Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.216
Peer-review started: April 22, 2021
First decision: June 16, 2021
Revised: June 22, 2021
Accepted: December 10, 2021
Article in press: December 10, 2021
Published online: January 15, 2022
Processing time: 263 Days and 13.7 Hours
Gastric cancer (GC) is a malignancy with a high incidence and mortality. The tumor immune microenvironment plays an important role in promoting cancer development and supports GC progression. Accumulating evidence shows that GC cells can exert versatile mechanisms to remodel the tumor immune microenvironment and induce immune evasion. In this review, we systematically summarize the intricate crosstalk between GC cells and immune cells, including tumor-associated macrophages, neutrophils, myeloid-derived suppressor cells, natural killer cells, effector T cells, regulatory T cells, and B cells. We focus on how GC cells alter these immune cells to create an immunosuppressive microenvironment that protects GC cells from immune attack. We conclude by compiling the latest progression of immune checkpoint inhibitor-based immunotherapies, both alone and in combination with conventional therapies. Anti-cytotoxic T-lymphocyte-associated protein 4 and anti-programmed cell death protein 1/programmed death-ligand 1 therapy alone does not provide substantial clinical benefit for GC treatment. However, the combination of immune checkpoint inhibitors with chemotherapy or targeted therapy has promising survival advantages in refractory and advanced GC patients. This review provides a comprehensive understanding of the immune evasion mechanisms of GC, and highlights promising immunotherapeutic strategies.
Core Tip: Gastric cancer (GC) is one of the most common malignancies with high incidence and mortality. GC can exert versatile mechanisms to induce immune evasion. Here, we systematically summarized the intricate crosstalk among GC cells and various immune cells and mainly focused on how GC cells educate immune cells to create an immunosuppressive microenvironment and facilitate GC cells from attack of the immune system. In addition, we retrieved the latest progression of immune checkpoint inhibitor-based immunotherapies and their combination with conventional therapies. This review provides a comprehensive understanding of the immune evasion mechanism and immunotherapeutic strategies in GC.
- Citation: Ma ES, Wang ZX, Zhu MQ, Zhao J. Immune evasion mechanisms and therapeutic strategies in gastric cancer. World J Gastrointest Oncol 2022; 14(1): 216-229
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/216.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.216
Gastric cancer (GC) is the fifth most common malignancy worldwide and causes the third most cancer-related deaths. Traditional treatment strategies, including gastrectomy, neoadjuvant chemotherapy, and targeted therapy, have made great progress in recent decades, all of which has markedly improved the prognosis of GC[1]. Recently, immune checkpoint inhibitors, such as programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) antibodies, have been approved for the treatment of refractory and metastatic GC patients[2,3]. However, only a small fraction of GC patients may benefit from immune checkpoint inhibitor treatment[4]. The overall and progression-free survival of GC remain disappointing. The median survival of refractory and advanced patients is usually less than 2 years[5]. Therefore, it is necessary to further explore the underlying mechanisms of GC development and to understand how GC cells escape from the antitumor immune response in order to identify novel biomarkers and develop effective therapeutic strategies for treating GC.
Accumulating evidence shows that the tumor microenvironment (TME) plays an important role in cancer development[6]. The TME is highly heterogeneous and includes a mix of stromal cells, macrophages, neutrophils, natural killer (NK) cells, T and B cells, and some negative regulatory cells. Intricate crosstalk between the cancer cells and immune cells can promote cancer progression, including in GC[7]. Theoretically, tumor cells have potential immunogenicity and should be recognized and eliminated by the host immune system. However, antitumor immunity is usually subverted by cancer cells[7]. It has been widely reported that GC cells can exert numerous mechanisms to evade immune attacks[8]. For instance, GC cells can release cytokines, chemokines, and growth factors to create an immunosuppressive microenvironment, recruit negative regulatory immune cells, or inhibit the antitumor activity of effector lymphocytes[8]. After exposure to GC cells, immune cells may lose their antitumor function and instead facilitate GC cell proliferation, metastasis, angiogenesis, and immune evasion. Successful cancer treatment should therefore both restore antitumor activity and block immunosuppression.
In the present review, we summarize the multiple mechanisms of immune evasion mediated by different immune cells and highlight the latest achievements in immunotherapy for treating GC. This systematic summary will provide a comprehensive understanding of cancer immunity and current immunotherapeutic strategies in GC.
The TME plays an important role in cancer development and progression. There is intricate crosstalk between cancer cells and the TME. Cancer cells can exert mechanisms that remodel the TME, thus triggering immune evasion and promoting the malignant progression of cancer. Several studies have delineated the interplay between GC cells and specific immune cell types, which will be discussed in detail below.
Macrophages are a major component of tumor-infiltrating lymphocytes. Tumor-associated macrophages (TAMs) play a crucial role in angiogenesis, metastasis, and immunosuppression. TAMs can be classified into two subtypes: M1 or classically activated macrophages, and M2 or alternatively activated macrophages. M1s have antitumor activity, whereas M2s support cancer development. M2-TAMs are remarkably enriched in GC samples and are closely associated with invasion, metastasis, peritoneal dissemination, and unfavorable prognosis[9,10]. TAMs can induce GC invasion through activating epidermal growth factor receptor, c-Src, Erk1/2, Akt, and small GTPase activity in GC cells[11]. Wang et al[9] showed that TAM-derived CCL5 bound to its receptor CCR5, resulting in signal transducer and activator of transcription 3 (STAT3) activation and increased DNMT1 expression, which epigenetically silenced the tumor suppressor GSN in GC. TAMs can also secrete CXCL1 and CXCL5 to trigger the CXCR2/STAT3 signaling cascade and increase tumor necrosis factor (TNF)-α release from GC cells[12]. Reciprocally, TNF-α can enhance CXCL1 and CXCL5 release from TAMs. The positive feedback loop between GC cells and TAMs promotes cancer metastasis[12].
Exosomes play a crucial role in mediating the crosstalk between GC and TAMs. GC-derived exosomes induced PD1+ TAM generation, which inhibited the function of CD8+ T cells and aggravated GC progression[13]. TAM-derived exosomes could transmit functional ApoE into GC cells, thereby activating the PI3K/Akt pathway to remodel the cytoskeleton and promote migration of GC cells[14]. In addition to inducing malignant features of GC cells, TAMs can also affect the antitumor function of immune cells. Peng et al[15] reported that TAMs impaired NK cell function through transforming growth factor (TGF)-β1 in GC, which decreased the expression of effectors including interferon (IFN)-γ, TNF-α, and Ki-67. TAM-derived CXCL8 abolished proliferation and infiltration of CD8+ T cells via autonomous PD-L1 expression in GC[16].
Although the immunosuppressive role of TAMs is widely substantiated, there remains a lack of effective approaches to target TAMs for cancer therapy. The underlying mechanisms and therapeutic implications of targeting TAMs still need to be explored.
Neutrophils are the predominant leukocytes in humans. The role of neutrophils in different cancer types is controversial because they can exert either tumor-promoting or tumor-suppressing effects depending on the cancer type. Neutrophils are abundant in peripheral blood and solid tumors, including in GC. Enriched neutrophils were significantly associated with larger tumor size, advanced TNM stage, and poor survival for patients with GC[17,18]. GC cells and the TME can exert multiple regulatory roles to remodel neutrophils and promote cancer development. GC cell-derived GM-CSF could activate and increase PD-L1 expression in neutrophils via activating the JAK/STAT3 signaling pathway. As a result, the PD-L1+ neutrophils inhibited proliferation and decreased IFN-γ expression in T cells, thereby inducing immunosuppression in GC[19]. Another study showed that the interleukin 17 (IL-17)+ neutrophil subpopulation was more abundant in the invasive margins of GC samples. This type of neutrophil can release IL-17 to recruit more neutrophils to the invasive frontier by CXC chemokines, which can in turn secrete matrix metallopeptidase 9 into the reprogrammed extracellular matrix and promote angiogenesis in GC[20].
Reciprocally, neutrophils can facilitate the acquisition of malignant phenotypes by cancer cells. The GC environment has high levels of CXCL5, which can stimulate the ERK pathway in GC cells to induce epithelial-mesenchymal transition. Conversely, CXCL5 also influences neutrophils to activate the ERK/P38 cascade and increase IL-6 and IL-23 expression, which in turn stimulates the invasion and metastasis of GC cells[21]. Recent studies have discovered that neutrophil extracellular traps (NETs) play an important role in cancer progression and may trap and protect cancer cells to facilitate distant metastasis. The DNA component of NETs can function as a chemotactic factor to attract cancer cells through its receptor CCDC25 on cancer cells and activate the ILK-β-parvin pathway to enhance cell migration[22]. NET levels were increased and linked to advanced tumor stage in GC. However, the mechanisms of NET formation and regulation remain unclear in GC and should be further investigated.
Myeloid-derived suppressor cells (MDSCs) are rapidly becoming a hotspot in the field of cancer immunity. MDSCs act as immunosuppressive cells to stimulate tumor growth and metastasis and modulate immune evasion. MDSCs are attracted to the tumor parenchyma by the interaction between CCL5 and CCR5 in GC[23]. Anti-CCR5 could effectively block the recruitment of MDSCs, and enhance the efficacy of anti-PD-L1 treatment in mice[23]. Some other chemokines, including CXCL12, CXCL5, and CCL2, are also responsible for the recruitment of MDSCs in GC[24]. Tumor-derived exosomes can affect MDSCs and thus exert a tumorigenic role. For example, GC-derived exosome miR-107 is taken up by MDSCs where it silences the expression of its targets PTEN and DICER and activates the PI3K/AKT pathway, leading to MDSC expansion[25].
There are various MDSC subsets in GC. A subset of MDSCs with CD45+CD33lowCD11bdim was specifically enriched in GC, which could effectively suppress CD8+ T cell proliferation and IFN-γ and granzyme B expression. Mechanistically, GC patient serum-derived IL-6 and IL-8 activated the PI3K/AKT signaling pathway in this MDSC subtype to increase AGRI expression and mediate T cell suppression. The presence of CD45+CD33lowCD11bdim cells, as well as IL-6, IL-8, and ARG I serum levels were positively correlated with GC progression and were negatively linked to overall patient survival[26].
In a mouse model of GC, Hsu et al[27] found that silencing STK24 expression could accelerate orthotopic tumor growth and induce MDSC infiltration into the tumor. Chemotherapeutic treatment could reduce MDSC enrichment in spontaneous gastric tumors in mice and improve the effects of anti-PD-1 therapy. Combining PD-1/PD-L1 blockade with MDSC targeting may be a promising strategy to prevent the progre
NK cells, as important effectors of host immunity, induce cancer cell apoptosis by secreting IFN-γ[29], TNF-α[30], or by forming the complexes Fas/FasL and TRAIL/ TRAILR[31]. NKG2D is an essential receptor for NK activation, and MICA and MICB are the well-known suppressive ligands of NKG2D that inhibit NK function[32,33]. Several studies have found that GC cells could reduce NKG2D expression and inhibit NK cell function through the release of soluble MICA and MICB. For example, Midkine could increase CHOP expression and form complexes with transcriptional factor AP-1, thereby increasing MICA/B expression and inhibiting NK cytotoxicity[34]. STA21 increased MICB expression and secretion by inhibiting the STAT3 pathway, which in turn repressed NKG2D expression and impaired NK function[35]. Matrix metallopeptidase inhibition could upregulate NKG2D ligand expression and increase NK activity in GC[36].
Cytokines, including IL-10, TGF-βl, and PGE2, could abolish the antitumor function of NK cells in GC[37]. There is mounting evidence that NK cells preferentially target cancer stem cells[38]. Recent research has found that the vital cancer stem cell marker CD133 can effectively activate NK cells in an NKG2D-dependent manner[39]. However, DKK3 inhibits CD133-induced NK cell activation by suppressing the ERK pathway and immune synapse formation[39]. Another recent study found that vinculin could induce epithelial-mesenchymal transition in GC cells and affect NK cell infiltration, which potentially predicts inferior prognosis and distant metastasis of GC[40]. It is critical to restore NK cell function and cytotoxicity to effectively treat GC, which should be carefully considered in combinatory treatment strategies.
T cell immunity is the most important component in the immune response to cancer. There are many subtypes of T cells, such as CD4, CD8, helper T (Th) 17, Th22, memory T, and regulatory T cells (Tregs). Each of the T cell subsets has its specific function, which include antitumor activity and immune evasion. CD8+ T cells and Tregs are two currently well-established lymphocytes that are involved in cancer immunity.
Metabolic reprogramming plays an important role in T cell-mediated immunity against cancer. CD155 on the surface of GC cells can bind to the immune checkpoint molecule TIGIT on the surface of T cells, which prevents T cells from metabolizing glucose, decreases IFN-γ production, and abolishes the cytotoxicity activity of CD8+ T cells[41]. Recently, it was determined that cancer cells can compete with T cells for nutrients, rendering T cells inactive. Lin et al[42] found that the infiltration of tissue-resident memory T cells (Trm) was markedly associated with a favorable prognosis in GC. Trm cells mainly rely on fatty acid oxidation, rather than glucose, for their energy supply. However, GC cells make use of a more efficient pathway to metabolize fatty acids than Trm cells, which results in Trm starvation and death[42].
Tregs are well-known immunoregulatory cells that can suppress the proliferation and cytokine secretion of T effector cells. However, the correlation between Treg infiltration and GC prognosis remains unclear, and there have been many contradictory results because of different Treg markers, location distances, and intracellular interactions. GC cells can release cytokines to recruit Tregs, including CCL17, CCL22, and CCL28. GC cells can also induce CD4+ naïve T cells to differentiate into Tregs via TGF-β and induced immunosuppression[43-45]. Tregs can exert mechanisms to abolish the cytotoxicity of T cells. Recently, Shi et al[46] reported that Tregs and the A2aR+/CD8+ T cell subpopulations were enriched in GC samples. Tregs can hydrolyze ATP into adenosine, which was taken up by CD8+ T cells through the adenosine A2aR pathway, inhibiting CD8+ T cell proliferation and inducing apoptosis[46]. An investigation on GC resistance to checkpoint blockade found that these patients frequently had an RHOA mutation. RHOA mutations activate the PI3K-AKT-mTOR signaling cascade, producing free fatty acids, which Tregs could consume more efficiently than effector T cells. This metabolic advantage of Tregs enabled them to accumulate within GC tissue and generate an immunosuppressive TME, thus limiting the efficacy of immune checkpoint blockade[47].
Understanding the regulatory mechanisms of T cell immunity is of critical importance to the goal of eliminating cancer cells, and there are still many unknown factors in this complex biological network.
B cells have a dual role in the immune system and can participate in antibody production and antigen presentation. CD20+ B cell infiltration is associated with better tumor prognosis[48]. A recent study found that the protective effect of CD20+ B cells may be related to the production of antibodies by sulfated glycosaminoglycan-induced functional B cells, which strongly inhibit the growth of GC[49]. In addition to the elimination of GC cells, a subpopulation of B cells with an inhibitory phenotype, known as Bregs, have recently come to the attention of researchers. Bregs can produce several inhibitory cytokines, including IL-10, TGF-β, and IL-35[50]. Furthermore, Bregs can express inhibitory molecules, such as FasL and PD-L1[51,52]. Bregs in GC can enhance IL-10 production by CD4+ and CD8+ T cells to accelerate tumor growth[53]. A different study showed that Bregs have no impact on the proliferation of CD3+ T cells or CD4+ Th cells but instead inhibit the secretion of IFN-γ and TNF-α by CD4+ Th cells and convert CD4+CD25 effector T cells to CD4+FoxP3+ Tregs via TGF-β1[54]. These findings demonstrate that Bregs can exert immunosuppressive effects in GC development, the detailed mechanisms of which require urgent clarification.
With the progression of high-throughput sequencing technology and multi-omics platforms, widespread cellular remodeling events have been identified in GC and the TME, including genomic alteration, transcriptional states, epigenetic reprogramming, intercellular interactions, and metabolic reprogramming. These new insights provide valuable knowledge that will explain cancer immune evasion and facilitate the development of novel immunotherapies.
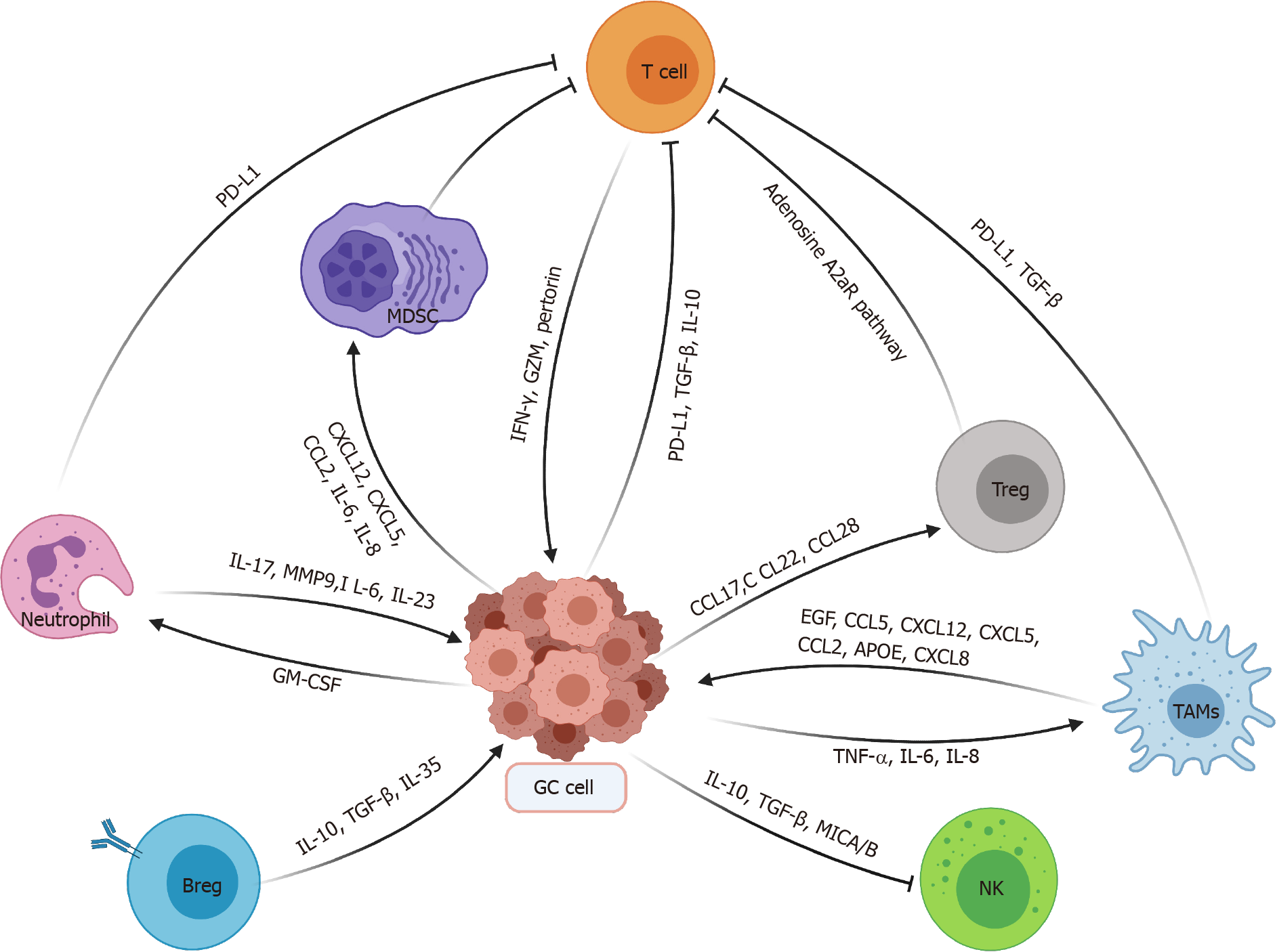
Collectively, there exists complex interplay between GC cells and various immune cells as described in Figure 1.
Immunotherapy has achieved impressive success in cancer treatment to date. A series of clinical trials of immunotherapeutic agents have been carried out for the treatment of GC. In this section, we will introduce the effects of checkpoint inhibitor-based immunotherapy in GC and focus on methods for selecting patients who will benefit from such therapy.
The immune checkpoint is a class of signaling molecule that regulates antigen recognition of T cell receptors during the immune response. Immune checkpoints can be categorized into co-stimulatory and co-inhibitory subtypes. The most common immune checkpoint blockers, which target co-inhibitory receptors of T cells, are antibodies against CTLA4, PD-1, and PD-L1 that can reinvigorate the anti-tumor immune activity of T cells.
The initial study of anti-CTLA-4 in GC was a small phase II trial with the antibody tremelimumab, which enrolled 18 patients with metastatic gastric and esophageal adenocarcinomas. Patients were treated with an intravenous infusion of tremelimumab. Although some patients showed stabilization or even remission, the objective response rate (ORR) for tremelimumab alone was unsatisfactory, as only in 1 of 18 patients (5.5%) reached the primary endpoint[55]. Another study using anti-CTLA-4 antibodies was a phase II trial of ipilimumab, which enrolled 57 patients with advanced/metastatic gastric or gastroesophageal junction cancer (GEJC). The clinical endpoints of the study were immune-related progression-free survival (PFS), PFS, overall survival (OS), and immune-related best overall response rate. The best supportive care group had an immune-related best overall response rate of 7.0% (n = 4/57), the median immune-related PFS was 4.90 mo, and the 12-mo immune-related PFS was about 10% without any improvement. Based on these findings, targeting CTLA-4 alone is not considered to be an effective remedy for GC[56].
Blockade of PD-1 and its ligand PD-L1 confers an encouraging survival advantage in several malignancies, including GC. KEYNOTE-012 was the first clinical trial of pembrolizumab, an antibody against PD-1, in 39 advanced GC patients. A sustained antitumor response was observed, and the median OS was 11.4 mo, which was better than the OS of 4-8 mo in the conventional chemotherapy group[57]. KEYNOTE-028 was another pembrolizumab trial that included 23 eligible patients with squamous cell carcinoma or adenocarcinoma of the esophagus or gastroesophageal junction who had failed standard therapy and had PD-L1-positive tumors. The ORR was 30%, and the median OS was 7.0 mo. In addition, this study developed a novel scoring system containing six immunomodulation-related IFN-γ-related genes, which significantly correlated with both PFS and ORR. Using this novel system, GC patients with higher scores frequently had a better response to pembrolizumab treatment[58].
Subsequently, a large-scale, randomized phase III trial, KEYNOTE-061, was carried out to compare the efficacy of pembrolizumab and paclitaxel in patients with advanced gastric or GEJC that progressed on first-line chemotherapy with platinum and fluoropyrimidine. In total, 196 patients were enrolled in the pembrolizumab cohort and 199 in the paclitaxel cohort. PD-L1 combined positive score (CPS) and microsatellite instability were two main criteria for subgroup analysis. As expected, the safety of pembrolizumab was superior to that of paclitaxel, and the OS in the CPS ≥ 1 group was better than in the low CPS group. Pembrolizumab had survival benefits in the long-term, with 12-mo and 18-mo survival rates of approximately 40% and 26%, respectively. Subgroup analysis suggested that the pembrolizumab response was more pronounced in patients with higher PD-L1 expression and high microsatellite instability levels[59].
Unlike pembrolizumab, which is an engineered humanized IgG4 antibody, nivolumab is a fully human IgG4 anti-PD-1 antibody. ATTRACTION-02, a phase III clinical trial, was conducted to evaluate the efficacy and safety of nivolumab in advanced and refractory GC or GEJC patients. Nivolumab showed a beneficial efficacy in GC and GEJC patients regardless of PD-L1 expression[2].
The efficacy of the PD-L1 monoclonal antibody avelumab has been compared with that of chemotherapy in chemotherapy-refractory GC or GEJC patients in the JAVELIN Gastric 300 trial. Unfortunately, avelumab failed to improve OS and PFS in this trial[60]. Subsequently, a global phase III clinical trial, named JAVELIN Gastric 100, was conducted to investigate the efficacy of avelumab after first-line induction chemotherapy for GC and GEJC. In line with previous results, avelumab alone seems to be slightly inferior in ORR, median PFS, and OS compared with chemotherapy[61]. A phase Ib clinical trial, named JAVELIN Solid Tumor trial, was conducted to investigate the antitumor activity and safety of avelumab as first-line switch-maintenance (1 L-mn) or second-line (2 L) treatment in patients with advanced GC/GEJC previously treated with chemotherapy. In this clinical trial avelumab showed clinical activity and an acceptable safety profile in patients with GC/GEJC[62]. However, avelumab was better tolerated, even in advanced-stage patients, than chemotherapy, suggesting that avelumab could be used as part of a combinatory therapy.
To explore whether combinations of different immune checkpoint inhibitors could synergistically resist cancer development, the CheckMate-032 trial was conducted to evaluate the safety and efficacy of nivolumab and nivolumab plus ipilimumab in chemotherapy-refractory GEJC patients. Nivolumab alone or combined with ipilimumab displayed a durable antitumor response and long-term OS benefits. Although increased toxicity was observed in the combination subgroup, the safety profile was manageable[63]. Recently, a phase Ib/II randomized controlled trial was performed to assess durvalumab and tremelimumab in combination or as monotherapy for chemotherapy-refractory GEJC patients. The response rates were low for both monotherapy or combination therapies. However, the combination therapy could significantly prolong the median OS and 12-mo OS. The tolerance of combination therapy was at an acceptable level. Therefore, this combination strategy may be an alternative option to improve the prognosis of these difficult-to-treat GC patients[64].
The clinical trial KEYNOTE-059 was initiated to evaluate the efficacy and safety of pembrolizumab alone or pembrolizumab combined with chemotherapy in patients with recurrent or metastatic GC and GEJC. The PD-L1 CPS score was found to be an effective tool to select patients who benefit from anti-PD-L1 treatment. Pembrolizumab monotherapy and in combination with chemotherapy displayed favorable antitumor activity and manageable tolerance as a first-line treatment[65]. Recently, the DURIGAST trial was designed to explore the efficacy of chemotherapy plus durvalumab (anti-PD-L1) vs chemotherapy plus durvalumab and tremelimumab (anti-CTLA-4) as second-line treatment of advanced GC and GEJC. At present, the safety profile is manageable, and further follow-up is ongoing. The trial results are eagerly anticipated[66].
Ramucirumab is an antibody targeting angiogenesis factor VEGFR2 that has shown promising efficacy in GC treatment. Recently, ramucirumab was combined with pembrolizumab in a Phase 1a/b JVDF Trial to treat naïve advanced GC and GEJC patients. PD-L1-positive patients acquired a better prognosis than PD-L1 negative patients; the median PFS was 8.6 mo vs 4.3 mo, and the median OS was 17.3 mo vs 11.3 mo, respectively. The adverse effects of ramucirumab plus pembrolizumab did not accumulate, suggesting a good safety profile[67]. Immunotherapy combined with targeted medicine may therefore be a novel option for treatment of advanced GC patients.
Overall, cancer immunotherapy has opened an exciting new avenue for cancer treatment. A series of immunotherapy and combination strategies have been conducted for the treatment of GC over the past few years as summarized in Table 1. Some of the clinical trials have achieved promising efficacy, and some have failed to improve prognosis. At present, there is still a lack of effective biomarkers to identify the patients that could potentially benefit from specific therapies. Novel strategies are needed to enhance the overall response rates and improve the prognosis of GC.
| Therapeutic strategy | Trial identifier | Drug name | Stage | Number | Type of cancer | Immune criterion | ORR (%) | Median PFS (mo) | Median OS (mo) | 6-mo PFS (%) | 1-year PFS (%) | 6-mo OS (%) | 1-year OS (%) |
| Anti-CTLA-4 | A Phase II trial of Tremelimumab[55] | Tremelimumab | II | 18 | Metastatic gastric and esophageal adenocarcinomas | 5.5 | 2.83 | 4.83 | - | - | - | 33 | |
| NCT01585987[56] | Ipilimumab | II | 57 | Advanced/metastatic gastric or gastroesophageal junction cancer | 1.8 (irBORR) | 2.72; 2.92 (irPFS) | 12.7 | 18.3; 22.3 (irPFS) | 8.4; 10.6 (irPFS) | - | - | ||
| Anti-PD-1 or Anti-PD-L1 alone | KEYNOTE-012 (NCT01848834)[57] | Pembrolizumab | Ib | 39 | PD-L1-positive adenocarcinoma of the stomach or gastroesophageal junction | 22 | 1.9 | 11.4 | 26 | - | 66 | 42 | |
| KEYNOTE-028 (NCT02054806)[58] | Pembrolizumab | Ib | 23 | Squamous cell carcinoma or adenocarcinoma of the esophagus or gastroesophageal junction in whom standard therapy failed and who had PD-L1–positive | 30 | 1.8 | 7 | 30 | 22 | 60 | 40 | ||
| KEYNOTE-061 (NCT02370498)[59] | Pembrolizumab | III | 296 | Advanced gastric or gastroesophageal junction cancer | PD-L1 CPS ≥ 1 | 16 | 1.5 | 9.1 | - | - | - | 40 | |
| JAVELIN solid tumor trial (NCT01772004) first-line switch-maintenance[62] | Avelumab | Ib | 90 | Advanced gastric or gastroesophageal cancer | 6.7 | 2.8 | 11.1 | 23 | 13 | - | 46.2 | ||
| ATTRACTION-2 (NCT02267343)[2] | Nivolumab | III | 330 | Advanced gastric or gastroesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens | - | - | 5.26 | - | - | 46.1 | 26.2 | ||
| JAVELIN Gastric 100 (NCT02625610)[61] | Avelumab | III | 249 | Locally advanced or metastatic gastric or gastroesophageal junction cancer | - | 3.2 | 10.4 | - | - | - | - | ||
| JAVELIN Gastric 300 (NCT02625623)[60] | Avelumab | III | 185 | Advanced gastric or gastroesophageal junction cancer | 2.2 | 1.4 | 4.6 | - | - | 41 | - | ||
| Immune checkpoints combination (Anti-PD-L1 and anti-CTLA4) | CheckMate-032 (NCT01928394)[63] | Nivolumab | I/II | 59 | Locally advanced or metastatic chemotherapy-refractory gastric, esophageal, or gastroesophageal junction cancer | 12 | 1.4 | - | - | 8 | - | 39 | |
| Nivolumab 1 mg/kg + ipilimumab 3 mg/kg | 49 | 24 | 1.4 | - | - | 17 | - | 35 | |||||
| Nivolumab 3 mg/kg + ipilimumab 1 mg/kg | 52 | 8 | 1.6 | - | - | 10 | - | 24 | |||||
| NCT02340975[64] | Durvalumab + Tremelimumab (second-line) | Ib/II | 44 | Chemotherapy-refractory gastric cancer or gastroesophageal junction cancer | 7.4 | - | 9.2 | 6.1 | - | - | 37 | ||
| Durvalumab (second-line) | 44 | 0 | - | 3.4 | 0 | - | - | 4.6 | |||||
| Tremelimumab (second-line) | 22 | 8.3 | - | 7.7 | 20 | - | - | 22.9 | |||||
| Durvalumab + Tremelimumab (third-line) | 25 | 4 | - | 9.2 | 15 | - | - | 38.8 | |||||
| Immune checkpoint combined with chemotherapy | KEYNOTE-059 (NCT02335411)[3,65] | Pembrolizumab | II | 259 | Previously treated gastric and gastroesophageal junction cancer | 11.6 | 2 | 5.6 | 14.1 | - | 46.5 | 23.4 | |
| PD-L1 CPS ≥ 1 | 15.5 | - | - | - | - | - | - | ||||||
| PD-L1 CPS < 1 | 6.4 | - | - | - | - | - | - | ||||||
| Pembrolizumab + chemotherapy | 25 | Advanced gastric or gastroesophageal junction cancer | 60 | 6.6 | 13.8 | 68 | - | - | 52 | ||||
| PD-L1 CPS ≥ 1 | 68.8 | - | 11.1 | - | - | - | - | ||||||
| PD-L1 CPS < 1 | 37.5 | - | 19.8 | - | - | - | - | ||||||
| Pembrolizumab | 31 | 25.8 | 3.3 | 20.7 | 34.9 | - | - | 63 | |||||
| Immune checkpoint combined with target angiogenesis | NCT02443324[67] | Ramucirumab + pembrolizumab | Ia/b | 28 | Advanced/metastatic gastric or gastroesophageal junction cancer | 25 | 5.6 | 14.6 | - | - | - | - | |
| PD-L1 CPS ≥ 1 | 32 | 8.6 | 17.3 | - | - | - | 66.7 | ||||||
| PD-L1 CPS < 1 | 17 | 4.3 | 11.3 | - | - | - | 41.7 |
As shown in Table 1, some of the immunotherapeutic effects are not statistically significant. To figure out which factor is critical for immunotherapeutic outcome, we performed an extensive analysis and found that PD-L1 CPS is an essential determinant because the prognosis of patients with PD-L1 CPS ≥ 1 was significantly better than patients with PD-L1 CPS < 1 and the ORR value in PD-L1 CPS ≥ 1 subgroup nearly reached to twice that compared with the PD-L1 CPS < 1 patients[3,65,67]. These findings suggest that it is necessary to carry out precise PD-L1 CPS and identify the potential GC patients who may benefit from immunotherapy.
Although immunotherapy may achieve a marvelous effect in some specific patients, the expensive cost has become an unneglectable financial burden for patient families and the whole society[68]. The term “financial toxicity” is referred to this particular side effect of drug therapy, which directly affects the prognosis of patients[69]. Financial toxicity may limit drug availability, result in poor qualities of life and care, account for lower obedience to treatment, and further lead to disease deterioration and poverty. The vicious circle formed by financial toxicity and malignant cancer ultimately aggravates the poorer prognostic outcomes and even higher mortality[69]. To objectively evaluate the effects of financial toxicity, de Souza et al[70] created the Comprehensive Score for Financial Toxicity, a quantitative measure of financial distress in cancer patients. The Comprehensive Score for Financial Toxicity associates with income, psychosocial stress, and health-related life quality[71]. Meeker et al[72] demonstrated that economic burden could cause grievous emotions such as worry, tension, and anxiety at the psychological level, which led to dismal life quality and poor prognosis in cancer patients.
Taken together, both the biological context of the immune criterion and the sociological context of the Comprehensive Score for Financial Toxicity should be fully considered to acquire better therapeutic effects for gastric cancer patients.
Our knowledge of cancer immunology has made great progress in recent years. Numerous novel and rational immunotherapeutic approaches have been designed and have achieved favorable clinical benefits in GC treatment. However, there are still some challenges that need to be conquered, such as identifying patients that could benefit from a specific therapy, improving the response rates, and decreasing adverse effects. These intractable challenges highlight the importance of systematically investigating the intricate and dynamic crosstalk between immune cells and tumor cells. Consistent effort is required to overcome the gaps in our knowledge in the fields of cancer biology and immunology. In the near future, more precise personalized immunotherapeutic strategies will be developed, which will provide survival advantages for refractory and advanced GC patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Geriatrics and Gerontology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pinheiro RN S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Wang SM, Zheng RS, Zhang SW, Zeng HM, Chen R, Sun KX, Gu XY, Wei WW, He J. [Epidemiological characteristics of gastric cancer in China, 2015]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1713] [Article Influence: 214.1] [Reference Citation Analysis (0)] |
| 3. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1461] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 4. | Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 5. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 6. | Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2019] [Cited by in RCA: 3819] [Article Influence: 545.6] [Reference Citation Analysis (0)] |
| 7. | Galon J, Bruni D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity. 2020;52:55-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 8. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 9. | Wang HC, Chen CW, Yang CL, Tsai IM, Hou YC, Chen CJ, Shan YS. Tumor-Associated Macrophages Promote Epigenetic Silencing of Gelsolin through DNA Methyltransferase 1 in Gastric Cancer Cells. Cancer Immunol Res. 2017;5:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One. 2017;12:e0170042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Cardoso AP, Pinto ML, Pinto AT, Oliveira MI, Pinto MT, Gonçalves R, Relvas JB, Figueiredo C, Seruca R, Mantovani A, Mareel M, Barbosa MA, Oliveira MJ. Macrophages stimulate gastric and colorectal cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt phosphorylation and smallGTPase activity. Oncogene. 2014;33:2123-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Zhou Z, Xia G, Xiang Z, Liu M, Wei Z, Yan J, Chen W, Zhu J, Awasthi N, Sun X, Fung KM, He Y, Li M, Zhang C. A C-X-C Chemokine Receptor Type 2-Dominated Cross-talk between Tumor Cells and Macrophages Drives Gastric Cancer Metastasis. Clin Cancer Res. 2019;25:3317-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, Chen L, Zhang P, Chen H, Liu Y, Dong P, Xie G, Ma Y, Jiang L, Yuan X, Shen L. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 15. | Peng LS, Zhang JY, Teng YS, Zhao YL, Wang TT, Mao FY, Lv YP, Cheng P, Li WH, Chen N, Duan M, Chen W, Guo G, Zou QM, Zhuang Y. Tumor-Associated Monocytes/Macrophages Impair NK-Cell Function via TGFβ1 in Human Gastric Cancer. Cancer Immunol Res. 2017;5:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Lv Y, Zuo L, Zhu X, Zhao J, Xue H, Jiang Z, Zhuge Y, Zhang C, Sun J, Ding P, Ren W, Li Y, Zhang K, Zhang W, He C, Zhong J, Peng Q, Ma F, Luo J, Zhang M, Wang G, Sun M, Dong J, Bai W, Guo W, Wang Q, Yuan X, Wang Z, Yu T, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Li K, Yin Z, Nie Y, Fan D, Han G. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut. 2019;68:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Li ZY, Wang JT, Chen G, Shan ZG, Wang TT, Shen Y, Chen J, Yan ZB, Peng LS, Mao FY, Teng YS, Liu JS, Zhou YY, Zhao YL, Zhuang Y. Expression, regulation and clinical significance of B7-H3 on neutrophils in human gastric cancer. Clin Immunol. 2021;227:108753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Shan ZG, Yan ZB, Peng LS, Cheng P, Teng YS, Mao FY, Fan K, Zhuang Y, Zhao YL. Granulocyte-Macrophage Colony-Stimulating Factor-Activated Neutrophils Express B7-H4 That Correlates with Gastric Cancer Progression and Poor Patient Survival. J Immunol Res. 2021;2021:6613247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, Fu XL, Yu PW, Guo G, Luo P, Zhuang Y, Zou QM. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 20. | Li TJ, Jiang YM, Hu YF, Huang L, Yu J, Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, Zhang Q, Li GX. Interleukin-17-Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin Cancer Res. 2017;23:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Mao Z, Zhang J, Shi Y, Li W, Shi H, Ji R, Mao F, Qian H, Xu W, Zhang X. CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis. 2020;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, Liu J, Xing Y, Chen X, Su S, Song E. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 632] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 23. | Yang L, Wang B, Qin J, Zhou H, Majumdar APN, Peng F. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol Immunotoxicol. 2018;40:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Zhou Y, Guo F. A selective sphingosine-1-phosphate receptor 1 agonist SEW-2871 aggravates gastric cancer by recruiting myeloid-derived suppressor cells. J Biochem. 2018;163:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, Rong H, Wang W, Zhang D, Zhang Z, Tu S, Zhu X, Zhang Q. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag Res. 2019;11:4023-4040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Mao FY, Zhao YL, Lv YP, Teng YS, Kong H, Liu YG, Wu XL, Hao CJ, Chen W, Duan MB, Han B, Ma Q, Wang TT, Peng LS, Zhang JY, Cheng P, Su CY, Fu XL, Zou QM, Guo G, Guo XL, Zhuang Y. CD45+CD33lowCD11bdim myeloid-derived suppressor cells suppress CD8+ T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis. 2018;9:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Hsu HP, Wang CY, Hsieh PY, Fang JH, Chen YL. Knockdown of serine/threonine-protein kinase 24 promotes tumorigenesis and myeloid-derived suppressor cell expansion in an orthotopic immunocompetent gastric cancer animal model. J Cancer. 2020;11:213-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Fabian KP, Padget MR, Donahue RN, Solocinski K, Robbins Y, Allen CT, Lee JH, Rabizadeh S, Soon-Shiong P, Schlom J, Hodge JW. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1764] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 31. | Martín-Antonio B, Suñe G, Perez-Amill L, Castella M, Urbano-Ispizua A. Natural Killer Cells: Angels and Devils for Immunotherapy. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | González S, López-Soto A, Suarez-Alvarez B, López-Vázquez A, López-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Saito H, Osaki T, Ikeguchi M. Decreased NKG2D expression on NK cells correlates with impaired NK cell function in patients with gastric cancer. Gastric Cancer. 2012;15:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Zhao S, Wang H, Nie Y, Mi Q, Chen X, Hou Y. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol Immunother. 2012;61:1745-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Garrido-Tapia M, Hernández CJ, Ascui G, Kramm K, Morales M, Ga Rate V, Zúñiga R, Bustamante M, Aguillón JC, Catala N D, Ribeiro CH, Molina MAC. STAT3 inhibition by STA21 increases cell surface expression of MICB and the release of soluble MICB by gastric adenocarcinoma cells. Immunobiology. 2017;222:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Shiraishi K, Mimura K, Kua LF, Koh V, Siang LK, Nakajima S, Fujii H, Shabbir A, Yong WP, So J, Takenoshita S, Kono K. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J Gastroenterol. 2016;51:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Szkaradkiewicz A, Karpiński TM, Drews M, Borejsza-Wysocki M, Majewski P, Andrzejewska E. Natural killer cell cytotoxicity and immunosuppressive cytokines (IL-10, TGF-beta1) in patients with gastric cancer. J Biomed Biotechnol. 2010;2010:901564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S, Monjazeb AM, Fragoso RC, Sayers TJ, Murphy WJ. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol. 2015;195:4010-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Xia P, Xu XY. DKK3 attenuates the cytotoxic effect of natural killer cells on CD133+ gastric cancer cells. Mol Carcinog. 2017;56:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Li H, Wang C, Lan L, Behrens A, Tomaschko M, Ruiz J, Su Q, Zhao G, Yuan C, Xiao X, Li B, Yan L, Wu W, Li W, Chen J, He Y, Zhang C. High expression of vinculin predicts poor prognosis and distant metastasis and associates with influencing tumor-associated NK cell infiltration and epithelial-mesenchymal transition in gastric cancer. Aging (Albany NY). 2021;13:5197-5225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | He W, Zhang H, Han F, Chen X, Lin R, Wang W, Qiu H, Zhuang Z, Liao Q, Zhang W, Cai Q, Cui Y, Jiang W, Wang H, Ke Z. CD155T/TIGIT Signaling Regulates CD8+ T-cell Metabolism and Promotes Tumor Progression in Human Gastric Cancer. Cancer Res. 2017;77:6375-6388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 42. | Lin R, Zhang H, Yuan Y, He Q, Zhou J, Li S, Sun Y, Li DY, Qiu HB, Wang W, Zhuang Z, Chen B, Huang Y, Liu C, Wang Y, Cai S, Ke Z, He W. Fatty Acid Oxidation Controls CD8+ Tissue-Resident Memory T-cell Survival in Gastric Adenocarcinoma. Cancer Immunol Res. 2020;8:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 43. | Lu X, Liu J, Li H, Li W, Wang X, Ma J, Tong Q, Wu K, Wang G. Conversion of intratumoral regulatory T cells by human gastric cancer cells is dependent on transforming growth factor-β1. J Surg Oncol. 2011;104:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Yuan XL, Chen L, Zhang TT, Ma YH, Zhou YL, Zhao Y, Wang WW, Dong P, Yu L, Zhang YY, Shen LS. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-β1. World J Gastroenterol. 2011;17:2019-2027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Hu JL, Yang Z, Tang JR, Fu XQ, Yao LJ. Effects of gastric cancer cells on the differentiation of Treg cells. Asian Pac J Cancer Prev. 2013;14:4607-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Shi L, Feng M, Du S, Wei X, Song H, Yixin X, Song J, Wenxian G. Adenosine Generated by Regulatory T Cells Induces CD8+ T Cell Exhaustion in Gastric Cancer through A2aR Pathway. Biomed Res Int. 2019;2019:4093214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity. 2020;53:187-203.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 48. | Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 49. | Katoh H, Komura D, Konishi H, Suzuki R, Yamamoto A, Kakiuchi M, Sato R, Ushiku T, Yamamoto S, Tatsuno K, Oshima T, Nomura S, Seto Y, Fukayama M, Aburatani H, Ishikawa S. Immunogenetic Profiling for Gastric Cancers Identifies Sulfated Glycosaminoglycans as Major and Functional B Cell Antigens in Human Malignancies. Cell Rep. 2017;20:1073-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 923] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 51. | Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, Ludwig-Portugall I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc Natl Acad Sci U S A. 2012;109:10468-10473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Dasgupta S, Dasgupta S, Bandyopadhyay M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020;352:104076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 53. | Hu HT, Ai X, Lu M, Song Z, Li H. Characterization of intratumoral and circulating IL-10-producing B cells in gastric cancer. Exp Cell Res. 2019;384:111652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Wang WW, Yuan XL, Chen H, Xie GH, Ma YH, Zheng YX, Zhou YL, Shen LS. CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget. 2015;6:33486-33499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 55. | Ralph C, Elkord E, Burt DJ, O'Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 56. | Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, Yamaguchi K, Balogh A, Sanchez T, Moehler M. Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin Cancer Res. 2017;23:5671-5678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 58. | Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol. 2018;36:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 59. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 998] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 60. | Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 61. | Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol. 2021;39:966-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 62. | Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, Kang YK, Lee KW, Infante JR, Lee SS, Kemeny M, Keilholz U, Melichar B, Mita A, Plummer R, Smith D, Gelb AB, Xiong H, Hong J, Chand V, Safran H. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 63. | Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, Le DT. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018;36:2836-2844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 64. | Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, Chung HC, Wainberg ZA, Gibson MK, Lee KW, Bendell JC, Denlinger CS, Chee CE, Omori T, Leidner R, Lenz HJ, Chao Y, Rebelatto MC, Brohawn PZ, He P, McDevitt J, Sheth S, Englert JM, Ku GY. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2020;26:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 65. | Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 66. | Evrard C, Louvet C, Hajbi FE, Fiore FD, Malicot KL, Aparicio T, Bouché O, Laurent-Puig P, Bibeau F, Lecomte T, Lièvre A, Guimbaud R, Kim S, Zaanan A, Sokol H, Chibaudel B, Desrame J, Pierre S, Gonzalez D, Lepage C, Tougeron D. PRODIGE 59-DURIGAST trial: A randomised phase II study evaluating FOLFIRI + Durvalumab ± Tremelimumab in second-line of patients with advanced gastric cancer. Dig Liver Dis. 2021;53:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Chau I, Penel N, Soriano AO, Arkenau HT, Cultrera J, Santana-Davila R, Calvo E, Le Tourneau C, Zender L, Bendell JC, Mi G, Gao L, McNeely SC, Oliveira JM, Ferry D, Herbst RS, Fuchs CS. Ramucirumab in Combination with Pembrolizumab in Treatment-Naïve Advanced Gastric or GEJ Adenocarcinoma: Safety and Antitumor Activity from the Phase 1a/b JVDF Trial. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, Abernethy AP. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 840] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 69. | Zafar SY. Financial Toxicity of Cancer Care: It's Time to Intervene. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 70. | de Souza JA, Yap BJ, Hlubocky FJ, Wroblewski K, Ratain MJ, Cella D, Daugherty CK. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer. 2014;120:3245-3253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 465] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 71. | de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araújo FS, Hlubocky FJ, Nicholas LH, O'Connor JM, Brockstein B, Ratain MJ, Daugherty CK, Cella D. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 634] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 72. | Meeker CR, Geynisman DM, Egleston BL, Hall MJ, Mechanic KY, Bilusic M, Plimack ER, Martin LP, von Mehren M, Lewis B, Wong YN. Relationships Among Financial Distress, Emotional Distress, and Overall Distress in Insured Patients With Cancer. J Oncol Pract. 2016;12:e755-e764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |