M, Charrier C, Téletchéa S, Heymann D. IL-34 and M-CSF form a novel heteromeric cytokine and regulate the M-CSF receptor activation and localization. Cytokine. 2015;76:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, Fan J, Huang XW, Zhou J. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. 2016;63:1560-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 18. | Zhang P, Zhang H, Dong W, Wang Z, Qin Y, Wu C, Dong Q. IL-34 is a potential biomarker for the treatment of papillary thyroid cancer. J Clin Lab Anal. 2020;34:e23335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kobayashi T, Baghdadi M, Han N, Murata T, Hama N, Otsuka R, Wada H, Shiozawa M, Yokose T, Miyagi Y, Takano A, Daigo Y, Seino KI. Prognostic value of IL-34 in colorectal cancer patients. Immunol Med. 2019;42:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Irie T, Yoshii D, Komohara Y, Fujiwara Y, Kadohisa M, Honda M, Suzu S, Matsuura T, Kohashi K, Oda Y, Hibi T. IL-34 in hepatoblastoma cells potentially promote tumor progression via autocrine and paracrine mechanisms. Cancer Med. 2022;11:1441-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kajihara N, Kitagawa F, Kobayashi T, Wada H, Otsuka R, Seino KI. Interleukin-34 contributes to poor prognosis in triple-negative breast cancer. Breast Cancer. 2020;27:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Franzè E, Di Grazia A, Sica GS, Biancone L, Laudisi F, Monteleone G. Interleukin-34 Enhances the Tumor Promoting Function of Colorectal Cancer-Associated Fibroblasts. Cancers (Basel). 2020;12:3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 989] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 24. | Sun L, Fang J. Epigenetic regulation of epithelial-mesenchymal transition. Cell Mol Life Sci. 2016;73:4493-4515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li J, Zhang Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 26. | Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, Zhao G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 27. | Xia H, Chen J, Shi M, Gao H, Sekar K, Seshachalam VP, Ooi LL, Hui KM. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol. 2015;63:863-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Hu J, Li L, Chen H, Zhang G, Liu H, Kong R, Wang Y, Li Y, Tian F, Lv X, Li G, Sun B. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Wang K, Ji W, Yu Y, Li Z, Niu X, Xia W, Lu S. FGFR1-ERK1/2-SOX2 axis promotes cell proliferation, epithelial-mesenchymal transition, and metastasis in FGFR1-amplified lung cancer. Oncogene. 2018;37:5340-5354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Franzè E, Dinallo V, Rizzo A, Di Giovangiulio M, Bevivino G, Stolfi C, Caprioli F, Colantoni A, Ortenzi A, Grazia AD, Sica G, Sileri PP, Rossi P, Monteleone G. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget. 2018;9:3432-3445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 32. | Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 911] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 33. | Franzè E, Monteleone I, Cupi ML, Mancia P, Caprioli F, Marafini I, Colantoni A, Ortenzi A, Laudisi F, Sica G, Sileri P, Pallone F, Monteleone G. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci (Lond). 2015;129:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Baghdadi M, Ishikawa K, Nakanishi S, Murata T, Umeyama Y, Kobayashi T, Kameda Y, Endo H, Wada H, Bogen B, Yamamoto S, Yamaguchi K, Kasahara I, Iwasaki H, Takahata M, Ibata M, Takahashi S, Goto H, Teshima T, Seino KI. A role for IL-34 in osteolytic disease of multiple myeloma. Blood Adv. 2019;3:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |