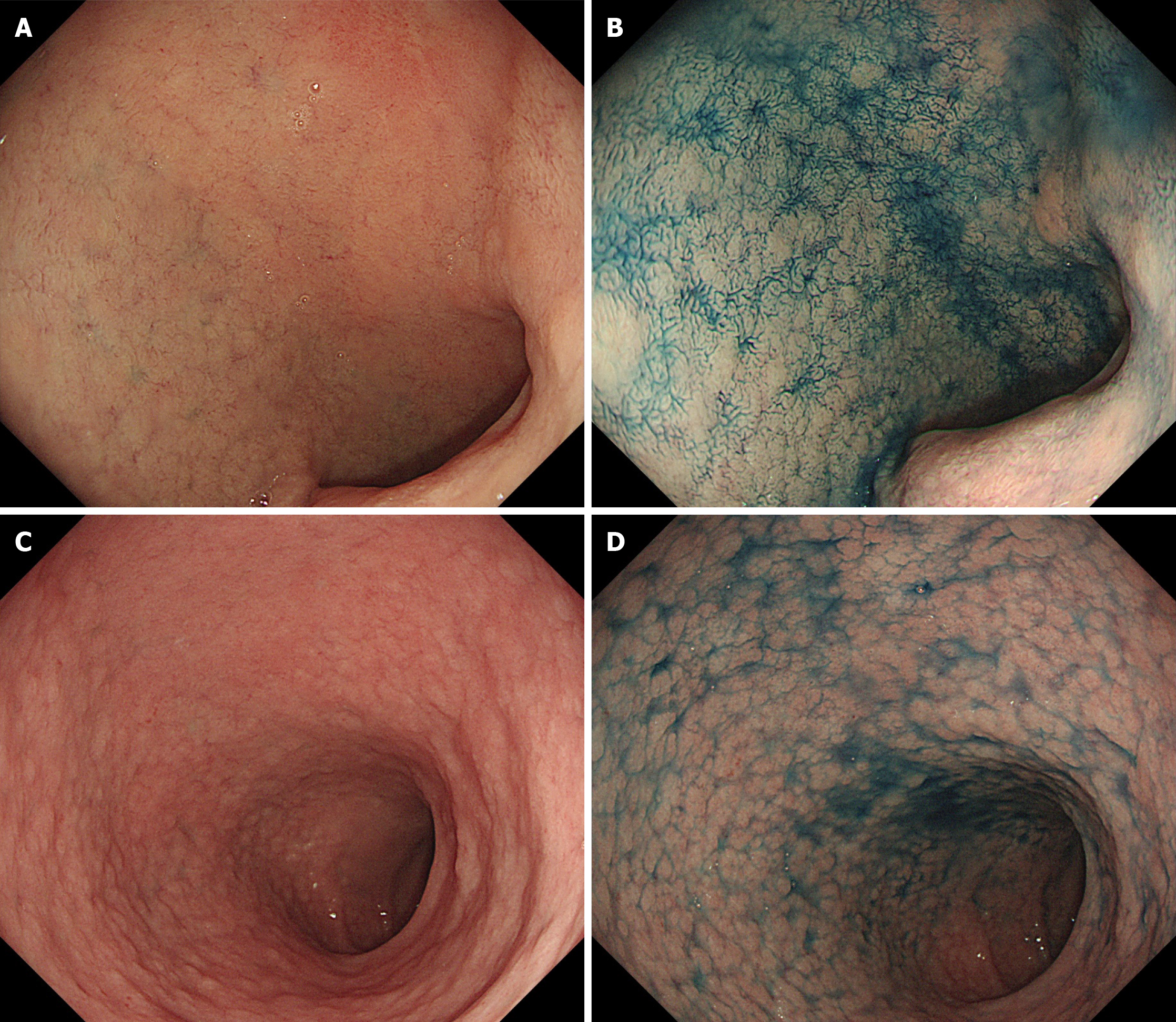
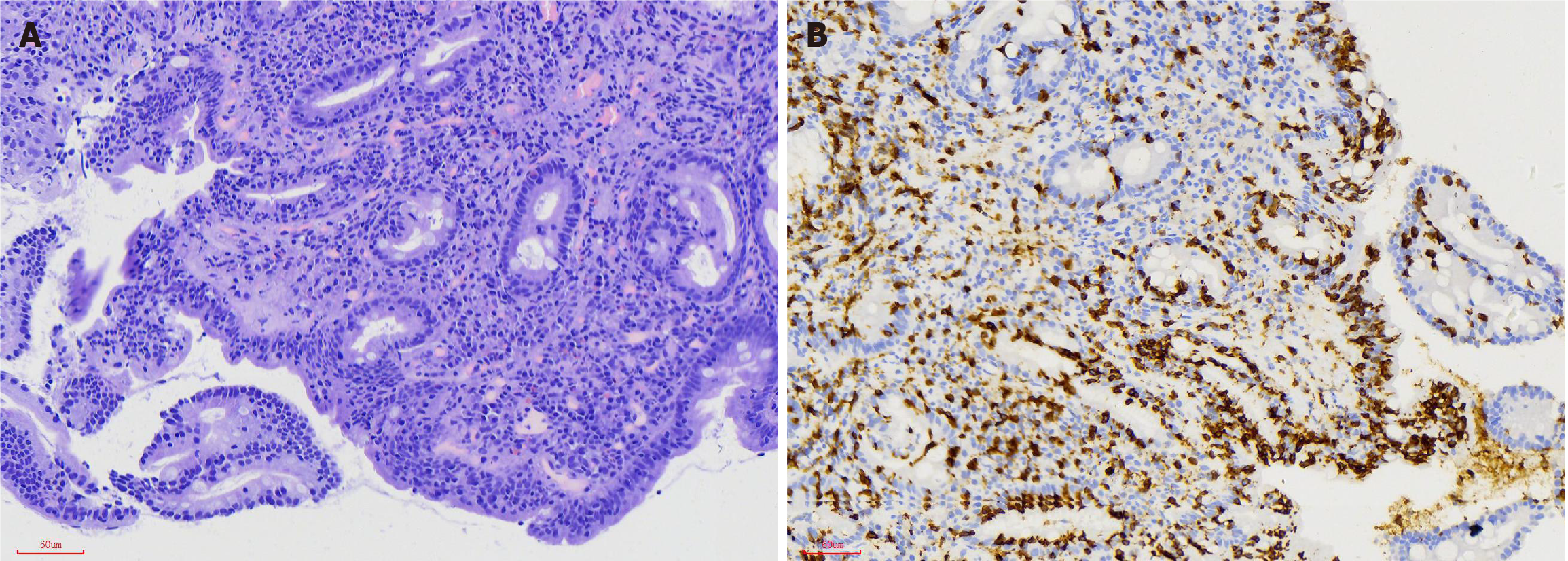
Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1017
Peer-review started: February 22, 2021
First decision: May 8, 2021
Revised: June 2, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: September 15, 2021
Processing time: 199 Days and 20.4 Hours
Celiac disease (CD) is a chronic immune-mediated intestinal disease with genetic susceptibility. It is characterized by inflammatory damage to the small intestine after ingestion of cereals and products containing gluten protein. In recent years, the global prevalence rate of CD has been approximately 1%, and is gradually increasing. CD patients adhere to a gluten-free diet (GFD) throughout their entire life. However, it is difficult to adhere strictly to a GFD. Untreated CD may be accompanied by gastrointestinal symptoms, such as diarrhea, abdominal pain, and extraintestinal symptoms caused by secondary malnutrition. Many studies have suggested that CD is associated with intestinal tumors such as enteropathy-associated T-cell lymphoma (EATL), small bowel cancer (SBC), and colorectal cancer. In this study, we reviewed related studies published in the literature to provide a reference for the prevention and treatment of intestinal tumors in patients with CD. Compared with the general population, CD patients had a high total risk of SBC and EATL, but not colorectal cancer. The protective effect of GFD on CD-related malignancies is controversial. Further studies are needed to confirm whether GFD treatment can reduce the risk of intestinal neoplasms in CD.
Core Tip: Celiac disease (CD) is an autoimmune intestinal disease caused by intake of gluten-containing cereals and their products by individuals with genetic susceptibility genes. The global prevalence rate is approximately 1% and is gradually increasing. CD can lead to intestinal mucosal damage and secondary malnutrition caused by extrain
- Citation: Wang M, Yu M, Kong WJ, Cui M, Gao F. Association between intestinal neoplasms and celiac disease: A review. World J Gastrointest Oncol 2021; 13(9): 1017-1028
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/1017.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.1017
Celiac disease (CD) is a chronic immune-mediated intestinal disease characterized by permanent intolerance to glutenin. Ingestion of glutathione by CD-sensitive indivi
According to a systematic review, the global prevalence of CD was 1.4% using serum samples and 0.7% using biopsy samples[13]. The prevalence of biopsy-con
The mortality rate in CD patients is higher than that of the general population[35-37]. An increased risk of death is mainly observed within a few years after diagnosis[38-40]. A cohort study from Scotland found a temporary increase in the risk of mortality among adults diagnosed with abdominal cancer, mainly malignant lym
Glutenin is mainly found in wheat, barley, rye, and oats[44]. Currently, the only effective treatment for CD is a lifelong strict gluten-free diet (GFD), which usually alleviates symptoms and improves intestinal mucosal damage[45,46]. There is evidence that early adoption of a GFD can prevent CD-related complications[35,47]. However, because of the extensive use of wheat in food, gluten may be inadvertently ingested[48-50]. Low doses of glutenin in the diet of patients with CD may also be harmful[51]. In addition, despite adherence to a GFD, up to 30% of patients have persistent symptoms. In 60% of patients, the villi of the small intestine atrophy, heal poorly, and the intestinal disease may persist[45,49,52]. Poor disease control has been associated with small bowel cancer (SBC), colorectal cancer, and enteropathy-associated T-cell lymphoma (EATL)[53-55]. However, the relationship between CD, GFD, and intestinal neoplasms is controversial. The purpose of this paper was to review the existing literature on CD and intestinal tumors and discuss their cor
Primary SBC is a rare malignant tumor, accounting for approximately 2%–3% of all gastrointestinal carcinomas[56-58]. Studies have confirmed that compared with the population without CD, patients with CD had a significantly increased risk of small intestinal adenomas and adenocarcinomas, but not carcinoids[59,60]. The risk of SBC in CD patients is 4–10 times higher than that in healthy individuals[55,61]. In a large questionnaire survey in the United States, 0.2% CD patients had SBC[62]. Compared with CD patients with nonmalignant tumors, CD patients with SBC were older[63]. SBC is also the main cause of death in young adults with early-onset CD triggered by chronic intestinal mucositis[64]. Chronic inflammation is associated with an increased risk of malignancy[65-67]. Compared with Crohn’s disease-related or sporadic SBC, CD-related SBC is more prone to mismatch repair defects[68,69]. In addition, CD-related SBC often contains a large number of tumor-infiltrating lymphocytes, particularly medullary-type lymphocytes[70]. Giuffrida et al[71] found that CD-associated SBC was often infiltrated by programmed death 1 (PD-1)-positive T cells, and PD-L1 was expressed in tumor/immune cells in more than one-third of cases. Some studies have reported that the mucosal lesions of CD are mainly located in the proximal small intestine, especially in the ileum, and CD-mediated SBC lesions have a similar distribution[72,73]. However, one study also showed that small intestinal adenocarcinoma in CD patients was more likely to occur in the jejunum[74]. Therefore, it is necessary to further study the location of CD-related SBC lesions in the small intestine.
Early detection of CD and early initiation of a GFD may inhibit or help to prevent chronic inflammation, thereby reducing the risk of SBC. However, a GFD may also alter the intestinal microbiota, thereby affecting the risk of cancer in CD patients[75,76].The main treatments for SBC include surgical resection and adjuvant chemotherapy for positive lymph nodes. Chemotherapy is recommended for the treatment of metastasis. Palascak-Juif et al[77] found that if preventive surgery (mainly ileectomy) is performed after 10 years of follow-up, 70% SBC cases can be prevented. However, the need for ileectomy requires further investigation. The prognosis of small intestinal adenocarcinoma is poor because the 5-year survival rate is 39%–46%[78]. However, the survival rate for CD-associated small intestinal adenocarcinoma is significantly higher than that for small intestinal adenocarcinoma without CD[68,79].
Colorectal neoplasms mainly include colorectal polyps, adenomas, advanced lesions, and cancers. The incidence and mortality rates of colorectal cancer are the third and second highest, respectively, among those of all malignancies[80]. Colorectal polyps, including adenomatous and non-adenomatous polyps, are abnormal protrusions on the surface of the large intestine. Colorectal adenomatous polyps are considered the most important precancerous lesions that develop into colorectal cancer through the adenomatous carcinoma sequence. Therefore, early screening, early detection, and early treatment of precancerous lesions can prevent the occurrence and development of colorectal cancer[81,82]. Although the incidence of SBC is high in CD patients, the relationship between CD and colorectal cancer is controversial. In 2002, a large, national population-based cohort study showed that CD patients had an increased risk of colorectal cancer, and that the cancers mainly occurred in the ascending and transverse colon[61]. A retrospective case-control study reported that adult patients with CD had an increased prevalence of colorectal adenomas compared with healthy controls[83]. In a population-based cohort study of patients with CD, the most common gastrointestinal cancer was colon cancer. Although the risk of colon cancer increased eight-fold during the first year of follow-up of a previous study, there was no increase in the risk after the first year of biopsy[54]. In 2014, an Italian study involving 1757 patients confirmed that CD was associated with a decreased risk of colon cancer[84]. However, in most studies, the risk of colorectal cancer in CD patients was not significantly correlated with the risk in the general population[35,85-87]. A meta-analysis conducted in 2015 found no significant association between CD and colon and rectal cancer[59]. A systematic review and meta-analysis by Lasa et al[88] found that there was no causal relationship between CD and colorectal adenoma. However, the incidence of colorectal cancer in patients with CD was similar to or lower than that in the general population[84]. It may be related to the increased utilization of medical care by patients with known CD, especially when gastroenterologists perform polypectomy during colonoscopy screening[81]. In addition, immune changes such as an increase in the number of intestinal intraepithelial lymphocytes (IELs) in CD patients may prevent the development of epithelial malignancies[89].
Whether GFD can reduce the risk of colon cancer in patients with CD is still inconclusive. A multicenter retrospective case-control study showed that CD was not associated with an increased risk of colorectal cancer and that adherence to a strict GFD was associated with the presence of adenoma[90]. Most studies of adults with CD have shown that a non-GFD diet does not increase the risk of colon cancer[91]. Untreated CD may have a protective effect against colon cancer because impaired absorption of fat or fat-soluble agents, including hydrocarbons and putative co-carcinogens, which are associated with the development of colon cancer. Some substances may be poorly absorbed and rapidly excreted. Further studies are required to clarify the relationship between colorectal cancer and CD.
EATL is a rare peripheral T-cell lymphoma that was first reported by O’Farrelly et al[92] in 1986. It is a CD-associated NHL of the upper small intestine. It is estimated that approximately 2%–3% CD patients will develop intestinal lymphoma, and EATL is currently considered the most common subtype of primary intestinal T-cell lymphoma[93,94]. There are two types of EATL. Classic EATL (type I) accounts for approximately 80%–90% of cases, and is related to CD. Type II EATL is not associated with CD. Here we mainly discuss the former. Severe complications, such as refractory celiac disease (RCD) or malignant tumors, occur in 2%–5% adult CD patients. There are two types of RCD. The phenotype of IELs is abnormal in RCD II patients and normal in RCD I patients. Approximately 50%–60% RCD II patients develop into EATL within 5 years after diagnosis[95]. Patients with EATL usually present with weight loss, anemia, abdominal pain, diarrhea, fever, and vomiting. Intestinal ulcers, stenosis, and perforation are typical manifestations of EATL. Multifocal involvement of the jejunum is the most common, followed by that of the ileum, duodenum, stomach, and colon[96].
Although the relationship between CD and EATL has been established, it is unclear whether a GFD can reduce the occurrence of EATL. Holmes et al[97] reported that compared with CD patients who followed a strict GFD for > 5 years, those with an unlimited or a low-gluten diet had an increased risk of intestinal NHL. One study reported that in a cohort of 335 patients who underwent early treatment for CD, 83% adhered to the GFD, and no NHL cases were found[98]. A Swedish study reported that for CD patients < 10 years of age, the risk of developing lymphoma was moderate and did not significantly increase[61]. A population-based prospective study in 1757 CD patients performed by Silano et al[99] found that a strict GFD had a protective effect on the development of EATL. In contrast, a retrospective study from the United Kingdom found that patients with good histological responses to a GFD did not have a reduced risk for intestinal lymphoma[100]. Green et al[101] reported the occurrence of intestinal NHL in patients with CD after years of following a GFD. The differences in the conclusions of these studies might be explained by a number of reasons. First, the relatively short dietary duration observed may not be sufficient to reverse the effects of years of gluten exposure[102]. Second, it is very difficult to comply with a strict GFD because of the small amount of gluten present in non-cereal foods[103]. Third, there is no non-invasive method to determine compliance with a GFD[104].
RCD II is characterized by the presence of a large number of abnormal clonal T cells, which are associated with poor prognosis. Because of the risk of conversion to EATL, RCD II is referred to as prelymphoma or low-grade lymphoma, with a high mortality rate. The 5-year survival rate of patients with RCD II is as high as 58%[50]. If EATL occurs, the 5-year survival rate is reduced to 8%. Fewer than 14% patients with RCD I develop EATL within 5 years of diagnosis[105]. The risk factors of EATL include older onset age, male sex, HLA-DQ2 homozygous, ulcerative jejunitis, and/or the presence of abnormal T cells[105,106]. The poor prognosis of EATL is associated with a large tumor volume and elevated levels of C-reactive protein and lactate dehydrogenase[94,107,108]. A comprehensive examination can improve the accuracy of EATL detection. For patients with suspected EATL, comprehensive evaluation, double-balloon enteroscopy biopsy, video capsule enteroscopy, magnetic resonance enteric examination, and 18F- fluorodeoxyglucose positron-emission tomography-computed tomography can be used for confirmation[95].
There is essentially no difference in the treatment of lymphoma in patients with and without CD. Surgery, radiotherapy, and chemotherapy are commonly used. For patients diagnosed early, the treatment effect is better[109]. Patients with RCD I generally respond well to corticosteroids and immunosuppressive drugs such as thiopurine and infliximab. However, patients with RCD II do not respond well to those drugs[50]. The key goal of RCD II therapy is to destroy the precancerous clonal T-cell population. Chemotherapy (e.g., with the purine analogue cladribine) and autologous stem cell transplantation (ASCT) have been used, and their success rates in patients with RCD II vary[110]. The cyclophosphamide, doxorubicin, vincristine, prednisone scheme is widely used, however, previous studies have shown that the overall median survival time was only 7 mo[111-113]. In a small case series, the combination of ifosfamide, etoposide, epirubicin/methotrexate-ASCT increased the 5-year survival rate to 60% compared with the anthracycline based chemotherapy[114]. CD disrupts cell-level regulation, leading to overexpression of IL-15 and chronic intestinal inflammation, which in turn leads to the proliferation of IELs. Recent developments include Janus kinase inhibitors that can block IL-15 and reduce IELs, which has been confirmed in animal models. Biologic drugs provide a new possible method for the treatment of RCD and EATL[115-118].
The available data show that the total risk of SBC and EATL, but not colorectal cancer, in CD patients is higher than that in the general population. The protective effect of a GFD on CD-related intestinal neoplasms is controversial. It is necessary to conduct more studies, especially prospective cohort and experimental studies, to further evaluate whether GFD treatment can reduce the risk for intestinal malignancies in patients with CD and explore the associated risk factors and biological relationships that may lead to CD-related intestinal malignancies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin Y S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Lindfors K, Ciacci C, Kurppa K, Lundin KEA, Makharia GK, Mearin ML, Murray JA, Verdu EF, Kaukinen K. Coeliac disease. Nat Rev Dis Primers. 2019;5:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1163] [Article Influence: 96.9] [Reference Citation Analysis (1)] |
| 3. | Lebwohl B, Ludvigsson JF, Green PH. Celiac disease and non-celiac gluten sensitivity. BMJ. 2015;351:h4347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 565] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 5. | D'Avino P, Serena G, Kenyon V, Fasano A. An updated overview on celiac disease: from immuno-pathogenesis and immuno-genetics to therapeutic implications. Expert Rev Clin Immunol. 2021;17:269-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 6. | Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J; European Genetics Cluster on Celiac Disease. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Størdal K, Kahrs C, Tapia G, Agardh D, Kurppa K, Stene LC. Review article: exposure to microbes and risk of coeliac disease. Aliment Pharmacol Ther. 2021;53:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Blázquez AB, Berin MC. Microbiome and food allergy. Transl Res. 2017;179:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | D'Argenio V, Casaburi G, Precone V, Pagliuca C, Colicchio R, Sarnataro D, Discepolo V, Kim SM, Russo I, Del Vecchio Blanco G, Horner DS, Chiara M, Pesole G, Salvatore P, Monteleone G, Ciacci C, Caporaso GJ, Jabrì B, Salvatore F, Sacchetti L. Metagenomics Reveals Dysbiosis and a Potentially Pathogenic N. flavescens Strain in Duodenum of Adult Celiac Patients. Am J Gastroenterol. 2016;111:879-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Lebwohl B, Blaser MJ, Ludvigsson JF, Green PH, Rundle A, Sonnenberg A, Genta RM. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol. 2013;178:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Kemppainen KM, Lynch KF, Liu E, Lönnrot M, Simell V, Briese T, Koletzko S, Hagopian W, Rewers M, She JX, Simell O, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Lernmark Å, Hyöty H, Triplett EW, Agardh D; TEDDY Study Group. Factors That Increase Risk of Celiac Disease Autoimmunity After a Gastrointestinal Infection in Early Life. Clin Gastroenterol Hepatol. 2017;15:694-702.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, Ernest JD, Iskarpatyoti JA, Costes LM, Lawrence I, Palanski BA, Varma M, Zurenski MA, Khomandiak S, McAllister N, Aravamudhan P, Boehme KW, Hu F, Samsom JN, Reinecker HC, Kupfer SS, Guandalini S, Semrad CE, Abadie V, Khosla C, Barreiro LB, Xavier RJ, Ng A, Dermody TS, Jabri B. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 13. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 950] [Article Influence: 135.7] [Reference Citation Analysis (1)] |
| 14. | Ashtari S, Najafimehr H, Pourhoseingholi MA, Rostami K, Asadzadeh-Aghdaei H, Rostami-Nejad M, Tavirani MR, Olfatifar M, Makharia GK, Zali MR. Prevalence of celiac disease in low and high risk population in Asia-Pacific region: a systematic review and meta-analysis. Sci Rep. 2021;11:2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Kivelä L, Kaukinen K, Lähdeaho ML, Huhtala H, Ashorn M, Ruuska T, Hiltunen P, Visakorpi J, Mäki M, Kurppa K. Presentation of Celiac Disease in Finnish Children Is No Longer Changing: A 50-Year Perspective. J Pediatr. 2015;167:1109-15.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Lebwohl B, Sanders DS, Green PH. Coeliac disease and dermatitis herpetiformis - Authors' reply. Lancet. 2018;392:917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Riznik P, De Leo L, Dolinsek J, Gyimesi J, Klemenak M, Koletzko B, Koletzko S, Korponay-Szabó IR, Krencnik T, Not T, Palcevski G, Sblattero D, Vogrincic M, Werkstetter KJ. Diagnostic Delays in Children With Coeliac Disease in the Central European Region. J Pediatr Gastroenterol Nutr. 2019;69:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Parzanese I, Qehajaj D, Patrinicola F, Aralica M, Chiriva-Internati M, Stifter S, Elli L, Grizzi F. Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol. 2017;8:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (4)] |
| 20. | Van Kalleveen MW, de Meij T, Plötz FB. Clinical spectrum of paediatric coeliac disease: a 10-year single-centre experience. Eur J Pediatr. 2018;177:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 21. | Nurminen S, Kivelä L, Huhtala H, Kaukinen K, Kurppa K. Extraintestinal manifestations were common in children with coeliac disease and were more prevalent in patients with more severe clinical and histological presentation. Acta Paediatr. 2019;108:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Nardecchia S, Auricchio R, Discepolo V, Troncone R. Extra-Intestinal Manifestations of Coeliac Disease in Children: Clinical Features and Mechanisms. Front Pediatr. 2019;7:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 599] [Article Influence: 99.8] [Reference Citation Analysis (1)] |
| 24. | Husby S, Murray JA, Katzka DA. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology. 2019;156:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Hujoel IA, Reilly NR, Rubio-Tapia A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol Clin North Am. 2019;48:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Alessio MG, Tonutti E, Brusca I, Radice A, Licini L, Sonzogni A, Florena A, Schiaffino E, Marus W, Sulfaro S, Villalta D; Study Group on Autoimmune Diseases of Italian Society of Laboratory Medicine. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Webb C, Norström F, Myléus A, Ivarsson A, Halvarsson B, Högberg L, Lagerqvist C, Rosén A, Sandström O, Stenhammar L, Carlsson A. Celiac disease can be predicted by high levels of anti-tissue transglutaminase antibodies in population-based screening. J Pediatr Gastroenterol Nutr. 2015;60:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Ortiz G, Messere G, Toca MDC, Fiorucci M, Bigliardi R, Vidal J, Reynoso R. IgA anti-tissue transglutaminase antibodies and IgG antibodies against deamidated gliadin peptides as predictors of celiac disease. Arch Argent Pediatr. 2019;117:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Smarrazzo A, Misak Z, Costa S, Mičetić-Turk D, Abu-Zekry M, Kansu A, Abkari A, Bouziane-Nedjadi K, Ben Hariz M, Roma E, Velmishi V, Legarda Tamara M, Attard T, Djurisic V, Greco L, Magazzù G. Diagnosis of celiac disease and applicability of ESPGHAN guidelines in Mediterranean countries: a real life prospective study. BMC Gastroenterol. 2017;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, Shamir R, Troncone R, Auricchio R, Castillejo G, Christensen R, Dolinsek J, Gillett P, Hróbjartsson A, Koltai T, Maki M, Nielsen SM, Popp A, Størdal K, Werkstetter K, Wessels M. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 694] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 31. | Shannahan S, Leffler DA. Diagnosis and Updates in Celiac Disease. Gastrointest Endosc Clin N Am. 2017;27:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | McCarty TR, O'Brien CR, Gremida A, Ling C, Rustagi T. Efficacy of duodenal bulb biopsy for diagnosis of celiac disease: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E1369-E1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Tye-Din JA, Galipeau HJ, Agardh D. Celiac Disease: A Review of Current Concepts in Pathogenesis, Prevention, and Novel Therapies. Front Pediatr. 2018;6:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1158] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 35. | Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Anderson LA, McMillan SA, Watson RG, Monaghan P, Gavin AT, Fox C, Murray LJ. Malignancy and mortality in a population-based cohort of patients with coeliac disease or "gluten sensitivity". World J Gastroenterol. 2007;13:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Holmes GKT, Muirhead A. Mortality in coeliac disease: a population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterol. 2018;5:e000201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabró A, Certo M; Club del Tenue Study Group. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 385] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 40. | West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 41. | Quarpong W, Card TR, West J, Solaymani-Dodaran M, Logan RF, Grainge MJ. Mortality in people with coeliac disease: Long-term follow-up from a Scottish cohort. United European Gastroenterol J. 2019;7:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Abdul Sultan A, Crooks CJ, Card T, Tata LJ, Fleming KM, West J. Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis. Gut. 2015;64:1220-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Lebwohl B, Green PHR, Söderling J, Roelstraete B, Ludvigsson JF. Association Between Celiac Disease and Mortality Risk in a Swedish Population. JAMA. 2020;323:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 44. | Balakireva AV, Zamyatnin AA. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 45. | Baggus EMR, Hadjivassiliou M, Cross S, Penny H, Urwin H, Watson S, Woodward JM, Sanders DS. How to manage adult coeliac disease: perspective from the NHS England Rare Diseases Collaborative Network for Non-Responsive and Refractory Coeliac Disease. Frontline Gastroenterol. 2020;11:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Yoosuf S, Makharia GK. Evolving Therapy for Celiac Disease. Front Pediatr. 2019;7:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Vilppula A, Kaukinen K, Luostarinen L, Krekelä I, Patrikainen H, Valve R, Luostarinen M, Laurila K, Mäki M, Collin P. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol. 2011;11:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Weisbrod VM, Silvester JA, Raber C, Suslovic W, Coburn SS, Raber B, McMahon J, Damast A, Kramer Z, Kerzner B. A Quantitative Assessment of Gluten Cross-contact in the School Environment for Children With Celiac Disease. J Pediatr Gastroenterol Nutr. 2020;70:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Daveson AJM, Popp A, Taavela J, Goldstein KE, Isola J, Truitt KE, Mäki M, Anderson RP; Group tRCS. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep. 2020;2:22-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Hujoel IA, Murray JA. Refractory Celiac Disease. Curr Gastroenterol Rep. 2020;22:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Makovicky P, Makovicky P, Caja F, Rimarova K, Samasca G, Vannucci L. Celiac disease and gluten-free diet: past, present, and future. Gastroenterol Hepatol Bed Bench. 2020;13:1-7. [PubMed] |
| 52. | Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, Green PH, Ludvigsson JF. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 53. | Biagi F, Schiepatti A, Maiorano G, Fraternale G, Agazzi S, Zingone F, Ciacci C, Volta U, Caio G, Tortora R, Klersy C, Corazza GR. Risk of complications in coeliac patients depends on age at diagnosis and type of clinical presentation. Dig Liver Dis. 2018;50:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol. 2012;10:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Ilus T, Kaukinen K, Virta LJ, Pukkala E, Collin P. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15470] [Article Influence: 2578.3] [Reference Citation Analysis (2)] |
| 57. | Paski SC, Semrad CE. Small bowel tumors. Gastrointest Endosc Clin N Am. 2009;19:461-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Raghav K, Overman MJ. Small bowel adenocarcinomas--existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 59. | Han Y, Chen W, Li P, Ye J. Association Between Coeliac Disease and Risk of Any Malignancy and Gastrointestinal Malignancy: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Emilsson L, Semrad C, Lebwohl B, Green PHR, Ludvigsson JF. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals With Celiac Disease. Gastroenterology. 2020;159:1686-1694.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 62. | Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 63. | Spijkerman M, Tan IL, Kolkman JJ, Withoff S, Wijmenga C, Visschedijk MC, Weersma RK. A large variety of clinical features and concomitant disorders in celiac disease - A cohort study in the Netherlands. Dig Liver Dis. 2016;48:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol. 2014;71:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 66. | Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11:e1004901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8180] [Article Influence: 545.3] [Reference Citation Analysis (0)] |
| 68. | Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073-7077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Vanoli A, Di Sabatino A, Furlan D, Klersy C, Grillo F, Fiocca R, Mescoli C, Rugge M, Nesi G, Fociani P, Sampietro G, Ardizzone S, Luinetti O, Calabrò A, Tonelli F, Volta U, Santini D, Caio G, Giuffrida P, Elli L, Ferrero S, Latella G, Ciardi A, Caronna R, Solina G, Rizzo A, Ciacci C, D'Armiento FP, Salemme M, Villanacci V, Cannizzaro R, Canzonieri V, Reggiani Bonetti L, Biancone L, Monteleone G, Orlandi A, Santeusanio G, Macciomei MC, D'Incà R, Perfetti V, Sandri G, Silano M, Florena AM, Giannone AG, Papi C, Coppola L, Usai P, Maccioni A, Astegiano M, Migliora P, Manca R, Martino M, Trapani D, Cerutti R, Alberizzi P, Riboni R, Sessa F, Paulli M, Solcia E, Corazza GR. Small Bowel Carcinomas in Coeliac or Crohn's Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study From the Small Bowel Cancer Italian Consortium. J Crohns Colitis. 2017;11:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Vanoli A, Di Sabatino A, Martino M, Klersy C, Grillo F, Mescoli C, Nesi G, Volta U, Fornino D, Luinetti O, Fociani P, Villanacci V, D'Armiento FP, Cannizzaro R, Latella G, Ciacci C, Biancone L, Paulli M, Sessa F, Rugge M, Fiocca R, Corazza GR, Solcia E. Small bowel carcinomas in celiac or Crohn's disease: distinctive histophenotypic, molecular and histogenetic patterns. Mod Pathol. 2017;30:1453-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Giuffrida P, Arpa G, Grillo F, Klersy C, Sampietro G, Ardizzone S, Fociani P, Fiocca R, Latella G, Sessa F, D'Errico A, Malvi D, Mescoli C, Rugge M, Nesi G, Ferrero S, Furlan D, Poggioli G, Rizzello F, Macciomei MC, Santini D, Volta U, De Giorgio R, Caio G, Calabrò A, Ciacci C, D'Armiento M, Rizzo A, Solina G, Martino M, Tonelli F, Villanacci V, Cannizzaro R, Canzonieri V, Florena AM, Biancone L, Monteleone G, Caronna R, Ciardi A, Elli L, Caprioli F, Vecchi M, D'Incà R, Zingone F, D'Odorico A, Lenti MV, Oreggia B, Reggiani Bonetti L, Astegiano M, Biletta E, Cantoro L, Giannone AG, Orlandi A, Papi C, Perfetti V, Quaquarini E, Sandri G, Silano M, Usai P, Barresi V, Ciccocioppo R, Luinetti O, Pedrazzoli P, Pietrabissa A, Viglio A, Paulli M, Corazza GR, Solcia E, Vanoli A, Di Sabatino A. PD-L1 in small bowel adenocarcinoma is associated with etiology and tumor-infiltrating lymphocytes, in addition to microsatellite instability. Mod Pathol. 2020;33:1398-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Rampertab SD, Forde KA, Green PH. Small bowel neoplasia in coeliac disease. Gut. 2003;52:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Freeman HJ. Malignancy in adult celiac disease. World J Gastroenterol. 2009;15:1581-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Brousse N, Meijer JW. Malignant complications of coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Wild D, Robins GG, Burley VJ, Howdle PD. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment Pharmacol Ther. 2010;32:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 76. | Pozo-Rubio T, Olivares M, Nova E, De Palma G, Mujico JR, Ferrer MD, Marcos A, Sanz Y. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Palascak-Juif V, Bouvier AM, Cosnes J, Flourié B, Bouché O, Cadiot G, Lémann M, Bonaz B, Denet C, Marteau P, Gambiez L, Beaugerie L, Faivre J, Carbonnel F. Small bowel adenocarcinoma in patients with Crohn's disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis. 2005;11:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Zar N, Holmberg L, Wilander E, Rastad J. Survival in small intestinal adenocarcinoma. Eur J Cancer. 1996;32A:2114-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Caio G, Volta U, Ursini F, Manfredini R, De Giorgio R. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol. 2019;19:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55823] [Article Influence: 7974.7] [Reference Citation Analysis (132)] |
| 81. | Wang M, Kong WJ, Zhang JZ, Lu JJ, Hui WJ, Liu WD, Kang XJ, Gao F. Association of Helicobacter pylori infection with colorectal polyps and malignancy in China. World J Gastrointest Oncol. 2020;12:582-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Wang M, Lu JJ, Kong WJ, Kang XJ, Gao F. Clinical characteristics of sentinel polyps and their correlation with proximal colon cancer: A retrospective observational study. World J Clin Cases. 2019;7:3217-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 83. | Lasa J, Rausch A, Bracho LF, Altamirano J, Speisky D, de Dávila MTG, Iotti A, Zubiaurre I. Colorectal Adenoma Risk Is Increased among Recently Diagnosed Adult Celiac Disease Patients. Gastroenterol Res Pract. 2018;2018:6150145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol. 2014;49:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Dickey W. Colon neoplasia co-existing with coeliac disease in older patients: coincidental, probably; important, certainly. Scand J Gastroenterol. 2002;37:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Lebwohl B, Stavsky E, Neugut AI, Green PH. Risk of colorectal adenomas in patients with coeliac disease. Aliment Pharmacol Ther. 2010;32:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | González R, Pereyra L, Mohaidle A, Mella JM, Fischer C, Medrano MA, Vizcaino B, Hadad AR, Luna P, Cimmino DG, Pedreira SC, Boerr LA. [Celiac disease and risk of colorectal neoplasia]. Acta Gastroenterol Latinoam. 2012;42:87-91. [PubMed] |
| 88. | Lasa J, Rausch A, Zubiaurre I. Risk of colorectal adenomas in patients with celiac disease: a systematic review and meta-analysis. Rev Gastroenterol Mex (Engl Ed). 2018;83:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Freeman HJ. Adult celiac disease and its malignant complications. Gut Liver. 2009;3:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Pereyra L, Gonzalez R, Mohaidle A, Fischer C, Mella JM, Panigadi GN, Manazzoni D, Matoso MD, Lasa JS, Novillo A, De Paula J, Soifer L, Nadales A, Cimmino DG, Pedreira S, Boerr L. Risk of colorectal neoplasia in patients with celiac disease: a multicenter study. J Crohns Colitis. 2013;7:e672-e677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Thies F, Masson LF, Boffetta P, Kris-Etherton P. Oats and bowel disease: a systematic literature review. Br J Nutr. 2014;112 Suppl 2:S31-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | O'Farrelly C, Feighery C, O'Briain DS, Stevens F, Connolly CE, McCarthy C, Weir DG. Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br Med J (Clin Res Ed). 1986;293:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Chott A, Haedicke W, Mosberger I, Födinger M, Winkler K, Mannhalter C, Müller-Hermelink HK. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol. 1998;153:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K, Rüdiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 95. | van de Water JM, Cillessen SA, Visser OJ, Verbeek WH, Meijer CJ, Mulder CJ. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Eigner W, Bashir K, Primas C, Kazemi-Shirazi L, Wrba F, Trauner M, Vogelsang H. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol Ther. 2017;45:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Holmes GK, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989;30:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 600] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 98. | Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease--associated disorders and survival. Gut. 1994;35:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 318] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 99. | Silano M, Volta U, Vincenzi AD, Dessì M, Vincenzi MD; Collaborating Centers of the Italian Registry of the Complications of Coeliac Disease. Effect of a gluten-free diet on the risk of enteropathy-associated T-cell lymphoma in celiac disease. Dig Dis Sci. 2008;53:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Swinson CM, Slavin G, Coles EC, Booth CC. Coeliac disease and malignancy. Lancet. 1983;1:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 264] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 101. | Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 102. | Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 103. | Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 104. | Martín-Pagola A, Ortiz-Paranza L, Bilbao JR, de Nanclares GP, Estevez EP, Castaño L, Vitoria JC. Two-year follow-up of anti-transglutaminase autoantibodies among celiac children on gluten-free diet: comparison of IgG and IgA. Autoimmunity. 2007;40:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 106. | Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 107. | Malamut G, Chandesris O, Verkarre V, Meresse B, Callens C, Macintyre E, Bouhnik Y, Gornet JM, Allez M, Jian R, Berger A, Châtellier G, Brousse N, Hermine O, Cerf-Bensussan N, Cellier C. Enteropathy associated T cell lymphoma in celiac disease: a large retrospective study. Dig Liver Dis. 2013;45:377-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99-107; quiz 352-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 109. | Ciccocioppo R, Perfetti V, Corazza GR. Treating ETTCL: A matter of early diagnosis and chemotherapy strategies. Dig Liver Dis. 2007;39:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Nijeboer P, van Wanrooij R, van Gils T, Wierdsma NJ, Tack GJ, Witte BI, Bontkes HJ, Visser O, Mulder C, Bouma G. Lymphoma development and survival in refractory coeliac disease type II: Histological response as prognostic factor. United European Gastroenterol J. 2017;5:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Novakovic BJ, Novakovic S, Frkovic-Grazio S. A single-center report on clinical features and treatment response in patients with intestinal T cell non-Hodgkin's lymphomas. Oncol Rep. 2006;16:191-195. [PubMed] |
| 112. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 2003;21:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 113. | Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 114. | Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P, Proctor SJ, Lennard AL. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. 2010;115:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 115. | Yokoyama S, Perera PY, Waldmann TA, Hiroi T, Perera LP. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J Clin Immunol. 2013;33:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Sestak K, Dufour JP, Liu DX, Rout N, Alvarez X, Blanchard J, Faldas A, Laine DJ, Clarke AW, Doyle AG. Beneficial Effects of Human Anti-Interleukin-15 Antibody in Gluten-Sensitive Rhesus Macaques with Celiac Disease. Front Immunol. 2018;9:1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Chibbar R, Nostedt J, Mihalicz D, Deschenes J, McLean R, Dieleman LA. Refractory Celiac Disease Type II: A Case Report and Literature Review. Front Med (Lausanne). 2020;7:564875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Cellier C, Bouma G, van Gils T, Khater S, Malamut G, Crespo L, Collin P, Green PHR, Crowe SE, Tsuji W, Butz E, Cerf-Bensussan N, Macintyre E, Parnes JR, Leon F, Hermine O, Mulder CJ; RCD-II Study Group Investigators. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol Hepatol. 2019;4:960-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |