Published online May 15, 2021. doi: 10.4251/wjgo.v13.i5.312
Peer-review started: December 27, 2020
First decision: February 14, 2021
Revised: February 24, 2021
Accepted: April 14, 2021
Article in press: April 14, 2021
Published online: May 15, 2021
Processing time: 130 Days and 16.6 Hours
The malfeasant role of the hypoxic tumour microenvironment (TME) in cancer progression was recognized decades ago but the exact mechanisms that augment the hallmarks of cancer and promote treatment resistance continue to be elucidated. Gastroesophageal cancers (GOCs) represent a major burden of worldwide disease, responsible for the deaths of over 1 million people annually. Disentangling the impact of hypoxia in GOCs enables a better overall understan
Core Tip: Improved methods in measuring the oxygen status in the tumour microenvironment have allowed for a better understanding of the role of hypoxia and how it contributes to tumour progression and treatment resistance. These methods include non-invasive imaging techniques as well as validated hypoxic molecular signatures. Specific hypoxia-targeted therapies have not matched their expectations but may have potential application in combination with traditional treatment approaches in gastroesophageal cancer.
- Citation: King R, Hayes C, Donohoe CL, Dunne MR, Davern M, Donlon NE. Hypoxia and its impact on the tumour microenvironment of gastroesophageal cancers. World J Gastrointest Oncol 2021; 13(5): 312-331
- URL: https://www.wjgnet.com/1948-5204/full/v13/i5/312.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i5.312
One of the major turning points in the study of solid tumours arose with the realization that a critical regulatory influence in the process of angiogenesis was an environmental feature; hypoxia[1,2]. Many studies have since demonstrated the oncogenic transforming power of hypoxia in the microenvironment of different tumour types and the observation that tumour oxygenation status could disrupt the anti-tumour effects of radiation therapy was published over 60 years ago[3-8]. This review will discuss the role of hypoxia in the tumour microenvironment (TME) of gastroesophageal cancers (GOCs) including gastric cancer (GC) and oesophageal cancer (OC), how it augments disease, and additionally its relevance in the setting of prognostication and therapeutic targeting.
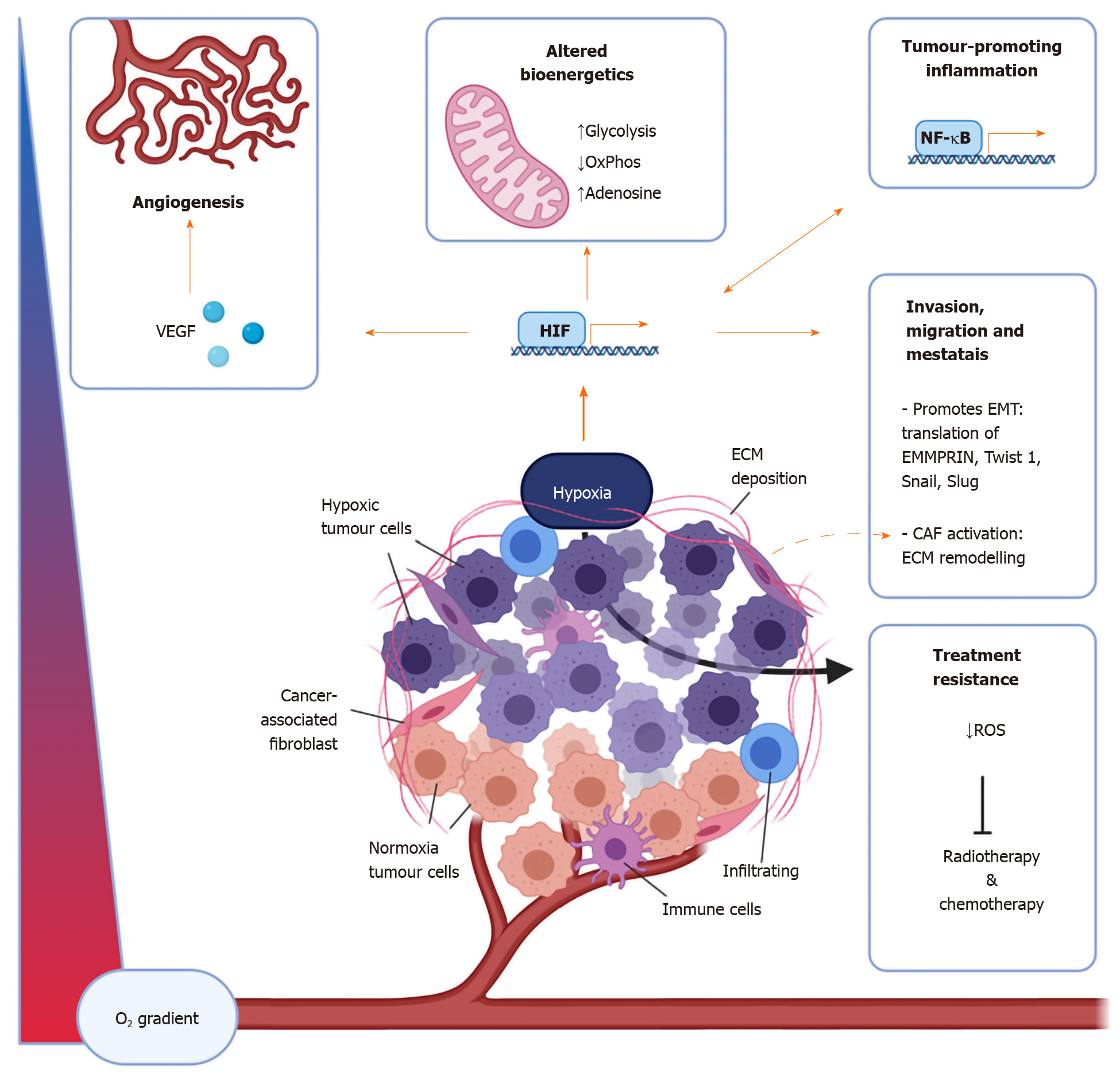
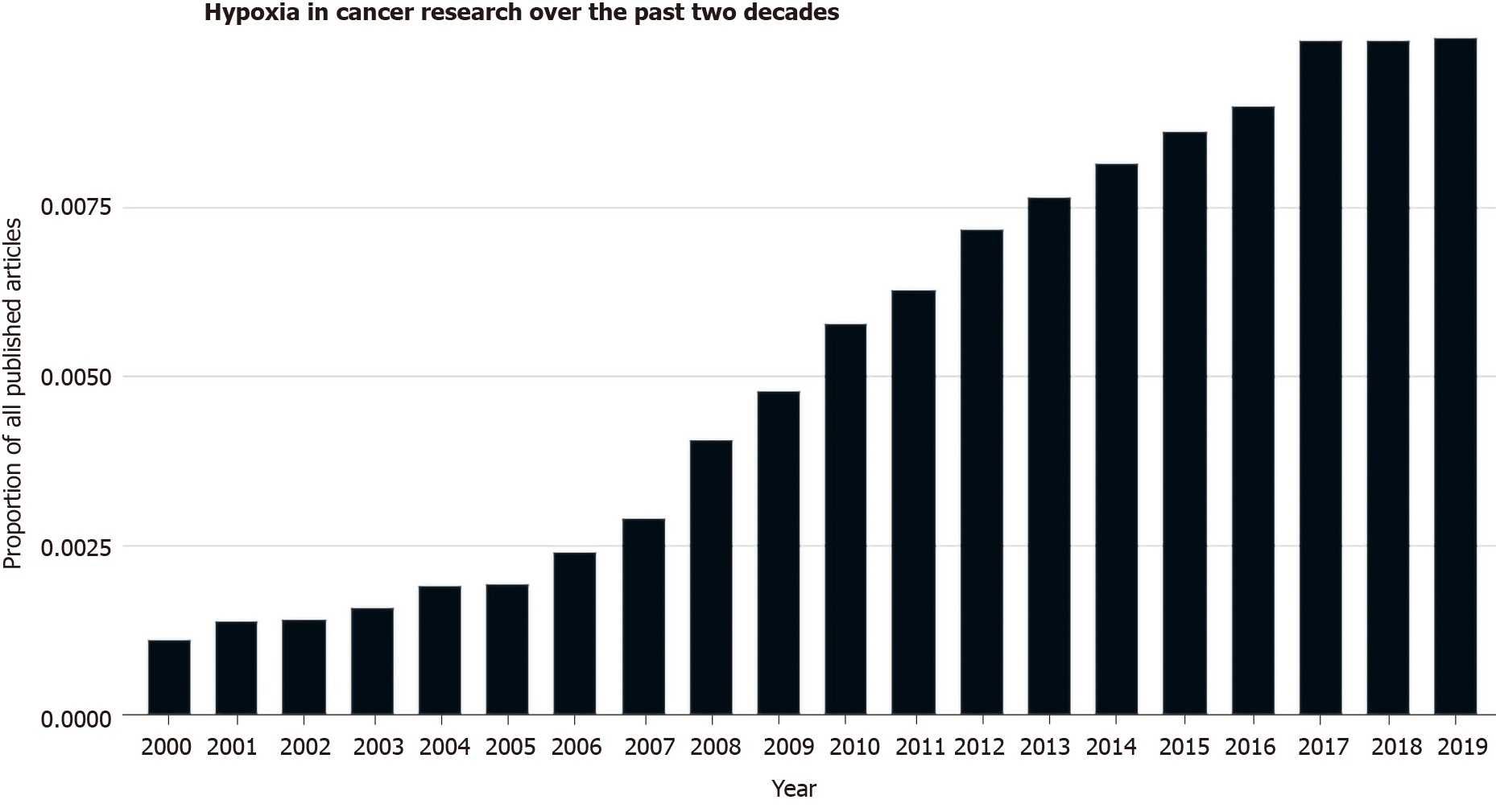
GOC is a substantial cause of morbidity and mortality, responsible for 1.2 million deaths per year globally[9-12]. An improved understanding of the risk factors for GC has seen a steady decline in both the incidence and mortality which is in sharp contrast to the rising incidence of OC, particularly oesophageal adenocarcinoma (OAC) globally[13,14]. GOCs develop insidiously and consequently, are commonly diagnosed at an advanced stage where chemotherapy with or without radiation remains the treatment of choice in the neoadjuvant setting[15]. Treatment at this stage is rarely curative and several mechanisms account for this resistance to treatment including tumour cell-intrinsic and extrinsic mechanisms. Hypoxia is a characteristic feature of the TME and a key mediator in conferring and enhancing treatment resistance[16-18]. The TME being the complex reciprocity between both the cellular (resident and infiltrating) and non-cellular components that surround, envelop, and make up the tumour mass, the components of which are summarized in Figure 1[19-21]. The exact mechanisms underlying resistance continue to be elucidated and as such, interest in the role of hypoxia in translational oncology research has garnered increasing interest recently as shown in Figure 2.
Hypoxia mediates aggressive, metastatic, and treatment-resistant disease by augmenting the hallmarks of cancer through various cellular and physiological events including; enhanced tumour cell proliferation, survival, immune evasion, inflamma
| Function | Gene |
| Enzymes | MMP1, MMP3, LOX, ADAMST1, ACE |
| Transcription factors | Twist1, Snail, Slug, β-Catenin, c-Myc, Oct4, NF-κB |
| Receptors | CXCR4, c-Met, TLR4, Notch |
| Growth factors | VEGF, TGFα |
| Transporters | Glut-1, MDR1 |
| Intracellular signalling | Cdc42, Rac1, RhoE |
| Bioenergetics | LDHA, PGK1, PKM2, GAPDH, GPI, ALDOC |
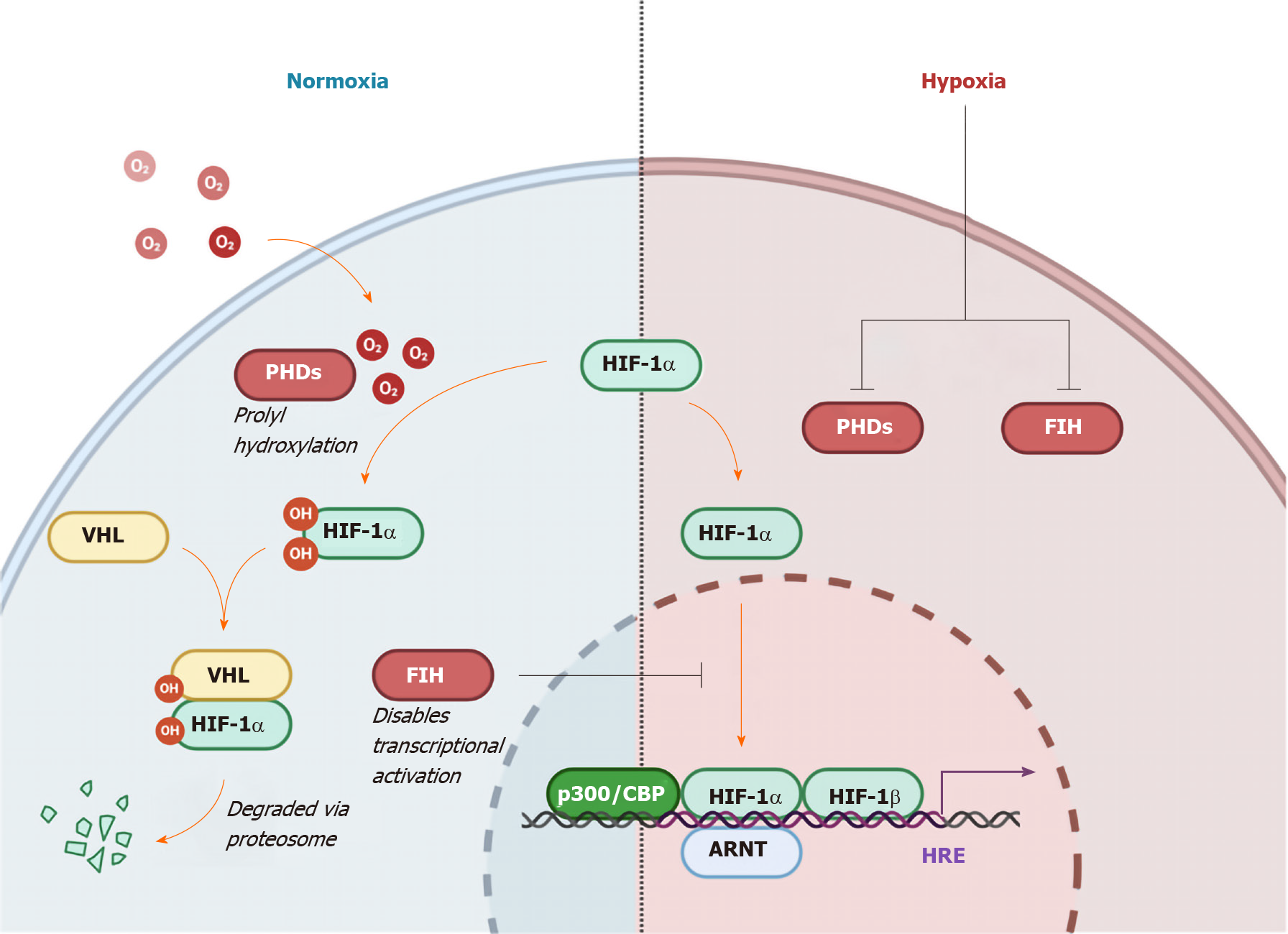
In the setting of normoxia, HIF1-α is regulated by two principal mechanisms; oxygen-dependent pVHL-dependent degradation, and oxygen-dependent non-pVHL-dependent inactivation (Figure 3)[25,27,28]. Hydroxylation by oxygen-dependent prolyl hydroxylase domain enzymes trigger recognition by the E3 ubiquitin ligase, pVHL, ensuring proteasomal degradation. In the non-pVHL dependent pathway, induction of factor inhibiting HIF leads to hydroxylation of an asparagine residue preventing HIF1-α from localizing with the co-activators p300 and CBP, hence disabling transcriptional activation[29].
The contribution of hypoxia to disease progression makes it an attractive thera
Cancer has long been described as a “wound that never heals”, in part due to inflammation, one of the enabling characteristics of cancer originally described by Hanahan and Weinberg[30-31]. Hypoxia and inflammation are intricately intertwined as illustrated through the fact that hypoxia has been shown to directly induce signalling via the inflammatory master transcription factor nuclear factor-kappa light chain enhancer of activated B cells (NF-κB), and likewise NF-κB induces HIFs[32-37]. In the context of malignancy, there exists a multitude of cancer implicated genes that are regulated by both HIFs and NF-κB, such as cyclooxygenase 2 and interleukin-6 (IL-6)[38]. This illustrates the complex crosstalk between signalling pathways and the difficulty involved in unravelling the net influence of certain factors in the network. In the setting of GOC, OAC has been described as “a model of inflammatory driven upper gastrointestinal cancer”[39,40]. The paramount importance of inflammation in the aetiology of OC is further validated by the risk reduction conferred by administration of the non-steroidal anti-inflammatory drugs such as aspirin, as demonstrated in a meta-analysis of 9 observational studies by Corley et al[41] and Farrow et al[42]. In a retrospective study of 53 patients with OAC and the metaplastic precursor lesion, Barrett’s oesophagus (BO), immunohistochemical staining of specimens revealed a significant increase in the expression of HIF1-α in OAC and BO compared to normal tissue but no further elevation between BO and OAC[43]. Furthermore, histological assessment of specimens’ inflammatory status, based on recruitment of neutrophils (reflecting acute inflammation) and monocytes (reflecting chronic inflammation) (known as the Sydney System), demonstrated a significant correlation with HIF1-α expression from normal tissue to metaplastic tissue but no association between other stages or between inflammatory status[43].
As previously mentioned, one of the defining discoveries involved in the study of the TME was the effect of hypoxia on angiogenesis[44-46]. This was originally demonstrated in HIF1-β deficient hepatoma cells having markedly reduced vascular endothelial growth factor (VEGF) mRNA levels when cultured under hypoxic conditions[24,47]. In the setting of GOC, a study of 92 oesophageal biopsy samples found a significant increase in the expression of HIF1-α in OAC vs dysplastic and metaplastic tissues but not between normal and metaplastic tissues[48]. These findings also reflected an increase in VEGF and HIF2-α expression in OAC vs dysplastic tissue. Several studies have revealed how hypoxia appears to drive tumour cell plasticity and hence vasculogenic mimicry, a process that allows malignant cells to impersonate endothelial cells and form a network of vessels, and in a sense bypass true angiogenic activity[49-54]. In an in vitro analysis of oral squamous cell carcinoma (OSCC) cells, transfection with siRNA targeting HIF1-α was shown to inhibit both vasculogenic mimicry (through three-dimensional culture) and proliferation (as measured by MTT assay)[55]. Validation of these results in a xenograft implant model was then performed; the HIF-1α knockout mice showed a longer time to tumour formation and had smaller tumours. In an experiment conducted by Chai et al[56] of 160 OSCC tumour tissues, both HIF1-α and the degree of vasculogenic mimicry correlated negatively with overall survival (OS). In a separate study, OSCC cell lines cultured under conditions of severe hypoxia (0.5% oxygen) for 5 d secreted exosomes which through tube formation assays, were shown to increase the angiogenic capacity of human umbilical vein endothelial cells when cultured together[57]. Vessel formation was significantly increased compared to umbilical vein endothelial cells cultured with exosomes obtained from OSCC cells exposed to normoxic conditions. When assessed in an in vivo implant model, findings reflected those found in the in vitro assay. As a consequence of these described phenomena, the blood vessels formed in tumours do not resemble those found in non-malignant tissues. The resulting network is disorganized and highly permeable and this limits the supply of blood and hence oxygen, nutrients, and anti-cancer drugs, further contributing to tumour hypoxia.
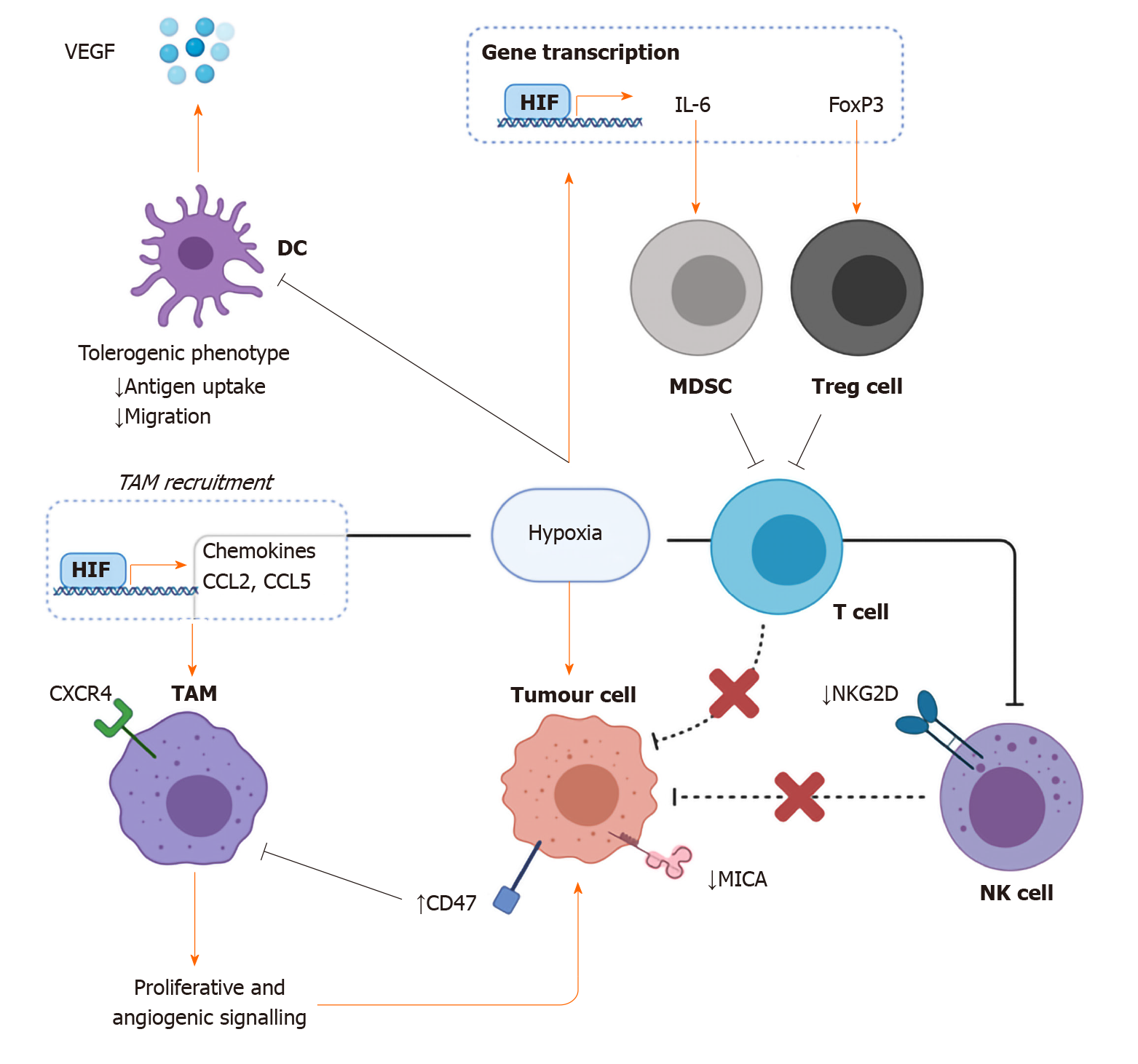
The cancer-immune set point refers to the equilibrium between factors that promote or suppress the anti-cancer immune response[58]. This is of great interest in GOC given the yet unrealized efficacy that was predicted of immune checkpoint inhibitor drugs in treating these cancer types, which are generally characterized as having high tumour mutational burden and evident immune cell infiltration[59]. A hypoxic TME promotes an immunosuppressive phenotype through actions on the diverse array of cellular and non-cellular entities across innate and adaptive immune arms and thus constitutes a vital host factor that may be contributing to a high cancer-immune set point and treatment failure. For example, in the context of cancer, the recruitment of myeloid-derived suppressor cells (MDSCs) is associated with less favourable patient outcomes which are likely mediated by their potent dampening of the anti-tumour immune response[60-62].
MDSCs are defined as “a heterogenous population of cells of myeloid origin that consist of myeloid progenitors, immature macrophages, immature granulocytes, and immature dendritic cells” (DCs)[63,64]. In a murine model of OSCC, intratumoural MDSC percentages were shown to correlate with the tumour progression sequence[65]. The role of IL-6 was then explored in the context of MDSCs and tumour progression. In patients with OSCC compared to healthy controls, serum IL-6 was significantly increased. Also, the percentage of intratumoural MDSCs correlated with general serum IL-6 levels. Delving further into this, the murine model of OSCC was utilized with 3 cohorts; IL-6 knockout, IL-6 stimulation (via 100 ng intraperitoneal injection twice weekly for 6 wk), and normal wild type. The cohort receiving IL-6 had a significant 3-fold increase in the percentage of MDSCs compared to the IL-6 deficient cohort (15% to 5% respectively). These findings were analogous when examining tumour invasiveness. As mentioned previously, HIF has been shown to upregulate the transcription of inflammatory factors including IL-6, and overall, the results demonstrate the importance of hypoxia in driving the pro-tumour immunosuppressive functions of MDSCs[38,66]. Others have shown the hypoxic TME to drive MDSC differentiation to tumour associated macrophages (TAMs), again in a manner that is orchestrated by HIF1-α[67].
TAMs comprise a large part of the cellular TME and as such are gaining further infamy for their role in driving tumour progression[68,69]. Studies have demonstrated how TAM recruitment and infiltration into the TME is in part mediated by the hypoxic response and HIF-driven regulation of chemoattractant including CCL2, CCL5, and receptors such as CXCR4[70-73] (Figure 4). There is strong evidence that macrophage infiltration and density are associated with worse patient outcomes in the setting of malignancy[74-76]. A meta-analysis of 16 OC cancer studies (n = 2292), found that M2-polarised pro-tumour macrophage density to be predictive of worse OS and disease stage[77,78]. In addition, in vitro evidence suggests that TAM density is significantly associated with an increase in programmed death-ligand 1 expression on OSCC cells[78]. Once infiltrated into the TME, low oxygen tension enhances the oncogenic role of TAMs via the promotion of proliferative and angiogenic growth signalling pathways[79,80]. Notably, while two studies have characterized the correlation between HIF1-α expression, TAM infiltration, and patient survival in the setting of gastric malignancy, the impact of hypoxia on the biology of TAMs could be further expanded in the context of GOC[81,82].
Signifying the potential of innate immune research in cancer, Gilead recently invested $4.8 billion for ownership of magrolimab[83], a monoclonal antibody that works through the disruption of CD47 which is expressed on cancer cells and acts to downregulate the anti-tumour phagocytic capability of macrophages. Targeting hypoxia-mediated CD47 function may also extend to cancers of the alimentary tract. Immunohistochemical staining and reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) of OSSC specimens taken from 14 patients demonstrated a significant increase in expression of CD47 while another preclinical study revealed an augmented response to immune checkpoint inhibition in combination with CD47 antagonism[84,85]. CD47 expression has also been shown to predict prognosis in OSCC[86].
Natural killer (NK) cells are a type of innate lymphoid cell that are capable of recognizing tumour cells through two principal mechanisms; altered expression of self or missing-self[87,88]. For example, in the absence of cellular stress, MHC class I chain-related molecules (MICA and MICB) are not normally expressed on cells. In one study of prostate cancer cells, culture under hypoxic conditions is shown to result in the shedding of MICA hence characterizing an immune evasive phenotype[89]. Hypoxia also affects both resting and activated NK cells directly by curtailing the expression of costimulatory NKG2D and other NK cell receptors (NKp46, NKp30) which enable NK cell function[90]. Furthermore, a low oxygen environment has revealed impaired NK cell differentiation in one in vitro study[91]. The density of infiltrating NK cells has been shown to be prognostic in OSCC[92]. In a study of OSCC xenografts implanted in nude mice, NK cell depletion was shown to restore tumour growth following treatment with an anti-PD-1 (programmed death-1) agent illustrating the important anti-tumour role of NK cells which is tightly regulated by the PD-1 pathway[93]. In human OC, NK cells that demonstrate high expression of a novel inhibitory regulator protein, T cell immunoglobulin domain and mucin domain 3 (Tim-3) are predisposed to apoptosis and hence fail to combat tumour progression[94]. Increased expression of Tim-3 in this context occurs through NF-κB signalling thus linking hypoxia to NK cell-mediated anti-tumour dysfunction. NK cells are also an important entity in GC. Tumour infiltrating NK cells expressing high levels of Tim-3 have been correlated with adverse prognosis in a study of 62 patients with the disease[95].
DCs present antigens to T cells including CD4+ T helper cells, resulting in the initiation of the adaptive anti-tumour immune response[96]. In cancer, impaired DC function is associated with defective anti-tumour immune responses and hence cancer progression[97-99]. While there are contrasting studies, the net effect of the hypoxic TME may be skewed towards a tolerogenic DC phenotype[100,101]. An in vitro study of peripheral blood mononuclear cells isolated from a healthy human cohort and cultured under hypoxic conditions (1% oxygen) showed that hypoxia impairs DC uptake of antigens and causes modulation of their cytokine expression patterns in both resting and activated states[100]. Hypoxia increased VEGF production and CXCR4 expression and lead to a reduction in DC production of tumor necrosis factor-α thereby revealing the pro-angiogenic and immunosuppressive effect of reduced oxygen tension on DCs. Lysosomal-associated membrane protein (LAMP3) is a marker of mature DCs and it has been shown to be induced by hypoxia in breast cancer both in vitro and in vivo[102]. It is thought to be implicated in metastasis[103]. RT-qPCR analysis of 157 OSCC tissues as well as immunohistochemical staining of 46 specimens reveal its expression to be correlated with poor patient outcomes, further emphasizing the tolerogenic capacity of DCs[104]. Again, in the context of OAC, co-culture with DCs has been shown to induce Treg (T regulatory) differentiation supporting the tolerogenic DC phenotype in these malignancies[105]. Given that successful activation of adaptive T cell responses is dependent on DC migration to peripheral lymphoid organs, further research and investigation of the effect of hypoxia in the TME on DCs is required to fully dissect the potential clinical impact regarding patient outcomes and treatment resistance[106].
Hypoxia-induced HIF1-α expression is also associated with the upregulation of the transcription factor Forkhead Box Protein P3 (FoxP3), highlighting the role of hypoxia in regulating the abundance and function of Treg cells, further illustrating the potential immunosuppressive effect of a hypoxic TME on anti-tumour immuni
The activation of cancer-associated fibroblasts (CAFs) in hypoxic TMEs has been implicated in the altered deposition, remodelling and degradation of the extracellular matrix (ECM) and hence invasion, migration, and metastasis[112-114]. In a study of 183 patients with OAC, characteristic expression of CAF marker α-SMA was found to be correlated with worse OS[115]. It was initially hypothesized that increased collagen production and fibrosis would present an obstacle to tumour cell invasion and metastasis, but evidence suggests that this is a lot more complex. In one study of pancreatic carcinoma cells, collagen has been shown to increase expression of the key epithelial to mesenchymal transition (EMT) transcription factor Snail in a transforming growth factor-β-mediated manner[116]. Thus, this series of events is thought to be involved in the activation of CAFs thereby, ensuring enhanced migratory capacity, invasiveness, survival, and ECM deposition in a positive feedback loop[117,118]. In the area of GOC, an in vitro assay revealed extracellular matrix metalloproteinase inducer (EMMPRIN) promoted EMT and hence invasion and migration of an OC cell line[119]. The authors followed up this study by showing, through HIF1-α interference and culture under hypoxic (1% oxygen) conditions, that EMMPRIN was regulated by HIF1-α. Further research probing the relationship between traditionally neglected components of the TME like CAFs and hypoxia in upper gastrointestinal cancers is required.
Cells deprived of oxygen promote tumour proliferation and survival through reprogramming of energy metabolism[30]. The observation that neoplastic cells shift their metabolism from aerobic to anaerobic respiration was first observed nearly 100 years ago by Otto Warburg[120,121]. This shift is orchestrated by the hypoxia master regulator HIF which upregulates enzymes involved in glycolysis such as pyruvate dehydrogenase kinase 1, and ultimately the production of lactate from pyruv
Lactate dehydrogenase is responsible for converting pyruvate to lactate under hypoxic conditions[126]. In a study of 152 patients with GC, immunohistochemical staining for lactate dehydrogenase (LDH) isoenzyme 5 demonstrated significant associations between immunoreactivity and a number of different tumour features such as tumour size, venous and lymphatic invasion, and tumour stage[127]. Inoculation of mice with LDH knock-out pancreatic cancer cells has been shown to result in reduced tumour size[128]. Furthermore, the quantity of MDSCs isolated from the LDH knock-out cancer mice both in tumour and spleen was significantly less in controls, and they demonstrated lower suppressive activity.
The effects of these processes are not restricted to neoplastic cells, as the evidence implicates hypoxia-driven metabolic shifts in other cellular components of the TME, particularly immune cells. Tissue hypoxia in cancerous or non-cancerous cells results in the build-up of the purine adenosine, extracellularly which augments a plethora of the hallmarks of cancer[129-134]. Evidence suggests this is conferred predominantly through the release and metabolism of ATP by the surface membrane nucleotidases CD39 and CD73[133,135,136]. In one in vitro experiment, an epithelial cell line demonstrated increased CD73 expression when exposed to hypoxic conditions, and examination of the CD73 gene has identified a binding site for HIF1[137]. Subsequent binding to purinergic receptors and adenosinergic signalling is known to mediate an anti-tumour immunosuppressive phenotype through effects on Tregs, MDSCs, TAMs, and B lymphocytes across various solid tumours types including OC[135,138-141]. In the context of GOC, a gene expression study of several radiotherapy resistant OC cell lines, CD73 expression was shown to be increased in TE-2, TE-13, and KYSE170 when compared to parent cell lines[142]. Once again, given the hypoxia-driven mechanism, this highlights the pro-inflammatory, tumour-promoting effect of the adenosine axis, thereby signifying another potential method of clinically targeting hypoxia pathways in the treatment of GOC.
Also, hypoxia (oxygen of 1.5%) driven reprogramming of energetic metabolism is linked to PD-1 immune checkpoint blockade resistance[143]. In vivo treatment with metformin, decreases OCR in tumour cells, while increasing consumption in T cells resulting in reduced hypoxia. The authors further examined the effect of anti-PD-1 agents in concert with metformin administration in vivo in a melanoma tumour type that traditionally fails to respond to immune checkpoint blockade. The synergistic effect demonstrated substantially increased tumour elimination[143]. These intricately woven hypoxia-mediated effects exist in concert with one another to contribute to an aggressive phenotype characterized by treatment resistance and poor prognosis.
Measuring tissue and tumour hypoxia is challenging. There are four principal methods for measuring oxygen levels in vivo; the Eppendorf oxygen electrode, exogenous markers, endogenous markers, and imaging techniques. The Eppendorf electrode quickly became the gold standard for measuring oxygen tension when it was introduced at the beginning of the millennium after studies confirmed that low tumour oxygenation status was associated with worse outcomes in cervical as well as head and neck cancer[144,145]. However, it fell out of favour just as quickly for a variety of reasons. It was notably limited to tumours that were accessible and it was steadfastly invasive. It was additionally prone to sampling error[146]. Although hypoxia can be arbitrarily classified as acute/perfusion limited or chronic/diffusion-limited, there remains significant spatiotemporal variation in tumour oxygen tension and hence multiple sampling observations must be taken[147]. The literature on the use of endogenous hypoxia markers in GOC is extensive and is discussed in the context of prognosis and treatment and resistance[148-152]. Exogenous markers such as pimonidazole are administered to a patient and undergo chemical modification in hypoxic cells and are then amenable to visualization in specimens. A summary of the major methods used to measure tumour hypoxia and their associated advantages and disadvantages can be found in Table 2.
| Technique | Advantages | Disadvantages |
| Needle Electrodes | Instrumental in establishing the link between hypoxia and treatment failure | Prone to sampling error due to poor spatial resolution |
| Real time direct measurement | Invasive and requires direct access to tumours | |
| Exogenous Markers | More sensitive than electrodes at lower oxygen levels | Requires biopsy and immunohistochemistry |
| Reproducible | ||
| Precise spatial resolution | ||
| Endogenous Markers | Precise spatial resolution | Requires biopsy and immunohistochemistry |
| Can be serological such as Osteopontin | ||
| Can be tissue based such as HIFs or carbonic anhydrase IX | ||
| Radiological | Non-invasive | Expensive |
| Reproducible | Radiation exposure | |
| Precise spatial resolution | Relatively less well established |
In the last decade, several studies have characterized gene expression signatures corresponding to oxygenation status[153-155]. Using a 15 gene expression panel derived from these studies, Ye et al[156] classified 24 cancer types from The Cancer Genome Atlas into a hypoxia score of high, low, and intermediate after adjusting for confounding factors such as sex and ethnicity. They were further able to validate this categorization with independent proteomic data where hypoxic status was known. 135/193 (70%) of GC samples had high hypoxic status while only 34/124 (27%) of OC samples fell into this category. There may be differences between OSCC and OAC but they were grouped together in this study. They further built on these findings by comparing molecular characteristics such as miRNA expression, highly mutated genes, and significant copy number alterations between the hypoxia score high and low tumours. In both OC and GC samples that had molecular signatures of high hypoxic status, a number of miRNAs that target the tumour suppressor gene tumour protein p53 inducible nuclear protein 1 (TP53INP1), were significantly downregulated[156].
Ionizing radiation generates free radicals from molecules of oxygen which then induce double-stranded DNA breaks resulting in mitotic catastrophe. This is one of the key mechanisms for radiation-induced tumour cell death and it is reliant on the presence of oxygen within the TME[144,157]. GC and OC cells cultured in vitro under hypoxic conditions (1% oxygen) were more resistant to radiation-induced cell death compared to GC and OC cells cultured under normoxic conditions, as assessed by colony formation assay[158]. The contribution of hypoxia to radiotherapy treatment resistance is relatively well established but its role in conventional chemotherapy and molecularly targeted therapy is less clear cut, particularly in GOC. Functional inactivation of HIF1-α in GC cell lines demonstrated increased susceptibility to 5-fluorouracil and cisplatin as determined by proliferation and apoptosis assays which lends support to the use of HIF1-α in predicting response to therapy[159]. Analysis of cell cycle distribution patterns following treatment with 5-fluorouracil revealed a greater proportion of senescent HIF1-α deficient cells compared with controls. Likewise, the apoptotic cell fraction as determined by caspase 3 cleavage of HIF1-α deficient cells was greatly increased. The mechanism for this is thought to be mediated by HIF1-α dependent suppression of P53 induction in response to 5-fluorou
There are a large number of studies that have investigated the prognostic value of hypoxia in OC. A systematic review carried out by Peerlings et al[152] evaluated 22 studies assessing various hypoxia-related markers and established that increased expression of HIF1-α in early-stage OSCC was associated with increased resistance to chemoradiotherapy treatment. They also conclude that radiologically, the positron emission tomography (PET) marker 18F-FETNIM was significantly predictive for response to combined chemoradiation in the setting of OSCC[164]. In brief, these tracers work by diffusing into cells non-specifically. In the absence of oxygen, they undergo a chemical reaction and their resultant physicochemical properties do not allow diffusion out of the cell[165]. PET with 18F-FAZA (18F-fluoroazomycin arabinoside) has been shown to predict radiotherapy response in OAC murine xenografts[166]. Validation of the tracer 18F-HX4 has been performed in OC but is yet to be studied as a potential prognostic factor[167]. Overall, imaging of hypoxia continues to be an attractive approach for studying the TME and subsequent patient outcomes.
The markers assessed in the systematic review by Peerlings et al[152] included HIF1-α, VEGF, carbonic anhydrase IX, GLUT1, Beclin-2, HIF2-α, as well as PET. The most common method used to assess these markers was immunohistochemical staining of surgical or biopsied specimens i.e. an invasive technique. The authors indicate that HIF1-α overexpression was associated with worse outcomes for OS and disease-free survival in OSCC but the evidence for its association in OAC was inconclusive, mainly due to the absence of data. VEGF expression correlated with patient outcomes in OSCC but not OAC[152]. In contrast, carbonic anhydrase IX appears to be an independent predictor of survival in OAC. Carbonic anhydrase IX is a glycoprotein expressed on the cell surface and its primary function is the catalytic conversion of carbon dioxide to bicarbonate and protons[150,168]. Under the transcriptional control of HIF1-α, the metalloenzyme is thought to contribute to tumour growth and proliferation through the regulation of pH, ECM degradation, and EMT[168,169]. In the majority of studies assessing endogenous markers, the determination of what constituted “hypoxic” was based on relatively arbitrary thresholds of immunohistochemical expression, with very little in the way of standardized protocols across studies. For example, Munipalle et al[151] defined “high” HIF-1α expression as greater than 10% of OSCC cells showing positive staining. Birner et al[170] devised a score based on intensity and percentage of cells showing positive expression in a cohort of 333 OCs. Anything above the median was then considered a "high" expression while those below were considered a "low" expression.
In a more recent systematic review and meta-analysis, Luo et al[148] examined the clinical predictive value of HIF2-α. It included 40 studies with 4345 cancer cases but only 2 of these studies assessed upper gastrointestinal cancers. Of these 2 studies, 1 was solely GC (n = 127), while the other was both GC and OC (n = 177)[149,171]. Based on the Newcastle Ottawa score, the authors determined that both of these papers were of high quality. Both of these studies demonstrated a statistically significant association between HIF2-α and OS on univariate analysis but not multivariate. In the pooled analysis, the authors conclude that high HIF2-α expression was associated with a lower OS.
While there is a non-insignificant aggregate of clinical evidence denoting a statistically significant association between endogenous markers of tumour oxygenation and clinical outcomes, the heterogeneity in study methods and contrasting results ultimately indicates a need for more prospective research with greater adherence to the standardization of reporting. The REMARK recommendations for tumour marker prognostic studies published by the Equator Network lay out a checklist for researchers to improve both quality and transparency in research[172]. The wealth of data as discussed above, demonstrating the correlation between outcomes or treatment resistance and tumour hypoxia further illustrates the importance of the development and clinical implementation of new techniques in measuring tumour hypoxia such as non-invasive imaging[148,149,151,170,171].
Hypoxic areas of the TME inherently suffer from poor perfusion and disorganized vasculature and this has been one of the primary limitations to systemically administered therapeutics[173]. Nevertheless, a number of agents have been tested in clinical studies. Hypoxia-targeted therapies mainly consist of bioreductive prodrugs (hypoxia-activated prodrugs) but molecularly targeted agents that inhibit effectors in hypoxia-responsive pathways such as HIF1-α target genes or receptor tyrosine kinases like the VEGF receptor could be grouped here as well[173].
Bioreductive agents such as tirapazamine work in a similar manner to exogenous markers of hypoxia; they undergo chemical modification in hypoxic cells resulting in hypoxia-selective cytotoxicity. The bioreductive alkylating agent apaziquone demonstrated efficacy as a first-line agent in early clinical studies of bladder cancer but in a phase II study in 20 patients with GC, there was no clinical benefit[174,175]. In a preclinical murine model of OSCC and OAC, administration of the bioreductive prodrug evofosfamide was shown to delay tumour growth in combination with radiotherapy vs radiotherapy alone[176]. This came with the added benefit of no additional toxicity. As of the time of writing, there have been no clinical trials investigating the potential use of evofosfamide or other bioreductive prodrugs in OC and although the efficacy of these agents has largely been disappointing as first-line treatment in other cancer types, they may potentially improve sensitivity when used in combination with conventional chemoradiation.
The myriad of components that comprise the TME and the effects imposed on them by oxygen deprivation ensures that researchers have yet to scratch the surface in disentangling the key processes amenable to overcoming treatment-refractory disease and prognostication. Hypoxia plays a role in promoting immunosuppressive cells and subverting anti-tumour immune responses within the TME. Hypoxia also promotes the additional hallmarks of cancer including inflammation, angiogenesis, and reprogramming of metabolism. The intricate nature of these hypoxia-mediated effects is very complex and further research is required to elucidate the mechanisms as they pertain to GOC. Standardization of methodology in hypoxia focused basic research and clinical reporting would be conducive to driving this area forward. This deeper understanding will hopefully reveal novel therapeutic targets to control disease progression in GOC but currently, this remains out of reach. However, hypoxia as a clinical marker to stratify patients into certain treatment pathways or aid prognosis is something that is firmly within our grasp.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang ZK S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 2. | Mazure NM, Chen EY, Yeh P, Laderoute KR, Giaccia AJ. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436-3440. [PubMed] |
| 3. | Rofstad EK, Rasmussen H, Galappathi K, Mathiesen B, Nilsen K, Graff BA. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase-type plasminogen activator receptor. Cancer Res. 2002;62:1847-1853. [PubMed] |
| 4. | Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1015] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 6. | Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1568] [Cited by in RCA: 1536] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1548] [Cited by in RCA: 1478] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Kolstad P. Intercapillary distance, oxygen tension and local recurrence in cervix cancer. Scand J Clin Lab Invest Suppl. 1968;106:145-157. [PubMed] |
| 9. | Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015; 149: 302-17. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 10. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1027] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 11. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2490] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 12. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 13. | Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 14. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1328] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 15. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 16. | Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 842] [Cited by in RCA: 1402] [Article Influence: 140.2] [Reference Citation Analysis (1)] |
| 18. | Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1691] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 19. | Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 958] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 20. | Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 525] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 21. | Belli C, Trapani D, Viale G, D'Amico P, Duso BA, Della Vigna P, Orsi F, Curigliano G. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 22. | Gillies RJ, Gatenby RA. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev. 2007;26:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4726] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 24. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2798] [Cited by in RCA: 2923] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 25. | Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1036] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 26. | Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2073] [Cited by in RCA: 2458] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 27. | Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4121] [Cited by in RCA: 4312] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 28. | Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1923] [Cited by in RCA: 1819] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 29. | Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1342] [Cited by in RCA: 1400] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 30. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47168] [Article Influence: 3369.1] [Reference Citation Analysis (5)] |
| 31. | Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 32. | Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425-1430. [PubMed] |
| 33. | Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol. 2010;30:4901-4921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 578] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 35. | van Uden P, Kenneth NS, Webster R, Müller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1β by NF-κB. PLoS Genet. 2011;7:e1001285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1563] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 37. | Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci USA. 2013;110:18351-18352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 436] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 39. | Picardo SL, Maher SG, O'Sullivan JN, Reynolds JV. Barrett's to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig Surg. 2012;29:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | O'Sullivan KE, Phelan JJ, O'Hanlon C, Lysaght J, O'Sullivan JN, Reynolds JV. The role of inflammation in cancer of the esophagus. Expert Rev Gastroenterol Hepatol. 2014;8:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 42. | Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST, Schoenberg JB, West AB, Rotterdam H, Fraumeni JF Jr, Blot WJ. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97-102. [PubMed] |
| 43. | Ling FC, Khochfar J, Baldus SE, Brabender J, Drebber U, Bollschweiler E, Hoelscher AH, Schneider PM. HIF-1alpha protein expression is associated with the environmental inflammatory reaction in Barrett's metaplasia. Dis Esophagus. 2009;22:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Witz IP. The tumor microenvironment: the making of a paradigm. Cancer Microenviron. 2009;2 Suppl 1:9-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 544] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 47. | Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem. 1996;271:15117-15123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Griffiths EA, Pritchard SA, McGrath SM, Valentine HR, Price PM, Welch IM, West CM. Increasing expression of hypoxia-inducible proteins in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer. 2007;96:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Zhang S, Li M, Zhang D, Xu S, Wang X, Liu Z, Zhao X, Sun B. Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Lab Invest. 2009;89:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, Tran MG, Sciot R, Hauben E, Hogendoorn PC, Delattre O, Maxwell PH, Hendrix MJ, Griffioen AW. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65:11520-11528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, Lv Y. Role of Twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Sun B, Zhang D, Zhang S, Zhang W, Guo H, Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 54. | Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 55. | Tang NN, Zhu H, Zhang HJ, Zhang WF, Jin HL, Wang L, Wang P, He GJ, Hao B, Shi RH. HIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World J Gastroenterol. 2014;20:17894-17904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Chai DM, Bao ZQ, Hu JG, Ma L, Feng ZZ, Tao YS. Vasculogenic mimicry and aberrant expression of HIF-lα/E-cad are associated with worse prognosis of esophageal squamous cell carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2013;33:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Mao Y, Wang Y, Dong L, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, Li X, Fu Z. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 58. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 3607] [Article Influence: 450.9] [Reference Citation Analysis (0)] |
| 59. | Power R, Lowery MA, Reynolds JV, Dunne MR. The Cancer-Immune Set Point in Oesophageal Cancer. Front Oncol. 2020;10:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 982] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 61. | Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 62. | Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 63. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5331] [Article Influence: 333.2] [Reference Citation Analysis (0)] |
| 64. | Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 958] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 65. | Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, Chen WC, Lee KD. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716-8728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 66. | Ambler DR, Fletcher NM, Diamond MP, Saed GM. Effects of hypoxia on the expression of inflammatory markers IL-6 and TNF-a in human normal peritoneal and adhesion fibroblasts. Syst Biol Reprod Med. 2012;58:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439-2453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 948] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 68. | Poh AR, Ernst M. Targeting Macrophages in Cancer: From Bench to Bedside. Front Oncol. 2018;8:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 384] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 69. | Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 545] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 70. | Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672-3679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 71. | Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 679] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 72. | Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 623] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 74. | Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1459] [Cited by in RCA: 1478] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 75. | Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3122] [Cited by in RCA: 3933] [Article Influence: 262.2] [Reference Citation Analysis (0)] |
| 76. | Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1051] [Cited by in RCA: 1005] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 77. | Li J, Xie Y, Wang X, Li F, Li S, Li M, Peng H, Yang L, Liu C, Pang L, Zou H, Zhao J, Qi Y, Cao Y, Hu J. Prognostic impact of tumor-associated macrophage infiltration in esophageal cancer: a meta-analysis. Future Oncol. 2019;15:2303-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Yagi T, Baba Y, Okadome K, Kiyozumi Y, Hiyoshi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Komohara Y, Baba H. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur J Cancer. 2019;111:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 79. | Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor beta-1. Ann Surg Oncol. 1998;5:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci USA. 1995;92:4606-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Zhang WJ, Chen C, Zhou ZH, Gao ST, Tee TJ, Yang LQ, Xu YY, Pang TH, Xu XY, Sun Q, Feng M, Wang H, Lu CL, Wu GZ, Wu S, Guan WX, Xu GF. Hypoxia-inducible factor-1 alpha Correlates with Tumor-Associated Macrophages Infiltration, Influences Survival of Gastric Cancer Patients. J Cancer. 2017;8:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Osinsky S, Bubnovskaya L, Ganusevich I, Kovelskaya A, Gumenyuk L, Olijnichenko G, Merentsev S. Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: interaction and impact on survival. Clin Transl Oncol. 2011;13:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Seven to Gilead: "Eat me". Nat Biotechnol. 2020;38:389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Zhao CL, Yu S, Wang SH, Li SG, Wang ZJ, Han SN. Characterization of cluster of differentiation 47 expression and its potential as a therapeutic target in esophageal squamous cell cancer. Oncol Lett. 2018;15:2017-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Tao H, Qian P, Wang F, Yu H, Guo Y. Targeting CD47 Enhances the Efficacy of Anti-PD-1 and CTLA-4 in an Esophageal Squamous Cell Cancer Preclinical Model. Oncol Res. 2017;25:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 86. | Suzuki S, Yokobori T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Caligiuri MA. Human natural killer cells. Blood. 2008;112:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 88. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2883] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 89. | Siemens DR, Hu N, Sheikhi AK, Chung E, Frederiksen LJ, Pross H, Graham CH. Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide. Cancer Res. 2008;68:4746-4753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 91. | Yun S, Lee SH, Yoon SR, Myung PK, Choi I. Oxygen tension regulates NK cells differentiation from hematopoietic stem cells in vitro. Immunol Lett. 2011;137:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Xu B, Chen L, Li J, Zheng X, Shi L, Wu C, Jiang J. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904-74916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 93. | Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, Peng J, Gao L, Liang X, Ma C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143-6153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 94. | Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao S, Qi Y, Zhang Y, Huang L. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med. 2019;17:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Wang Z, Zhu J, Gu H, Yuan Y, Zhang B, Zhu D, Zhou J, Zhu Y, Chen W. The Clinical Significance of Abnormal Tim-3 Expression on NK Cells from Patients with Gastric Cancer. Immunol Invest. 2015;44:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4:36-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 97. | Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol. 2011;2011:430394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 98. | Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 99. | Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Elia AR, Cappello P, Puppo M, Fraone T, Vanni C, Eva A, Musso T, Novelli F, Varesio L, Giovarelli M. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84:1472-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 101. | Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723-3734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 103. | Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, Koritzinsky M, Wouters BG. Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126-6137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 104. | Liao X, Chen Y, Liu D, Li F, Li X, Jia W. High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Int J Mol Sci. 2015;16:17655-17667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Somja J, Demoulin S, Roncarati P, Herfs M, Bletard N, Delvenne P, Hubert P. Dendritic cells in Barrett's esophagus carcinogenesis: an inadequate microenvironment for antitumor immunity? Am J Pathol. 2013;182:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | MartIn-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 692] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 107. | Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 108. | Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784-E2793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 446] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 109. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL, Shen LS, Xu D. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 111. | Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 112. | Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482-497. [PubMed] |
| 113. | Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed). 2010;15:166-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 558] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 114. | Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 725] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 115. | Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M, Uzoho C, Primrose JN, Blaydes JP, Thomas GJ. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 2015;235:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 116. | Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J Biol Chem. 2011;286:10495-10504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 117. | Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123-10128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 765] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 118. | Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 119. | Wu X, Qiao B, Liu Q, Zhang W. Upregulation of extracellular matrix metalloproteinase inducer promotes hypoxia-induced epithelial-mesenchymal transition in esophageal cancer. Mol Med Rep. 2015;12:7419-7424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11818] [Article Influence: 738.6] [Reference Citation Analysis (0)] |
| 121. | Nobel Prize Outreach. The Nobel Prize in Physiology or Medicine 1931. [cited 24 February 2021]. In: Nobel Prize Outreach [Internet]. Available from: https://www.nobelprize.org/prizes/medicine/1931/summary/. |
| 122. | Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 522] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 123. | Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118:3835-3837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 124. | Isidoro A, Martínez M, Fernández PL, Ortega AD, Santamaría G, Chamorro M, Reed JC, Cuezva JM. Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J. 2004;378:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 125. | Lynam-Lennon N, Maher SG, Maguire A, Phelan J, Muldoon C, Reynolds JV, O'Sullivan J. Altered mitochondrial function and energy metabolism is associated with a radioresistant phenotype in oesophageal adenocarcinoma. PLoS One. 2014;9:e100738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 898] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 127. | Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15:2336-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 128. | Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 590] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 129. | Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J Neurochem. 1987;49:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 331] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 130. | Ballarín M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 131. | Zetterström T, Vernet L, Ungerstedt U, Tossman U, Jonzon B, Fredholm BB. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett. 1982;29:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 272] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 132. | Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 566] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 133. | Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602-2605. [PubMed] |
| 134. | Vaupel P, Mayer A. Hypoxia-Driven Adenosine Accumulation: A Crucial Microenvironmental Factor Promoting Tumor Progression. Adv Exp Med Biol. 2016;876:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 135. | Ohta A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol. 2016;7:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 136. | Chambers AM, Matosevic S. Immunometabolic Dysfunction of Natural Killer Cells Mediated by the Hypoxia-CD73 Axis in Solid Tumors. Front Mol Biosci. 2019;6:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 137. | Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 360] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 138. | Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 139. | Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 140. | Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, Thayer M, Rodig S, Kutok JL, Jackson EK, Karger B, Podack ER, Ohta A, Sitkovsky MV. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7:277ra30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 141. | Wang MX, Ren LM, Shan BE. Inhibitory effects of extracellular adenosine triphosphate on growth of esophageal carcinoma cells. World J Gastroenterol. 2005;11:5915-5919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 142. | Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 143. | Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol Res. 2017;5:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 396] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 144. | Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, Chapman W, Levin W, Manchul L, Keane TJ, Hill RP. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 443] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 145. | Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 728] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 146. | Olive PL, Banáth JP, Aquino-Parsons C. Measuring hypoxia in solid tumours--is there a gold standard? Acta Oncol. 2001;40:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 147. | Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P. Acute vs chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys. 2011;80:965-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 148. | Luo D, Liu H, Lin D, Lian K, Ren H. The Clinicopathologic and Prognostic Value of Hypoxia-Inducible Factor-2α in Cancer Patients: A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev. 2019;28:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Tong WW, Tong GH, Chen XX, Zheng HC, Wang YZ. HIF2α is associated with poor prognosis and affects the expression levels of survivin and cyclin D1 in gastric carcinoma. Int J Oncol. 2015;46:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 150. | Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, Nafteux P, Penninckx F, Geboes K, Lerut T, Ectors N. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 151. | Munipalle PC, Viswanath YK, Davis PA, Scoones D. Prognostic value of hypoxia inducible factor 1α in esophageal squamous cell carcinoma. Dis Esophagus. 2011;24:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 152. | Peerlings J, Van De Voorde L, Mitea C, Larue R, Yaromina A, Sandeleanu S, Spiegelberg L, Dubois L, Lambin P, Mottaghy FM. Hypoxia and hypoxia response-associated molecular markers in esophageal cancer: A systematic review. Methods. 2017;130:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 153. | Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. 2010;102:428-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 154. | Harris BH, Barberis A, West CM, Buffa FM. Gene Expression Signatures as Biomarkers of Tumour Hypoxia. Clin Oncol (R Coll Radiol). 2015;27:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 155. | Fox NS, Starmans MH, Haider S, Lambin P, Boutros PC. Ensemble analyses improve signatures of tumour hypoxia and reveal inter-platform differences. BMC Bioinformatics. 2014;15:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 156. | Ye Y, Hu Q, Chen H, Liang K, Yuan Y, Xiang Y, Ruan H, Zhang Z, Song A, Zhang H, Liu L, Diao L, Lou Y, Zhou B, Wang L, Zhou S, Gao J, Jonasch E, Lin SH, Xia Y, Lin C, Yang L, Mills GB, Liang H, Han L. Characterization of Hypoxia-associated Molecular Features to Aid Hypoxia-Targeted Therapy. Nat Metab. 2019;1:431-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 157. | Rofstad EK, Sundfør K, Lyng H, Tropé CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 158. | Kato Y, Yashiro M, Fuyuhiro Y, Kashiwagi S, Matsuoka J, Hirakawa T, Noda S, Aomatsu N, Hasegawa T, Matsuzaki T, Sawada T, Ohira M, Hirakawa K. Effects of acute and chronic hypoxia on the radiosensitivity of gastric and esophageal cancer cells. Anticancer Res. 2011;31:3369-3375. [PubMed] |
| 159. | Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, Cramer T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5:e12038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 160. | Hammond EM, Giaccia AJ. Hypoxia-inducible factor-1 and p53: friends, acquaintances, or strangers? Clin Cancer Res. 2006;12:5007-5009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 161. | Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 162. | Shi WJ, Gao JB. Molecular mechanisms of chemoresistance in gastric cancer. World J Gastrointest Oncol. 2016;8:673-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 163. | Xu HW, Xu L, Hao JH, Qin CY, Liu H. Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res. 2010;38:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 164. | Yue J, Yang Y, Cabrera AR, Sun X, Zhao S, Xie P, Zheng J, Ma L, Fu Z, Yu J. Measuring tumor hypoxia with ¹⁸F-FETNIM PET in esophageal squamous cell carcinoma: a pilot clinical study. Dis Esophagus. 2012;25:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, Domarkas J, Lord S, Baldry C, Gilbert FJ. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112:238-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 166. | Melsens E, De Vlieghere E, Descamps B, Vanhove C, Kersemans K, De Vos F, Goethals I, Brans B, De Wever O, Ceelen W, Pattyn P. Hypoxia imaging with 18F-FAZA PET/CT predicts radiotherapy response in esophageal adenocarcinoma xenografts. Radiat Oncol. 2018;13:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Klaassen R, Bennink RJ, van Tienhoven G, Bijlsma MF, Besselink MG, van Berge Henegouwen MI, Wilmink JW, Nederveen AJ, Windhorst AD, Hulshof MC, van Laarhoven HW. Feasibility and repeatability of PET with the hypoxia tracer [(18)F]HX4 in oesophageal and pancreatic cancer. Radiother Oncol. 2015;116:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 168. | Pastorekova S, Gillies RJ. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019;38:65-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 291] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 169. | Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 170. | Birner P, Jesch B, Friedrich J, Riegler M, Zacherl J, Hejna M, Wrba F, Schultheis A, Schoppmann SF. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol. 2011;18:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 171. | Griffiths EA, Pritchard SA, Valentine HR, Whitchelo N, Bishop PW, Ebert MP, Price PM, Welch IM, West CM. Hypoxia-inducible factor-1alpha expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastro-oesophageal adenocarcinomas. Br J Cancer. 2007;96:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 172. | Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J Natl Cancer Inst. 2018;110:803-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 173. | Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2384] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 174. | Phillips RM, Hendriks HR, Sweeney JB, Reddy G, Peters GJ. Efficacy, pharmacokinetic and pharmacodynamic evaluation of apaziquone in the treatment of non-muscle invasive bladder cancer. Expert Opin Drug Metab Toxicol. 2017;13:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 175. | Dirix LY, Tonnesen F, Cassidy J, Epelbaum R, ten Bokkel Huinink WW, Pavlidis N, Sorio R, Gamucci T, Wolff I, Te Velde A, Lan J, Verweij J. EO9 phase II study in advanced breast, gastric, pancreatic and colorectal carcinoma by the EORTC Early Clinical Studies Group. Eur J Cancer. 1996;32A:2019-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 176. | Spiegelberg L, van Hoof SJ, Biemans R, Lieuwes NG, Marcus D, Niemans R, Theys J, Yaromina A, Lambin P, Verhaegen F, Dubois LJ. Evofosfamide sensitizes esophageal carcinomas to radiation without increasing normal tissue toxicity. Radiother Oncol. 2019;141:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 177. | R Core Team. R: A Language and Environment for Statistical Computing. [cited 24 February 2021]. In: The R Foundation [Internet]. 2019; Available from: https://www.r-project.org/. |
| 178. | Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci. 2012;19:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 179. | Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 625] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 180. | Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587-4602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 181. | Hammond EM, Asselin MC, Forster D, O'Connor JP, Senra JM, Williams KJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol). 2014;26:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 182. | Rademakers SE, Span PN, Kaanders JH, Sweep FC, van der Kogel AJ, Bussink J. Molecular aspects of tumour hypoxia. Mol Oncol. 2008;2:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 183. | Le QT, Courter D. Clinical biomarkers for hypoxia targeting. Cancer Metastasis Rev. 2008;27:351-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |