Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2161
Peer-review started: May 12, 2021
First decision: July 14, 2021
Revised: July 25, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 15, 2021
Processing time: 216 Days and 14.2 Hours
Current tumor regression grade (TRG) evaluations are based on various systems which brings confusion for oncologists and pathologists when interpreting results. The recent six-tier system (JGCA2017-TRG) recommended by the Japanese Gastric Cancer Association (JGCA) is worth investigating, as four-tier TRG systems are favored in various parts of the world.
To compare the predictive accuracies of five published TRG systems.
Data were retrospectively collected from patients with locally advanced gastric cancer (LAGC) who underwent neoadjuvant chemotherapy followed by D2 Lymphadenectomy between January 2005 and January 2014 at our institution. Outcomes were overall survival (OS) and disease-free survival (DFS), which were evaluated separately using the following TRG systems: JGCA2017, JGCA, Becker, AJCC/CAP, and Mandard.
All five published TRG systems were independent predictors for OS and DFS. Concordance indices of the JGCA2017, JGCA, Becker, AJCC/CAP-TRG, and Mandard systems were 0.651/0.648 0.652/0.649, 0.693/0.695, 0.688/0.685, and 0.674/0.675 for OS and DFS, respectively. The four-tier Becker system showed the highest c-index, which was significantly greater than that of the six-tier JGCA2017 and five-tier JGCA systems (P < 0.05 in OS and DFS). When residual tumor percentages were reset as: “no residual tumor”, < 10%, < 100%, and “no respon
The newly introduced six-tier JGCA-TRG system cannot increase prognostic stratification. The four-tier Becker system is more suitable for LAGC patients. A population-based study is warranted to define the optimal criterion for TRG in LAGC patients.
Core Tip: Current Tumor regression grade (TRG) evaluations are based on various systems bringing confusion to oncologists and pathologists when interpreting results in similar clinical contexts. On the other hand, the recent six-tier system tumor regression grade (JGCA2017-TRG) recommended by Japanese Gastric Cancer Association (JGCA) is investigational. This is the first report of the use of the c-index to evaluate predictive accuracies of five published TRG systems in gastric cancer. With a satisfying sample size, our results gave clinicians a better understanding of the TRG, especially the residual tumor percentage, in gastric cancer and furthermore alleviates the oncologists and pathologist’s workload.
- Citation: Liu ZN, Wang YK, Zhang L, Jia YN, Fei S, Ying XJ, Zhang Y, Li SX, Sun Y, Li ZY, Ji JF. Comparison of tumor regression grading systems for locally advanced gastric adenocarcinoma after neoadjuvant chemotherapy. World J Gastrointest Oncol 2021; 13(12): 2161-2179
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2161.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2161
Although surgical resection is the mainstay therapy of locally advanced gastric cancer (LAGC), neoadjuvant chemotherapy (NACT) has now been widely adopted for LAGC in Europe and most recently in China due to the solid evidence that it reduces the risk of recurrence and improves overall survival[1-3]. Theoretical benefits of NACT are downstaging the primary tumor, increasing the R0 resection rate, and treatment of potential micrometastases.
The effects of NACT on the tumor can be histopathologically evaluated in sub
Currently, most pathologists favor four-tier TRG systems in gastrointestinal cancer. There are the Becker system and the American Joint Committee on Cancer (AJCC)/ College of American Pathologists (CAP) system, as these have superior inter-rater agreement with no loss of discriminatory ability[4,6]. In October 2017, the 15th Japanese Classification of Gastric Carcinoma proposed a new six-tier pathological regression evaluation for GC based on its previous Japanese Gastric Cancer Association (JGCA) TRG system[7]. This added the following sub-groupings of JGCA-TRG grade 2 (residual tumor 1%-33%): grade 2a (residual tumor 10%-33%) and 2b (residual tumor < 10%) according to the result of JCOG1004-A[8,9]. This new classification did not draw much attention in Western countries, only in East Asia[10]. However, as both the JGCA and AJCC/CAP criteria obtained good consistency in Chinese patients, exten
Therefore, the present study sought to validate the utility of the new JGCA-TRG system (JGCA2017-TRG). This was achieved by comparing JGCA2017-TRG with different TRG systems and exploring meaningful cutoff values of residual tumor percentage based on a current dataset comprising 413 LAGC patients who received D2 Lymphadenectomy following NACT.
Data were obtained from a retrospective database of all patients receiving NACT followed by curative gastrectomy at the Peking University Cancer Hospital and Institute (“The Institute”) from January 1, 2005 to January 1, 2014.
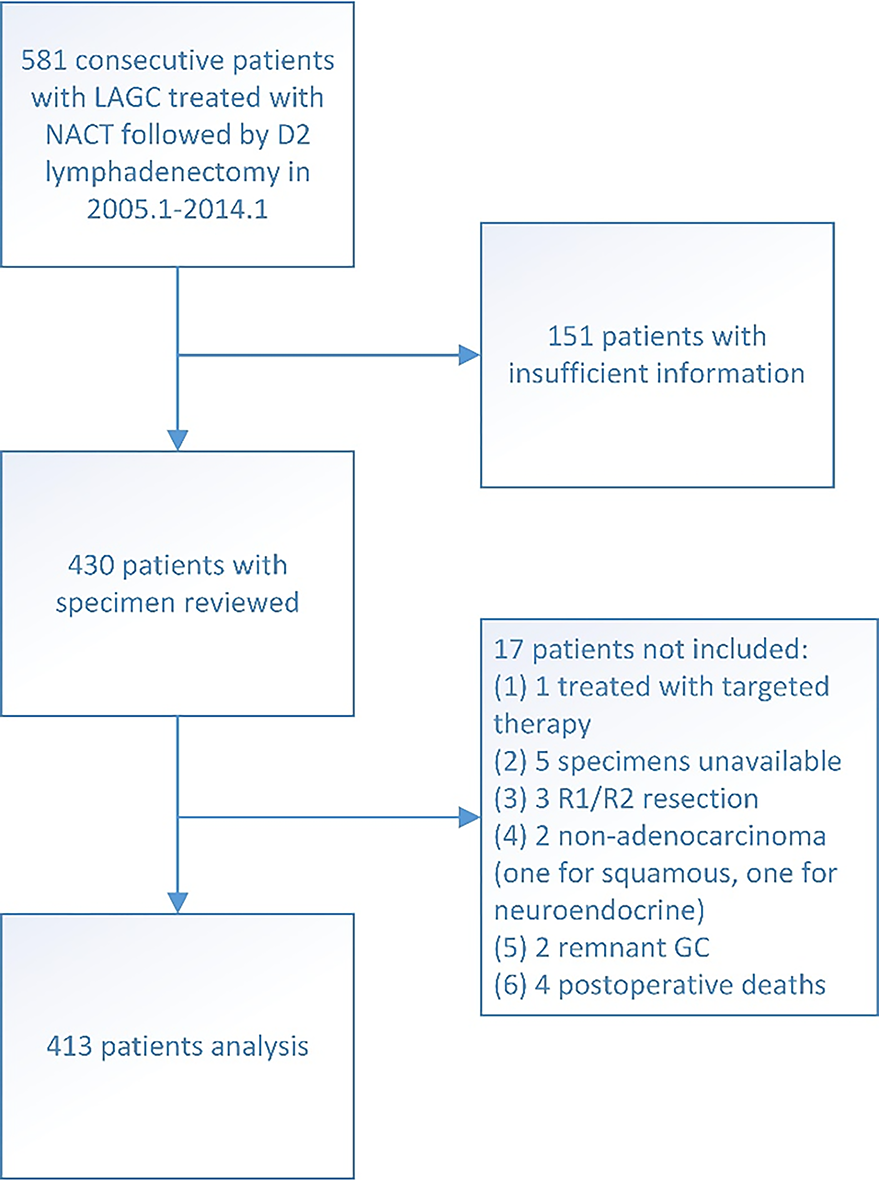
The inclusion criteria included: (1) Proven diagnosis of gastric adenocarcinoma by preoperative pathology; (2) No signs of distant metastasis at first visit; (3) Complete perioperative medical record and documentation of NACT in the Institute; and (4) Curative gastrectomy with D2 Lymph node resection performed at the Institute.
The exclusion criteria were as follows: (1) Insufficient record of clinicopathological information; (2) Patients who received radiotherapy or targeted therapy before surgery; (3) Specimen information was not available; (4) Patients with R1/R2 resection or suspected of having metastasis when surgery was performed; (5) Non-adenocarcinoma diagnosis based on postoperative histological findings (except for complete response cases); (6) Remnant gastric cancer; and (7) Died within 30 d post-surgery.
Except for eight patients with logistic reasons, e.g., poor economic status or severe adverse events, all patients received at least two cycles of chemotherapy. In summary, 364 patients received platin-based doublet regimens, 25 patients received Taxol-based doublet regimens, and 24 patients received Taxol-platin-based triplet regimens. Supplementary Table 1 describes the detailed dosing regimens.
To assess the influence of the treatment duration, three 14 d cycles of FOLFOX or POS were regarded as two 21 d cycles of treatment. Dosage reduction or withdrawal was applied in cases of severe adverse events during chemotherapy; this was determined by the clinician according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, as in our previous study[12]. After two to three chemotherapy cycles, the antitumor effect was evaluated using abdominal computed tomography (CT). In most cases, two or three alignment cycles were performed. The therapy was prematurely terminated in cases of disease progression. Otherwise, gastrectomy or continued NACT was considered after obtaining informed consent and approval from patients. Subtotal or total gastrectomy plus D2 Lymphadenectomy was performed according to the JGCA guideline[13].
The pathological preparation of the surgical specimens was commenced immediately after the operation. After recording the localization, measurement, and complete inclusion of visible tumor or suspected tumor areas, a surgeon identified the lymph node groups in the specimen. They were dissected and labeled separately from the main stomach specimen. Generally, the stomach tissue was fixed in 10% neutral buffered formalin overnight and then embedded in paraffin wax. Sections of 5 μm thickness were cut and stained with hematoxylin and eosin (H&E) for microscopic examination, all according to standard procedures. The histological patterns, degrees of differentiation, the extent of tumor invasion, number of regional lymph node metastases, and lymphovascular invasion (LVI), were recorded in each patient’s pathology report. This information was then integrated according to the 8th AJCC Cancer Staging Manual and World Health Organization pathologic classifications by two oncologists (Liu ZN and Wang YK)[14,15].
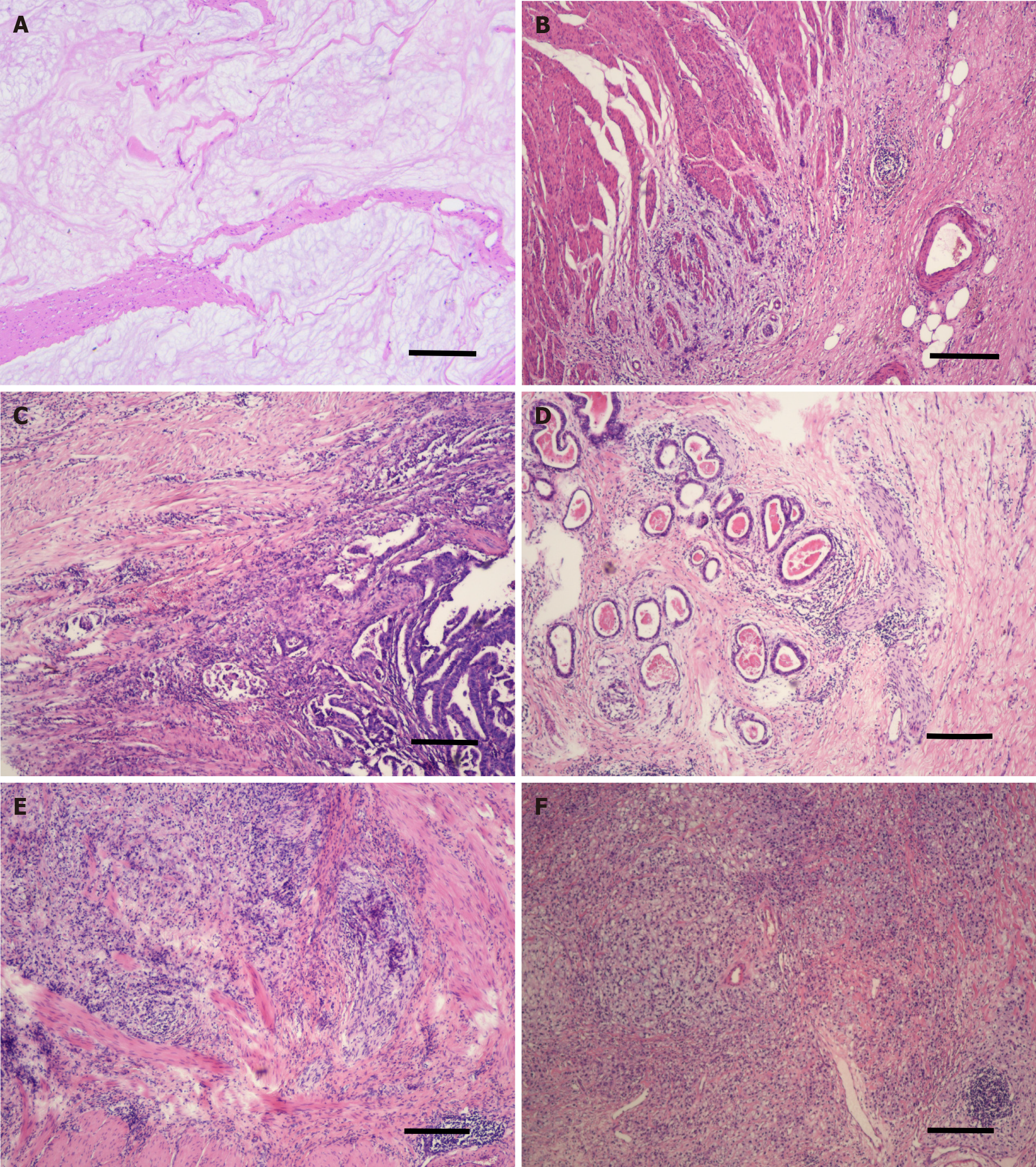
All normal sections were stained with H&E and preserved in paraffin. From December 2017, two designated pathologists (Zhang L and Sun Y) were responsible for reviewing the extent of tumor regression. All patients’ H&E slides were re-examined using bright-field fluorescence microscopy for discrimination between necrotic or heat-fixed tissue and viable tissue. The extent of regressive tumors was evaluated and recorded according to: (1) The amount of viable tumor vs fibrotic tissue, which ranged from a total lack of tumor regression to complete response with no viable tumor identified; and (2) The percentage of the viable residual tumor, which was calculated by dividing the viable residual tumor area by the total tumor area. Tumor regression grades were then allocated according to the JGCA2017, JGCA, Becker, AJCC/CAP, and Mandard systems (Table 1)[7,8,16-18]. As for the tumor regression grade, the JGCA2017 criteria example for each grade is shown in Figure 1. The review task ended in December 2019.
| TRG system | Description |
| JGCA/JGCA2017-TRG | |
| 0 | No response |
| 1a | 67%-99% residual tumor/tumor bed |
| 1b | 34%-66% residual tumor/tumor bed |
| 2/2a | 10%-33% residual tumor/tumor bed |
| /2b | < 10% residual tumor/tumor bed |
| 3 | Complete response |
| Becker-TRG | |
| 1a | Complete response |
| 1b | < 10% residual tumor/tumor bed |
| 2 | 10%-50% residual tumor/tumor bed |
| 3 | > 50% residual tumor/tumor bed |
| AJCC/CAP-TRG | |
| 0 | No residual tumor |
| 1 | Single cells or rare small groups of cancer cells |
| 2 | More than single cells or rare small groups of cancer cells with evident tumor regression |
| 3 | Extensive residual tumor or no response |
| Mandard-TRG | |
| 1 | No residual tumor |
| 2 | Rare residual tumor |
| 3 | Fibrosis outgrowing residual tumor |
| 4 | Residual tumor outgrowing fibrosis |
| 5 | No response |
In addition to histopathological features, other included patient characteristics were age, sex, body mass index (BMI), American Society of Anesthesiologists score (ASA), ECOG performance status, tumor location, tumor diameter (on short axis), type of resection, type of NACT regimens, complications grade by Clavien-Dindo classification, NACT cycles, survival time, and survival status[19]. The follow-up methods were described in our earlier study[20]. Disease-free survival (DFS) was calculated from the date of surgery to the date of recurrence or metastasis.
Continuous variables were summarized as the median (IQR) and were compared across groups using the Kruskal-Wallis test. Categorical variables were analyzed using the Chi-squared test. The relationships between clinical and pathological factors and long-term DFS and OS were assessed using univariate log-rank tests and a multivariate Cox proportional hazard model. Tumor or treatment characteristics that achieved a P value < 0.10 in univariate analysis were included in the multivariate analysis. The prognostic strength and the discrimination ability of each TRG system were assessed using the concordance index (c-index ± SE), with a concordance index of 1 indicating perfect prediction and 0.5 indicating no discrimination. The c-index was calculated and compared using the “survcomp” R package[21]. Testing for trends was based on various statistical hypotheses when necessary. For all analyses, P < 0.05 was considered to be statistically significant. Statistical analyses were performed using SE STATA (Stata Statistical Software, release 15.1; Stata Corp, College Station, TX, United States).
A total of 413 patients met the inclusion criteria and were included in this study (Figure 2). All achieved total tumor clearance (R0). The patients had a median age of 61 years (range 24-82) and were predominantly male (73.61%). Tumor localization was proximal (including esophagogastric junction Siewert III) in 166 cases, body in 51 cases, distal in 170 cases, and 26 patients had tumor involvement in the whole stomach (linitis plastica). Most patients received preoperative therapy of 5-Fu-based oxaliplatin doublet regimen (88.14%) and 105 patients did not receive adjuvant treatment after complete resection (25.42%). The demographic data of these patients are shown in Table 2, stratified by the JGCA2017-TRG system.
| Characteristics | n (%) |
| No. of patients | 413 |
| Age, median (IQR), yr | 61 (54-67) |
| BMI, median (IQR), (kg/m2) | 23.04 (20.83-25.10) |
| Male | 304 (73.61) |
| ASA score | |
| 1 | 102 (24.70) |
| 2 | 255 (61.74) |
| 3 | 56 (13.56) |
| ECOG | |
| 0 | 229 (55.45) |
| 1 | 168 (40.68) |
| 2 | 16 (3.87) |
| Location | |
| Upper | 166 (40.19) |
| Middle | 51 (12.35) |
| Lower | 170 (41.16) |
| Diffuse | 26 (6.30) |
| Diameter (cm) | 3.0 (1.5-4.0) |
| Differentiation | |
| Well | 27 (6.54) |
| Moderate | 176 (42.86) |
| Poor | 209 (50.61) |
| Mucinous or signet cell | 85 (20.58) |
| LVI | 132 (31.96) |
| Cycles of treatment | 2 (2-3) |
| ypT | |
| ypT0 | 37 (8.96) |
| ypT1 | 25 (6.05) |
| ypT2 | 55 (13.32) |
| ypT3 | 66 (15.98) |
| ypT4 | 230 (55.69) |
| ypN | |
| N0 | 169 (40.92) |
| N1 | 64 (15.50) |
| N2 | 70 (16.95) |
| N3 | 110 (26.63) |
| ypStage | |
| pCR | 32 (7.75) |
| I | 57 (13.80) |
| II | 116 (28.09) |
| III | 208 (50.36) |
| Total gastrectomy | 180 (43.58) |
| Regimen | |
| Platin-based | 364 (88.14) |
| Taxol-based | 25 (6.05) |
| Triplet | 24 (5.81) |
| Adjuvant chemotherapy | 308 (74.58) |
| Postoperative complications | |
| Grade 0-1 | 277 (67.07) |
| Grade 2 | 78 (18.89) |
| Grade 3-4 | 58 (14.04) |
| JGCA2017-TRG | |
| Grade 3 (no residual) | 36 (8.72) |
| Grade 2b (< 10%) | 39 (9.44) |
| Grade 2a (10%-33%) | 29 (7.02) |
| Grade 1b (34%-66%) | 78 (18.89) |
| Grade 1a (67%-99%) | 205 (49.64) |
| Grade 0 (no response) | 26 (6.30) |
| JGCA-TRG | |
| Grade 3 (no residual) | 36 (8.72) |
| Grade 2 (< 33%) | 68 (16.46) |
| Grade 1b (34%-66%) | 78 (18.89) |
| Grade 1a (67%-99%) | 205 (49.64) |
| Grade 0 (no response) | 26 (6.30) |
| Becker-TRG | |
| 1a (no residual) | 36 (8.72) |
| 1b (< 10%) | 39 (9.44) |
| 2 (10%-50%) | 65 (15.74) |
| 3 (> 50%) | 273 (66.10) |
| AJCC-TRG | |
| 0 (complete response) | 36 (8.72) |
| 1 (moderate response) | 48 (11.62) |
| 2 (minimal response) | 89 (21.55) |
| 3 (poor response) | 240 (58.11) |
| Mandard-TRG | |
| 1 (complete response) | 36 (8.72) |
| 2 (Fibrosis + scattered tumor cells) | 48 (11.62) |
| 3 (Fibrosis predominance + tumor cells) | 89 (21.55) |
| 4 (Tumor cells preponderance + fibrosis) | 214 (51.82) |
| 5 (No response) | 26 (6.30) |
According to the JGCA system, 26 cases were grade 0 (6.30%), 205 were grade 1a (49.64%), 78 were grade 1b, 68 were grade 2 (16.46%) including 29/39 (7.02%/9.44%) in grades 2a/2b according to the JGCA2017 classification, and 36 patients were grade 3 (8.72%; Table 2, Supplementary Table 2). Similarly, the subgroup frequencies according to the Becker, AJCC/CAP, and Mandard systems are presented in Supplementary Table 3-6, respectively. Significant differences were found in the ypT, ypN, ypTNM, and LVI stages in all five systems. The correlation coefficients of ypT were 0.619, 0.587, 0.662, 0.639, and 0.616 for the JGCA017, JGCA, Becker, AJCC/CAP and Mandard systems, respectively. On the other hand, no statistical significance was found between the NACT regimen and the TRG grade or between the duration of NACT and the TRG grade in any system.
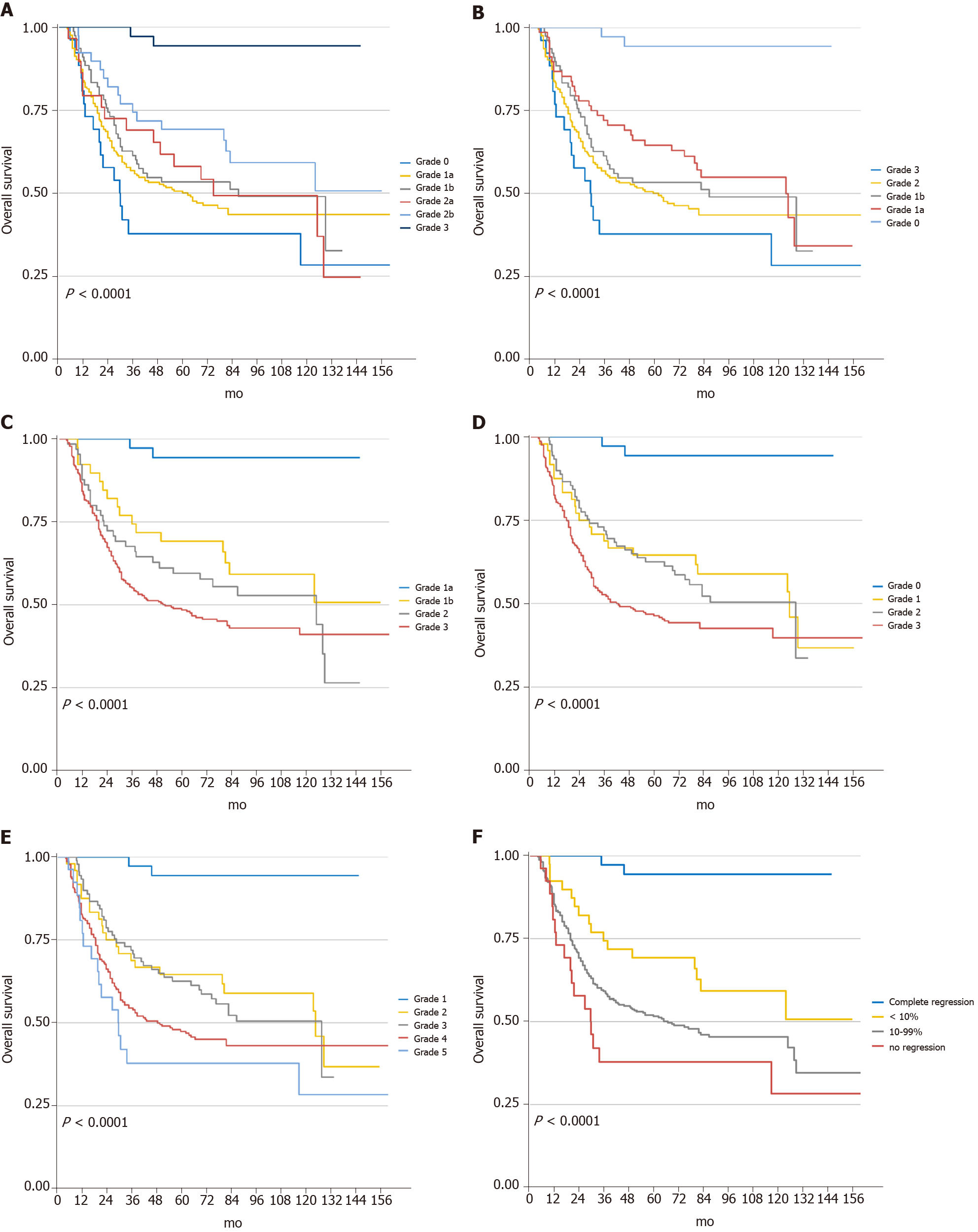
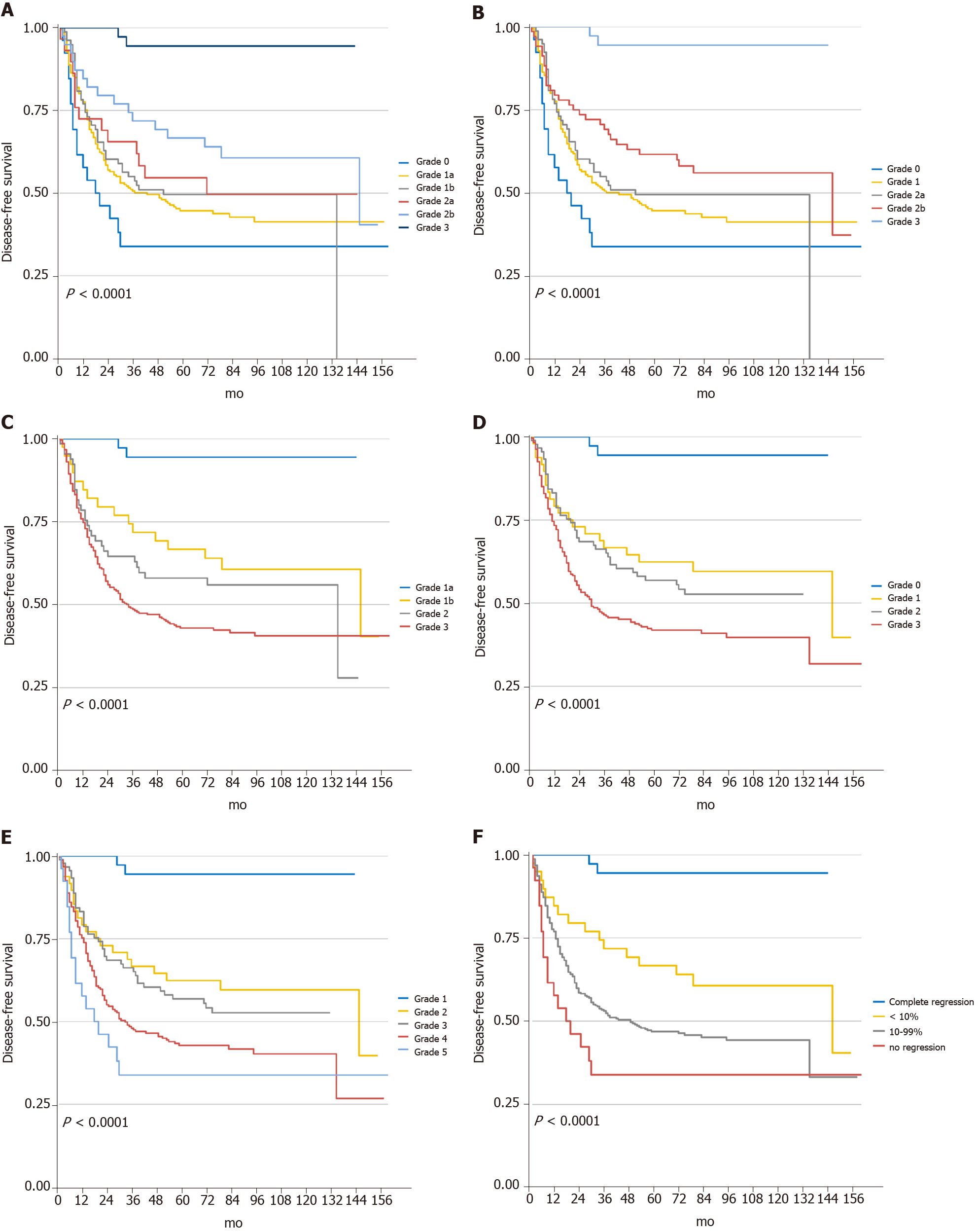
The median follow-up was at 62 mo, with an IQR of 4.5 to 210 mo. At the final follow-up, 209 patients had recurrence, and 200 died due to cancer. Kaplan-Meier curves for OS and DFS based on each system are presented in Figures 3 and 4. In the univariate analyses, all five regression classification systems had prognostic relevance (Table 3). Although all five systems revealed statistical trends towards an increase in the risk of OS and DFS (Ptrend < 0.001), JGCA2017 grade 2a showed a higher OS risk compared with grade 1b despite no statistical intergroup significance (HR: 1.06; 95%CI: 0.59-1.89; P = 0.855). The C-index for the six-tier JGCA2017, five-tier JGCA, four-tier Becker, four-tier AJCC/CAP, and five-tier Mandard systems was 0.651 ± 0.027, 0.652 ± 0.027, 0.693 ± 0.033, 0.688 ± 0.031, and 0.674 ± 0.028, respectively, for OS, and 0.648 ± 0.028, 0.649 ± 0.028, 0.695 ± 0.034, 0.685 ± 0.031, and 0.675 ± 0.028, respectively, for DFS. The four-tier Becker system had the highest c-index and was statistically significantly more accurate in predicting survival and recurrence than the six- or five-tier JGCA systems (Becker vs JGCA2017, P = 0.006 for OS, P = 0.002 for DFS; Becker vs JGCA, P = 0.007 for OS, P = 0.003 for DFS). The c-indices were comparable between the Becker and AJCC/CAP systems (P = 0.397 for OS and P = 0.273 for DFS), and between the Becker and Mandard systems (P = 0.148 for OS and P = 0.136 for DFS), while the predictive ability of the four-tier AJCC/CAP system was more accurate than the five-tier Mandard system for OS (P = 0.039) under similar evaluation principles.
| Variables | OS | DFS | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | ||||
| ≤ 65 | ||||
| > 65 | 1.12 (0.84-1.51) | 0.439 | 1.12 (0.84-1.49) | 0.4351 |
| BMI | ||||
| ≤ 23.9 | 1.39 (1.04-1.87) | 0.027 | 1.31 (0.98-1.74) | 0.065 |
| > 23.9 | ||||
| Gender | ||||
| Male | ||||
| Female | 1.14 (0.84-1.56) | 0.393 | 1.06 (0.78-1.45) | 0.690 |
| ASA score | ||||
| 1-2 | ||||
| 3 | 1.00 (0.67-1.49) | 0.993 | 0.97 (0.65-1.43) | 0.874 |
| ECOG | ||||
| 0 | ||||
| 1-2 | 1.29 (0.98-1.70) | 0.073 | 1.30 (0.99-1.71) | 0.056 |
| Location | ||||
| Diffuse vs Upper | 3.32 (2.07-5.31) | < 0.001 | 2.97 (1.86-4.73) | < 0.001 |
| Diffuse vs Middle | 2.63 (1.51-4.56) | < 0.001 | 2.28 (1.32-3.94) | 0.003 |
| Diffuse vs Lower | 3.94 (2.45-6.35) | < 0.001 | 3.52 (2.19-5.65) | < 0.001 |
| Diffuse | 1.00 | 1.00 | ||
| Diameter (cm) | ||||
| ≤ 5 | ||||
| > 5 | 2.79 (2.00-3.88) | < 0.001 | 2.99 (2.17-4.13) | < 0.001 |
| Differentiation | ||||
| Well-Moderate | ||||
| Poor | 1.43 (1.08-1.90) | 0.012 | 1.51 (1.15-1.99) | 0.003 |
| Histology | ||||
| Non-mucinous | ||||
| Mucinous or signet cell | 1.86 (1.39-2.53) | < 0.001 | 1.77 (1.31-2.40) | < 0.001 |
| Lymphovascular invasion | ||||
| No | ||||
| Yes | 2.75 (2.08-3.64) | < 0.001 | 2.91 (2.21-3.83) | < 0.001 |
| ypT | ||||
| ypT0-2 | ||||
| ypT3-4 | 3.54 (2.35-5.36) | < 0.001 | 3.66 (2.44-5.49) | < 0.001 |
| ypN | ||||
| ypN0 | ||||
| ypN+ | 3.50 (2.50-4.90) | < 0.001 | 3.59 (2.58-4.98) | < 0.001 |
| Resection type | ||||
| Subtotal | ||||
| Total | 1.79 (1.35-2.36) | < 0.001 | 1.74 (1.32-2.28) | < 0.001 |
| Cycle of NACT | ||||
| ≤ 2 | ||||
| > 2 | 1.18 (0.89-1.56) | 0.247 | 1.18 (0.90-1.55) | 0.233 |
| NACT regimen | ||||
| Platin-based | 1.00 | 1.00 | ||
| Paclitaxel-based | 1.10 (0.62-1.92) | 0.752 | 1.27 (0.75-2.15) | 0.373 |
| Triplet drug | 1.05 (0.59-1.89) | 0.862 | 1.03 (0.57-1.84) | 0.930 |
| Adjuvant chemotherapy | ||||
| Received | ||||
| Not received | 1.36 (1.00-1.85) | 0.050 | 1.18 (0.87-1.60) | 0.286 |
| Complications | ||||
| Clavien-dindo 0-2 | ||||
| Clavien-dindo 3-4 | 1.15 (0.78-1.69) | 0.491 | 1.11 (0.76-1.63) | 0.585 |
| JGCA2017-TRG | ||||
| Grade 3 (no residual) | 1.00 | 1.00 | ||
| Grade 2b (< 10%) | 8.97 (2.06-39.02) | 0.003 | 8.75 (2.01-38.09) | 0.004 |
| Grade 2a (10%-33%) | 13.55 (3.11-58.93) | 0.001 | 14.03 (3.23-61.06) | < 0.001 |
| Grade 1b (34%-66%) | 12.83 (3.10-53.18) | < 0.001 | 14.05 (3.40-58.09) | < 0.001 |
| Grade 1a (67%-99%) | 15.15 (3.74-61.42) | < 0.001 | 15.55 (3.84-62.97) | < 0.001 |
| Grade 0 (no response) | 20.24 (4.67-87.68) | < 0.001 | 21.15 (4.88-91.67) | < 0.001 |
| JGCA-TRG | ||||
| Grade 3 (no residual) | 1.00 | 1.00 | ||
| Grade 2 (< 33%) | 10.79 (2.59-45.05) | 0.001 | 10.79 (2.58-45.05) | 0.001 |
| Grade 1b (34%-66%) | 12.83 (3.10-53.18) | < 0.001 | 14.04 (3.40-58.05) | < 0.001 |
| Grade 1a (67%-99%) | 15.15 (3.74-61.42) | < 0.001 | 15.54 (3.84-62.93) | < 0.001 |
| Grade 0 (no response) | 20.24 (4.67-87.66) | < 0.001 | 21.18 (4.89-91.78) | < 0.001 |
| Becker-TRG | ||||
| 1a (no residual) | 1.00 | 1.00 | ||
| 1b (< 10%) | 8.98 (2.06-39.06) | 0.003 | 8.74 (2.01-38.05) | 0.004 |
| 2 (10%-50%) | 12.19 (2.92-50.87) | 0.001 | 12.72 (3.05-53.06) | < 0.001 |
| 3 (> 50%) | 15.50 (3.84-62.62) | < 0.001 | 16.15 (4.00-65.22) | < 0.001 |
| AJCC-TRG | ||||
| 0 (complete response) | 1.00 | 1.00 | ||
| 1 (moderate response) | 10.46 (2.46-44.48) | 0.001 | 10.31 (2.42-43.90) | 0.002 |
| 2 (minimal response) | 11.21 (2.71-46.34) | 0.001 | 11.67 (2.83-48.22) | 0.001 |
| 3 (poor response) | 16.31 (4.03-65.97) | < 0.001 | 16.94 (4.19-68.49) | < 0.001 |
| Mandard-TRG | ||||
| 1 (complete response) | 1.00 | 1.00 | ||
| 2 (Fibrosis + scattered tumor cells) | 10.46 (2.46-44.48) | 0.001 | 10.33 (2.43-43.95) | 0.002 |
| 3 (Fibrosis predominance + tumor cells) | 11.20 (2.71-46.30) | 0.001 | 11.66 (2.82-48.16) | 0.001 |
| 4 (Tumor cells preponderance + fibrosis) | 15.85 (3.91-64.19) | < 0.001 | 16.48 (4.07-66.71) | < 0.001 |
| 5 (No response) | 20.27 (4.68-87.81) | < 0.001 | 21.22 (4.90-91.96) | < 0.001 |
Multivariate analysis for overall OS and DFS were then performed, including features that were related to poorer survival prognosis in univariate analysis (P < 0.10): BMI, ECOG, tumor location, diameter in short axis, differentiation, histology type, LVI, resection type, adjuvant chemotherapy, and ypT and ypN stages. After adjusting for potential confounders in the multivariate Cox regression model, BMI, histology type, LVI, and the ypN stage were independent predictors for OS, while LVI and the ypN stage were independent risk factors for DFS. All five TRG systems showed significant differences when setting the “complete response” group as a reference (Table 4). However, the increase in the hazard ratio was not entirely in accord with the increase in the TRG grade in the JGCA2017, AJCC/CAP, and Mandard systems. In fact, the intergroup differences were not statistically significant when the “complete response” group was absent in each system. Only a marginal difference was found between JGCA2017-TRG grade 0 (no response) vs 2b (< 10%) for OS (HR: 1.84; 95%CI: 0.90-3.75; P = 0.096) and DFS (HR: 1.87; 95%CI: 0.92-3.83; P = 0.085).
| Whole patients (n = 413) | ||||
| Covariates | OS | DFS | ||
| HR | P value | HR | P value | |
| BMI ≤ 23.9 | 1.37 (1.01-1.87) | 0.045 | 1.28 (0.95-1.72) | 0.109 |
| ECOG > 0 | 1.19 (0.87-1.61) | 0.271 | 1.18 (0.88-1.60) | 0.272 |
| Linitis plastica | 1.74 (0.97-3.13) | 0.063 | 1.30 (0.74-2.30) | 0.362 |
| Diameter > 5 cm | 1.20 (0.77-1.88) | 0.426 | 1.43 (0.93-2.19) | 0.102 |
| Poorly differentiated | 1.16 (0.85-1.57) | 0.345 | 1.24 (0.92-1.66) | 0.160 |
| Mucinous or signet cell | 1.45 (1.03-2.05) | 0.036 | 1.32 (0.94-1.84) | 0.111 |
| Lymphovascular invasion | 1.53 (1.12-2.10) | 0.008 | 1.61 (1.18-2.19) | 0.002 |
| ypT3-4 | 1.45 (0.92-2.28) | 0.113 | 1.52 (0.97-2.37) | 0.065 |
| ypN+ | 1.96 (1.35-2.85) | < 0.001 | 1.94 (1.34-2.82) | < 0.001 |
| Total gastrectomy | 1.30 (0.94-1.79) | 0.118 | 1.23 (0.90-1.69) | 0.202 |
| Without AC | 1.35 (0.98-1.87) | 0.066 | Not included | NA |
| JGCA2017-TRG (Model 1) | ||||
| Grade 3 (no residual) | 1.00 | 1.00 | ||
| Grade 2b (< 10%) | 4.69 (1.04-21.08) | 0.044 | 4.50 (1.00-20.27) | 0.050 |
| Grade 2a (10%-33%) | 5.48 (1.19-25.23) | 0.029 | 5.50 (1.20-25.26) | 0.028 |
| Grade 1b (34%-66%) | 5.32 (1.22-23.30) | 0.026 | 5.73 (1.32-24.88) | 0.020 |
| Grade 1a (67%-99%) | 6.69 (1.55-28.96) | 0.011 | 6.22 (1.44-26.81) | 0.014 |
| Grade 0 (no response) | 8.60 (1.87-39.58) | 0.006 | 8.44 (1.84-38.76) | 0.006 |
| JGCA-TRG (Model 2) | ||||
| Grade 3 (no residual) | 1.00 | |||
| Grade 2 (< 33%) | 5.00 (1.15-21.78) | 0.032 | 4.90 (1.13-21.30) | 0.034 |
| Grade 1b (34%-66%) | 5.27 (1.21-23.05) | 0.027 | 5.67 (1.30-24.61) | 0.021 |
| Grade 1a (67%-99%) | 6.63 (1.53-28.66) | 0.011 | 6.16 (1.43-26.52) | 0.015 |
| Grade 0 (no response) | 8.48 (1.84-39.00) | 0.006 | 8.31 (1.81-38.15) | 0.006 |
| Becker-TRG (Model 3) | ||||
| 1a (no residual) | 1.00 | 1.00 | ||
| 1b (< 10%) | 4.74 (1.05-21.30) | 0.043 | 4.57 (1.02-20.57) | 0.047 |
| 2 (10%-50%) | 5.11 (1.16-22.51) | 0.031 | 5.13 (1.17-22.49) | 0.030 |
| 3 (> 50%) | 6.77 (1.57-29.14) | 0.010 | 6.64 (1.55-28.46) | 0.011 |
| AJCC-TRG (Model 4) | ||||
| 0 (complete response) | 1.00 | 1.00 | ||
| 1 (moderate response) | 5.39 (1.22-23.78) | 0.026 | 5.34 (1.21-23.50) | 0.027 |
| 2 (minimal response) | 5.01 (1.15-21.85) | 0.032 | 5.05 (1.16-21.93) | 0.031 |
| 3 (poor response) | 6.72 (1.56-28.97) | 0.011 | 6.53 (1.52-28.05) | 0.012 |
| Mandard-TRG (Model 5) | ||||
| 1 (complete response) | 1.00 | 1.00 | ||
| 2 (Fibrosis + scattered tumor cells) | 5.37 (1.22-23.68) | 0.026 | 5.31 (1.21-23.39) | 0.027 |
| 3 (Fibrosis predominance + tumor cells) | 4.95 (1.14-21.60) | 0.033 | 4.98 (1.15-21.62) | 0.032 |
| 4 (Tumor cells preponderance + fibrosis) | 6.44 (1.49-27.87) | 0.013 | 6.26 (1.45-26.93) | 0.014 |
| 5 (No response) | 8.44 (1.83-38.86) | 0.006 | 8.41 (1.83-38.60) | 0.006 |
According to the previous analysis, a comparison of the five systems revealed the Becker system to enable the best prognostic differentiation between subgroups across the whole patient cohort. The AJCC/CAP system, although having the second-highest c-index, did not provide better intergroup discrimination in multivariate analysis. According to the JGCA2017 criteria, two cutoff values of residual tumor percentage - 10% and 100% - were of more clinical significance than any other commonly used cutoff percentages except for total regression. Despite the intergroup differences being marginal, a higher c-index of 0.728 ± 0.035 for OS and 0.737 ± 0.035 for DFS, could be achieved based on the following rearranged residual tumor percentage cutoffs: 0 (no residual tumor; reference), < 10% (HR: 4.61; 95%CI: 1.02-20.73; P = 0.047 for OR; HR: 4.46; 95%CI: 0.99-20.08; P = 0.051 for DFS), 10-99% (HR: 5.98; 95%CI: 1.40-25.63; P = 0.016 for OS; HR: 5.93; 95%CI-25.29; P = 0.016 for DFS), no response (HR: 8.36; 95%CI: 1.82-38.44; P = 0.006 for OS; HR: 8.33; 95%CI: 1.82-38.23; P = 0.006 for DFS). There was a significant difference in the prognostic ability for DFS (P = 0.046) and a borderline significance for OS (P = 0.073) between the rearranged cutoffs and the Becker system (Table 5).
| JGCA2017 | JGCA | Becker | AJCC/CAP | Mandard | Modified | |
| Overall survival | ||||||
| JGCA2017 | 1.000 | 0.308 | 0.006 | 0.018 | 0.053 | < 0.001 |
| JGCA | 1.000 | 0.007 | 0.021 | 0.063 | < 0.001 | |
| Becker | 1.000 | 0.397 | 0.148 | 0.073 | ||
| AJCC/CAP | 1.000 | 0.039 | 0.062 | |||
| Mandard | 1.000 | 0.005 | ||||
| Modified | 1.000 | |||||
| Disease-free survival | ||||||
| JGCA2017 | 1.000 | 0.320 | 0.002 | 0.021 | 0.033 | < 0.001 |
| JGCA | 1.000 | 0.003 | 0.025 | 0.040 | < 0.001 | |
| Becker | 1.000 | 0.273 | 0.136 | 0.046 | ||
| AJCC/CAP | 1.000 | 0.112 | 0.024 | |||
| Mandard | 1.000 | 0.002 | ||||
| Modified | 1.000 |
Neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy is the current standard treatment for LAGC[1,2]. Although the benefit of this multimodality treatment was first confirmed by the MAGIC trial in 2006, the use of NACT had been adopted in GC for 30 years[22,23]. To assess the treatment response, despite the widespread use of the TRG system for gastrointestinal tract tumors, the response rates are always poor in GC compared with esophageal or colorectal cancer[5]. This might be due to the lack of chemoradiation and sensitive regimens in preoperative settings. Due to the currently limited preoperative therapies and the limited number of responsive patients, findings on the value of TRG prognostic systems in LAGC are varied. Additional complexities arise when the study contexts are based on different TRG systems, especially on the comparison between TRG and ypTNM systems as independent predictors of patients survival[24-29]. Becker et al[16] investigated 480 patients with LAGC undergoing surgical resection and found TRG 2-3 grade (10%-100% residual tumor) to be an independent risk factor for patient OS; this reinforced the efficacy of the Becker TRG system. Ikoma et al[25] reviewed 356 LAGC patients receiving D0-D2 Lymphadenectomy following NACT or NACRT, finding that the residual tumor < 50% group was associated with a shorter OS but not as an independent predictor[25]. And Derieux first proved the predictive value of the Mandard system in GC, observing a poorer OS and DFS in patients with a high proportion of residual cancer cells (Mandard TRG 4) and no response (Mandard TRG 5)[29].
Therefore, when verifying the prognostic value of the histological response, considerable work should be done on determining an optimal tumor response classification for GC. Currently, two major principles are common to these systems for grading tumor regression: (1) estimating residual tumor in relation to fibrotic changes, e.g., the Mandard, AJCC/CAP, and Dworak systems[17,18,30]; and (2) proportioning the resi
A comparison of different histological response systems for GC was conducted by Zhu et al[28]. This study included 192 patients and found that five TRG systems - including Mandard, JGCA, AJCC/CAP, Becker, and China - were not independent predictors for patient survival. Although the predictive abilities of each system were not measured, the Mandard and JGCA systems were recommended due to their superior prognosis prediction abilities. This was because a higher hazard ratio was discovered in the “no response” patients. In JCOG1004-A, 173 patients who received surgery following NACT were stratified according to different residual tumor cutoff percentages of 10%, 33%, 50%, and 67%. The 10% cutoff was found to be the best predictor of survival for various pathological types[9]. While this 10% cutoff finding was remarkable and coincided with Becker’s cutoff method (described above)[35], whether the current five- or six-tier JGCA standard provided the optimal discrimination value was not further investigated.
In the present study, c-index analysis was used to compare the discrimination value of five TRG systems including the most recent JGCA2017-TRG system. Both the five-tier JGCA and the six-tier JGCA2017 systems scored significantly lower c-indexes than the four-tier AJCC/CAP and Becker systems. Because both the JGCA2017 and the JGCA have overlapped measuring spacing compared with the four-tier Becker system, the results of the present study indicated that five- or six-tier grading systems performed no better (and even worse) than four-tier systems in evaluating GC patients. The c-index comparison suggested that the four-tier Becker system had the best predictive value for GC patients. Because of their relatively wide measuring distance, four-tier systems based on residual percentages also mean a lower workload, easier understanding of protocols, and less inter-observer disagreement propagation[4,36].
On the other hand, based on the JCGA2017 criteria, the present study revealed that grade 2b (1%-10%) was likely to predict longer OS and DFS than grade 0 (no response). Interestingly, when the percentages of residual tumor were reset to “no residual tumor”, < 10%, < 100%, and “no response”, the c-index of the rearranged cutoff values scored significantly higher than the Becker system for patient survival. Similar results using these revised cutoffs were reported by Zhu et al[28], wherein an overt higher HR was observed for grade 3 among the other JGCA grades, and by Becker et al[35] who demonstrated the independent predictive ability of the Becker system by using a cutoff of < 10% residual tumor. The results of the present study suggested that among moderate-to-poor (residual tumor 10-99%) responders, the response rate may not have a decisive impact on hazard stratification because NACT or chemotherapy only accounted for a small part of improving the prognosis among significant covariates in this group of GC patients. Meanwhile, a complete or subtotal response (0%-10%) often indicated a fairly good sensitivity to chemotherapy and vice versa for non-responders (no regression), who cannot receive any benefit but toxicity. Although the non-responders only accounted for 6.3% of the total patient number, it is suggested that this “break off both ends” approach provides a way for screening chemosensitivity and predicting prognosis in GC patients. However, a larger sample size is required to verify this proposal.
There were some limitations to this study. First, it was restricted by its single-center retrospective nature. Second, although histopathology was performed by two pathologists with over 10 years of experience, analysis of the inter- and intra-observer variability of the actual TRG classification was not conducted. Third, despite the involvement of many covariates, the macroscopic information may not be sufficient. According to JCOG1004-A, the TRG cutoff standard may not be recommended for Bormann type IV patients, for which a current dataset is not available[9]. Furthermore, this study did not consider intestinal and diffuse types according to the Lauren classification, which are thought to be independent prognostic factors for survival[37]. Statistically, collinearity between the TRG and ypT categories is inevitable but would have affected the multivariable analysis results: the Pearson's coefficients with ypT were 0.619, 0.587, 0.662, and 0.639 for the JGCA2017, JGCA, Becker, and AJCC/CAP systems, respectively. To reduce the impact of multicollinearity, studies with an increased sample size are warranted.
In conclusion, it was demonstrated that although all five TRG systems could be used as independent predictors for LAGC patient survival, the six-tier JGCA-TRG system did not increase prognostic stratification but may reduce the reproducibility and increase the working load on histological response evaluation. Patient survival can be effectively discriminated by the Becker system using the residual tumor percentage rather than by estimating the fibrosis/residual tumor ratio. Apart from when using the Becker classification, the group of non-responders with no regression was predicted to have a poorer prognosis. A large population-based study is still required to find the optimal criteria and validate the boundary settings of current TRG systems for LAGC patients.
The tumor regression grade systems for gastric cancer (GC) are various, while the most suitable one is yet to be known.
We aimed to investigate the most accurate criteria for TRG in predicting patient’s prognosis.
To collect 413 locally advanced GC (LAGC) patient’s clinical data and their post-treatment pathological samples after neoadjuvant chemotherapy treatment.
This is a retrospectively clinical study in which the LAGC patient’s specimens were reviewed by two pathologists and the TRG grades were revalued. Then, the predictive abilities of five TRG criteria were assessed and statistically compared based on survival/risk prediction model.
The four-tier Becker system showed the highest predictive ability, among the five common TRG criteria. The TRG criteria could achieve an optimal prediction when the residual tumor percentages were reset as: “no residual tumor”, < 10%, < 100%, and “no response”.
The four-tier Becker system is more suitable and should be recommended for LAGC patients.
A population-based study is warranted to define the optimal criterion for TRG for GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kao JT, Mohamed SY S-Editor: Wang LL L-Editor: Filipodia P-Editor: Guo X
| 1. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4606] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 2. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 3. | Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 4. | Westerhoff M, Osecky M, Langer R. Varying practices in tumor regression grading of gastrointestinal carcinomas after neoadjuvant therapy: results of an international survey. Mod Pathol. 2020;33:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Fanelli GN, Loupakis F, Smyth E, Scarpa M, Lonardi S, Pucciarelli S, Munari G, Rugge M, Valeri N, Fassan M. Pathological Tumor Regression Grade Classifications in Gastrointestinal Cancers: Role on Patients' Prognosis. Int J Surg Pathol. 2019;27:816-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Tong Y, Liu D, Zhang J. Connection and distinction of tumor regression grading systems of gastrointestinal cancer. Pathol Res Pract. 2020;216:153073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 8. | Association JGC. Japanese Classification of Gastric Carcinoma, the 15th Edition (in Japanese). Tokyo: Kanehara Shuppan, 2017: 43-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 9. | Nakamura K, Kuwata T, Shimoda T, Mizusawa J, Katayama H, Kushima R, Taniguchi H, Sano T, Sasako M, Fukuda H. Determination of the optimal cutoff percentage of residual tumors to define the pathological response rate for gastric cancer treated with preoperative therapy (JCOG1004-A). Gastric Cancer. 2015;18:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Liang H. Reports of plenary session from the 89th Annual Meeting of the Japanese Gastric Cancer Association. Zhongguo Shiyong Waike Zazhi. 2017;37:390-393. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (1)] |
| 12. | Liu Z, Wang Y, Shan F, Ying X, Zhang Y, Li S, Jia Y, Li Z, Ji J. 5-Fu-Based Doublet Regimen in Patients Receiving Perioperative or Postoperative Chemotherapy for Locally Advanced Gastric Cancer: When to Start and How Long Should the Regimen Last? Cancer Manag Res. 2021;13:147-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 14. | In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24:3683-3691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 15. | Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 16. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Chen HY, Feng LL, Li M, Ju HQ, Ding Y, Lan M, Song SM, Han WD, Yu L, Wei MB, Pang XL, He F, Liu S, Zheng J, Ma Y, Lin CY, Lan P, Huang MJ, Zou YF, Yang ZL, Wang T, Lang JY, Orangio GR, Poylin V, Ajani JA, Wang WH, Wan XB. College of American Pathologists Tumor Regression Grading System for Long-term Outcome in Patients with Locally Advanced Rectal Cancer. Oncologist 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8608] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 20. | Li Z, Wang Y, Shan F, Ying X, Wu Z, Xue K, Miao R, Zhang Y, Ji J. ypTNM staging after neoadjuvant chemotherapy in the Chinese gastric cancer population: an evaluation on the prognostic value of the AJCC eighth edition cancer staging system. Gastric Cancer. 2018;21:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Schröder MS, Culhane AC, Quackenbush J, Haibe-Kains B. survcomp: an R/Bioconductor package for performance assessment and comparison of survival models. Bioinformatics. 2011;27:3206-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 22. | Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, Solej M, Degiuli M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018;24:274-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Songun I, Keizer HJ, Hermans J, Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van Krieken JH, van de Velde CJ. Chemotherapy for operable gastric cancer: results of the Dutch randomised FAMTX trial. The Dutch Gastric Cancer Group (DGCG). Eur J Cancer. 1999;35:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Stark AP, Estrella JS, Chiang YJ, Das P, Minsky BD, Blum Murphy MA, Ajani JA, Mansfield P, Badgwell BD, Ikoma N. Impact of tumor regression grade on recurrence after preoperative chemoradiation and gastrectomy for gastric cancer. J Surg Oncol. 2020;122:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ikoma N, Estrella JS, Blum Murphy M, Das P, Minsky BD, Mansfield P, Ajani JA, Badgwell BD. Tumor Regression Grade in Gastric Cancer After Preoperative Therapy. J Gastrointest Surg. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Blackham AU, Greenleaf E, Yamamoto M, Hollenbeak C, Gusani N, Coppola D, Pimiento JM, Wong J. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol. 2016;114:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Xu X, Zheng G, Zhang T, Zhao Y, Zheng Z. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? Cancer Chemother Pharmacol. 2019;84:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Zhu Y, Sun Y, Hu S, Jiang Y, Yue J, Xue X, Yang L, Xue L. Comparison of five tumor regression grading systems for gastric adenocarcinoma after neoadjuvant chemotherapy: a retrospective study of 192 cases from National Cancer Center in China. BMC Gastroenterol. 2017;17:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Derieux S, Svrcek M, Manela S, Lagorce-Pages C, Berger A, André T, Taieb J, Paye F, Voron T. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard's Tumor Regression Grade (TRG) in gastric adenocarcinoma. Dig Liver Dis. 2020;52:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1061] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 31. | Ninomiya Y, Yanagisawa A, Kato Y, Kitagawa T, Ishihara S, Nakajima T. Histological indications of a favorable prognosis with far-advanced gastric carcinomas after preoperative chemotherapy. J Cancer Res Clin Oncol. 1999;125:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Karamitopoulou E, Thies S, Zlobec I, Ott K, Feith M, Slotta-Huspenina J, Lordick F, Becker K, Langer R. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol. 2014;38:1551-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Puetz K, Bollschweiler E, Semrau R, Mönig SP, Hölscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer - which response grading system best impacts prognostic discrimination? Histopathology. 2019;74:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 35. | Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 36. | Tong Y, Zhu Y, Zhao Y, Shan Z, Liu D, Zhang J. Evaluation and Comparison of Predictive Value of Tumor Regression Grades according to Mandard and Becker in Locally Advanced Gastric Adenocarcinoma. Cancer Res Treat. 2021;53:112-122. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Reim D, Gertler R, Novotny A, Becker K, zum Büschenfelde CM, Ebert M, Dobritz M, Langer R, Hoefler H, Friess H, Schumacher C. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol. 2012;19:2108-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |