Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2219
Peer-review started: May 31, 2021
First decision: June 26, 2021
Revised: July 15, 2021
Accepted: November 5, 2021
Article in press: November 5, 2021
Published online: December 15, 2021
Processing time: 197 Days and 16.3 Hours
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer and one of the major causes of cancer-related death. The development of specific non-invasive or diagnostic markers from blood, urine and feces may represent a valuable tool for detecting HCC at an early stage. Biomarkers are considered novel potential targets for therapeutic intervention. It helps in the prediction of prognosis or recurrence of HCC, and also assist in the selection of appropriate treatment modality. We summarize the most relevant existing data about various biomarkers that play a key role in the progression of HCC.
Core Tip: Hepatocellular carcinoma (HCC) ranks fourth among the leading causes of cancer-related mortality. The development of specific noninvasive or diagnostic markers from blood, urine and feces may represent a valuable tool for detecting HCC at an early stage. Biomarkers help in the prediction of prognosis or recurrence, selection of appropriate treatment modality, and signify novel potential targets for therapeutic interventions. We summarize the most relevant existing data about various biomarkers involved in the progression of HCC.
- Citation: Nath LR, Murali M, Nair B. Critical biomarkers of hepatocellular carcinoma in body fluids and gut microbiota. World J Gastrointest Oncol 2021; 13(12): 2219-2222
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2219.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2219
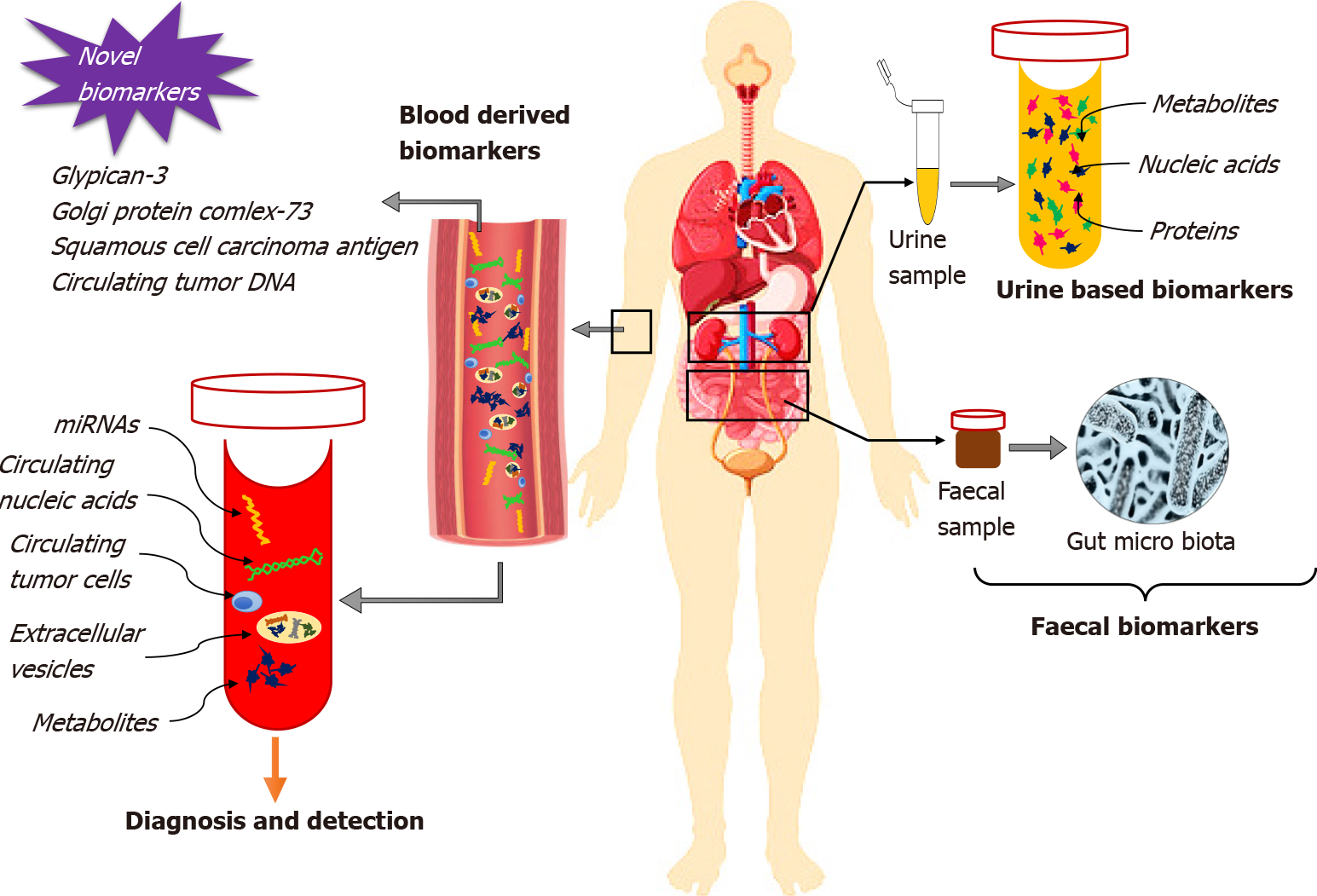
We were interested to read the review reported by Guan et al[1] that clearly emphasized the substantial role of biomarkers from different body fluids such as blood, urine and feces for the early detection of primary and recurrent hepatocellular carcinoma (HCC). From the study reports, detection of biomarkers through screening of body fluids or feces is regarded as beneficial due to the quick and easy extraction procedures, stability, proper time management, cost-effectiveness and accessibility in comparison with conventional screening methods. The review highlights the clinical significance of several diagnostic biomarkers of HCC, including proteins, metabolites, circulating nucleic acids, circulating tumor cells (CTCs), extracellular vesicles (EVs), and gut microbiota from blood, urine and feces.
A large pool of evidence suggests the presence of elevated serum blood levels of bilirubin, albumin, α-fetoprotein (AFP), Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) and des-γ-carboxy prothrombin (DCP) at the time of diagnosis of HCC. These biomarkers exhibit a close relation with HCC staging and prognosis of overall survival and disease-free survival. Elevated levels of AFP in cases of liver injury above the reference range (400-500 ng/mL) can be considered crucial for the prognosis of HCC. AFP-L3 possesses better sensitivity but low specificity for the early detection of HCC. To its expression in small tumors (< 2 cm in diameter) of aggressive types, the prognosis of early-stage HCC is relevant if the AFP-L3 level is greater than 10% in comparison with AFP. DCP can be regarded as an excellent prognostic biomarker since it can differentiate nonmalignant cirrhosis and HCC with a specificity of 93% and sensitivity of 92% at a cut-off value of about 150 mAU/mL[2]. With disease pro
The progression of HCC involves invasion, migration, proliferation and metastasis. Studies have shown that drug resistance is mainly mediated through the functional activation of miRNAs. Clinicians can predict the overall survival of patients based on the expression of miRNA. Single miRNAs like miR-130b, miR-150, miR-182, miR-215 and miR-96 are considered key candidates among all miRNAs but the use of multiple miRNAs as promising biomarkers for the prediction of early as well as recurring HCC is recent[5]. CTCs play a significant role in the prediction of HCC recurrence, prognostic evaluation for surveillance, and promotion of suitable adjuvant therapy. CTCs are generally categorized as a small subpopulation of malignant cells secreted from primary malignant tissue and they are usually expressed at the aggressive malignancy stage; therefore, liquid biopsy of CTCs facilitates timely diagnosis of HCC[6]. Another important category of biomarker with a functional role in the prediction of HCC progression is EVs. Increased circulating levels of EVs have contributed to poor survival and disease-free survival in HCC patients. Despite their high capability of being absorbed into host cells, EVs are considered an efficient tool for targeted approaches. This is by the incorporation of therapeutic agents to improve therapeutic efficacy and reduce side effects. The incorporation of sodium/iodide symporter protein to EVs has been used as one of the systemic targeted approaches to cancer treatment with the promotion of cytotoxicity and radioiodine therapy[7].
Another potential category of biomarkers for HCC are urine-based. Among the biomarkers, higher levels of 8-oxodeoxyguanosine improve DNA repair mechanisms by overcoming oxidative DNA damage with a reduction in risk of developing HCC. Enhanced levels of 15-F2t-isoprostane are also correlated with the risks of HCC. Urinary proteins such as urinary DJ-1, chromatin assembly factor-1, heat shock protein 60 and orosomucoid, and metabolites such as ethanolamine, lactic acid, aconitinic acid, phenylalanine and ribose were found to be effective predictors for early HCC recurrence. Additionally, the overexpression of urinary trypsin inhibitor in HCC was revealed to be a risk factor for HCC recurrence[1]. In a study reported by Hann et al[8], detection of urinary markers such as TP53m, mSGTP and mRASSF1A were potential tools for the early detection of HCC recurrence (Figure 1).
Inflammation significantly decreases the expression of beneficial microflora which, in turn, enhances the risk of liver malignancy by accumulating harmful compounds. Translocated bacterial products such as lipopolysaccharides, peptidoglycans, muramyl-dipeptides and bacterial DNA from the infectious stage of the gut stimulate an inflammatory cascade by activation of signaling through Toll-like receptors (TLRs). Stimulation of interleukin-6, either directly or via the JAK/STAT3 pathway forces the gut microbiota to induce proliferation and progression of HCC. Gut microbiota can stimulate the generation of reactive free radical oxygen species indirectly via small molecular motifs derived from a pathogenic class of microbes by the activation of NADPH-oxidase (NOX1–NOX4). Microbial imbalance and enhancement of inflammation are directly correlated with fluctuating redox status. Modulation of farnesoid X receptor activation by gut microbiota enhances bile acid accumulation in the liver. This leads to damage of hepatocyte plasma membranes, resulting in activation of an inflammatory response and production of reactive oxygen species through stimulating the MAPK pathway. As a result, the secretion of inflammatory cytokines via the nuclear factor-B pathway is increased by induction of proliferation and immortalization of HCC cells directly or via the JAK/STAT3 pathway. Gut microbiota can exhaust the surveillance of the immune system within the tumor microenvironment of HCC through macrophage polarization via the activation of TLRs. This results in further diversification and progression of the tumor[9]. Additionally, several other biomarkers namely, glypican-3, Golgi protein complex-73, squamous cell carcinoma antigen and circulating tumor DNA are useful for early diagnosis of HCC, and might be clinically validated in the near future[10].
The supporting evidence gives an insight into novel biomarkers for early prediction and prevention of HCC. HCC accounts for almost 90% of primary liver malignancies and has a poor prognosis due to rapid metastasis and multidrug resistance. Diagnosis of HCC at an early stage is important for overcoming the hurdles associated with the disease[11]. To conclude, it is important to identify and develop promising biomarkers for early diagnosis and prognosis as well as therapy of HCC.
We thank Dr. Abraham R, JHSPH, Baltimore for the proofreading and Ms. Diers AR, University of Florida, United States for the language editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: European Association for Cancer Research, No. EACR26534.
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Li X, Li Y, Sitkin S, Xu X S-Editor: Gao CC L-Editor: Kerr C P-Editor: Gao CC
| 1. | Guan MC, Ouyang W, Wang MD, Liang L, Li N, Fu TT, Shen F, Lau WY, Xu QR, Huang DS, Zhu H, Yang T. Biomarkers for hepatocellular carcinoma based on body fluids and feces. World J Gastrointest Oncol. 2021;13:351-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Wang X, Zhang Y, Yang N, He H, Tao X, Kou C, Jiang J. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020;2020:5087643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Kim DJ, Cho EJ, Yu KS, Jang IJ, Yoon JH, Park T, Cho JY. Comprehensive Metabolomic Search for Biomarkers to Differentiate Early Stage Hepatocellular Carcinoma from Cirrhosis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 5. | Ning S, Liu H, Gao B, Wei W, Yang A, Li J, Zhang L. miR-155, miR-96 and miR-99a as potential diagnostic and prognostic tools for the clinical management of hepatocellular carcinoma. Oncol Lett. 2019;18:3381-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018;67:2204-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Costanzi E, Simioni C, Varano G, Brenna C, Conti I, Neri LM. The Role of Extracellular Vesicles as Shuttles of RNA and Their Clinical Significance as Biomarkers in Hepatocellular Carcinoma. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Hann HW, Jain S, Park G, Steffen JD, Song W, Su YH. Detection of urine DNA markers for monitoring recurrent hepatocellular carcinoma. Hepatoma Res. 2017;3:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Gupta H, Youn GS, Shin MJ, Suk KT. Role of Gut Microbiota in Hepatocarcinogenesis. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Pandyarajan V, Govalan R, Yang JD. Risk Factors and Biomarkers for Chronic Hepatitis B Associated Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 701] [Article Influence: 140.2] [Reference Citation Analysis (0)] |