Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.1031
Peer-review started: April 8, 2020
First decision: May 5, 2020
Revised: May 13, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: September 15, 2020
Processing time: 154 Days and 19.5 Hours
Primary hepatic neuroendocrine tumors (PHNETs), a group of neuroendocrine neoplasms, are extremely rare. There are only few case reports about PHNETs in the literature. The lack of large samples and multicenter research results in poor diagnostic and therapeutic approaches.
To discuss the clinical characteristics, diagnosis, and treatment of PHNETs and risk factors related to survival.
We retrospectively analyzed the clinical data, imaging features, immunohistochemistry data, and treatment efficacy of 40 patients who were pathologically diagnosed with PHNETs and admitted to The First Affiliated Hospital of Zhengzhou University from January 1, 2014 to November 15, 2019. Finally, survival analysis was performed to identify the risk factors for survival.
The main symptoms and signs included intermittent abdominal pain (19 patients, 47.5%) and bloating (8 patients, 20.0%). The positive rates of tested tumor markers were recorded as follows: Carbohydrate antigen 19-9 (CA19-9) (6 patients, 15.0%), CA72-4 (3 patients, 7.5%), carcinoembryonic antigen (7 patients, 17.5%), and alpha-fetoprotein (6 patients, 15.0%). Immunohistochemical staining results showed positivity for Syn in 38 (97.4%) of 39 patients, for chromogranin A in 17 (65.4%) of 26 patients, for CD56 in 35 (94.6%) of 37 patients, for AE1/AE3 in 28 (87.5%) of 32 patients, and for Ki-67 in all 40 (100.0%) patients. The overall survival rate was significantly related to the tumor grade, AE1/AE3, and Ki-67. No significant correlation was found between other parameters (age, gender, tumor number, tumor size, metastasis, and treatment) and overall survival.
Higher grade, negative AE1/AE3, and higher Ki-67 are associated with a worse survival rate. Kinds of treatment and other parameters have no significant influence on overall survival.
Core Tip: Neuroendocrine tumors (NETs) are originally mainly from the gastrointestinal and bronchopulmonary tracts. Liver neuroendocrine tumors mostly metastasize from other organs, and primary hepatic NETs (PHNETs), which were first reported by Edmonson in 1958, are extremely rare and account for only 0.3% of all NETs. Furthermore, there are less than 150 PHNET cases described in English-language articles because of the low incidence. The longer-term, larger sample multi-center studies are urgently needed to figure out their clinicopathological features and prognostic factors, and finally facilitate their diagnosis and treatment.
- Citation: Wang HH, Liu ZC, Zhang G, Li LH, Li L, Meng QB, Wang PJ, Shen DQ, Dang XW. Clinical characteristics and outcome of primary hepatic neuroendocrine tumors after comprehensive therapy. World J Gastrointest Oncol 2020; 12(9): 1031-1043
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/1031.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.1031
Neuroendocrine tumors (NETs), mostly originating from bronchopulmonary and gastrointestinal sites, are uncommon low-malignancy tumors that affect 6.98/100000 individuals annually, however, the incidence rate was in upward trend from 2000 to 2014 worldwide[1,2]. According to the World Health Organization 2019 classification of gastroenteropancreatic NETs, NETs are divided into four grades: NET G1 (low grade), NET G2 (intermediate grade), NET G3 (high grade), and neuroendocrine carcinomas (NEC), based on mitotic count and Ki-67 index of the tumor[3,4]. More than 80% of hepatic NETs are metastatic for the dual blood supply to the liver provides an advantage for tumor metastasis to the liver. The most common primary site of metastatic hepatic NETs is the lungs (44%), followed by the pancreas (19%) and the small intestine (8%)[5].
However, primary hepatic NETs (PHNETs), which were first reported by Edmonson in 1958, are extremely rare and account for only 0.3% of all NETs[6,7]. Furthermore, there are less than 150 PHNET cases described in English-language articles because of the low incidence[8]. Case reports comprise a large proportion of these articles. The longer-term, larger sample multi-center studies are urgently needed to figure out their clinicopathological features and prognostic factors, and finally facilitate their diagnosis and treatment.
In this study, we studied the clinicopathological characteristics of 40 patients with a definite diagnosis of PHNET. The various therapies used to treat these patients and corresponding outcomes were also explored in this article, and survival analysis was utilized to identify specific prognostic risk factors, finally making further improvement in treatments and outcomes.
We retrospectively analyzed the clinical data, image features, treatments, outcomes, and immunohistochemistry results of 40 patients with a definite diagnosis of PHNET who were admitted to The First Affiliated Hospital of Zhengzhou University from January 2014 to November 2019. The patients consisted of 30 with NETs (4 cases of G1, 21 cases of G2, and 5 cases of G3) and 10 with NECs. None of the described patients had a history of long-term drinking or hepatitis. There were 18 males (45.0%) and 22 females (55.0%). The mean age was 51.35 ± 12.52 years old (range from 26 to 73 years old); 50.0% of the patients were over 50 years old. The group-based parameters are described in Table 1, and correlation of tumor grade with clinicopathological characteristics was detected by Pearson χ2 test (Table 1).
| Parameter | Tumor grade | P value | |
| G1/G2 | G3/NEC | ||
| Age (yr) | 0.804 | ||
| < 50 | 11 | 6 | |
| ≥ 50 | 14 | 9 | |
| Gender | 0.622 | ||
| Male | 12 | 6 | |
| Female | 13 | 9 | |
| Hypertension | 0.914 | ||
| Yes | 5 | 2 | |
| No | 20 | 13 | |
| Diabetes | 0.711 | ||
| Yes | 4 | 1 | |
| No | 21 | 14 | |
| Syn | 1.000 | ||
| Positive | 23 | 15 | |
| Negative | 1 | 0 | |
| CgA | 0.810 | ||
| Positive | 11 | 6 | |
| Negative | 7 | 2 | |
| CD56 | 1.000 | ||
| Positive | 21 | 14 | |
| Negative | 1 | 1 | |
| AE1/AE3 | 0.341 | ||
| Positive | 18 | 10 | |
| Negative | 1 | 3 | |
| Ki-67 | 0.000 | ||
| ≤ 20% | 25 | 2 | |
| > 20% | 0 | 13 | |
| NSE | 0.585 | ||
| Positive | 7 | 6 | |
| Negative | 10 | 4 | |
| Tumor number | 0.334 | ||
| Single | 6 | 1 | |
| Multiple | 19 | 14 | |
| Tumor size (cm) | 1.000 | ||
| ≤ 3 | 3 | 2 | |
| > 3 | 16 | 8 | |
| Metastasis | 0.884 | ||
| Yes | 5 | 4 | |
| No | 19 | 10 | |
| Treatment | 0.470 | ||
| Non-surgical treatment | 10 | 9 | |
| Surgical treatment | 10 | 4 | |
| Mixed treatment | 5 | 2 | |
Enhanced computed tomography (CT), magnetic resonance imaging (MRI), gastroenterological endoscopy, positron emission tomography-CT (PET-CT), and/or somatostatin receptor antagonist single-photon emission CT (SPECT) imaging were performed to rule out the possibility of extrahepatic primary lesions. Based on the histological and immunohistochemical examinations of the tumor tissue by liver needle biopsy, a primary liver neuroendocrine tumor diagnosis was made.
Hepatectomy and liver transplantation: To ensure that the liver was free of any remaining tumor in the surgical margin, we completely removed the tumor to meet the 2017 standard established criteria[9].
Radiofrequency ablation: Before the operation of radiofrequency ablation (RFA), careful ultrasound scanning was performed to locate the point and target access with the assistance of patients. Under general anesthesia, an 18G biopsy needle was pierced into the lesions to obtain tissue, and the tissue was fixed in 10% formalin solution for further pathological examination. The radiofrequency needle (exposed section 3 cm) was controlled to go straight into the lesions, and perform ablation until the lesions were covered by strong echoes[10].
Transcatheter arterial chemoembolization: Transcatheter arterial chemoembolization (TACE) was performed through the right groin under local anesthesia. The procedures were composed of locating the specific artery supplying tumors and injecting chemotherapy. The wire-guided catheter was inserted into the artery supplying the tumors, and the catheter mainly went through the abdominal aorta, the celiac trunk, and the common hepatic artery. Then, the branches of the hepatic artery supplying nutrition for the tumors were discovered under the more selective angiogram. Finally, chemotherapy particles were injected into the tumors through the catheter.
Long-acting repeatable octreotide: Long-acting repeatable octreotide (LAR), formed by incorporating octreotide into the microspheres of a biodegradable polymer, is a long-acting somatostatin analog and was used for monthly intramuscular injection. Injections at 4-wk intervals provided a constant and stable serum drug concentration. There are three doses used in routine clinical practice: 10 mg, 20 mg, and 30 mg[11]. In our study, the LAR dose received by all patients was 20 mg.
Chemotherapy: Chemotherapy drugs (temozolomide, tegafur, capecitabine, etc.) were taken on a regular basis.
Mixed treatment: Surgical treatments and non-surgical treatments were all used in some patients.
We followed the study patients through outpatient follow-up or hospitalization follow-up, and the last date of follow-up was November 15, 2019.
Symptoms and signs: The main symptoms and signs included intermittent abdominal pain (19 patients, 47.5%), acid reflux (4 patients, 10.0%), vomiting (6 patients, 15.0%), bloating (8 patients, 20.0%), diarrhea (5 patients, 12.5%), and dizziness (1 patient, 2.5%).
Laboratory tests: The levels of alanine transaminase, aspartate aminotransferase, total bilirubin, direct bilirubin, and indirect bilirubin were in the normal range for all patients. We detected five tumor markers [NSE, carbohydrate antigen 19-9 (CA19-9), CA72-4, carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP)], and the rate of NSE positivity was 48.1% in the 27 patients who received NSE test. The remaining four tumor markers were evaluated in all 40 patients, and the rates of positivity were recorded as follows: CA19-9 (6 patients, 15.0%), CA72-4 (3 patients, 7.5%), CEA (7 patients, 17.5%), and AFP (6 patients, 15.0%). The immunohistochemistry indices worth noting were Syn (38 positive patients, 97.4%) in 39 patients, chromogranin A (CgA) (17 positive patients, 65.4%) in 26, CD56 (35 positive patients, 94.6%) in 37, AE1/AE3 (28 positive patients, 87.5%) in 32, and Ki-67 (40 positive patients, 100.0%) in 40.
CT/MRI: Totally 30 patients received enhanced CT imaging. The numbers of low-density shadow lesions and slightly high-density shadow on the abdominal CT scans were 29 and 1, respectively, and the intensity of lesions included enhancement (29 patients, 96.7%) or no enhancement (1 patient, 3.33%). The features of enhancement were annular (10 patients, 34.5%), self (9 patients, 31.0%), and uneven (10 patients, 34.5%). Nine patients received MRI imaging: The lesions showed high signal on T1WI (9 patients, 100.0%), high signal on T2WI (8 patients, 88.9%), and limited diffusion with high signal on DWI (7 patients, 77.8%). High signal on T1WI, T2WI, and DWI existed in most patients who received MRI. Except for two patients who lack the specific MRI image, the enhancement features of seven patients in dynamic MRI were listed as follows: In the arterial phase, the enhancement features were obvious (5 patients, 71.4%), moderate (1 patient, 14.3%), and slight (1 patient, 14.3%), respectively. Consistent enhancement in the portal phase (2 patients, 28.6%) and in the delayed phase (1 patient, 14.3%) were observed. Collectively, the results of CT showed that most PHNETs were enhanced with diverse patterns, and the results of MRI showed that most PHNETs displayed high signal on T1WI, T2WI, and DWI, and that enhancement existed in the arterial phase of all patients.
PET-CT/SPECT: Fifteen patients received 18F-FDG PET-CT imaging, and all (100.0%) showed low density shadows. Among them, four (26.7%) had no anomalous radioactive distribution, while 11 (73.3%) had radioactivity gathering. Six patients received 99mTc-Octreotide SPECT imaging, of whom five (83.3%) had radioactivity gathering in the lesions, and one (16.7%) had no anomalous radioactive distribution. The sensitivities of PET-CT and SPECT for PHNETs were 73.3% and 83.3%, respectively.
In the PHNETs, single liver tumors accounted for 17.5%, and multiple liver tumors accounted for 82.5%.
The metastasis condition in two patients was uncertain due to the lack of examination information. In the 38 patients, there were 29 patients (76.3%) without definite distant metastasis, and 9 patients (23.7%) had distant metastasis. Metastasis sites included the lung (1 patient, 12.5%), pelvic lymph node (1 patient, 12.5%), left supraclavicular lymph node (1, 12.5%), retroperitoneal lymph node (1 patient, 12.5%), and bone (5 patients, 62.5%).
Nineteen patients were treated non-surgically (untreated: 5; chemotherapy: 10; LAR: 2; and LAR + chemotherapy: 2), fourteen were treated surgically (TACE: 5; RFA: 3; radical operation: 4; and RFA + TACE: 2), and seven received mixed treatment.
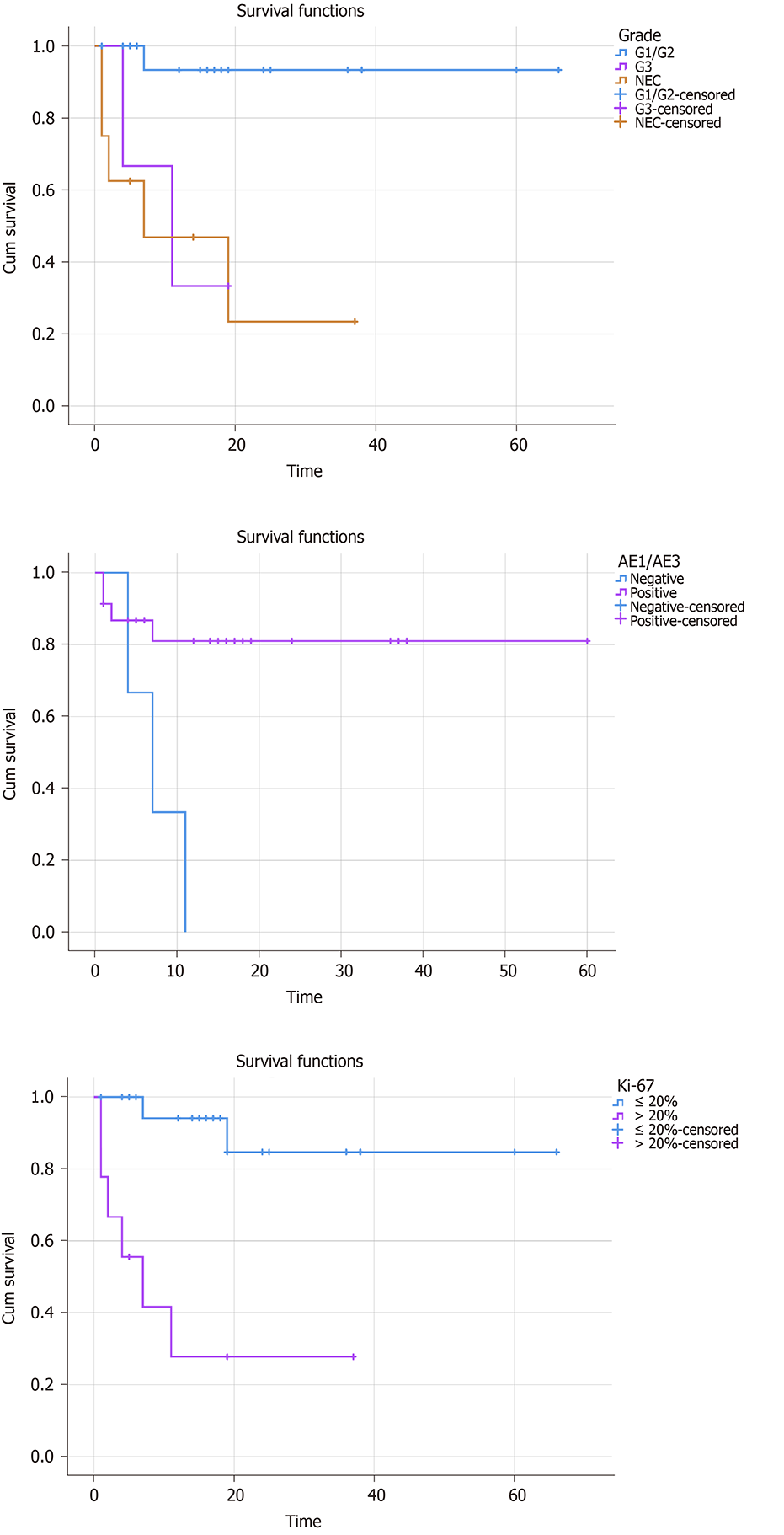
Pearson χ2 test was used to detect the correlation between tumor grade and clinicopathological characteristics. All patients were divided into two groups, G1/G2 group and G3/NEC group. The data about age, gender, hypertension, diabetes, NSE, Syn, CgA, CD56, AE1/AE3, Ki-67, tumor number, tumor size, metastasis, and treatment were collected to detect the correlation with tumor grade. The tumor grade was divided into three group (G1/G2, G3, and NEC) in survival analysis. For further investigating the prognostic factors, Kaplan-Meier survival analysis was performed to analyze these above parameters, and the results showed that the survival time was significantly related to the tumor grade, AE1/AE3, and Ki-67 (Figure 1, P < 0.05). The other indexes did not display a significant correlation. Collectively, negative AE1/AE3, lower tumor grade, and Ki-67 ≤ 20% were associated with a better overall survival rate.
The median follow-up was 16.63 ± 16.06 mo (range from 0.1 to 66 mo). At the last time of follow-up, eight patients had been dead due to the PHNETs, ten were lost to follow-up, and 22 were alive and received significant relief from their clinical symptoms (paroxysmal abdominal pain, acid reflux, vomiting, bloating, diarrhea, and dizziness).
The symptoms of PHNETs are non-specific. In a previous report, abdominal pain was the main characteristic (approximately 80% of patients), and only a few patients (approximately 6.8%) had typical carcinoid syndrome-like skin flushing and diarrhea[12]. In our research, the rates of paroxysmal abdominal pain, acid reflux, vomiting, bloating, diarrhea, and dizziness were 47.5%, 10.0%, 15.0%, 20.0%, 12.5%, and 2.5%, respectively. We did not find that our patients were experiencing skin flushing. In our study, 55.0% of the participants were female, and 45.0% were male, which is comparable with other studies[13]. Most PHNETs were enhanced on CT followed by washout in the late phase, which was similar to hepatocellular carcinoma (HCC). As we all know, the enhancements of typical HCC are well-defined in the arterial phase and display rapid loss in the delayed phases. Also, HCC patients have hepatitis, hepatic cirrhosis, and elevated serum AFP, which do not exist in most PHNETs patients[14,15]. Research showed that further analysis of CT washout process also helps to distinguish PHNET from HCC[16]. On MRI, HCC lesions are commonly hypointense in T1WI, which is different from the features of PHNETs in our study[17]. It is still challenging to distinguish between PHNETs and HCC, even with the assistance of these factors. Therefore, it is necessary to perform hematoxylin-eosin staining and immunohistochemistry for a definitive diagnosis[18,19]. PHNETs were usually misdiagnosed as HCCs or hepatic hemangiomas due to their uncharacteristic enhancement on CEUS and enhanced MRI and CT. Thus, CEUS and enhanced MRI and CT have a low sensitivity for PHNETs[16]. Fortunately, the development of somatostatin receptor agonist SPECT imaging improved the diagnostic sensitivity and predicted the response to octreotide analogues for treating tumors. The level of sensitivity ranges from 85%-90%[19]. In our research, the sensitivities of PET-CT and somatostatin receptor agonist SPECT were 73.3% and 83.3%, respectively. Notably, recent studies have shown that somatostatin receptor antagonist SPECT/PET-CT may perform better than somatostatin receptor agonist SPECT alone in tumors and metastases regarding location accuracy and guiding treatments of NETs. However, somatostatin receptor antagonist SPECT/PET-CT is not yet used in clinic[20,21]. As we know, only a few specific indicators help us to diagnose NETs, including CgA, 5-HIAA, and NSE[13]. However, these three assessments are often not routinely ordered at initial admission because of the low incidence of NETs. In the study, NSE was examined, and the positivity rates was 48.1%. The tumor markers routinely evaluated on initial admission, such as AFP, CEA, CA19-9, CA125, and CA72-4, are nonspecific, and they are often within the normal range. Tumor biopsy is the gold standard for the diagnosis of NETs[22]. Iimuro et al[23] and Shetty et al[24] showed that CgA and Syn were more effective in the pathological diagnosis of PHNETs. However, Lv et al[25] hold the opposing opinion that there are no differences between the positive rates of Syn, NSE, CgA, and AFP. In our study, the positive rates of Syn, CgA, CD56, AE1/AE3, and Ki-67 were 97.4%, 65.4%, 94.6%, 87.5%, and 100.0%, respectively. Therefore, the effectiveness of CgA, NSE, and Syn is a moot point. The diagnosis of PHNETs is difficult to make.
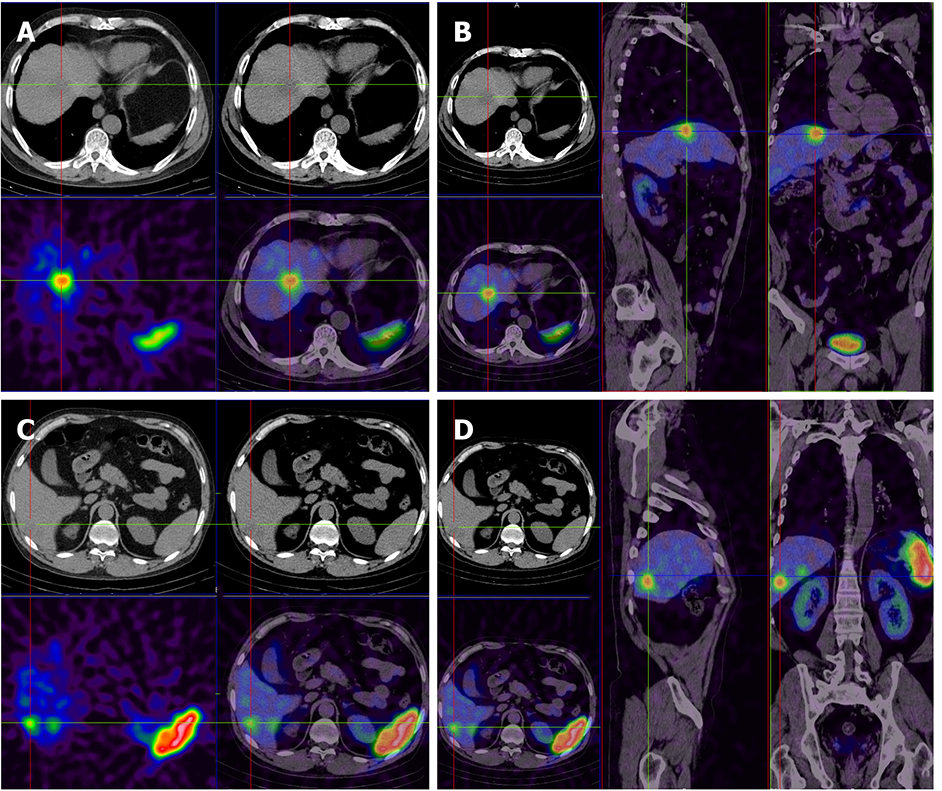
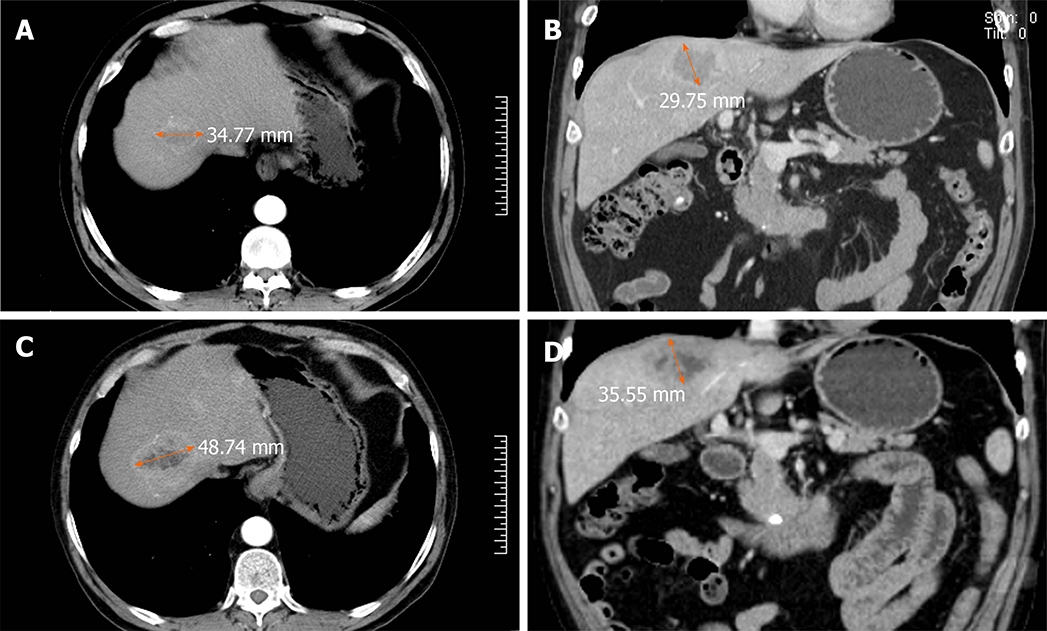
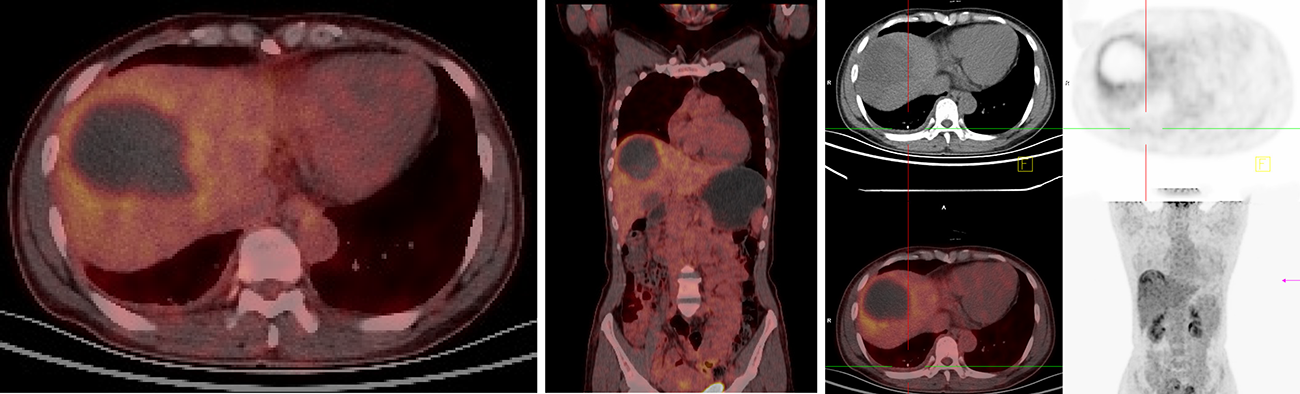
In this study, a 60-year-old man who was in the hospital for paroxysmal abdominal pain lasting approximately 7 mo, without sickness or vomiting, represented an example of the misdiagnosis as hepatic hemangioma. The hepatitis, AFP, CA19-9, CEA, and CA72-4 tests were negative. CT imaging revealed multiple liver lesions with atypical enhancement. Combined with the test results, the liver lesions were diagnosed as hepatic hemangiomas, and the patient was discharged without further treatment. Eleven months later, the patient was admitted to the hospital for sudden and unbearable abdominal pain. A full abdominal CT scan still showed the same results as before and the tumor size was unchanged. To make a definitive diagnosis, a liver needle biopsy was carried out. The biopsy results confirmed a NET diagnosis, which was further graded as G1, with the possibility of G2 not excluded. Tumors in the tissue were scattered, and the Ki-67 index was 3%. Immunohistochemically, the tumor cells were positive for AE1/AE3, hepatocytes (cancer nests), CK19, Syn, and CD56. The octreotide (somatostatin receptor analogue) 99m-Tc somatostatin receptor agonist SPECT showed that the multiple liver lesions were positive without extrahepatic lesions (Figure 2). The patient was diagnosed with PHNET and treated with LAR for about 21 mo. A recent CT scan showed that the tumor did not show obvious changes in size (Figure 3). We will continue to follow this patient to acquire long-term outcomes.
The main treatments for PHNETs include surgery, TACE, RFA, LAR, and liver transplantation. In our study, five patients refused further treatment resulting from the personal desire, ten were treated with chemotherapy, three with RFA, two with LAR, five with TACE, two with LAR + chemotherapy, two with RFA + TACE, four with radical operation, and mixed treatment was used in the remaining seven (1 with TACE + LAR, 2 with chemotherapy + LAR + TACE, and 4 with radical operation + chemotherapy). The clinical symptoms in the three groups were relieved, and the remission rate of the surgical treatment group was higher than those of the other two groups (Table 2).
| Treatment/symptoms | Pre-treatment (n) | Post-treatment (n) | Remission rate |
| Non-surgical treatment (19 cases) | 23 | 7 | 69.6% |
| Abdominal pain | 10 | 4 | |
| Acid reflux | 2 | 0 | |
| Vomiting | 3 | 1 | |
| Bloating | 3 | 1 | |
| Diarrhea | 5 | 1 | |
| Surgical treatment (14 cases) | 12 | 2 | 83.3% |
| Abdominal pain | 5 | 1 | |
| Acid reflux | 2 | 0 | |
| Vomiting | 3 | 0 | |
| Bloating | 1 | 1 | |
| Dizziness | 1 | 0 | |
| Mixed treatment (7 cases) | 8 | 2 | 75.0% |
| Abdominal pain | 4 | 1 | |
| Bloating | 4 | 1 |
Surgery is preferred as the first-line treatment for patients with no metastases; it can improve the survival rate and bring greater benefit compared with conservative treatment[18]. The 5-year survival rate is as high as 74-78%[13,26], and surgery can even result in better outcomes for large tumors (27 cm × 13 cm)[27], but the recurrence rate is still high (19.8%)[13]. Some research shows that we can choose liver transplantation for the treatment of some unresectable tumors[13,18].
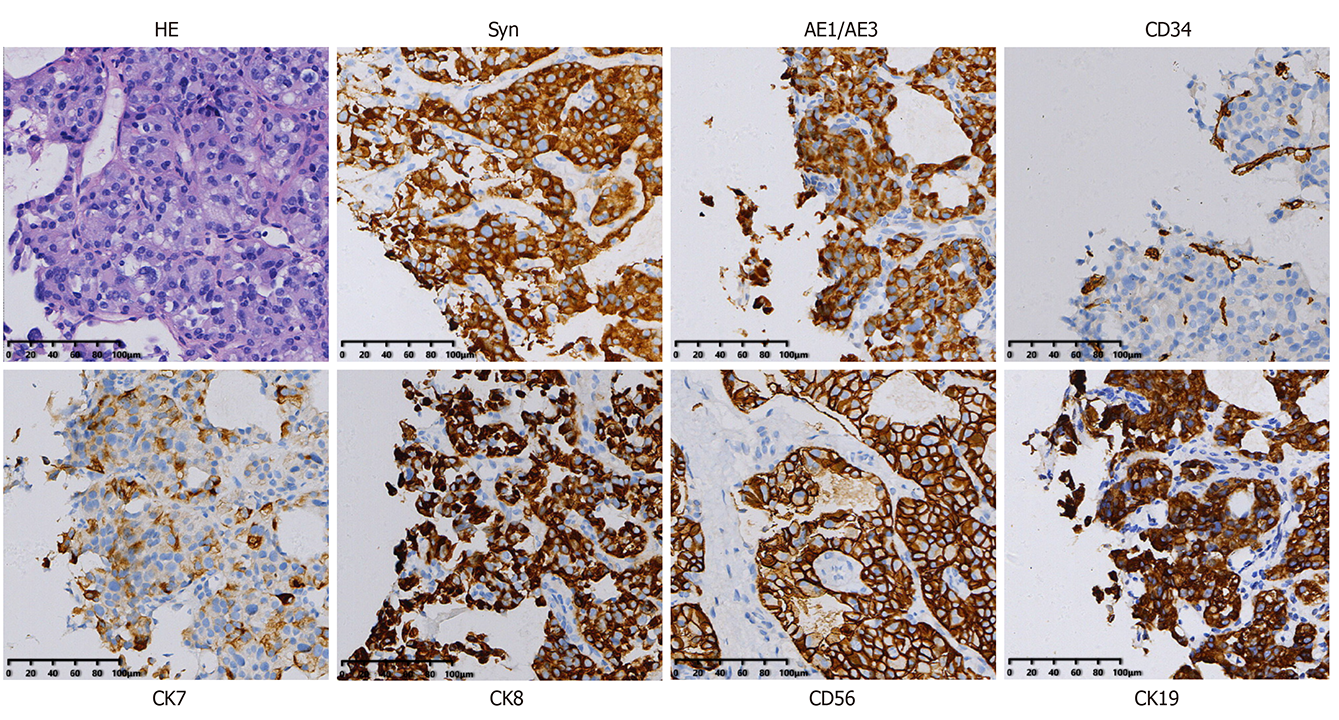
With the wide use and development of RFA technology, an increasing number of liver lesions can be treated, regardless of whether they are a benign or malignant form. RFA is rarely used in PHNETs[7]. The indications for RFA are a tumor number less than or equal to three and a diameter less than or equal to 5 cm or an existing tumor diameter less than or equal to 3 cm[6,12]. In this study, a patient was treated by RFA, and MRI showed a single 42 mm × 41 mm × 56 mm liver lesion in the right lobe that was considered a possible atypical HCC. To obtain a clear and definitive diagnosis and treatment plan, an ultrasound-guided liver biopsy and RFA were carried out under local anesthesia. The pathology results of the biopsy suggested a highly differentiated neuroendocrine tumor, and the diagnosis of a typical carcinoid was made after excluding the possibility of gastrointestinal and pancreatic metastases. Immunohistochemistry showed AE1/AE3 (+), CK8/18 (+), CD34 (+), Ki-67 (2%+), CK7 (+), CK19 (+), CDX-2 (+), Syn (+), CgA (+), and CD56 (+) (Figure 4). Postoperative PET-CT was carried out to exclude an extrahepatic metastatic tumor. The result showed that the margin of the low-density shadow in the right hepatic lobe was more active, with more consideration for postoperative changes. No other obvious abnormalities were observed (Figure 5). Finally, the patient successfully recovered and was discharged.
For tumors that are in an inoperable condition and express somatostatin (positive for somatostatin receptor agonist SPECT), somatostatin and its analogues can be used not only to reduce the hypersecretion of hormones but also to inhibit rapid tumor growth[21]. In our study, 10.0% of the patients received LAR or LAR + chemotherapy due to the SPECT findings. The clinical symptoms were relieved in three (75.0%) cases and exacerbated in one (25.0%). And the tumor sizes in the four cases were unchanged.
Another typical treatment is TACE. Some articles show that the rate of symptom improvement is up to 90% by TACE[28], and the effect is persistent. At the same time, preoperative alkaline phosphatase levels have predicted worse outcomes[29]. Therefore, it is necessary to detect the level of alkaline phosphatase. In our study, one patient was first treated with TACE, followed by a one-time LAR injection after 1 mo, one patient was treated by TACE eight times, and one patient alternately received LAR, chemotherapy, and TACE. Nakatake et al[29] showed that combination therapies for PHNETs could produce better results than single modality therapies.
This study plays an important role in facilitating the diagnosis and treatment of PHNETs, and provides novel insights into the risk factors related to overall survival. However, there are several limitations in this study as it was designed as a retrospective analysis based on a small sample size due to the rare incidence of PHNETs. In addition, several patients were lost to follow-up, which leads to the incompleteness of survival status. Thus, there is clearly a need for prospective, multicenter, large-scale trials to aid surgical decision-making in the future regarding the treatment of PHNETs.
PHNETs are extremely rare, and it is difficult to make a definitive diagnosis and choose the appropriate treatment for an individual person. For patients without metastasis, surgery is the preferred first choice of method due to the high survival rate. For patients with metastasis and unresectable tumors, RFA, LAR, TACE, chemotherapy, and combination therapy can be selected to inhibit the growth of tumors, and relieve clinical symptoms at the same time. The survival rates of PHNETs have no significant correlation with the kinds of treatments. Finally, higher grade, negative AE1/AE3, and higher Ki-67 are associated with a worse survival rate. Kinds of treatment and other parameters have no significant influence on overall survival rate.
Neuroendocrine tumors (NETs), mostly originating from bronchopulmonary and gastrointestinal sites, are uncommon low-malignancy tumors that affect 6.98/100000 individuals annually, however, the incidence rate was in upward trend from 2000 to 2014 worldwide. Primary hepatic NETs (PHNETs), a group of NENs, are extremely rare and account for only 0.3% of all NETs. There are only few case reports about PHNETs in the literature. The lack of large sample and multicenter research results in poor diagnostic and therapeutic approaches.
Longer-term, larger sample multi-center studies are urgently needed to figure out the clinicopathological features and prognostic factors of PHNETs to facilitate their diagnosis and treatment.
This study aimed to explore the clinical characteristics, diagnosis, treatment of PHNETs and risk factors related to survival.
We analyzed the clinical data, imaging features, immunohistochemistry results, and treatment efficacy of 40 patients who were pathologically diagnosed with PHNETs from January 1, 2014 to November 15, 2019, and the survival analysis was performed to identify the risk factors for survival.
The main symptoms and signs included intermittent abdominal pain (19 patients, 47.5%) and bloating (8 patients, 20.0%). The positive rates for tumor markers included carbohydrate antigen 19-9 (CA19-9) (6 patients, 15.0%), CA72-4 (3 patients, 7.5%), carcinoembryonic antigen (7 patients, 17.5%), and alpha-fetoprotein (6 patients, 15.0%). The results of immunohistochemistry staining revealed positivity for Syn in 38 (97.4%) of 39 patients, for chromogranin A in 17 (65.4%) of 26 patients, for CD56 in 35 (94.6%) of 37 patients, for AE1/AE3 in 28 (87.5%) of 32 patients, and for Ki-67 in all 40 (100.0%) patients . Finally, the overall survival rate was significantly related to the tumor grade, AE1/AE3, and Ki-67.
Higher grade, negative AE1/AE3, and higher Ki-67 are associated with a worse survival rate. Kinds of treatment and other parameters have no significant influence on overall survival rate, but they can inhibit the growth of tumors, and relieve clinical symptoms.
The results of the study display the features of PHNETs patients, and reveal the relationship of the clinicopathological features and treatments with overall survival, which may facilitate the diagnosis and treatment of PHNETs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azoulay L, Kastelein F, Yamada A S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Li JH
| 1. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 2. | Zhong Q, Chen QY, Xie JW, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Li P, Zheng CH, Huang CM. Incidence trend and conditional survival estimates of gastroenteropancreatic neuroendocrine tumors: A large population-based study. Cancer Med. 2018;7:3521-3533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Burad DK, Kodiatte TA, Rajeeb SM, Goel A, Eapen CE, Ramakrishna B. Neuroendocrine neoplasms of liver - A 5-year retrospective clinico-pathological study applying World Health Organization 2010 classification. World J Gastroenterol. 2016;22:8956-8966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 5. | Saeed OAM, Cramer H, Wang X, Wu HH. Fine needle aspiration cytology of hepatic metastases of neuroendocrine tumors: A 20-year retrospective, single institutional study. Diagn Cytopathol. 2018;46:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with review of the literature. World J Gastroenterol. 2015;21:3132-3138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Camargo ÉS, Viveiros Mde M, Corrêa Neto IJ, Robles L, Rezende MB. Primary hepatic carcinoid tumor: case report and literature review. Einstein (Sao Paulo). 2014;12:505-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Park CH, Chung JW, Jang SJ, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 10. | Zhang W, Luo E, Gan J, Song X, Bao Z, Zhang H, Chen M. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J Surg Oncol. 2017;15:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Keskin O, Yalcin S. A review of the use of somatostatin analogs in oncology. Oncotargets Ther. 2013;6:471-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Chen QC, Chen X, Lu HZ, Zhao H, Zhao JJ, Bi XY, Zhao DB, Chi YHBL, Li ZY, Huang Z, Zhang YF, Zhou JG, Cai JQ. Primary hepatic neuroendocrine tumors: retrospective analysis of seven cases and literature review. Transl Cancer Res. 2018;7:428-440. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | DeLuzio MR, Barbieri AL, Israel G, Emre S. Two Cases of Primary Hepatic Neuroendocrine Tumors and a Review of the Current Literature. Ann Hepatol. 2017;16:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chen Z, Xiao HE, Ramchandra P, Huang HJ. Imaging and pathological features of primary hepatic neuroendocrine carcinoma: An analysis of nine cases and review of the literature. Oncol Lett. 2014;7:956-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Song T, Jia Z, Guo X, Zhao H, Bao W, Han D, Zhou X, Qi X. Does Hepatic Impairment Influence Renal Function Parameters in Liver Cirrhosis? J Transl Int Med. 2018;6:90-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma KS, Guo DY, Ding SY. Primary hepatic neuroendocrine tumors: clinical characteristics and imaging features on contrast-enhanced ultrasound and computed tomography. Abdom Radiol (NY). 2016;41:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Cho ES, Choi JY. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol. 2015;16:449-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Sethi S, Kulkarni P. A rare case of a primary hepatic neuroendocrine tumor. Transl Gastroenterol Hepatol. 2016;1:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Fani M, Nicolas GP, Wild D. Somatostatin Receptor Antagonists for Imaging and Therapy. J Nucl Med. 2017;58:61S-66S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 21. | Mikołajczak R, Maecke HR. Radiopharmaceuticals for somatostatin receptor imaging. Nucl Med Rev Cent East Eur. 2016;19:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Lambrescu IM, Martin S, Cima L, Herlea V, Badiu C, Fica S. Primary hepatic neuroendocrine tumor after 4 years tumor-free follow-up. J Gastrointestin Liver Dis. 2015;24:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Iimuro Y, Deguchi Y, Ueda Y, Tanaka A, Iwasa Y, Ishihara M, Mizuta K, Yamamoto Y, Ikai I, Shimahara Y, Yamaoka Y. Primary hepatic carcinoid tumor with metachronous lymph node metastasis after long-term follow up. J Gastroenterol Hepatol. 2002;17:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Shetty PK, Baliga SV, Balaiah K, Gnana PS. Primary hepatic neuroendocrine tumor: an unusual cystic presentation. Indian J Pathol Microbiol. 2010;53:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Lv Y, Huang C, Xu H, Han X, Zhang L, Mao W, Ji Y, Jin D, Lou W, Xu X. Clinicopathological Characteristics of the primary and metastatic Hepatic Neuroendocrine Tumors and the relevant Prognosis-Related Factors: A Retrospective Study of 81 Cases in a Single Chinese Center. J Cancer. 2018;9:479-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Shinkawa H, Takatsuka S, Kaizaki R, Fujiwara Y, Kurai O, Yamazaki O. Postoperative outcomes of primary hepatic neuroendocrine carcinomas: review article. Osaka City Med J. 2013;59:105-113. [PubMed] |
| 27. | Sotiropoulos GC, Charalampoudis P, Delladetsima I, Stamopoulos P, Dourakis S, Kouraklis G. Surgery for giant primary neuroendocrine carcinoma of the liver. J Gastrointest Surg. 2014;18:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Yao KA, Talamonti MS, Nemcek A, Angelos P, Chrisman H, Skarda J, Benson AB, Rao S, Joehl RJ. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130:677-82; discussion 682-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Nakatake R, Ishizaki M, Matui K, Yanagimoto H, Inoue K, Kaibori M, Kawaguchi Y, Kon M. Combination therapies for primary hepatic neuroendocrine carcinoma: a case report. Surg Case Rep. 2017;3:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |