Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.1919
Peer-review started: March 12, 2021
First decision: April 6, 2021
Revised: April 17, 2021
Accepted: October 18, 2021
Article in press: October 18, 2021
Published online: December 15, 2021
Processing time: 277 Days and 0.3 Hours
Hepatocellular carcinoma (HCC) is the sixth most common primary malignancy worldwide, and the third most common cause of death among cancers world
Core Tip: Hepatocellular carcinoma (HCC) is the sixth most common cancer world
- Citation: Ezzat R, Eltabbakh M, El Kassas M. Unique situation of hepatocellular carcinoma in Egypt: A review of epidemiology and control measures. World J Gastrointest Oncol 2021; 13(12): 1919-1938
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/1919.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.1919
Hepatocellular carcinoma (HCC) is the sixth most common primary malignancy worldwide[1] with higher incidence and prevalence in Africa and Asia[2]. The in
In Egypt, the relation between hepatitis C virus (HCV) and HCC is an important research area. Firstly, Egypt has a high recorded HCV transmission rate, with around 416000 new infections each year[4]. Secondly, there is known to be a relationship between HCV and HCC development. Thirdly, the programmed screening and follow up that was initiated by the government increased the number of known cases of individuals having both diseases. According to a study carried out by Ziada et al[5], 108 out of 514 patients diagnosed with HCV infection (21%) had focal lesions detected by ultrasound. In another study carried out by Abd-Elsalam et al[6], HCC occurred more frequently in patients with HCV than in those with hepatitis B virus (HBV) infection. These results may indicate the main predisposing factor for the development of HCC in Egypt.
A possible cause for the increase in detection of HCC in Egypt is the mass screening program that was implemented by the government for detecting and treating HCV. Due to this program, many patients were diagnosed and treated for HCC. According to a study carried out by Shaker et al[7], 75% of identified HCC cases came from rural areas in Egypt, with 45.7% of individuals ranging in age between 51-60 years.
According to the global cancer observatory, liver cancer represented 19% of all newly diagnosed cases in all ages and both sexes in 2018, with an incidence rate of 32% and a mortality rate of 31%[8].
HCV protein expression in infected hepatic cells causes mutation and malignant transformation leading to the development of HCC[9-11]. Repeated inflammation, damage and regeneration are believed to be the main cause of malignant transfor
Another review was published by Reig et al[17], debating about revising the published data, and they concluded that no solid evidence could be reached about the relationship between HCC recurrence and DAA therapy[22]. El Kassas et al[23] concluded that there is a possible role of DAAs in HCC recurrence.
Egypt recorded the highest prevalence of HCV worldwide, as a consequence of unsafe IV treatment of schistosomiasis in 1950s until the 1980s[24]. A decline was recorded in the prevalence of HCV infection from 14.7% in 2008 to 10% in 2015. This was attributed to the aging of the group who received antischistosomal treatment[25,26].
In Egypt, genotype 4 is the main genotype, occurring in up to 92.5% of infected patients, followed by genotype 1 (3.6%)[27-30]. A study demonstrated that at least in Egypt, the lymphotoxin alpha gene mutation may have a role in susceptibility to HCV infection, and the subsequent development of clinical manifestations[31].
DNA viruses can be incorporated into a host genome[32], inducing malignant trans
In Egypt, the population prevalence of HBV was 1.4%, with an HBV-HCV co-infection rate of 0.06%[38]. The nationwide vaccination program has decreased the prevalence of HBV infection considerably[39,40]. The HBeAg negative variant was found to be highly prevalent in Egypt, and represents a late phase of HBV infection with persistent viral replication. This situation will lead to early development of cirrhosis[41]. However, 16% of patients with HCV have an occult B infection[42] A study carried out by Fouad et al[43] found that 81.9% of their chronic HBV cohort were HBeAg negative.
Of patients with liver cirrhosis, 3%-5% develop HCC annually[44]. In Egypt, HCC represents nearly 70% of all liver tumors[45].The increased incidence in Egypt may be related to the increased screening carried by the government. and a greater focus on HBV and HCV as predisposing factors in the past few years[46].
The liver is the main organ involved in the metabolism of chemical agents[47]. It has a characteristic blood supply, and is involved in many metabolic and excretory pro
In Egypt, nearly 26% of the population works in agriculture[48], and thus have a high risk of exposure to pesticides. A study carried out by Abou El Azm et al[49] found that 13.87 % of the total HCC in Egypt was associated with risk factors other than HVB or HCV, predominantly pesticides, and superphosphate and ammonium sulfate fertilizers (94.87%, P < 0.001) with significant exposure occurring in industry, farming, and residences. The HCC in these cases had specific criteria, being solitary, of smaller size, and having lower alpha fetoprotein (AFP) titers[49].
Aflatoxins are known to have a major role in the development of HCC in Egypt. They are known carcinogenic metabolites of molds, mainly Aspergillus flavus, and parasites that contaminate many agricultural products, such as peanuts, maize, and cotton seed[50].
Beside molds, a study conducted on desserts in Egypt showed that aflatoxin B1 (AFB1) was detected at above the acceptable limits of 2 ppb in 70% of samples of one of the dairy desserts, and Aflatoxin M1 exceeded the limits in 10% of each type of sample[51]. High serum levels were detected in Egyptians with HCC by a study that was carried out by Dilber et al[52]. AFB1 is the main metabolite produced, and is the most carcinogenic, teratogenic, and mutagenic metabolite[53]. It was present in high levels in those presenting with multiple hepatic focal lesions over 5 cm in diameter[54]. Anwar et al[55] found that presence of Aflatoxins and HCV is connected to hepatic disease progression to G3S3 which indicates HCC. Aflatoxin levels were found to be significantly higher in HCC patients than in cirrhotic individuals and controls in a study conducted by Sharaf-Eldin et al[56].
Non-alcoholic fatty liver disease (NAFLD) produces abnormal fat accumulation in the liver, without significant alcohol ingestion. NAFLD includes a broad spectrum of liver conditions ranging from steatosis and reaching up to cirrhosis. It is considered to be the most common liver disease related to obesity[57], and is a condition that can progress to HCC[58]. HCC development is related to disease progression from NAFLD to non-alcoholic steatohepatitis (NASH). NAFLD can accelerate the disease burden of HCV in terms of morbidity and mortality[59]. A study that was carried out on school children in Egypt, fatty liver was prevalent in 15.8% of the study group, and increased significantly with age (P = 0.004)[60]. NAFLD (56.8%) was a predominant feature among the study population in a study that was conducted by Abd El-Wahab EW et al[61] on 190 adults seeking health check-ups at the outpatient clinic of a tertiary care hospital in Alexandria, Egypt. Fatty liver was detected in 47 (65.3%) children, and in 52 (62.7%) adults in another Egyptian study by Wafaa et al[59]. A study concluded that NASH is present in 5.3% of Egyptian patients presenting with HCC[62]. This finding reflects the high prevalence of the condition in Egypt, and the subsequent increased risk of HCC transformation. Screening and early detection of the condition indicates the importance of avoiding further burdens on public health, as in the campaign carried out by the Egyptian government last year with respect to the detection of obesity, diabetes, and hypertension as predisposing causes for NAFLD.
Excessive alcohol consumption is a well-known risk factor for developing HCC[62]. In the European Union, 60%-80% of liver-related mortality is caused by excessive drinking[63] and alcohol-related chronic disease is considered to be the second most common indication for liver transplantation, accounting for approximately 40% of all primary liver transplants[64]. In Egypt, this risk is low[48,65-67]. Heavy alcohol consumption increases the risk of HCC by up to 16%[68]. The risk is increased by 5- to 7-fold with heavy ethanol consumption for more than 10 years[69,70].
Smoking is another factor that may lead to HCC, due to the tobacco[71]. A Korean study reported a connection between primary liver cancer and smoking with the risk increased by up to 50% compared with non-smokers[72]. Bakir and Ali-Eldin[73] concluded that 64% of Egyptian patients with HCC are smokers. Abou El Azm et al[49] reported that heavy smoking is one of the primary risk factors for non-B non-C HCC in Egypt. Another study mentioned smoking as one of the main causative agents for HCC in Egypt[5]. Another Egyptian study documented an increased risk of HCC development in patients with a smoking pattern of 20 cigarettes per day for more than 29 years[74].
Around 1.9 billion people around the world are overweight, and 600 million are suffering from obesity[75]. Obesity is related to the development of many metabolic disorders, including diabetes mellitus and hypertension, with an increased burden of HCC development. Premorbid obesity is associated with up to a two-fold risk of HCC related mortality[75]. It has been suggested that for every 5 unit increase in body mass index (BMI), there is a 39% increased risk of HCC[76]. In another study, carried out by Calle et al[77], the HCC related mortality in obese men (BMI, 30-34.9 kg/m2) was 1.9 times the number in men with normal BMI (BMI 18.5-24.9 kg/m2).
In Egypt, a study on primary school students showed that the overall prevalence of obesity and overweight was 13.9% and 16.2% respectively[78]. In adults, it is estimated to be present in 61%70% of the whole population aged 20 and above, with a prevalence of 18%22% in men and 39%48% in women[79]. Aitsi-Selmi et al[80] investigated the relationship between wealth, education, and obesity among 49058 Egyptian women using the Demographic and Health Surveys’ datasets. Obesity was mainly recorded among women with a primary education or less, and whether they are poor or wealthy. A survey of young people in Egypt[81] found that consuming more white bread and carbonated drinks is directly related to their economic state.
Some hereditary liver diseases with genetic mutations are believed to carry a risk for HCC development. These diseases are Wilson disease, hemochromatosis, alpha-1 antitrypsin deficiency, tyrosinemia, glycogen storage diseases, and porphyrias. The same is true for polymorphisms with increased risk for HCC. Polymorphisms in UGT1A7, MnSOD, and IL-1B were reported to be significantly associated with risk[82]. HCV and HBV infection are reported to increase the risk of gene mutation, leading to the development of HCC[83-87].
In an Egyptian study, the TNF-α-308 G > A polymorphism was associated with increased HCC risk in an Egyptian population, but no significant difference was found for cytokines interleukin (IL)-1β and IL-10[88]. In another study on Egyptian patients, XRCC1 G28152A (rs25487) and XRCC7 G6721T (rs7003908) polymorphisms were found to have a role in susceptibility to HCC in the Egyptian population[89].
Epidermal growth factor gene polymorphism 61*G was found to be positively associated with HCC risk in Egyptians. Uncreased concentration of EGF was asso
The prevalence of hereditary hemochromatosis in Egypt is reported to be 0.5%[50]. This indicates that hereditary disorders are not a major cause of HCC.
In 2015, 10% of the population tested positive for HCV antibodies, which would amount to around 5.5 million persons at that time[91]. As a major cause of HCC in Egypt, after the World Health Assembly’s decision in 2016 to eliminate HCV, the Egyptian government decided to begin a nationwide campaign for the detection and treatment of HCV in Egypt[92]. More than two million individuals were treated by the year 2018 under the umbrella of this campaign, with cure rates reaching 90%. Disease elimination was achieved mostly by the decrease in the cost of direct-acting antiviral drugs implemented by the Egyptian government. This process was first applied to identified chronic patients. The government then began mass screening of the population, to facilitate rapid and effective elimination of the disease. Screening was done in all of the hospitals related to universities, military hospitals, rural health units, and police hospitals. This was achieved by moving teams to other areas, using gathering spaces, factories, and open places to aid in the screening. Finger prick rapid diagnostic tests were used. Patients reported positive were scheduled for evaluation and treatment plans. Between October 2018 and April 2019, 79.4% of the targeted population participated spontaneously in the screening, with higher female than male participation (84.5% vs 74.6%). By the end of September 2019, 1148346 (76.5%) of screened individuals were reported to have viremia, and treatment was started in 91.8% of them. Out of these people, 465992 reached 12-wk follow up after ending treatment. At this stage, 386103 (82.9%) had a known treatment outcome, and 381491 (98.8%) of those with a known outcome had a SVR. Of the 93651 patients with viremia who did not show up for treatment, 53445 who were reached reported having treatment in private[93].
There have been no screening programs for HCC in Egypt until now. Because HCC in Egypt is mostly diagnosed early, as more patients are diagnosed under surveillance, the survival duration is longer than in other African countries[94]. The effect of treatment itself is controversial. A study carried by El Kassas et al[23] reported: “Our data point to a high (i.e., almost 4 times) increased rate of recurrence after DAA treatment for patients with a history of successfully treated HCC, when compared to similar patients who were not given DAAs”.
After HCV elimination, decreased rates of HCC were expected, but Reig et al[17] found exactly the opposite tendency after using direct-acting antiviral drugs for HCV treatment.
This work was followed by a paper that emphasized the early occurrence of HCC in patients receiving DAAs for HCV[18]. Another study produced different results, in which no difference in the cumulative incidence was found in developing de novo HCC in patients with HCV and those treated by DAAs or interferon-based therapy[95]. Similar results were reached by Cabibbo et al[96]. A study on patients with HCV-related cirrhosis treated with DAAs and subsequently developing HCC reported a relation between age, Child-Pugh classification, liver stiffness, history of HCC, and the development of HCC[18]. In 2019, a study on 7344 patients concluded that DAAs decrease the risk of developing HCC[97].
An Egyptian study concluded that DAAs do not increase the risk of HCC recurrence, but still did not recommend abolishing it, rather implementing close follow up[98]. Another study denied the occurrence of HCC after DAAs although a high incidence of recurrence was still found. This study also suggested that high AFP before treatment is a good predictor for developing HCC[99].
Immunization for HBV and protection against HCC was discussed in a study on an analysis of 1509 patients with HCC in Taiwan. The study concluded that risk reduction of HCC is obvious after immunization of infants against HBV[100]. The HBV vaccination program in Egypt began in 1992 with a schedule of 2, 4, and 6 mo of age. This program was not associated with simultaneous screening for pregnant women[101]. A multicenter study was carried on 3600 children aging from 9 mo to 16 years old to assess the effectiveness of the Egyptian vaccination program. The study concluded that the vaccination is protective from 1 years to 16 years post vaccination[40]. Another study assessed the benefit of follow up post vaccination response and seroprotection persistence, to determine the importance of booster doses in healthy subjects. A protective level of HBsAb was found (> 10 IU/mL) among 66.7% of all individuals studied[102]. The risk of HCC danger is escalated by co-infection with occult HBV in HCV patients[103].
Screening programs gain value when the benefits from screening are greater than the expected harm. A large randomized controlled trial showed benefits for screening noncirrhotic HBV patients for the development of HCC, leading to improved early detection, better treatment, and better survival rates[104]. An association between screening for HCC and improvement in three-year survival rates is well established[105]. A study observed the difference between the survival rates of HCC in Japan and in Hong Kong. Japan has an intensive screening program unlike Hong Kong. The survival rate was 52 vs 17.8 mo[106]. In spite of the psychological or financial harm that could result from screening for HCC, the benefits overweigh the harm. Cirrhotic patients show an annual risk of 2%-4% of developing HCC which makes screening highly recommended in all cirrhotic patients whatever the etiology[107,108].
The risk of progression to HCC in non-cirrhotic patients has ranges from 7% to 54%, varying according to etiology and geographic distribution[109]. The most common etiological factors for this condition are obesity, aflatoxins, NAFLD, genetic mutations, smoking, inherited diseases, and sex hormones[107,109-112]. Non-cirrhotic liver HCC has a better prognosis and better results following surgical intervention than cirrhotic liver HCC[113]. In the European Association for the Study of the Liver 2018 report, a risk stratification model was recommended for non-cirrhotic HCC patients, namely PAGE-B (platelet, age, gender, hepatitis B), that is currently used in non-cirrhotic HBV patients[114,115].
The risk of HCC development in cirrhotic patients is from 2% to 4% annually. This high risk makes screening an obligation for all cirrhotic patients, whatever their etiology[107,111]. Screening is mainly to be done for compensated cirrhosis with Child-Pugh class A and B, while class C is to be offered liver transplantation[116].
Ultrasound is the most widely used imaging technique for regular screening for HCC. It has many advantages, being easy, readily available, non-invasive, and inexpensive. The sensitivity of ultrasound in detecting HCC is not more than 45%[117], especially in lesions less than 1 cm in diameter[118]. It is affected by the operator, the patient ability to hold their breath during examination, and the nodularity of the liver, which makes the detection of new lesions difficult, with some areas unreachable, like the dome of the liver. Obesity and NASH renders examination difficult which, decreasing the efficacy of the procedure[119]. In such cases, magnetic resonance imaging (MRI) and computed tomography (CT) scanning can replace ultrasound[120,121], but they are not cost effective, so they are not considered as first-line screening methods for HCC[119,121].
AFP is the biomarker most widely used in screening for HCC[122]. Although it is readily available, inexpensive, and easy to perform, its addition in the guidelines along with ultrasound was debatable. The American Association for the Study of Liver Diseases recommends using ultrasound, with the use of AFP to be judged by the clinician according to the patient’s condition[107]. However, European guidelines recommend using ultrasound with no AFP needed[123].
In Egypt, HCV is the main etiological factor for liver cirrhosis, followed by HCC. Liver elastography is a documented method for assessing liver stiffness. A study investigated its role in the early detection of HCC in HCV cirrhotic patients. It recorded cutoff value of 24 kPa for diagnostic prediction of HCC produced sensitivity 100%, specificity 83.3%, PPV 94.5%, NPV 77.3%, and AUC 89%[124]. Another study discussed the superiority of an abbreviated MRI protocol over AFP and ultrasound in detecting small hepatic focal lesions in post HCV cirrhotic patients[125].
A scoring system was suggested by Abdelaziz et al[126]. The HCC Multidisciplinary Clinic-Cairo University (HMC-CU) score (Logit probability of HCC = -2.524 + 0.152 × age -0.121 × Hb -0.696 × INR -1.059 × Alb + 0.022 × AFP + 0.976 × Sex. Male = 1, Female = 0), with a cutoff of 0.559 was superior to other scores for predicting HCC, having a sensitivity of 90% and a specificity of 80.6%. In 2010, El-Zayadi et al[127] investigated the effect of surveillance of HCC on tumor staging and treatment options in Egypt. The study divided the patients into two groups: (1) For those who followed screening regularly; and (2) Who were diagnosed as HCC as first presentation with no screening program followed. They produced variable results three months after interval screening was suggested, as the doubling time of the tumor size is from 1 mo to 19 mo, and as HCV is the main predisposing factor in Egypt. The study reported that surveillance increased the detection of small lesions in the absence of vascular invasion.
A prospective study carried out by Gomaa et al[46] on 2000 patients diagnosed with HCC reported that BCLC has the best prognostic stratification for Egyptians with HCC. Salama et al[128] suggested adding leptin to AFP for HCC screening in Egyptians. All of these studies were trials from separate centers to detect and screen for HCC in Egypt.
HCC is a disease with different modalities of treatment. Surgical resection comes in the first place, followed by liver transplantation. Ablative techniques come next, including ethanol (percutaneous ethanol injection), microwave (MWA) or radiofrequency (RFA), catheter-directed trans arterial chemoembolization (TACE) or radioembolization (TARE). Last comes external beam radiation therapy in the form of stereotactic body radiation therapy or proton beam therapy, systemic targeted small molecule tyrosine kinase inhibitors (TKIs), check-point inhibitor immunotherapy, and investigational agents.
A multidisciplinary approach has been now widely recognized and is the mainstay in managing HCC in different health centers all over Egypt. This approach includes a scientific committee with the patient of HCC presented to it, and through discussion is performed, along with counseling the patient with different treatment options.
Surgery for HCC includes tumor resection or liver transplantation. Liver transplan
In Arab countries, 3804 liver transplants were done between the period 1990–2013, of which living donor liver transplantation (LDLT) was 80%, and deceased donor liver transplantation was 20%. Fifty-six percent of the reported cases were in Egypt[137]. In Egypt, the only source for a liver graft is from a living donor. From 2001 to 2019, 1230 cases of liver transplantation were reported from three transplantation centers in Egypt. Of them, 394 cases were HCC transplanted patients. In a retrospective study done by the surgical team in Dar ALfouad, Egypt, 60 patients with HCC who had undergone liver transplant within and beyond the Milan criteria were investigated for their clinical outcome. The results were as follows: “Overall 1-, 3-, and 5-year survival rates were 98.3%, 93.5%, and 71.4%. Overall disease-free survival rates at 1, 3, and 5 years were 96.6%, 93.5%, and 64.2%. There was no statistically significant difference in overall survival time between patients within and beyond the Milan criteria. Factors affecting recurrence were the tumor grade, lobar distribution, size of the largest nodule, and the total tumor burden in the explanted liver”[138]. In a study done by Galal et al[139], the researchers concluded that AFP may predict HCC recurrence after LDLT (area under the curve = 0.806) at cutoff values of more than 66 ng/mL, with 60% sensitivity, 94.3% specificity, 42.9% positive predictive value, and 97.1% negative predictive value.
We in Egypt have certain constants regarding liver transplantation as an option for HCC treatment, the major issue being the high cost of the operation, and the difficulty of finding a proper matched donor, as only living donor transplant is allowed in Egypt. Nevertheless, the success rate of liver transplant in Egypt is comparable to international results. So, it became of importance to allow health insurance coverage for liver transplantation operations in public health centers as a better treatment option for Egyptian HCC patients.
Using thermal ablation for hepatic focal lesions has many advantages, such as the ability to repeating the maneuver, low morbidity and very few complications[140]. MWA ablation provides better results in areas with high blood flow, or near vessels, because it is not affected by the heat sink effect[141]. An Egyptian study carried out by Soliman et al[142] aimed to investigate the efficacy of MWA ablation in risky areas adjacent to other organs, near the diaphragm, and near blood vessels. In the study group, MWA reached ablation rates of 100%, 75%, and 87.5% for lesions close to the gall bladder, perivascular lesions, and subcapsular lesions, respectively. Another study done at Menoufia university, Egypt, compared single local ablative and combined techniques in HCC. The combined locoregional method provided better results[143]. However, Kamal et al[144] found no difference between MWA ablation and RFA ablation in treating HCC. Due to the high incidence of HCC related HCV in Egypt, the high risk of recurrence in those patients was investigated by Sharaf-Eldin et al[145]. The study concluded that in those patients, the presence of hepatomegaly, heterogenous liver, and splenomegaly, a sign of portal hypertension, together with tumor factors such as large size, bilobar affliction, and lesions near the liver capsule, showed a significant association with tumor recurrence.
TACE is the treatment of choice for patients with intermediate stage HCC, according to BCLC[123]. It is also the standard treatment in non-resectable HCC[107]. It is considered to be a palliative treatment, with positive impacts on survival and quality of life[146]. Since Seldinger described his technique in 1953, many intravascular procedures have been used[147]. This was followed by percutaneous selective an
Guidelines recommend TACE as the standard line of treatment for BCLC-B, but the results are still not very satisfactory[157]. Radiation from external beams to the liver is not effective in delivering lethal doses, as HCC is radio-resistant[158]. Radioembolization with Yttrium-90 microspheres is a recently used catheter-based treatment for HCC. It can be performed safely in patients with portal vein thrombosis, due to its low embolic effect[159]. TARE has the advantages of short hospital stay[160], prolonged time until progression[148], and long progression free survival[161].
Hamed et al[162] investigated the efficacy of Yttrium-90 on 20 Egyptian patients with intermediate and advanced HCC, with good outcomes even in the presence of compromised liver functions. Similar results were produced by Hetta et al[163], in a study in which TARE was investigated in advanced HCC with or without portal vein thrombosis. TARE Y90 showed the best results, especially in advanced stage disease, when compared to TACE in a study on 86 Egyptian patients with intermediate HCC[164].
Treatment for advanced HCC is now based on systemic therapy relying on TKIs, anti-angiogenesis agents, and immunotherapy[123]. Before the development of sorafenib, no drug was available that could provide this improved overall survival in such patients[165]. Sorafenib is an oral multi-kinase inhibitor with anti-proliferative and anti-angiogenic properties. It acts by inhibiting vascular endothelial growth factor receptor (VEGFR) -2 and -3 tyrosine kinases, platelet-derived growth factor receptor (PDGFR)-β tyrosine kinases, and rapidly accelerated fibrosarcoma kinases[166]. Sorafenib was first used in cases with well-preserved liver function, but results from the Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its treatment with sorafenib (GIDEON) found a similar safety profile, irrespective of Child-Pugh staging[167]. Routine use of sorafenib in patients with underlying liver dysfunction is not recommended.
Lenvatinib (Lenvima, Eisai) is an oral TKI of fibroblast growth factor receptor (FGFR), VEGFR, PDGFR-α, rearranged during transfection, and KIT. It has been accepted as a first-line therapy for unresectable HCC since August 2018[168]. Regorafenib (Stivarga, Bayer) came next. It is a potent oral inhibitor of angiopoietin-1 receptor (Tie2), VEGFR, PDGFR, and FGFR, and was studied by Bruix and colleagues in patients who did not respond to sorafenib. It was approved by the FDA based on this multinational study[169].
A study on sorafenib in Egypt claimed that it cannot be used except in patients with Child A and low disease burden[170]. The same recommendation was made by Abdel-Rahman et al[171]. When sorafenib was studied in Egyptian patients with advanced HCC, it gave better outcomes, overall survival, and progression free survival when compared to no treatment[172]. It is, however, considered to be a costly treatment for the Egyptian patients, as was found in a study carried out by Hamdy Elsisi et al[173], in which they concluded that “sorafenib does offer increased survival and quality of life at an increased cost but at an incremental cost effective ratio that exceeds the nationally accepted cost-effectiveness threshold”. Hanafy showed that a combination of sorafenib and low dose capecitabine is effective in advanced HCC in an Egyptian population[174].
A comprehensive summary of studies discussing the results of different treatment modalities for HCC in Egypt is presented in Table 1.
| Treatment modality | Ref. | Design | Sample size | Summary of the most important results |
| Resection | Senbel et al[134] | Retrospective | 84 | Median OS was 50 mo |
| Zakaria et al[132] | Retrospective | 204 | Predictors of decreased survival: serum AFP level > 400 ng/mL, TTV > 65.5 cm³, microvascular invasion, postoperative decompensation | |
| Makhlouf et al[135] | Retrospective | 28 | Predictors for developing post-resection liver failure: low serum albumin-higher child score | |
| Liver transplant | Kamal et al[144] | Retrospective | 60 | Overall disease-free survival rates at 1, 3, and 5 yr were 96.6%, 93.5%, and 64.2%; Overall, 1-, 3-, and 5-yr survival rates were 98.3%, 93.5%, and 71.4%. Factors affecting recurrence were the tumor grade, lobar distribution, size of the largest nodule, and the total tumor burden in the explanted liver |
| Galal et al[139] | Retrospective | 75 | AFP may predict HCC recurrence after LDLT (area under the curve = 0.806) at cutoff values of more than 66 ng/mL | |
| MWA | Soliman et al[142] | Prospective | 88 | MWA reached ablation rates of 100%, 75%, and 87.5% for lesions close to the GB, perivascular lesions, and subcapsular lesions, respectively |
| Radio frequency | Sharaf-Eldin et al[145] | Retrospective | 45 | Hepatomegaly, heterogenous liver, and splenomegaly, a sign of portal hypertension, together with tumor factors such as large size, bilobar affliction, and lesions near the liver capsule, showed a significant association with tumor recurrence |
| Nouh et al[143] | Prospective | 60 | Combined techniques (RFA and percutaneous ethanol injection) give the best results for management of HCCs in comparison with individual techniques | |
| TACE | Farouk et al[154] | Retrospective | 27 | Successful TACE for down-staging of HCC can be achieved in the majority of carefully selected patients and is associated with excellent post transplantation outcome |
| Fouad et al[155] | Prospective | 99 | Improved quality of life after three months of TACE | |
| TARE | Hamed et al[162] | Prospective | 20 | The complete response, partial response, stable disease and disease progression rates for the study sample after 3 mo using the conventional RECIST criteria was 0%, 55%, 30% and 10%, while after 6 mo it became 0, 50%, 20% and 25% respectively |
| Hetta et al[163] | Prospective | 40 | The overall response (complete or partial response) was exhibited by 9% of patients, stable disease exhibited by 80% of patients, progressive disease seen in 11% of patients after one month of TARE | |
| El Fouly et al[164] | Prospective | 86 | The median OS (TACE: 18 mo vs TARE Y-90: 16.4 mo) and the median TTP (TACE: 6.8 mo vs TARE Y-90: 13.3 mo) were not statistically different between TACE and TARE group | |
| Systemic therapy | Nada et al[170] | Retrospective | 130 | The median overall survival of patients with HCC treated with sorafenib was 5 mo (CI: 4.166-5.834), and progression free survival was 4 mo (CI: 3.479-4.521) |
| El Baghdady et al[172] | Prospective | 55 | The one-year OS was 0.0% vs 75.5% (P = 0.008) in control and sorafenib respectively. Median PFS was 5 mo vs 12 mo in control group and sorafenib respectively (P = 0.008). Sorafenib treatment showed a better outcome OS, PFS and QOL as compared to no-treatment in Egyptian patients with advanced Hepatocellular Carcinoma |
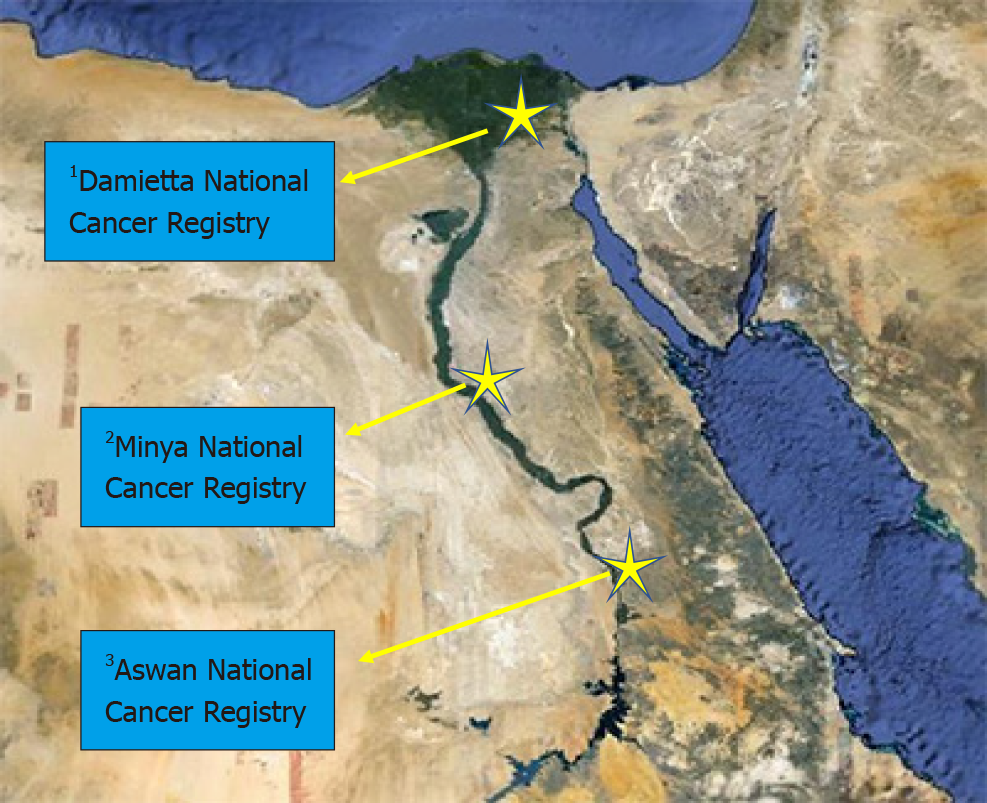
The Egyptian National Cancer Registry Program (NCRP) was launched in 2008 to represent a source for cancer incidence figures in Egypt[175]. NCRP stratified Egypt into 3 geographical areas: lower, middle, and upper. Data are regularly collected from specialized cancer treatment centers that are scattered all over the country map. Results of NCRP showed that HCC was the first among the most frequently observed cancers in lower and middle Egypt and the 2nd in upper Egypt (Figure 1).
A major breakthrough was noted after the national campaigns of fighting and screening HCV, in which all of the population was screened for HCV, and basic laboratory results and ultrasonography were performed[93,176]. Many HCC patients were discovered and provided with treatment options. Despite the high safety profile of DAAs therapy, which enabled treatment of advanced cases and with expected lower incidence rate of HCC post-treatment, there were some contradictory reports on HCC incidence rates post SVR[177].
The major drawback in our campaign in Egypt was lack of a program after achieving SVR for continued screening for HCC after cure of HCV, with a resultant faulty impression of the patient that they were completely cured, with no need for lifetime follow up and screening for HCC.
This is why it is important to highlight the importance of screening for HCC for all individuals with SVR for cirrhotic features for life. Increasing public awareness of the importance of the screening is warranted just as in the national screening campaign for breast cancer in Egypt 2020[178].
Major screening programs in Egypt, like the National Initiative of 100 Million Healthy Individuals and Breast Cancer 2020 have produced high success rates[178]. Now it is time for proper guidance and screening programs for HCC in Egypt.
HCC is a disease posing a rising burden in Egyptian society. HCV is the main etiology in our country, with an expected decline following the decline in HCV incidence. HBV is the second most important etiology in Egypt. Mass vaccination campaigns are the only way to stop the disease and ameliorate its effects. A registry of the different modalities for management for HCC is still lacking in Egypt, and will require a more systematized effort between different centers. A national campaign is crucial for early diagnosis and management.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, No. 001, President.
Specialty type: Oncology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu Y S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4108] [Article Influence: 586.9] [Reference Citation Analysis (6)] |
| 2. | Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 3. | Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 4. | Kandeel AM, Talaat M, Afifi SA, El-Sayed NM, Abdel Fadeel MA, Hajjeh RA, Mahoney FJ. Case control study to identify risk factors for acute hepatitis C virus infection in Egypt. BMC Infect Dis. 2012;12:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 5. | Ziada DH, El Sadany S, Soliman H, Abd-Elsalam S, Salama M, Hawash N, Selim A, Hamisa M, Elsabagh HM. Prevalence of hepatocellular carcinoma in chronic hepatitis C patients in Mid Delta, Egypt: A single center study. J Egypt Natl Canc Inst. 2016;28:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Abd-Elsalam S, Elwan N, Soliman H, Ziada D, Elkhalawany W, Salama M, Hawash N, Arafa M, Badawi R, Shehata WM, Khalil HS, Elmashad N. Epidemiology of liver cancer in Nile delta over a decade: A single-center study. South Asian J Cancer. 2018;7:24-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Shaker MK, Abdella HM, Khalifa MO, El Dorry AK. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int. 2013;33:1601-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4901] [Article Influence: 700.1] [Reference Citation Analysis (1)] |
| 9. | Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 10. | El-Houseini ME, Ismail A, Abdelaal AA, El-Habashy AH, Abdallah ZF, Mohamed MZ, El-Hadidi M, Cho WCS, Ahmed H, Al-Shafie TA. Role of TGF-β1 and C-Kit Mutations in the Development of Hepatocellular Carcinoma in Hepatitis C Virus-Infected Patients: in vitro Study. Biochemistry (Mosc). 2019;84:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Neamatallah M, El-Bendary M, Elalfy H, Besheer T, El-Maksoud MA, Elhammady D, Abed S, Elegezy M, Kandeel L, Eldeib D, Mousa N, Abd El-Hafeez M, El-Gilany AH, Esmat G. Impact of Toll-like Receptors 2(TLR2) and TLR 4 Gene Variations on HCV Susceptibility, Response to Treatment and Development of Hepatocellular Carcinoma in Cirrhotic HCV Patients. Immunol Invest. 2020;49:462-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Borgia M, Dal Bo M, Toffoli G. Role of Virus-Related Chronic Inflammation and Mechanisms of Cancer Immune-Suppression in Pathogenesis and Progression of Hepatocellular Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Doi AM, Hill G, Seely J, Hailey JR, Kissling G, Bucher JR. alpha 2u-globulin nephropathy and renal tumors in national toxicology program studies. Toxicol Pathol. 2007;35:533-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol. 2021;12:S361-S373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 15. | Chang KC, Wu YY, Hung CH, Lu SN, Lee CM, Chiu KW, Tsai MC, Tseng PL, Huang CM, Cho CL, Chen HH, Hu TH. Clinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapy. Br J Cancer. 2013;109:2481-2488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | El Kassas M, Elbaz T, Salaheldin M, Abdelsalam L, Kaseb A, Esmat G. Impact of treating chronic hepatitis C infection with direct-acting antivirals on the risk of hepatocellular carcinoma: The debate continues - A mini-review. J Adv Res. 2019;17:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 18. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 19. | Ohki T, Sato K, Kondo M, Goto E, Sato T, Kondo Y, Akamatsu M, Sato S, Yoshida H, Koike Y, Obi S. Effectiveness of direct acting antiviral agents for hepatitis C virus related recurrent hepatocellular carcinoma patients who had multiple courses of recurrence. J Viral Hepat. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Imai K, Takai K, Hanai T, Suetsugu A, Shiraki M, Shimizu M. Sustained virological response by direct-acting antivirals reduces the recurrence risk of hepatitis C-related hepatocellular carcinoma after curative treatment. Mol Clin Oncol. 2020;12:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Lui FH, Moosvi Z, Patel A, Hussain S, Duong A, Duong J, Nguyen DL. Decreased risk of hepatocellular carcinoma recurrence with direct-acting antivirals compared with no treatment for hepatitis C: a meta-analysis. Ann Gastroenterol. 2020;33:293-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sapena V, Enea M, Torres F, Celsa C, Rios J, Rizzo GEM, Nahon P, Mariño Z, Tateishi R, Minami T, Sangiovanni A, Forns X, Toyoda H, Brillanti S, Conti F, Degasperi E, Yu ML, Tsai PC, Jean K, El Kassas M, Shousha HI, Omar A, Zavaglia C, Nagata H, Nakagawa M, Asahina Y, Singal AG, Murphy C, Kohla M, Masetti C, Dufour JF, Merchante N, Cavalletto L, Chemello LL, Pol S, Crespo J, Calleja JL, Villani R, Serviddio G, Zanetto A, Shalaby S, Russo FP, Bielen R, Trevisani F, Cammà C, Bruix J, Cabibbo G, Reig M. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 23. | El Kassas M, Funk AL, Salaheldin M, Shimakawa Y, Eltabbakh M, Jean K, El Tahan A, Sweedy AT, Afify S, Youssef NF, Esmat G, Fontanet A. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: A comparative analysis. J Viral Hepat. 2018;25:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti Infect Ther. 2018;16:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med. 2017;9:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 26. | Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017;37:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 27. | Roudot-Thoraval F. Epidemiology of hepatitis C virus infection. Clin Res Hepatol Gastroenterol. 2021;45:101596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Leumi S, El Kassas M, Zhong J. Hepatitis C virus genotype 4: A poorly characterized endemic genotype. J Med Virol. 2021;93:6079-6088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Ghaderi-Zefrehi H, Gholami-Fesharaki M, Sharafi H, Sadeghi F, Alavian SM. The Distribution of Hepatitis C Virus Genotypes in Middle Eastern Countries: A Systematic Review and Meta-Analysis. Hepat Mon. 2016;16:e40357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep. 2018;8:1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 31. | Elsammak MY, Al-Sharkaweey RM, Ragab MS, Amin GM, Kandil MH. In Egyptians, a mutation in the lymphotoxin-alpha gene may increase susceptibility to hepatitis C virus but not that to schistosomal infection. Ann Trop Med Parasitol. 2008;102:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 653] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 33. | Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V, Boscarino JA, Henkle EM, Oja-Tebbe N, Lu M; CHeCS Investigators. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 35. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 36. | Hsu YC, Wu CY, Lane HY, Chang CY, Tai CM, Tseng CH, Lo GH, Perng DS, Lin JT, Mo LR. Determinants of hepatocellular carcinoma in cirrhotic patients treated with nucleos(t)ide analogues for chronic hepatitis B. J Antimicrob Chemother. 2014;69:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Nahon P, Vo Quang E, Ganne-Carrié N. Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Alavian SM, Haghbin H. Relative Importance of Hepatitis B and C Viruses in Hepatocellular Carcinoma in EMRO Countries and the Middle East: A Systematic Review. Hepat Mon. 2016;16:e35106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Allison RD, Teleb N, Al Awaidy S, Ashmony H, Alexander JP, Patel MK. Hepatitis B control among children in the Eastern Mediterranean Region of the World Health Organization. Vaccine. 2016;34:2403-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Salama II, Sami SM, Said ZN, El-Sayed MH, El Etreby LA, Rabah TM, Elmosalami DM, Abdel Hamid AT, Salama SI, Abdel Mohsen AM, Emam HM, Elserougy SM, Hassanain AI, Abd Alhalim NF, Shaaban FA, Hemeda SA, Ibrahim NA, Metwally AM. Effectiveness of hepatitis B virus vaccination program in Egypt: Multicenter national project. World J Hepatol. 2015;7:2418-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 41. | Fung J, Lai CL, Yuen MF. New paradigms for the treatment of chronic hepatitis B. J Gastroenterol Hepatol. 2008;23:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Atti EA. HCC Burden in Egypt. Gastroenterol Hepatol. 2015;2:00045. [DOI] [Full Text] |
| 43. | Fouad R, Abdo M, Eldeen HG, Sabry D, Atef M, Ahmed R, Zayed N. Influence of delta virus infection on the virologic status in Egyptian patients with chronic hepatitis B virus genotype D. J Med Virol. 2016;88:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Fares N, Péron JM. [Epidemiology, natural history, and risk factors of hepatocellular carcinoma]. Rev Prat. 2013;63:216-217, 220. [PubMed] |
| 45. | Mokhtar N, Gouda I, Adel I. Cancer pathology registry 2003-2004 and time trend analysis. Malign Digest Sys Tumors. 2007;55-67. |
| 46. | Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One. 2014;9:e90929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Ledda C, Loreto C, Zammit C, Marconi A, Fago L, Matera S, Costanzo V, Fuccio Sanzà G, Palmucci S, Ferrante M, Costa C, Fenga C, Biondi A, Pomara C, Rapisarda V. Noninfective occupational risk factors for hepatocellular carcinoma: A review (Review). Mol Med Rep. 2017;15:511-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Omar A, Abou-Alfa GK, Khairy A, Omar H. Risk factors for developing hepatocellular carcinoma in Egypt. Chin Clin Oncol. 2013;2:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 49. | Abou El Azm AR, Yousef M, Mansour N, Awad A, El Dardiry S, Abdel Aziz I. New insights on non-B non-C hepatocellular carcinoma in mid Delta Region, Egypt. J Gastrointest Cancer. 2014;45:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (1)] |
| 51. | Khalifa MI, Shata RR. Mycobiota and Aflatoxins B1 and M1 Levels in Commercial and Homemade Dairy Desserts in Aswan City, Egypt. J Adv Vet Res. 2018;8:43-48. |
| 52. | Dilber MS, Phelan A, Aints A, Mohamed AJ, Elliott G, Smith CI, O'Hare P. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Ismaiel A, Papenbrock J. Mycotoxins: producing fungi and mechanisms of phytotoxicity. Agriculture. 2015;5:492-537. [DOI] [Full Text] |
| 54. | El-Farrash MA, Abdel-Wahab M, Rizk MS. Serum Aflatoxin level as a predictor of Hepatocarcinogenesis in HCV-infected Egyptians. Egypt J Med Microbiol. 2008;17:83-90. |
| 55. | Anwar WA, Khaled HM, Amra HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res. 2008;659:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Sharaf-Eldin M, Salah R, Soliman HH, Abdou SH, Abd-Elsalam S, Elkhalawany W, Mansour L, Elsabagh HM, Khalil H. Aflatoxin As An Environmental Risk Factor Attributable To Liver Cancer In Nile Delta. Indian J Med Res Pharm Sci. 2016;3:19-26. [DOI] [Full Text] |
| 57. | Hazlehurst JM, Tomlinson JW. Non-alcoholic fatty liver disease in common endocrine disorders. Eur J Endocrinol. 2013;169:R27-R37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Michelotti A, de Scordilli M, Palmero L, Guardascione M, Masala M, Roncato R, Foltran L, Ongaro E, Puglisi F. NAFLD-Related Hepatocarcinoma: The Malignant Side of Metabolic Syndrome. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Wafaa ME, Shadia R, Nagwa AI, Yasser AE, Abeer M. Nour EA, Hebatallah F, Inas AR. Frequency of non-alcoholic fatty liver disease in overweight/obese children and adults: clinical, sonographic picture and biochemical assessment. J Genet Eng Biotechnol. 2012;10:221-227. |
| 60. | Alkassabany YM, Farghaly AG, El-Ghitany EM. Prevalence, risk factors, and predictors of nonalcoholic fatty liver disease among schoolchildren: a hospital-based study in Alexandria, Egypt. Arab J Gastroenterol. 2014;15:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Abd El-Wahab EW, Zein El-Abedin RA, Ahmed WM, Shatat HZ. Validation of a Non-Laboratory Based Screening Tool for Predicting Non-Alcoholic Fatty Liver Disease in an Egyptian Setting. Am J Med Sci. 2020;360:662-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 876] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 63. | Cojocariu CE, Trifan AV, Gîrleanu I, Stanciu C. Alcoholic liver disease--epidemiology and risk factors. Rev Med Chir Soc Med Nat Iasi. 2014;118:910-917. [PubMed] |
| 64. | Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Neuberger J; ELITA; ELTR Liver Transplant Centers. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 65. | Ezzat S, Abdel-Hamid M, Eissa SA, Mokhtar N, Labib NA, El-Ghorory L, Mikhail NN, Abdel-Hamid A, Hifnawy T, Strickland GT, Loffredo CA. Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health. 2005;208:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Badawi AF, Michael MS. Risk factors for hepatocellular carcinoma in Egypt: the role of hepatitis-B viral infection and schistosomiasis. Anticancer Res. 1999;19:4565-4569. [PubMed] |
| 67. | Lehman EM, Soliman AS, Ismail K, Hablas A, Seifeldin IA, Ramadan M, El-Hamzawy H, Shoushtari CS, Wilson ML. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based cancer registry. Hepatol Res. 2008;38:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, Boffetta P, La Vecchia C. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 69. | Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603-3615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4267] [Article Influence: 237.1] [Reference Citation Analysis (2)] |
| 71. | Rashed WM, Kandeil MAM, Mahmoud MO, Ezzat S. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J Egypt Natl Canc Inst. 2020;32:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 72. | Yun YH, Jung KW, Bae JM, Lee JS, Shin SA, Min Park S, Yoo T, Yul Huh B. Cigarette smoking and cancer incidence risk in adult men: National Health Insurance Corporation Study. Cancer Detect Prev. 2005;29:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Bakir AS, Ali-Eldin ZA. Is diabetes mellitus a risk factor for hepatocellular carcinoma in Egyptian patients? J Am Sci. 2012;8:353-358. |
| 74. | Abdou Moustafa EF, Galal GM, Aly A, Mohammed K. Smoking and the risk of hepatocellular carcinoma among Egyptian patients. A preliminary case-control study. Arab J Gastroenterol. 2009;10:AB53-AB60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 75. | Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Singh S. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer-related Mortality: A Systematic Review and Meta-Analysis. Am J Clin Oncol. 2018;41:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Wang B, Shen F, Fan J, Cao H. Body mass index and risk of primary liver cancer: a meta-analysis of prospective studies. Oncologist. 2012;17:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 78. | Hamed A, Hassan A, Younis M, Kamal A. Prevalence of Obesity and Overweight among Primary Schools Children in Qena, Egypt. Egypt J Hosp Med. 2019;77:4899-4905. |
| 79. | Ellabany E, Abdel Nasser MA. Non-Communicable Disease Surveillance System, Egypt 2006. Ministry of Health and Population. Preventive and Primary Health Care Sector Preventive Sector. [cited 10 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/ncds/surveillance/steps/EgyptSTEPSPresentation.pdf. |
| 80. | Aitsi-Selmi A, Chandola T, Friel S, Nouraei R, Shipley MJ, Marmot MG. Interaction between education and household wealth on the risk of obesity in women in Egypt. PLoS One. 2012;7:e39507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Population Council. Survey of Young People in Egypt. West Asia and North Africa Office. [cited 12 February 2021]. In: Population Council [Internet]. Available from: https://www.popcouncil.org/uploads/pdfs/2010PGY_SYPEFinalReport.pdf. |
| 82. | Jin F, Xiong WJ, Jing JC, Feng Z, Qu LS, Shen XZ. Evaluation of the association studies of single nucleotide polymorphisms and hepatocellular carcinoma: a systematic review. J Cancer Res Clin Oncol. 2011;137:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 84. | Clifford RJ, Zhang J, Meerzaman DM, Lyu MS, Hu Y, Cultraro CM, Finney RP, Kelley JM, Efroni S, Greenblum SI, Nguyen CV, Rowe WL, Sharma S, Wu G, Yan C, Zhang H, Chung YH, Kim JA, Park NH, Song IH, Buetow KH. Genetic variations at loci involved in the immune response are risk factors for hepatocellular carcinoma. Hepatology. 2010;52:2034-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Jiang DK, Sun J, Cao G, Liu Y, Lin D, Gao YZ, Ren WH, Long XD, Zhang H, Ma XP, Wang Z, Jiang W, Chen TY, Gao Y, Sun LD, Long JR, Huang HX, Wang D, Yu H, Zhang P, Tang LS, Peng B, Cai H, Liu TT, Zhou P, Liu F, Lin X, Tao S, Wan B, Sai-Yin HX, Qin LX, Yin J, Liu L, Wu C, Pei Y, Zhou YF, Zhai Y, Lu PX, Tan A, Zuo XB, Fan J, Chang J, Gu X, Wang NJ, Li Y, Liu YK, Zhai K, Hu Z, Liu J, Yi Q, Xiang Y, Shi R, Ding Q, Zheng W, Shu XO, Mo Z, Shugart YY, Zhang XJ, Zhou G, Shen H, Zheng SL, Xu J, Yu L. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet. 2013;45:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 86. | Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, Koike K, Kamatani N, Kubo M, Nakamura Y, Matsuda K. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 87. | Li S, Qian J, Yang Y, Zhao W, Dai J, Bei JX, Foo JN, McLaren PJ, Li Z, Yang J, Shen F, Liu L, Li S, Pan S, Wang Y, Li W, Zhai X, Zhou B, Shi L, Chen X, Chu M, Yan Y, Wang J, Cheng S, Shen J, Jia W, Liu J, Wen Z, Li A, Zhang Y, Zhang G, Luo X, Qin H, Chen M, Wang H, Jin L, Lin D, Shen H, He L, de Bakker PI, Zeng YX, Wu M, Hu Z, Shi Y, Zhou W. GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet. 2012;8:e1002791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 88. | Abdel-Azyem H, Abdel-Aziz A, Elbaz R, Eldesoky A And Abdel-Mageed WS. Single Nucleotide Polymorphism In Cytokines And Risk Of Hepatocellular Carcinoma In Egyptian Patients. Egypt J Genet Cytol. 2016;45:245-259. |
| 89. | Khaled IA, Zahran N, Saeed ME, Abdel-Aziz OA. Study of the relation between Egyptian patients with hepatocellular carcinoma and the genetic variations in DNA repair genes. J Blood Disord Transfus. 2019;10. |
| 90. | EI Sergany HF, Mohamed AM, Madkour NK, Elsebeaey MA, Fared AM, Elshaer SS, Zahran FE, EI Deeb HH. Epidermal Growth Factor Gene Polymorphism in Egyptian Patients with Hepatocellular carcinoma related to Hepatitis C. J Gastroenterol Hepatol Res. 2017;6:2481-2485. |
| 91. | Ministry of Health and Population; El-Zanaty and Associates; The DHS Program ICF International. Egypt health issues survey 2015. Rockville, MD: Ministry of Health and Population, ICF International, October 2015. [cited 9 March 2021]. In: Dhsprogram [Internet]. Available from: https://dhsprogram.com/pubs/pdf/FR313/FR313.pdf. |
| 92. | World Health Organization. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. 2016. [cited 15 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en. |
| 93. | Waked I, Esmat G, Elsharkawy A, El-Serafy M, Abdel-Razek W, Ghalab R, Elshishiney G, Salah A, Abdel Megid S, Kabil K, El-Sayed MH, Dabbous H, El Shazly Y, Abo Sliman M, Abou Hashem K, Abdel Gawad S, El Nahas N, El Sobky A, El Sonbaty S, El Tabakh H, Emad E, Gemeah H, Hashem A, Hassany M, Hefnawy N, Hemida AN, Khadary A, Labib K, Mahmoud F, Mamoun S, Marei T, Mekky S, Meshref A, Othman A, Ragab O, Ramadan E, Rehan A, Saad T, Saeed R, Sharshar M, Shawky H, Shawky M, Shehata W, Soror H, Taha M, Talha M, Tealaab A, Zein M, Hashish A, Cordie A, Omar Y, Kamal E, Ammar I, AbdAlla M, El Akel W, Doss W, Zaid H. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N Engl J Med. 2020;382:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 94. | Yang JD, Altekruse SF, Nguyen MH, Gores GJ, Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017;123:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Yoo SH, Kwon JH, Nam SW, Kim HY, Kim CW, You CR, Choi SW, Cho SH, Han JY, Song DS, Chang UI, Yang JM, Lee HL, Lee SW, Han NI, Kim SH, Song MJ, Hwang S, Sung PS, Jang JW, Bae SH, Choi JY, Yoon SK. Early development of de novo hepatocellular carcinoma after direct-acting agent therapy: Comparison with pegylated interferon-based therapy in chronic hepatitis C patients. J Viral Hepat. 2018;25:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tinè F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbàra M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV). Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? Aliment Pharmacol Ther. 2017;46:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 97. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 98. | Musa NI, Mohamed IE, Abohalima AS. Impact of treating chronic hepatitis C infection with direct-acting antivirals on the risk of hepatocellular carcinoma recurrence. Egypt Liver J. 2020;10:26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 99. | Lashen SA, Shamseya MM, Madkour MA. Hepatocellular Carcinoma Occurrence/Recurrence after Direct-Acting Antivirals for Hepatitis C in Egyptian Cohort: Single-Center Experience. Dig Dis. 2019;37:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 100. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472-480.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 101. | Mansour E, Abdul-Rahim S, Batouty G, Zaghloul I, Abdel-Hadi S. Integration of hepatitis B immunization in the Expanded Program on Immunization of the Child Survival Project. J Egypt Public Health Assoc. 1993;68:487-494. [PubMed] |
| 102. | El-Deen Mohamed NM, Abuo-El-Yazed AH, El-Deen Mohamed HM. Follow up of hepatitis b virus vaccine response in healthy individuals. Sci J Al-Azhar Med Fac Girls. 2018;2:58-63. |
| 103. | Gaballah A, Shawky S, Elsawaf G, Shamsia M, Al Makdad A, Abd El Rahman M, Osman NA, Islim H, Alhaifi A, Kader O. Virological profiles of HBV and HCV in hepatocellular carcinoma in Egypt and Yemen. Egypt J Med Microbiol. 2018;27:7-17. |
| 104. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 945] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 105. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 106. | Johnson P, Berhane S, Kagebayashi C, Satomura S, Teng M, Fox R, Yeo W, Mo F, Lai P, Chan SL, Tada T, Toyoda H, Kumada T. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br J Cancer. 2017;116:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 107. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 108. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 109. | Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 110. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1499] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 111. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1645] [Article Influence: 205.6] [Reference Citation Analysis (0)] |
| 112. | Sastre J, Díaz-Beveridge R, García-Foncillas J, Guardeño R, López C, Pazo R, Rodriguez-Salas N, Salgado M, Salud A, Feliu J. Clinical guideline SEOM: hepatocellular carcinoma. Clin Transl Oncol. 2015;17:988-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 114. | Fateen W, Ryder SD. Screening for hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Seo YS, Jang BK, Um SH, Hwang JS, Han KH, Kim SG, Lee KS, Kim SU, Kim YS, Lee JI. Validation of risk prediction models for the development of HBV-related HCC: a retrospective multi-center 10-year follow-up cohort study. Oncotarget. 2017;8:113213-113224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Frenette CT, Isaacson AJ, Bargellini I, Saab S, Singal AG. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin Proc Innov Qual Outcomes. 2019;3:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 117. | Marrero JA. Surveillance for Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 118. | França AV, Elias Junior J, Lima BL, Martinelli AL, Carrilho FJ. Diagnosis, staging and treatment of hepatocellular carcinoma. Braz J Med Biol Res. 2004;37:1689-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 120. | Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 121. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 122. | Harding JJ, Khalil DN, Abou-Alfa GK. Biomarkers: What Role Do They Play (If Any) for Diagnosis, Prognosis and Tumor Response Prediction for Hepatocellular Carcinoma? Dig Dis Sci. 2019;64:918-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6060] [Article Influence: 865.7] [Reference Citation Analysis (3)] |
| 124. | Ebrahim AE, Shehata MAH, Abou-saif S, Hamisa MF, Abd-Elsalam S & Yousef M. Role of Fibroscan for early detection of hepatocellular carcinoma (HCC) in hepatitis C cirrhotic patients. Egypt J Radiol Nucl Med. 2020;51:134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Ahmed NNA, El Gaafary SM, Elia RZ, Abdulhafiz EM. Role of abbreviated MRI protocol for screening of HCC in HCV related cirrhotic patients prior to direct-acting antiviral treatment. Egypt J Radiol Nucl Med. 2020;51:102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Abdelaziz AO, Nabil MM, Omran DA, Abdelmaksoud AH, Asem N, Shousha HI, Elbaz TM, Leithy R. Hepatocellular Carcinoma Multidisciplinary Clinic-Cairo University (HMC-CU) score: A new simple score for diagnosis of HCC. Arab J Gastroenterol. 2020;21:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | El-Zayadi AR, Badran HM, Shawky S, Emara S, El-Bareedy A, Sobhi M. Effect of surveillance for hepatocellular carcinoma on tumor staging and treatment decisions in Egyptian patients. Hepatol Int. 2010;4:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Salama MM, Allam AS, Nasser HM, Kabiel YWA, Elsayed EH. Role of Serum Leptin in the Diagnosis and Prognosis of Hepatocellular Carcinoma in Egyptian Cirrhotic Patients. Med J Cairo Univ. 2020;88:259-266. |
| 129. | Bryant R, Laurent A, Tayar C, van Nhieu JT, Luciani A, Cherqui D. Liver resection for hepatocellular carcinoma. Surg Oncol Clin N Am. 2008;17:607-633, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery. 2003;133:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Zakaria HM, Macshut M, Gaballa NK, Sherif AE, Abdel-Samea ME, Abdel-Samiee M, Marwan I, Yassein T. Total tumor volume as a prognostic value for survival following liver resection in patients with hepatocellular carcinoma. Retrospective cohort study. Ann Med Surg (Lond). 2020;54:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 133. | Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 134. | Senbel A, Elmahdy Y, Roshdy S, Khater A, Shehatoo F, Farouk O, Fathi A, Hamed E, Kotb S, Denwer A. Role of Hepatic Resection for HCC in the era of Transplantation; an Experience of Two Tertiary Egyptian Centers. Indian J Surg Oncol. 2017;8:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 135. | Makhlouf NA, Abdel-Malek MO, Hassany SM, Abd-Elmawgood AM, Taha AM, Ibraheem TM, Fadel BA. Risk of liver failure after major hepatectomy for patients with hepatocellular carcinoma. Egypt J Surg. 2020;39:81-85. |
| 136. | Shehta A, Farouk A, Fouad A, Aboelenin A, Elghawalby AN, Said R, Elshobary M, El Nakeeb A. Post-hepatectomy liver failure after hepatic resection for hepatocellular carcinoma: a single center experience. Langenbecks Arch Surg. 2021;406:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 137. | Amer KE, Marwan I. Living donor liver transplantation in Egypt. Hepatobiliary Surg Nutr. 2016;5:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 138. | Kamel R, Hatata Y, Hosny K, Nabil A, El-Deen Abd-Allah A, Mostafa A, Abdel-Aal A, Elganzoury MZ, Elmalt O, Marwan I, Hosny A. Outcome of Living-Donor Liver Transplant for Hepatocellular Carcinoma: 15-Year Single-Center Experience in Egypt. Exp Clin Transplant. 2017;15:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Galal M, Bahaa M, Ebrahim WA, El-Shafei AE, Sedrak CR. Pretransplantation alpha fetoprotein level as a predictor of hepatocellular carcinoma recurrence after adult living donor liver transplantation within milan criteria in Egyptian patients. Egypt J Intern Med. 2019;31:203-207. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 140. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 897] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 141. | Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38:65-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 142. | Soliman AF, Abouelkhair MM, Hasab Allah MS, El-Kady NM, Ezzat WM, Gabr HA, Elsayed EH, Saleh AI, Kamel A. Efficacy and Safety of Microwave Ablation (MWA) for Hepatocellular Carcinoma (HCC) in Difficult Anatomical Sites in Egyptian Patients with Liver Cirrhosis. Asian Pac J Cancer Prev. 2019;20:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 143. | Nouh MA, El Sharkawy MK, El Deeb GS, Badawy AM, Azab HM. Comparative study of radiofrequency ablation combined with either percutaneous ethanol injection or percutaneous acetic acid injection in the management of hepatocellular carcinoma. Menoufia Med J. 2020;33:819-823. |
| 144. | Kamal A, Elmoety AAA, Rostom YAM, Shater MS, Lashen SA. Percutaneous radiofrequency vs microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol. 2019;10:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 145. | Sharaf-Eldin MA, El-Yamany SA, Salah RA, Kohla M, Habba E, Fattah HA, Ghazy MS. Risk factors for recurrence of hepatocellular carcinoma after radiofrequency ablation in a cohort of Egyptian patients with hepatitis C virus-induced cirrhosis: a multicenter analysis. Egypt Liver J. 2014;4:13-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 146. | Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, Cella D, Burns JL, Ganger D, Benson AB 3rd, Mulcahy MF, Kulik L, Lewandowski R. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358-1365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 147. | Seldinger technique. Reprint from Acta Radiologica 1953. AJR Am J Roentgenol. 1984;142:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 148. | Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, Mamone G, Vizzini G, Luca A, Gridelli B. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol. 2007;13:2952-2955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Kang IK, Kim SW, Hahn SH, Cho SC, Gham CW, Lee DH. [A comparison of patients with hepatocellular carcinoma between a short-term (less than 6 mo) survival group and a long-term (over 24 mo) survival group after treatment with transcatheter arterial chemoembolization]. Taehan Kan Hakhoe Chi. 2002;8:189-200. [PubMed] |
| 150. | Huang YH, Wu JC, Chau GY, Lui WY, King KL, Chiang JH, Yen SH, Sheng WY, Hou MC, Lu CL, Chang FY, Lee SD. Supportive treatment, resection and transcatheter arterial chemoembolization in resectable hepatocellular carcinoma: an analysis of survival in 419 patients. Eur J Gastroenterol Hepatol. 1999;11:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Eltawil KM, Berry R, Abdolell M, Molinari M. Analysis of survival predictors in a prospective cohort of patients undergoing transarterial chemoembolization for hepatocellular carcinoma in a single Canadian centre. HPB (Oxford). 2012;14:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011;7:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 153. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 154. | Farouk Ahmed AL, Nasser HM, Abo-Elmaaty ME, Montasser IF. Role of trans arterial chemoembolization (TACE) in down staging of hepatocellular carcinoma (HCC) before liver transplantation. Egypt J Hosp Med. 2018;72:5578-5583. |
| 155. | Fouad YM, Aboalela1 AS, Mokarrab H, abdelghany W, Abdelhamid W, Essawy MG. Prospective Evaluation of Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Radiofrequency or TACE. Indian J Public Health Res Dev. 2020;11:882-887. |
| 156. | Hassan H, Eman MB, El-Folly RF, Amr MA, El-Hariri H, Abdelghany RS, El-Fouly NF. Assessment of health-related quality of life after hepatocellular carcinoma management (radiofrequency ablation or transarterial chemoembolization): a pilot Egyptian study. Egypt Liver J. 2017;7:51-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 157. | Dufour JF, Bargellini I, De Maria N, De Simone P, Goulis I, Marinho RT. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol. 2013;24 Suppl 2:ii24-ii29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 158. | Kim YH, Kim DY. Yttrium-90 radioembolization for hepatocellular carcinoma: what we know and what we need to know. Oncology. 2013;84 Suppl 1:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 159. | Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, Wang D, Gao H. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 160. | Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, Larusso NF, Alberts SR, Gores GJ, Fleming CJ, Slettedahl SW, Harmsen WS, Therneau TM, Wiseman GA, Andrews JC, Roberts LR. Efficacy and safety of transarterial radioembolization vs chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 161. | Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, Vaidya S, Monsky WL, Park JO, Bhattacharya R, Hippe DS, Harris WP. Segmental Yttrium-90 Radioembolization vs Segmental Chemoembolization for Localized Hepatocellular Carcinoma: Results of a Single-Center, Retrospective, Propensity Score-Matched Study. J Vasc Interv Radiol. 2017;28:777-785.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (37)] |
| 162. | Hamed MM, Abdelhay AA, Abd Alfattah MH, Gameel GA. Efficacy of Transarterial Y90 Radioembolization in Management for Unresectable-Intermediate and Locally Advanced-HCC. Med J Cairo Univ. 2019;87:3147-3156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 163. | Hetta MO, Hetta MW, Shebrya NH, El Ghazaly HA. Radioembolization with Yttrium-90 resin microspheres in treatment of HCC with or without PVT: Initial Egyptian experience. Egypt J Radiol Nuc Med. 2013;44:215-222. |
| 164. | El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, Mueller S, Barakat E, Lauenstein T, Bockisch A, Gerken G, Schlaak JF. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 165. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 166. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3147] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 167. | Keating GM. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol. 2017;12:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 168. | Javan H, Dayyani F, Abi-Jaoudeh N. Therapy in Advanced Hepatocellular Carcinoma. Semin Intervent Radiol. 2020;37:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 169. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2714] [Article Influence: 339.3] [Reference Citation Analysis (0)] |
| 170. | Nada Y, Rashad N, Eissa M, Ghonaim A, Farag K, Saadawi I, Sheha A, El Gewaity M, Abdel-Rahman O. Outcomes of treatment with sorafenib in Egyptian patients with hepatocellular carcinoma: a retrospective cohort study. Expert Rev Gastroenterol Hepatol. 2018;12:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 171. | Abdel-Rahman O, Abdelwahab M, Shaker M, Abdelwahab S, Elbassiony M, Ellithy M. Sorafenib for Egyptian patients with advanced hepatocellular carcinoma; single center experience. J Egypt Nat Cancer Insti. 2014;26:9-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 172. | El Baghdady NS, El Wakeel L, Ellithy MA, Eltohamy N, Shaheen SM, El Naggar AER. Assessment of efficacy and safety of sorafenib vs no treatment in Egyptian hepatocellular carcinoma patients. Ann Oncol. 2019;30:ix42-ix67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 173. | Hamdy Elsisi G, Nada Y, Rashad N, Carapinha J. Cost-effectiveness of sorafenib vs best supportive care in advanced hepatocellular carcinoma in Egypt. J Med Econ. 2019;22:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Hanafy AS. Efficacy of low dose capecitabine and sorafenib in patients with advanced alfa-fetoprotein secreting hepatocellular carcinoma: a 1 year experience. Springerplus. 2016;5:1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 175. | Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 176. | Omran D, Alboraie M, Zayed RA, Wifi MN, Naguib M, Eltabbakh M, Abdellah M, Sherief AF, Maklad S, Eldemellawy HH, Saad OK, Khamiss DM, El Kassas M. Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations. World J Gastroenterol. 2018;24:4330-4340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 177. | El Kassas M, Tawheed A, Eltabbakh M, Kaseb A. Hepatitis C Antiviral Therapy In Patients With Successfully Treated Hepatocellular Carcinoma: Dancing With Wolves. J Hepatocell Carcinoma. 2019;6:183-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 178. | Wahdan IH. Cost-Effectiveness of National Breast Cancer Screening Programs in Developing Countries, with Reference to the Recent Egyptian Initiative. J High Institute Public Health. 2020;50:1-9. |