Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2076
Peer-review started: March 29, 2021
First decision: June 16, 2021
Revised: July 8, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: December 15, 2021
Processing time: 260 Days and 14.5 Hours
The phase III clinical trial of the novel molecular targeted agent (MTA) lenvatinib for patients with advanced hepatocellular carcinoma (HCC) (REFLECT trial) found that lenvatinib was non-inferior to sorafenib in overall survival. Recently, the efficacy of multiple MTAs, including lenvatinib, in practice has been reported, and therapeutic strategies for Barcelona Clinic Liver Cancer (BCLC) intermediate stage HCC are undergoing major changes. Based on these results, lenvatinib could be recommended for patients with transcatheter arterial chemoembolization (TACE)-refractory, ALBI grade 1, within the up-to-seven criteria in the BCLC intermediate stage. Lenvatinib provides a more favorable outcome than TACE, even in cases with large or multinodular HCC beyond the up-to-seven criteria with Child-Pugh grade A. When patients meet the definitions of TACE-refractory or TACE-unsuitable, switching to systemic chemotherapy, including lenvatinib, is for favorable for preserving liver function. If initial treatment, including MTA, has a significant therapeutic effect and downstaging of HCC is obtained, additional TACE or surgical resection should be considered. Lenvatinib also has a therapeutic effect for poorly differentiated type and non-simple nodular type HCC thanks to the survival-prolonging effect of this drug. Furthermore, a significant therapeutic effect is expected in tumors with more than 50% liver involvement or main portal vein invasion, which have traditionally been considered to have a poor prognosis in patients. This suggests that at the start of lenvatinib treatment, HCC patients with ALBI grade 1 may be able to maintain liver functional reserve.
Core Tip: For about 10 years, first-line systemic chemotherapy for patients with advanced hepatocellular carcinoma (HCC) had been limited to sorafenib. The Phase III clinical trial of lenvatinib for patients with advanced HCC showed lenvatinib to be non-inferior to sorafenib with respect to overall survival (OS). The OS of patients is still far from satisfactory, and there is a great unmet medical need for more effective therapies. This review focuses on the current understanding of the therapeutic efficacy and safety of lenvatinib in the world and outlines the role of lenvatinib in the new era of chemotherapy for HCC.
- Citation: Sho T, Morikawa K, Kubo A, Tokuchi Y, Kitagataya T, Yamada R, Shigesawa T, Kimura M, Nakai M, Suda G, Natsuizaka M, Ogawa K, Sakamoto N. Prospect of lenvatinib for unresectable hepatocellular carcinoma in the new era of systemic chemotherapy. World J Gastrointest Oncol 2021; 13(12): 2076-2087
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2076.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2076
Hepatocellular carcinoma (HCC) is one of the most common solid cancers and a major cause of cancer-related deaths globally[1]. According to the Global Cancer Observatory in 2020, HCC is ranked third in mortality, causing over 830000 deaths per year. Despite increasing global incidence as a major cause of cancer death, the development of new anticancer drugs for HCC has been inadequate. Traditionally, HCC has a poor prognosis. However, this might be partly due to the confined treatment options for patients with advanced HCC[2,3].
Since the publication of the practice guidelines of the American Association for the Study of Liver Diseases (AASLD) on the management of HCC in 2005, the Barcelona Clinic Liver Cancer (BCLC) staging system has been widely accepted and is also being used in many clinical trials of new drugs to treat HCC. These take into account factors including tumor burden, liver function, and general health conditions to determine prognosis and the best treatment. Accordingly, patients at an early stage are those with HCC ≤ 5 cm or up to three nodules < 3 cm each (BCLC stage A). Patients exceeding these limits, without vascular invasion or extrahepatic spread, fit into the intermediate stage (BCLC stage B). Patients with evidence of a performance status ≤ 2 or an aggressive tumor pattern (vascular invasion or extrahepatic spread) correspond to the advanced stage (BCLC stage C)[4].
Systemic chemotherapy is the only therapeutic option for patients with Child-Pugh grade A at BCLC stage B with unresectable HCC and stage C. Prior to the development of the molecular targeted agent (MTA), patients in the advanced stage had a survival time of approximately 6 mo. Systemic chemotherapy for HCC has changed since the introduction of MTA sorafenib in 2007. The SHARP trial demonstrated that sorafenib prolonged median overall survival (OS) compared to placebo in patients who had not received systemic chemotherapy [10.7 mo vs 7.9 mo, hazard ratio (HR) = 0.69, 95%CI: 0.55-0.87, P < 0.001][5]. The subsequent Asia-Pacific trial confirmed these results in Asian patients[6]. Therapeutic options for extrahepatic metastases (e.g., lung, lymph node, or bone) and vascular invasion (e.g., portal vein tumor thrombus) have been demonstrated, and relatively long survival has been achieved for patients with BCLC stage C.
However, sorafenib does not shrink or induce necrosis in tumors and has relatively severe adverse events (AEs), including hand-foot-skin reactions. Therefore, the development of a novel MTA that can substitute for sorafenib is much anticipated.
For the past 10 years, sorafenib has been the only available first-line systemic chemotherapy for patients with advanced HCC. Many clinical trials of candidate systemic chemotherapeutic agents for advanced HCC have failed to demonstrate superiority or non-inferiority to sorafenib[7-9]. The phase III clinical trial of the lenvatinib for patients with advanced HCC (REFLECT trial)[10] showed lenvatinib to be non-inferior to sorafenib with respect to OS (13.6 mo vs 12.3 mo, HR = 0.92, 95%CI: 0.79-1.06). Furthermore, the secondary efficacy endpoints [progression-free survival (PFS) and objective response rate (ORR)] in the lenvatinib group showed a significant improvement compared with sorafenib. Based on the REFLECT trial, lenvatinib has been approved in the United States, European Union, and other countries as a first-line treatment option alongside sorafenib for advanced HCC, making it the first such drug to be used in Japan. A recent Phase III trial (IMbrave150) showed that combination immunotherapy with atezolizumab plus bevacizumab improved outcomes, including OS, PFS, ORR, and disease control rate, compared with sorafenib monotherapy[11]. Based on these results, both the 2020 AASLD and the 2021 European Society for Medical Oncology liver treatment options depends on BCLC staging and treatment guidelines which recommended atezolizumab+bevacizumab as first-line systemic therapy in stage B with transcatheter arterial chemoembolization (TACE)-unsuitable HCC and stage C. In addition, even with the latest version of the treatment algorithm in the Clinical Practice Guidelines for HCC 2020 in Japan, the first-line drug therapy for unresectable HCC is atezolizumab+bevacizumab combination therapy. Sorafenib and lenvatinib, which were previously the first-line treatments, are now second-line treatments. Regorafenib, ramucirumab, and cabozantinib can be used as third-line treatments (Figure 1).
Although various systemic chemotherapies are available for HCC, the OS of patients is still far from satisfactory, and there is a great unmet medical need for more effective therapies. Furthermore, in the future, issues are likely to arise regarding the order and combination of treatment options for HCC.
This review focuses on the current understanding of the therapeutic efficacy and safety of lenvatinib and outlines the role of lenvatinib in the new era of chemotherapy for HCC.
Lenvatinib is an MTA that suppresses vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), proto-oncogene tyrosine-protein kinase receptor RET, platelet-derived growth factor receptor α, and stem cell factor receptor[12]. Because these targets act as drivers in cancer, lenvatinib has been reported to exhibit antitumor and immunomodulatory activities in a variety of preclinical cancer models[13].
Angiogenesis, mostly regulated by the VEGF pathway, is an essential event in tumor growth and metastasis[14]. In particular, VEGFR-2 is a high-affinity VEGF receptor in vascular endothelial cells[15,16]. Ligand binding activates certain signaling pathways, such as the phospholipase-Cγ, phosphoinositide 3-kinase (PI3K)/V-akt murine thymoma viral oncogene homolog (AKT) pathways, and rat sarcoma (Ras)/mitogen-activated protein kinase (MAPK)[16]. These signaling pathways have been implicated in endothelial cells and vascular permeability during tumor enlargement[17]. Moreover, VEGFR is highly expressed in HCC cells. Lenvatinib has been shown to inhibit tumor angiogenesis in various preclinical models. In patient-derived and PLC/PRF/5 cell-transplanted tumor models, Lenvatinib administration resulted in a reduction in microvessel density of tumor[18]. In addition, lenvatinib had been found to suppress various types of cancers[19-23], by blocking the VEGFR pathway. These data indicate that lenvatinib exhibits potent anti-angiogenic activity and may have a stronger effect than sorafenib in preclinical models.
Activated fibroblast growth factor (FGF) signaling can directly facilitate cell proliferation and survival, as well as promote tumor angiogenesis and progression[24]. The binding of FGF to FGFR leads to the activation of the RAS/MAPK and PI3K/AKT signaling pathways[25,26]. FGF and FGFR are typically overexpressed in HCC, and the expression of FGF19/FGFR4 contributes to HCC progression[27]. Analysis of the effects of selective FGFR inhibitors and FGFR small interfering RNAs on cancer stem-like cells (CSCs) in HCC showed that lenvatinib diminished CSCs in HCC by inhibiting FGFR1-3 signaling; however, FGFR4 signaling was not affected. FGF2 and FGF19 are involved in maintaining CD44High/CD133High CSCs in HCC, potentially via FGFR1-3[28]. Preclinical studies have shown that lenvatinib inhibits the proliferation of FGF19 and FGFR-overexpressing cell lines[18,28,29].
RET activates downstream signaling pathways through mutations or chromosome rearrangements, promoting tumor cell growth[30]. The autophosphorylation of specific tyrosine residues of RET allows for the recruitment adaptor proteins that connect the RET receptor to RAS/MAPK and PI3K/AKT signaling pathways, thereby promoting cell growth, proliferation, survival, and differentiation[31]. Lenvatinib can inhibit cell proliferation by blocking RET autophosphorylation[12].
The immune escape of tumor cells is the main mechanism of tumorigenesis[32]. It has been suggested that VEGF-A has immunosuppressive properties[33]. VEGF-A produced by tumor cells enhances the expression of immunosuppressive receptors in CD8+ T cells and promotes immune escape[34]. Immune inhibitory receptors cause CD8+ T cell exhaustion by recognizing tumor antigens[32]. Lenvatinib reduced the infiltration of tumor-associated macrophages and increased the percentage of activated CD8+ T cells in HCC[13].
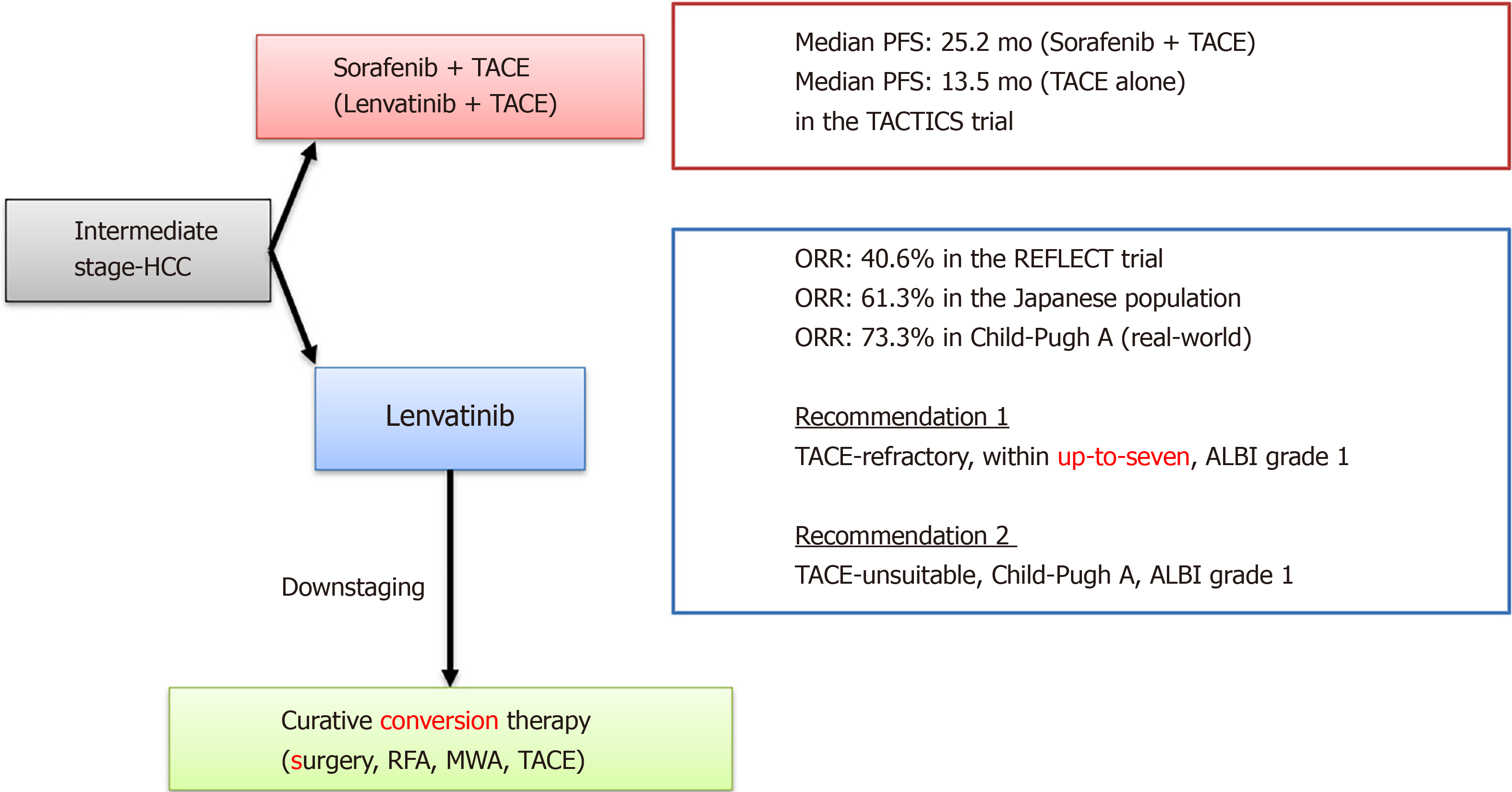
In recent years, the efficacy of multiple MTAs, including lenvatinib, has been reported. As a result, therapeutic strategies for BCLC intermediate-stage HCC are undergoing major changes. In the REFLECT trial, the ORR of lenvatinib was 40.6% in the mRECIST evaluation, and in the sub-analysis, the ORR of BCLC intermediate stage HCC in the Japanese population was 61.3%[35]. Furthermore, Kudo et al[36] reported that a very high response rate (RR) of 73.3% was obtained for Child-Pugh A in BCLC intermediate stage HCC. Tomonari et al[37] reported from their real-world data that BCLC intermediate stage HCC cases had fewer AEs, could maintain the dosage amount of lenvatinib, and had a good therapeutic effect. Many reports have demonstrated the high therapeutic effect of lenvatinib in BCLC intermediate stage HCC.
TACE is the guideline-recommended standard of care for intermediate stage HCC. The AASLD Consensus Conference showed that locoregional TACE may still be the best approach if the patient’s tumor volume is small and nodules can be accessed superselectively[38]. According to a systematic review of patients treated with lipi
Recently, the characteristics of TACE-resistant tumors, which are prone to being TACE-refractory and to exacerbate to Child-Pugh grade B by TACE, have been clarified[36,44]. In cases in which the tumor is a non-simple nodular type, occupying multiple lobes, a large tumor mass, such as beyond the up-to-seven criteria, and of a histopathologically poorly differentiated type, TACE has little effect. As a result, these cases may become TACE-refractory at an early stage[45].
Therapeutic alternatives are needed for patients who are TACE-refractory. Repeated TACE could worsen liver function, thereby narrowing the time window for a switch to MTAs, which is recommended for patients with Child-Pugh grade A. In the era of sorafenib, TACE failure/refractoriness was proposed for switching to systemic chemotherapy[46]. The OPTIMIS trial showed that the survival time in TACE-refractory patients was longer in patients who received sorafenib than in those who continued TACE.
A recent study showed that the median PFS times in TACE-refractory patients treated with lenvatinib, sorafenib, and TACE was 5.8, 3.2, and 2.4 mo, respectively[47]. In a Cox regression analysis, lenvatinib treatment and being within the up-to-seven criteria were identified as independent factors for PFS (lenvatinib, P < 0.0001; within the up-to-seven criteria, P = 0.001). Similarly, decision-tree analysis showed that patients treated with ALBI grade 1 beyond the up-to-seven criteria had longer PFS than patients treated with ALBI grade 2 beyond the up-to-seven criteria. Therefore, lenvatinib could be recommended to patients with TACE-refractory, ALBI grade 1, and within the up-to-seven criteria in the BCLC intermediate stage. Thus, treatment with lenvatinib could give rise to good outcomes in TACE-refractory intermediate-stage HCC patients.
In patients beyond the up-to-seven criteria, TACE treatment was reported to be likely to worsen liver function, potentially resulting in losing the opportunity to be treated with MTA[42,48]. Recently, the Asia-Pacific Primary Liver Cancer Expert (APPLE) consensus statement proposed the criteria for TACE unsuitability[49].
A proof-of-concept study demonstrated that, in patients with intermediate stage HCC who exceeded the up-to-seven criteria, the lenvatinib group showed a significantly higher ORR (73.3% vs 33.3%; P < 0.001) and a significantly longer median PFS than the conventional TACE group (16.0 mo vs 3.0 mo; P < 0.001)[50]. The ALBI score was maintained in the lenvatinib group during treatment, whereas it worsened in the TACE group. Therefore, in the case of large or multinodular intermediate stage HCC with Child-Pugh grade A, lenvatinib provides a better outcome than TACE.
When patients meet the definitions of TACE-refractory or TACE-unsuitable, switching to systemic chemotherapy including lenvatinib may be advisable in order to preserve liver function. Upfront lenvatinib therapy may also be suitable for patients with a high tumor burden or those who are considered TACE-refractory. If the tumor responds well and downstaging is possible with lenvatinib, additional TACE (or surgical resection or ablation) could be considered. However, the efficacy and safety of this strategy have not yet been validated.
Recently, a randomized, controlled trial comparing the efficacy and safety of TACE plus sorafenib to TACE alone (TACTICS trial) found that median PFS was significantly longer in the TACE plus sorafenib group than in the TACE alone group (25.2 mo vs 13.5 mo; P = 0.006)[51]. MTA treatment is thought to improve the clinical outcome of TACE by promoting the normalization of tumor vessels and contributing to a higher density of lipiodol deposition[49]. Lenvatinib has a higher RR than sorafenib, suggesting that Len-TACE sequential therapy may be a promising treatment for patients with moderately differentiated HCC.
In the REFLECT study, poorly differentiated HCC was diagnosed in 42 patients (4.4%), with 21 in the lenvatinib group and 21 in the sorafenib group. The ORR was 47.6% in the lenvatinib group and 14.3% in the sorafenib group. Thus, lenvatinib is expected to have an effect on poorly differentiated HCC[49].
HCC shows great diversity of tumor differentiation even in the same nodule, and it is difficult to examine the heterogeneity of all tumors by liver tumor biopsy. Therefore, it is extremely important to evaluate the degree of tumor differentiation using a non-invasive method. Poorly differentiated HCC is characterized by tumors that show a heterogeneous enhancement pattern with irregularly shaped ring structures in the arterial phase of dynamic computed tomography (CT)[52] and tumors in which lesions are detected on FDG-positron emission tomography[53]. Based on the prediction of tumor differentiation by the enhancement pattern of dynamic CT[54], the therapeutic effect of lenvatinib is also recognized for poorly differentiated type and non-simple nodular type, and it has been reported that it has a survival-prolonging effect for all of them[54]. Tumors with a heterogeneous enhancement pattern and irregularly shaped ring structures had an RR of 84%, which was significantly better than the RR of tumors with a homogeneous enhancement pattern, which was 53%. Furthermore, there was no significant difference in the PFS between the two groups. The therapeutic effect of lenvatinib, regardless of the degree of tumor differentiation, will have a significant impact on future HCC treatment[54].
Traditionally, non-simple nodular, poorly differentiated HCC has a poor prognosis with existing treatments, including hepatectomy. In particular, it was difficult to control the progression of poorly differentiated HCC using TACE. Therefore, these factors are considered to be among those that are TACE-unsuitable. It is important to identify TACE-unsuitable cases even during the course of TACE treatment and to identify Len-suitable cases while maintaining hepatic reserve.
A sub-analysis of the REFLECT trial examined the time to progression of Child-Pugh score 6 before lenvatinib treatment to a Child-Pugh score of 7. The median time to progression to Child-Pugh score 7 was 23.7 mo in the sorafenib group, 23.9 mo in the lenvatinib 8 mg group, and 15.9 mo in the lenvatinib 12 mg group, respectively. There was no significant difference between the sorafenib and lenvatinib groups. Both sorafenib and lenvatinib affected liver functional reserve, but the difference was not significant[35].
Terashima et al[55] reported that patients treated with lenvatinib had a Child-Pugh score that was maintained or improved after 4 and 12 wk compared with those treated with sorafenib (P = 0.048 and P = 0.036, respectively) in clinical settings. Lenvatinib was identified as one of the factors associated with maintaining Child-Pugh scores. On multivariate analysis, a worse Child-Pugh score after 4 wk was an independent predictor of poor OS. Patients treated with lenvatinib for advanced HCC maintained their liver functional reserves better than those treated with sorafenib.
Uchikawa et al[56] assessed the ALBI score as an index of liver function during sorafenib and lenvatinib treatment. The median ALBI score was -2.53 before MTA treatment and -2.45, -2.44, and -2.36 post-2, -4, and -6 mo, respectively. The ALBI scores tended to increase during MTA treatment. When examined separately in the sorafenib and lenvatinib groups, no significant difference was observed between the two groups. However, the ALBI scores of the sorafenib group increased 2 mo after treatment initiation, and at 4 and 6 mo, significant differences were observed (P < 0.01). Based on the above, although the ALBI score may gradually decrease with the course of MTA treatment, lenvatinib may have a lower effect on the deterioration of the ALBI score than sorafenib.
Hiraoka et al[57] compared the ALBI score at the start of lenvatinib with scores after 2 and 4 wk; decreased liver function was common in the early stages after starting lenvatinib (within 4 wk, especially within 2 wk). It is important to introduce MTA to patients with as good liver function as possible, taking into account the early decrease in liver function due to lenvatinib. These results suggest that ALBI grade 1 and lenvatinib at the start of MTA treatment may be related to the maintenance of liver functional reserve.
In the REFLECT trial, patients were excluded if they had a treatment history of MTA, large HCC with more than 50% liver occupation, HCC with main portal vein invasion, Child–Pugh grade B, platelet count < 75 × 109/L, or apparent bile duct invasion.
We reported the efficacy and safety of lenvatinib in patients with unresectable HCC who did not meet the REFLECT eligibility criteria in clinical settings[58,59]. The ORR and the median PFS was similar between patients who met the REFLECT inclusion criteria and those who did not. Thus, the study results support the use of lenvatinib for patients with unresectable HCC who do not meet the REFLECT inclusion criteria.
But, the efficacy and tolerability of lenvatinib treatment differed according to the eligibility criteria of the REFLECT trial.
Chuma et al[60] reported that lenvatinib treatment offers benefits in highly advanced HCC (tumors with more than 50% liver occupation or main portal vein invasion) patients with good liver function or nodular-type tumors. Maruta et al[61] also reported that lenvatinib had potential profits for patients with advanced HCC with second- or later-line therapies and a high burden of intrahepatic lesions. The various characteristics identified in these studies may be useful as indicators for lenvatinib treatment in highly advanced HCC cases, which are considered treatment-resistant cancers.
In the REFLECT trial, among the 954 patients randomized to receive first-line lenvatinib (n = 478) or sorafenib (n = 476), 340 patients received subsequent anticancer medication during the survival follow-up period: 156 patients (32.6%) had received first-line lenvatinib, and 184 patients (38.7%) had received first-line sorafenib[62]. Of the patients who were treated with first-line lenvatinib, the most common subsequent carcinostatic substance was sorafenib (25.3%), and the most common subsequent non-anticancer medication treatment was TACE (56.6%). The OS of patients who were initially randomized to first-line lenvatinib (versus first-line sorafenib) and who received any subsequent anticancer medication was 20.8 mo vs 17.0 mo (HR = 0.87; 95%CI: 0.67-1.14). The OS of patients who initially received first-line lenvatinib (versus first-line sorafenib) and who did not receive any subsequent carcinostatic substance was 11.5 mo vs 9.1 mo (HR = 0.90, 95%CI: 0.75-1.09). In the -hoc analysis of all patients in the REFLECT study, the OS of those who received subsequent anticancer medication was prolonged compared with patients who did not receive any subsequent anticancer medication. In the REFLECT trial, the lenvatinib group had significantly longer PFS than the sorafenib group, but superiority in OS could not be demonstrated because of the effect of post-treatment on post-progression survival prolongation.
Lenvatinib is the preferred agent for TACE-unsuitable patients in the intermediate stage based on the high RR, survival benefit over TACE, and the possibility of conversion to resection or ablation therapy[49]. Sorafenib combined with surgical resection is a feasible option in advanced HCC patients; if sorafenib is effective, long-term survival may be achieved[63]. Additional surgery was the most significant factor predicting survival exceeding 3 years (P < 0.0001) and represents an independent prognostic factor (HR = 0.07, 95%CI: 0.003-0.40, P = 0.01)[64]. Long-term survival may be obtained for select patients with HCC receiving adequate additional surgical treatment, even after sorafenib induction. Therefore, lenvatinib treatment, which has a higher RR than sorafenib, is expected to increase the number of cases that can be switched to conversion therapy.
Many MTAs and immunotherapies have become available as treatment options for advanced HCC. Lenvatinib has been shown to have a good therapeutic effect, even in TACE-refractory and TACE-unsuitable cases. Intermediate-stage HCC beyond the up-to-seven criteria with Child-Pugh grade A/ALBI grade 1 usually does not benefit from TACE, whereas lenvatinib provides a better outcome than TACE.
Lenvatinib also has good therapeutic performance even in cases of non-simple nodular, poorly differentiated, tumor masses with more than 50% involvement of the liver and main portal vein invasion, which are generally recognized as having a poor prognosis with existing treatments. To maximize the therapeutic effect of lenvatinib in such cases, it is necessary to preserve liver function in patients with Child-Pugh grade A and ALBI grade 1 at the start of treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: The Japanese Society of Internal Medicine; The Japanese Society of Gastroenterology; Japan Gastroenterological Endoscopy Society; the Japan Society of Hepatology; Japan Society for Occupational Health.
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Antwi SO, Madian A S-Editor: Chang KL L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64276] [Article Influence: 16069.0] [Reference Citation Analysis (174)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3591] [Article Influence: 276.2] [Reference Citation Analysis (4)] |
| 3. | Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, Kuwahara A, Okusaka T. Current status of hepatocellular carcinoma in Japan. Chin Clin Oncol. 2013;2:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2870] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 5. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10244] [Article Influence: 602.6] [Reference Citation Analysis (2)] |
| 6. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4644] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 7. | Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S. Linifanib vs Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 8. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib vs sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 9. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Aviña J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib vs sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 10. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3808] [Article Influence: 544.0] [Reference Citation Analysis (1)] |
| 11. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4652] [Article Influence: 930.4] [Reference Citation Analysis (2)] |
| 12. | Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, Minoshima Y, Iwata M, Funahashi Y. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 358] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y, Ghosh S, Kitano H, Nomoto K, Matsui J, Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e0212513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 14. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2184] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 15. | Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9 Suppl 1:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 407] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 16. | Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 632] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 17. | Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005;109:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 663] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 19. | Matsuki M, Adachi Y, Ozawa Y, Kimura T, Hoshi T, Okamoto K, Tohyama O, Mitsuhashi K, Yamaguchi A, Matsui J, Funahashi Y. Targeting of tumor growth and angiogenesis underlies the enhanced antitumor activity of lenvatinib in combination with everolimus. Cancer Sci. 2017;108:763-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 21. | Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 22. | Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 23. | Ferrari SM, Bocci G, Di Desidero T, Elia G, Ruffilli I, Ragusa F, Orlandi P, Paparo SR, Patrizio A, Piaggi S, La Motta C, Ulisse S, Baldini E, Materazzi G, Miccoli P, Antonelli A, Fallahi P. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol Rep. 2018;39:2225-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 2033] [Article Influence: 135.5] [Reference Citation Analysis (1)] |
| 25. | Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1344] [Cited by in RCA: 1453] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 26. | Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Raja A, Park I, Haq F, Ahn SM. FGF19-FGFR4 Signaling in Hepatocellular Carcinoma. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 28. | Shigesawa T, Maehara O, Suda G, Natsuizaka M, Kimura M, Shimazaki T, Yamamoto K, Yamada R, Kitagataya T, Nakamura A, Suzuki K, Ohara M, Kawagishi N, Umemura M, Nakai M, Sho T, Morikawa K, Ogawa K, Ohnishi S, Sugiyama M, Mizokami M, Takeda H, Sakamoto N. Lenvatinib suppresses cancer stem-like cells in HCC by inhibiting FGFR1-3 signaling, but not FGFR4 signaling. Carcinogenesis. 2021;42:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Ogasawara S, Mihara Y, Kondo R, Kusano H, Akiba J, Yano H. Antiproliferative Effect of Lenvatinib on Human Liver Cancer Cell Lines In Vitro and In Vivo. Anticancer Res. 2019;39:5973-5982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 31. | Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 32. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4531] [Article Influence: 323.6] [Reference Citation Analysis (0)] |
| 33. | Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 34. | Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 901] [Article Influence: 90.1] [Reference Citation Analysis (1)] |
| 35. | Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 36. | Kudo M. Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma. Liver Cancer. 2018;7:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Tomonari T, Sato Y, Tanaka H, Tanaka T, Fujino Y, Mitsui Y, Hirao A, Taniguchi T, Okamoto K, Sogabe M, Miyamoto H, Muguruma N, Kagiwada H, Kitazawa M, Fukui K, Horimoto K, Takayama T. Potential use of lenvatinib for patients with unresectable hepatocellular carcinoma including after treatment with sorafenib: Real-world evidence and in vitro assessment via protein phosphorylation array. Oncotarget. 2020;11:2531-2542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, Zhu AX, Finn RS, Roberts LR; AASLD Panel of Experts on Trial Design in HCC. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1:158-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 39. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 40. | Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015;33:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 41. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1568] [Article Influence: 92.2] [Reference Citation Analysis (1)] |
| 42. | Yasui Y, Tsuchiya K, Kurosaki M, Takeguchi T, Takeguchi Y, Okada M, Wang W, Kubota Y, Goto T, Komiyama Y, Higuchi M, Takaura K, Hayashi T, Takada H, Tamaki N, Nakanishi H, Itakura J, Takahashi Y, Asahina Y, Enomoto N, Himeno Y, Izumi N. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol Res. 2018;48:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, Trevisani F, Bolondi L; Bologna Liver Oncology Group (BLOG). Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013;24:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer. 2019;8:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 45. | Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, Sakurai T, Nishida N, Kudo M. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer. 2015;4:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 46. | Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T; Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87 Suppl 1:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 47. | Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E, Suzuki H, Niizeki T, Shirono T, Nakano M, Suga H, Yamaguchi T, Yokokura Y, Noguchi K, Koga H, Torimura T. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol Lett. 2020;20:2257-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Eso Y, Takai A, Takahashi K, Ueda Y, Taura K, Marusawa H, Seno H. Combination of Mac-2 Binding Protein Glycosylation Isomer and Up-To-Seven Criteria as a Useful Predictor for Child-Pugh Grade Deterioration after Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 50. | Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Wada Y, Ikeda M, Ishii H, Izumi N, Nishida N. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 51. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 499] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 52. | Kawamura Y, Ikeda K, Hirakawa M, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F, Suzuki Y, Arase Y, Kumada H. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatol Res. 2010;40:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, Iwaisako K, Ikai I, Uemoto S. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, Fujiyama S, Hosaka T, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ikeda K, Arase Y, Hashimoto M, Kumada H. Pretreatment Heterogeneous Enhancement Pattern of Hepatocellular Carcinoma May Be a Useful New Predictor of Early Response to Lenvatinib and Overall Prognosis. Liver Cancer. 2020;9:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Terashima T, Yamashita T, Takata N, Toyama T, Shimakami T, Takatori H, Arai K, Kawaguchi K, Kitamura K, Sakai Y, Mizukoshi E, Honda M, Kaneko S. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatol Res. 2020;50:871-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Uchikawa S, Kawaoka T, Ando Y, Yamaoka K, Kosaka Y, Suehiro Y, Fujii Y, Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M, Takahashi S, Chayama K, Aikata H. Trends in Hepatic Functional Reserve of Patients with Hepatocellular Carcinoma Treated with Tyrosine Kinase Inhibitors. Oncology. 2020;98:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y, Michitaka K; On behalf of the Real-Life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Early Relative Change in Hepatic Function with Lenvatinib for Unresectable Hepatocellular Carcinoma. Oncology. 2019;97:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Sho T, Suda G, Ogawa K, Kimura M, Shimazaki T, Maehara O, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Baba M, Yamamoto Y, Kobayashi T, Meguro T, Saga A, Miyagishima T, Yokoo H, Kamiyama T, Taketomi A, Sakamoto N. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open. 2020;4:54-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Sho T, Suda G, Ogawa K, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Baba M, Ito J, Yamamoto Y, Kobayashi T, Meguro T, Saga A, Miyagishima T, Terasita K, Takagi T, Kamiyama T, Taketomi A, Sakamoto N. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol Res. 2020;50:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Chuma M, Uojima H, Hiraoka A, Kobayashi S, Toyoda H, Tada T, Hidaka H, Iwabuchi S, Numata K, Itobayashi E, Itokawa N, Kariyama K, Ohama H, Hattori N, Hirose S, Shibata H, Tani J, Imai M, Tajiri K, Moriya S, Wada N, Iwasaki S, Fukushima T, Ueno M, Yasuda S, Atsukawa M, Nouso K, Fukunishi S, Watanabe T, Ishikawa T, Nakamura S, Morimoto M, Kagawa T, Sakamoto M, Kumada T, Maeda S. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis. Hepatol Res. 2021;51:201-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Maruta S, Ogasawara S, Ooka Y, Obu M, Inoue M, Itokawa N, Haga Y, Seki A, Okabe S, Azemoto R, Itobayashi E, Atsukawa M, Sugiura N, Mizumoto H, Koroki K, Kanayama K, Kanzaki H, Kobayashi K, Kiyono S, Nakamura M, Kanogawa N, Saito T, Kondo T, Suzuki E, Nakamoto S, Tawada A, Chiba T, Arai M, Kanda T, Maruyama H, Kato N. Potential of Lenvatinib for an Expanded Indication from the REFLECT Trial in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2020;9:382-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Alsina A, Kudo M, Vogel A, Cheng AL, Tak WY, Ryoo BY, Evans TRJ, López López C, Daniele B, Misir S, Ren M, Izumi N, Qin S, Finn RS. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer. 2020;9:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Yoshimoto T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Iwahashi S, Saito YU, Takasu C, Ishikawa D, Teraoku H, Bando Y, Shimada M. The Outcome of Sorafenib Therapy on Unresectable Hepatocellular Carcinoma: Experience of Conversion and Salvage Hepatectomy. Anticancer Res. 2018;38:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Takeyama H, Beppu T, Higashi T, Kaida T, Arima K, Taki K, Imai K, Nitta H, Hayashi H, Nakagawa S, Okabe H, Hashimoto D, Chikamoto A, Ishiko T, Tanaka M, Sasaki Y, Baba H. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surg Today. 2018;48:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |