Published online Jun 15, 2020. doi: 10.4251/wjgo.v12.i6.677
Peer-review started: February 24, 2020
First decision: March 24, 2020
Revised: April 11, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: June 15, 2020
Processing time: 112 Days and 2.2 Hours
The occurrence and development of colon cancer are complex, involving a variety of genetic changes, such as mutation and activation of oncogenes, inactivation of tumour suppressor genes, and aberrant proliferation and apoptosis regulation mechanisms. Fibrous sheath interacting protein 1 (FSIP1) is a newly discovered oncogene that is frequently activated in a variety of tumours such as breast cancer and bladder cancer. However, the clinical significance of FSIP1 in colon cancer is unclear. In this study, we analysed the clinical significance of expression of FSIP1 in human colon cancer, aimed to clarify the biological role of FSIP1 in the development and progression of colon cancer.
To investigate the clinical significance of expression of FSIP1 in colon cancer.
From March 2011 to March 2014, 302 specimens of tumour tissues and paracancerous tissues were obtained from patients pathologically diagnosed with colon cancer at Shengjing Hospital of China Medical University. Immunohistochemistry was used to detect FSIP1 expression in colon cancer tissues and adjacent normal tissues. Spearman correlation coefficient and Cox regression analyses were used to determine the relationship between FSIP1 expression and clinicopathological factors and prognosis, as well as the impact on survival.
Compared with its expression in adjacent normal tissues, FSIP1 was expressed at higher levels in colon cancer tissues. Spearman correlation analysis showed that high expression of FSIP1 was positively correlated with clinicopathological stage, lymph node metastasis, and poor prognosis in colon cancer; it was negatively correlated with the degree of tumour differentiation. Cox regression analysis showed that high FSIP1 expression was an independent risk factor for the prognosis of colon cancer patients.
High expression of FSIP1 may be one of the important factors affecting the clinical outcome of colon cancer patients and leading to poor prognosis.
Core tip: This study examined the expression of fibrous sheath interacting protein 1 (FSIP1) in 302 patients with colon carcinoma and analysed the follow-up data. The results showed that FSIP1 expression was not correlated with sex, age, tumour size, or other factors but was positively correlated with tumour pathological T/N stages and negatively correlated with tumour differentiation. Univariate and multivariate Cox regression analyses and survival analysis showed that high expression of FSIP1 was an independent risk factor for poor prognosis, and overall survival was worse in the FSIP1-high expression group than in the FSIP1-low expression group. So we speculate that FSIP1 plays a role in the carcinogenesis of colon cancer.
- Citation: Wu HY, Yang B, Geng DH. Clinical significance of expression of fibrous sheath interacting protein 1 in colon cancer. World J Gastrointest Oncol 2020; 12(6): 677-686
- URL: https://www.wjgnet.com/1948-5204/full/v12/i6/677.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i6.677
Colon cancer is one of the most common malignant tumours of the digestive system. Approximately 1.2 million patients worldwide are diagnosed with colorectal cancer each year, and more than 600000 patients die directly or indirectly from colorectal cancer. In China, colon cancer accounts for approximately 12%-15% of malignant tumours. At present, approximately 130000 people are newly diagnosed with colon cancer every year in China, and the proportion of patients that are younger than 30 years old can reach 15%[1-6], which is a serious threat to the lives and health of Chinese people.
The main treatment for colon cancer is surgical resection supplemented with postoperative chemotherapy and radiotherapy. However, the early detection rate of colon cancer in China is low, and the treatment effect on invasive or metastatic colon cancer is poor. Therefore, the biological characteristics of invasion and metastasis of malignant tumours are important factors affecting prognosis and are also key to tumour treatment. The occurrence and development of colon cancer are complex, involving a variety of genetic changes, such as mutation and activation of oncogenes, inactivation of tumour suppressor genes, and aberrant proliferation and apoptosis regulation mechanisms.
Fibrous sheath-interacting protein 1 (FSIP1), also known as 17-β-hydroxysteroid dehydrogenase X (HSD10), is encoded by the HSD17B10 gene located at the human 15q14 locus (GenBank ID: 161835). The FSIP1-encoding gene is highly conserved, and its protein product is a mitochondrial enzyme that catalyses the oxidation of various fatty acids, alcohols, and steroids, precisely regulates chromosome segregation, and maintains microtubule system stability. Missense or silent mutations in the FSIP1-encoding gene may be associated with degenerative diseases such as Alzheimer's disease and aspirin-intolerant asthma[8,9]. FSIP1 is also involved in the early development of spermatogonia, and the transcription of its family member FISP2 begins in the late development of spermatogonia and is related to the assembly of skeletal proteins in sperm flagellar cells[10,11]. In the field of oncology, single nucleotide polymorphisms in FSIP1, including GG homozygous and AG heterozygous variants, are associated with a risk of arsenic exposure-related bladder cancer compared with the AA homozygous wild type[12]. High expression of FSIP1 is also associated with malignant biological behaviour and poor prognosis in bladder cancer, and this process may be related to the PI3K/Akt pathway[13,14]. FSIP1 may also be involved in the synthetic lethality of paclitaxel drugs in non-small-cell lung cancer; FSIP1 knockdown promotes the formation of multipolar spindles, prolongs the mitotic cycle, and sensitizes cells to the micronucleation effect of paclitaxel[15]. Liu et al[16] found that FSIP1 expression was related to the biological behaviour of breast cancer cells and the prognosis of breast cancer. In normal tissues, FSIP1 is minimally expressed except for in the testes. By investigating the relationship between FSIP1 and the development, progression, and clinicopathological factors of colon cancer using immunohistochemistry, we verified the abnormal expression of FSIP1 in colon cancer with a large sample of tissues and analysed the correlation between FSIP1 expression and pathological factors and prognosis in colon cancer patients. This study aimed to clarify the biological role of FSIP1 in the development and progression of colon cancer and open a new path for its clinical diagnosis, treatment, and prognosis evaluation.
Pathological histological sections of tumour tissues and paracancerous tissues were obtained from 302 patients who were diagnosed by pathology and underwent radical resection of colon cancer from March 2011 to March 2014 at the General Hospital of Shengjing Hospital of China Medical University. The relevant clinical information of the patients was collected, including sex, age, tumour size, T stage, N stage, and degree of differentiation. The inclusion criteria were as follows: (1) All patient tumour tissue specimens were identified as primary colon cancer by pathological examination; (2) All patients did not receive anti-tumour treatment before surgery, such as radiotherapy, chemotherapy, and targeted therapy; (3) Patients had no distant metastasis (M0), and the surgical margin was negative (R0); and (4) Patients were healthy in the past and did not have other tumours, severe chronic diseases, or immune system diseases. Patients were followed for at least 5 years to record recurrence and survival.
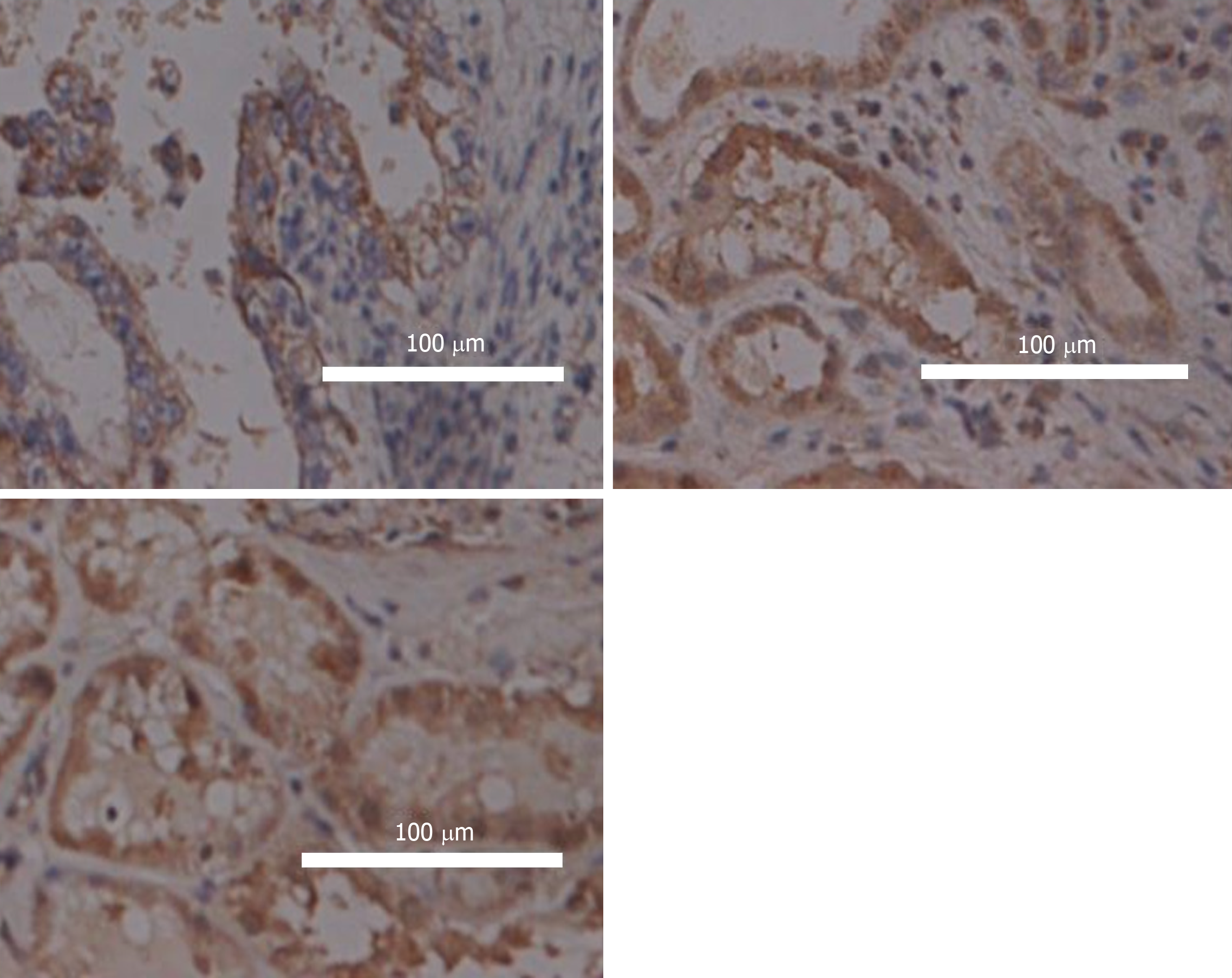
Tissue specimens were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned. Conventional dewaxing and hydration were carried out. The sections were immersed in sodium citrate antigen repair solution and heated at high pressure for 15 min; then 3% hydrogen peroxide solution was added and incubated at room temperature for 10 min. After that, 5% bovine serum albumin solution was added and incubated at room temperature for 1 h. Rabbit polyclonal FSIP1 antibody (1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, United States) was added and incubated overnight at 4 °C. The sections were washed three times with phosphate buffer saline (PBS), and horseradish peroxidase-labelled goat anti-rabbit secondary antibody (1:5000 dilution, Biyuntian Biotechnology Co., Ltd., China) was added and incubated for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was added (1:5000 dilution, Beyotime Biotechnology Co., Ltd., China) and incubated for 1 h at room temperature. The sections were washed three times with PBS, and the DAB staining solution was used to develop colour (Fuzhou Maixin Biotech. Co., Ltd., China). Haematoxylin staining solution was used for counterstaining. Then, the sections were dehydrated, cleared, and sealed with a neutral gum. The staining solution with the primary antibody was replaced with PBS in the negative control. Samples were observed under a microscope. All slides were independently examined blindly by two pathologists. Each slide was scored for FSIP1 staining in five randomly selected views. Cytoplasmic or membrane staining was considered positive for FSIP1 expression. The proportion of positive cells and the intensity of staining were determined using a semi-quantitative scale. The proportion of positive cells was graded as follows: < 25%, 1 point; 25%-50%, 2 points; 51%-75%, 3 points; and > 75%, 4 points. The staining intensity was graded as follows: No colouring, 0 points; light yellow, 1 point; and brown yellow, 2 points. The staining intensity score was multiplied by the score of the positive cell proportion to determine whether FSIP1 expression was negative (total score < 4) or positive (total score ≥ 4).
Data processing and statistical analyses were performed using SPSS 24.0 software (SPSS Inc., Chicago, IL, United States). The count data are expressed as the mean ± SD, and an independent or paired Student’s t test was used; the measurement data were expressed as a rate, and the χ2 test was used. Survival analysis was performed using the Kaplan-Meier method. Correlations between FSIP1 expression and various clinicopathological factors were identified using Spearman correlation analysis. Univariate and multivariate Cox regression analyses were used to determine the risk factors that influenced patient outcomes. P < 0.05 was considered statistically significant.
FSIP1 expression in tissues sections and the clinicopathological data of 302 patients with colon cancer were statistically analysed. It was found that FSIP1 was mainly localized in the cytoplasm, and the expression in colon cancer tissues was significantly higher than that in adjacent tissues, and it was related to the degree of tumour differentiation. (Figure 1).
Of all the 302 patients in this group, 203 were negative for FSIP1 expression and 99 had positive FSIP1 expression. FSIP1 expression was not associated with age, sex, or tumour size, whereas positive FSIP1 expression was closely associated with tumour T stage, N stage, and degree of differentiation. That is, patients with positive expression of FSIP1 had worse tumour T and N stages and a lower degree of tumour differentiation than patient with negative FSIP1 expression (Table 1).
| Characteristic (%) | FSIP1 negative (n = 203) | FSIP1 positive (n = 99) | P value |
| Median age | 63 ± 17.6 | 59 ± 20.8 | 0.521 |
| Sex | |||
| Male | 121 (60) | 54 (55) | 0.438 |
| Female | 82 (40) | 45 (45) | |
| Tumor size | 0.313 | ||
| < 5 | 85 (41) | 39 (39) | |
| 5-10 | 97 (48) | 49 (49) | |
| > 10 | 21 (5) | 11 (12) | |
| Pathological tumor stage | 0.0011 | ||
| T1 | 89 (44) | 30 (30) | |
| T2 | 46 (23) | 17 (17) | |
| T3 | 44 (22) | 25 (25) | |
| T4 | 24 (11) | 27 (28) | |
| Pathological nodal stage | 0.0001 | ||
| N0 | 65 (32) | 20 (20) | |
| N1 | 78 (38) | 33 (33) | |
| N2 | 39 (19) | 28 (28) | |
| N3 | 21 (11) | 18 (19) | |
| Tumor differentiation | 0.0001 | ||
| Well | 71 (35) | 45 (46) | |
| Moderate | 85 (42) | 31 (31) | |
| Poor | 47 (23) | 23 (23) | |
The Spearman correlation coefficient was used to analyse the correlation between FSIP1 expression and various clinicopathological parameters. The results showed that there was no significant correlation between FSIP1 expression and patient sex, age, or tumour size. FSIP1 expression was significantly positively correlated with tumour pathological stage (T stage) and lymph node metastasis (N stage) (Table 2, Spearman correlation coefficient greater than 0, P < 0.05). FSIP1 expression was negatively correlated with tumour differentiation (Table 2, Spearman correlation coefficient less than 0, P < 0.05).
The risk factors for death in this group were evaluated by univariate and multivariate Cox regression analyses (Table 3). A total of 92 patients died during the follow-up period. The hazard ratio (HR) of death for FSIP1 expression was 2.933 [95% confidence interval (CI): 2.067-4.559, P = 0.000]; after adjusting for the other six baseline factors (age, sex, tumour size, pathological tumour stage, pathological nodal stage, and tumour differentiation), the HR of death for positive expression of FSIP1 was 2.661 (95%CI: 1.979-5.635, P = 0.001) according to multivariate regression analysis. There was a significant difference, so positive expression of FSIP1 was an independent risk factor for death in this group of patients.
| Characteristic | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.899 | 0.675 | ||
| ≤ 60 | 1 (Ref) | 1 (Ref) | ||
| > 60 | 0.972 (0.721-1.322) | 0.899 | 1.019 (0.732–1.418) | 0.675 |
| Sex | 0.674 | 0.739 | ||
| Male | 1 (Ref) | 1 (Ref) | ||
| Female | 1.558 (0.731-2.235) | 0.674 | 2.339 (0.610-3.886) | 0.739 |
| Tumor size | 0.003 | 0.219 | ||
| < 5 | 1 (Ref) | 1 (Ref) | ||
| 5-10 | 1.427 (0.973-1.886) | 0.043 | 1.339 (0.921-1.992) | 0.198 |
| > 10 | 1.661 (1.095-2.291) | 0.005 | 1.431 (0.695-1.832) | 0.399 |
| Pathological tumor stage | 0.000 | |||
| T1 | 1 (Ref) | 1 (Ref) | 0.000 | |
| T2 | 2.521 (1.567-4.631) | 0.001 | 1.330 (1.009-2.461) | 0.002 |
| T3 | 3.213 (2.288–9.198) | 0.000 | 3.445 (1.925-4.901) | 0.000 |
| T4 | 4.883 (1.528–5.438) | 0.000 | 4.870 (2.062-9.126) | 0.000 |
| Pathological nodal stage | 0.000 | 0.000 | ||
| N0 | 1 (Ref) | 1 (Ref) | ||
| N1 | 1.916 (1.379-3.221) | 0.003 | 1.687 (1.563-6.019) | 0.002 |
| N2 | 4.422 (1.837-7.622) | 0.000 | 6.819 (3.421-12.567) | 0.000 |
| N3 | 9.230 (4.399-12.257) | 0.000 | 7.909 (3.963-16.878) | 0.000 |
| Tumor differentiation | 0.000 | 0.000 | ||
| Well | 1 (Ref) | 1 (Ref) | ||
| Mediate | 4.696 (1.565-8.912) | 0.000 | 5.229 (4.442-9.887) | 0.000 |
| Poor | 7.129 (3.126-11.703) | 0.000 | 6.443 (2.126-12.556) | 0.000 |
| FSIP1 expression | ||||
| Negative | 1 (Ref) | 1 (Ref) | ||
| Positive | 2.933 (2.067-4.559) | 0.000 | 2.661 (1.979-5.635) | 0.001 |
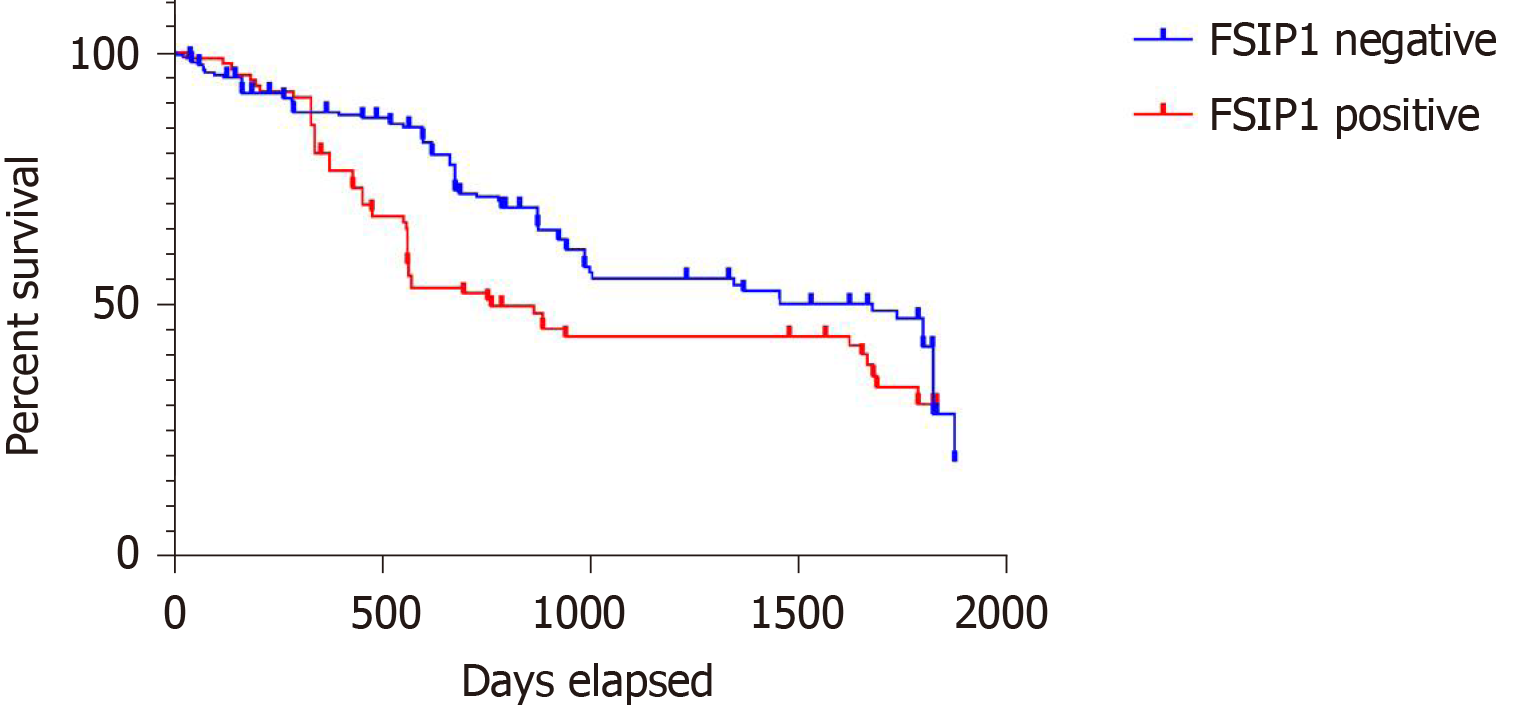
Patients were grouped according to FSIP1 expression (FSIP1-negative group, n = 203; FSIP1-positive group, n = 99), and survival analysis was performed according to the follow-up results using the Kaplan-Meier method. The results showed that the overall prognosis of patients in the FSIP1-positive group was significantly worse than that of patients in the FSIP1-negative group (Figure 2, P = 0.0014).
The occurrence and development of colon cancer involve in a variety of genetic changes. The prognosis of colon cancer also varies widely, depending on factors such as tumour TNM stage, pathological type, and degree of differentiation, in addition to whether an oncogene mutation is involved. Over the years, many scholars have been constantly looking for markers related to the diagnosis and prognosis of colon cancer. Currently, only EGFR/K-ras/BRAF[17-19] and related targeted therapies are commonly accepted for they have some significance in determining treatments and prognosis. Targeted immunotherapy based on immunological checkpoints, such as PD1/PDL1 inhibitors, has also gradually been included in clinical applications in recent years[20,21]. However, the long-term effects lack the support of large-sample multicentre clinical data. Therefore, markers of broad significance to guide clinical diagnosis and treatment are urgent needed. In the field of tumour biology, FSIP1 is currently considered a cancer antigen that is highly expressed in various tumours, such as breast cancer, lung cancer, and bladder cancer, and is associated with a poor prognosis[9,22,23]. Research on FSIP1 is relatively scarce worldwide. It is currently recognized that FSIP1 is a protein involved in sperm flagella assembly and the assembly of A-kinase anchoring protein 4 (AKAP4). AKAP4 can target cAMP-dependent binding protein kinase A (PKA), forming a complex with it, and anchoring PKA to the fibre sheath; studies have reported that AKAP4 is a substrate for ERK1/2 and a protein that catalyses the switch between cAMP/PKA and PKC/ERK1/2 in human sperm[24,25]. It is well known that PKA and PKC play important roles in tumour proliferation, angiogenesis, drug resistance, biological behaviour, and prognosis in colorectal cancer[24,26-28]. Therefore, FSIP1 is likely to participate in the biological activities of tumour cells, such as proliferation and invasion, through the abovementioned pathways and may also promote tumour angiogenesis and affect prognosis. It has been confirmed that FSIP1 is overexpressed in various breast cancers and is associated with prognosis. High expression of FSIP1 can promote the biological activity of breast cancer cells and reduce the sensitivity of tumours to chemotherapeutic drugs such as paclitaxel, which may be related to FSIP1 binding multidrug resistance protein 1 and making it stable. FSIP1 also regulates the proliferation and invasion of ER+ or HER2+ breast cancer cells by interacting with HER2[22]. It has also been reported that FSIP1 binds to PKA and SRC-3[31] and participates in chromosome segregation[16]. In addition, knockdown of FSIP1 in triple-negative breast cancer promotes autophagy, enhances AMP-activated protein kinase signalling, and reduces mTOR and Wnt/β-catenin pathway effects, thus reducing the sensitivity of tumour cells to chemotherapy drugs[32]. There are also a few reports in other tumours. For example, Sun et al[13,14] found that high expression of FSIP1 was positively correlated with a poor prognosis in bladder cancer, and in vitro cytological experiments confirmed that knockdown of FSIP1 expression inhibited PI3K/AKT pathway activity and phosphorylation levels in T24 cells, thereby inducing tumour cell apoptosis. However, there is few report on the expression of FSIP1 in colorectal cancer and its relationship with clinicopathological factors and prognosis, as well as its effects on related biological behaviour.
In this study, we examined the expression of FSIP1 in the pathological tissues of 302 patients with colon adenocarcinoma and statistically analysed at least 5 years of follow-up data. The results showed that compared with the expression in tumour adjacent tissues, the expression of FSIP1 was significantly increased in colon cancer tissues, and the high expression of FSIP1 was positively correlated with colon cancer stage and lymph node metastasis and negatively correlated with tumour differentiation. Spearman correlation analysis was used to determine the correlation between the expression of FSIP1 and various clinicopathological factors. The results showed that FSIP1 expression was not correlated with sex, age, tumour size, or other factors but was positively correlated with tumour pathological T stage and lymph node metastasis (N stage) and negatively correlated with tumour differentiation. Univariate and multivariate Cox regression analyses and survival analysis showed that high expression of FSIP1 was an independent risk factor for poor prognosis, and overall survival was worse in the FSIP1-high expression group than in the FSIP1-low expression group. The above results indicate that the FSIP1-encoding gene plays a role in promoting the carcinogenesis and progression of human colon cancer and is closely related to the occurrence and development of colon cancer. High FSIP1 expression often indicates more active biological behaviour in tumour cells and poor prognosis. Therefore, we speculate that FSIP1 may become an important molecular marker for the diagnosis, prognosis, and treatment of colon cancer.
However, this study only performed preliminary histological validation in human samples. We look forward to further in vitro cell experiments exploring the effects of FSIP1 on many factors of colon cancer cells, such as biological behaviour, epithelial to mesenchymal transition, drug sensitivity, immune tolerance, and the tumour microenvironment, as well as the specific signalling pathways and related interacting proteins. These studies will fully reveal the role of FSIP1 in the development of colon cancer, further enrich the understanding of the molecular mechanism of colon cancer pathogenesis, and provide a theoretical basis and new ideas for future methods for gene detection, diagnosis, prognosis determination, and drug treatment in colon cancer.
The occurrence and development of colon cancer are complex, involving a variety of genetic changes. Fibrous sheath interacting protein 1 (FSIP1) is a newly discovered oncogene that is frequently activated in a variety of tumours. However, the clinical significance of FSIP1 in colon cancer is unclear. In this study, the authors analysed the clinical significance of expression FSIP1 in human colon cancer, with an aim to clarify the biological role of FSIP1 in the development and progression of colon cancer.
Over the years, many scholars have been constantly looking for markers related to the diagnosis and prognosis of colon cancer. However, only EGFR/K-ras/BRAF and related targeted therapies are commonly accepted. Therefore, markers of broad significance to guide clinical diagnosis and treatment are still urgent needed.
In the study, the authors aimed to clarify the biological function of FSIP1 in the development and progression of colon cancer and its relationship with the clinical parameters.
A total of 302 specimens of tumour tissues and paracancerous tissues from patients with a pathological diagnosis of colon cancer were analyzed. FSIP1 expression in colon cancer tissues and adjacent normal tissues was detected by immunohistochemistry. Spearman correlation coefficient and Cox regression analyses were used to determine the relationship between FSIP1 expression and clinicopathological factors and prognosis, as well as the impact on survival.
Compared with the expression in benign adjacent tissues, the expression of FSIP1 was significantly increased in colon cancer tissues, and the high expression of FSIP1 was positively correlated with colon cancer stage and lymph node metastasis and negatively correlated with tumour differentiation. Spearman correlation analysis was used to determine the correlation between the expression of FSIP1 and various clinicopathological factors. The FSIP1 expression was not correlated with sex, age, tumour size, or other factors but was positively correlated with tumour pathological T stage and lymph node metastasis (N stage) and negatively correlated with tumour differentiation.
High expression of FSIP1 may be one of the important factors affecting the clinical outcome of colon cancer patients and leading to poor prognosis.
From the results, the authors speculate that FSIP1 may become an important molecular marker for the diagnosis, prognosis, and treatment of colon cancer. Although this study only performed preliminary histological validation in human samples, we look forward to further in vitro cell experiments exploring the effects of FSIP1 on many factors of colon cancer cells.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arisawa T, Guerin A, Majewski M S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Li BX, Zhang XS. Mechanism of Beclin1 as a favorable molecules marker is that Beclin1 inhibits tumor proliferation and cell cycle arrest in stage IIIB colon cancers. Zhonghua Linchuang Yishi Zazhi. 2010;4:23-27. |
| 2. | Akhter A, Walker A, Heise CP, Kennedy GD, Benson ME, Pfau PR, Johnson EA, Frick TJ, Gopal DV. A tertiary care hospital's 10 years' experience with rectal ultrasound in early rectal cancer. Endosc Ultrasound. 2018;7:191-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Cartana ET, Gheonea DI, Cherciu IF, Streaţa I, Uscatu CD, Nicoli ER, Ioana M, Pirici D, Georgescu CV, Alexandru DO, Şurlin V, Gruionu G, Săftoiu A. Assessing tumor angiogenesis in colorectal cancer by quantitative contrast-enhanced endoscopic ultrasound and molecular and immunohistochemical analysis. Endosc Ultrasound. 2018;7:175-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Uberoi AS, Bhutani MS. Has the role of EUS in rectal cancer staging changed in the last decade? Endosc Ultrasound. 2018;7:366-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Ersan V, Kutlu R, Erdem C, Karagul S, Kayaalp C. Colorectal Stenting for Obstruction due to Retrorectal Tumor in a Patient Unsuitable for Surgery. J Transl Int Med. 2017;5:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wu D, Li JN, Qian JM. Endoscopic Diagnosis and Treatment of Precancerous Colorectal Lesions in Patients with Inflammatory Bowel Disease: How Does the Latest SCENIC International Consensus Intersect with Our Clinical Practice? J Transl Int Med. 2017;5:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kim MS, Lee J, Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010;29:181-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Kim JY, Kim JH, Park TJ, Bae JS, Lee JS, Pasaje CF, Park BL, Cheong HS, Park JS, Park SW, Uh ST, Kim MK, Choi IS, Cho SH, Choi BW, Park CS, Shin HD. Positive association between aspirin-intolerant asthma and genetic polymorphisms of FSIP1: a case-case study. BMC Pulm Med. 2010;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Karagas MR, Andrew AS, Nelson HH, Li Z, Punshon T, Schned A, Marsit CJ, Morris JS, Moore JH, Tyler AL, Gilbert-Diamond D, Guerinot ML, Kelsey KT. SLC39A2 and FSIP1 polymorphisms as potential modifiers of arsenic-related bladder cancer. Hum Genet. 2012;131:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Cappell KM, Larson B, Sciaky N, Whitehurst AW. Symplekin specifies mitotic fidelity by supporting microtubule dynamics. Mol Cell Biol. 2010;30:5135-5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Brown PR, Miki K, Harper DB, Eddy EM. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol Reprod. 2003;68:2241-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Lesseur C, Gilbert-Diamond D, Andrew AS, Ekstrom RM, Li Z, Kelsey KT, Marsit CJ, Karagas MR. A case-control study of polymorphisms in xenobiotic and arsenic metabolism genes and arsenic-related bladder cancer in New Hampshire. Toxicol Lett. 2012;210:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Sun M, Zhao W, Zeng Y, Zhang D, Chen Z, Liu C, Wu B. Fibrous sheath interacting protein 1 overexpression is associated with unfavorable prognosis in bladder cancer: a potential therapeutic target. Onco Targets Ther. 2017;10:3949-3956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Sun M, Chen Z, Tan S, Liu C, Zhao W. Knockdown of fibrous sheath interacting protein 1 expression reduces bladder urothelial carcinoma cell proliferation and induces apoptosis via inhibition of the PI3K/AKT pathway. Onco Targets Ther. 2018;11:1961-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Cappell KM, Sinnott R, Taus P, Maxfield K, Scarbrough M, Whitehurst AW. Multiple cancer testis antigens function to support tumor cell mitotic fidelity. Mol Cell Biol. 2012;32:4131-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Luo M, Jin Z, Wang D, Sun M, Zhao X, Zhao Z, Lei H, Li M, Liu C. Expression and clinicopathological significance of FSIP1 in breast cancer. Oncotarget. 2015;6:10658-10666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R, Wright G, Lynch P, Patterson S, Lynch H, Gallinger S, Buchbinder A, Gordon G, Hawk E, Kirsch IR. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755-762. [PubMed] |
| 18. | Ohhara Y, Fukuda N, Takeuchi S, Honma R, Shimizu Y, Kinoshita I, Dosaka-Akita H. Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8:642-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7734] [Article Influence: 368.3] [Reference Citation Analysis (1)] |
| 20. | Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 21. | Passardi A, Canale M, Valgiusti M, Ulivi P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int J Mol Sci. 2017;18:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Liu T, Zhang H, Sun L, Zhao D, Liu P, Yan M, Zaidi N, Izadmehr S, Gupta A, Abu-Amer W, Luo M, Yang J, Ou X, Wang Y, Bai X, Wang Y, New MI, Zaidi M, Yuen T, Liu C. FSIP1 binds HER2 directly to regulate breast cancer growth and invasiveness. Proc Natl Acad Sci USA. 2017;114:7683-7688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Mao Y, Xu R, Liu X, Shi W, Han Y. Elevated fibrous sheath interacting protein 1 levels are associated with poor prognosis in non-small cell lung cancer patients. Oncotarget. 2017;8:12186-12193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Chiriva-Internati M, Ferrari R, Yu Y, Hamrick C, Gagliano N, Grizzi F, Frezza E, Jenkins MR, Hardwick F, D'Cunha N, Kast WM, Cobos E. AKAP-4: a novel cancer testis antigen for multiple myeloma. Br J Haematol. 2008;140:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Rahamim Ben-Navi L, Almog T, Yao Z, Seger R, Naor Z. A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci Rep. 2016;6:37922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Wang M, Li Y, Wang R, Wang Z, Chen K, Zhou B, Zhou Z, Sun X. PKA RIα/A-kinase anchoring proteins 10 signaling pathway and the prognosis of colorectal cancer. J Gastroenterol Hepatol. 2015;30:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Dükel M, Tavsan Z, Erdogan D, Erkan Gök D, Ayar Kayali H. Protein kinase C Inhibitors selectively modulate dynamics of cell adhesion molecules and cell death in human colon cancer cells. Cell Adh Migr. 2019;13:83-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Chiriva-Internati M, Yu Y, Mirandola L, D'Cunha N, Hardwicke F, Cannon MJ, Cobos E, Kast WM. Identification of AKAP-4 as a new cancer/testis antigen for detection and immunotherapy of prostate cancer. Prostate. 2012;72:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Chapman KB, Prendes MJ, Kidd JL, Sternberg H, West MD, Wagner J. Elevated expression of cancer/testis antigen FSIP1 in ER-positive breast tumors. Biomark Med. 2013;7:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Yan M, Wang J, Ren Y, Li L, He W, Zhang Y, Liu T, Li Z. Over-expression of FSIP1 promotes breast cancer progression and confers resistance to docetaxel via MRP1 stabilization. Cell Death Dis. 2019;10:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Labhart P, Karmakar S, Salicru EM, Egan BS, Alexiadis V, O'Malley BW, Smith CL. Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci USA. 2005;102:1339-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Liu C, Sun L, Yang J, Liu T, Yang Y, Kim SM, Ou X, Wang Y, Sun L, Zaidi M, New MI, Yuen T, Guo Q. FSIP1 regulates autophagy in breast cancer. Proc Natl Acad Sci USA. 2018;115:13075-13080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |