Published online Oct 15, 2020. doi: 10.4251/wjgo.v12.i10.1209
Peer-review started: March 18, 2020
First decision: May 28, 2020
Revised: July 6, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 15, 2020
Processing time: 210 Days and 11.5 Hours
Sarcomatoid hepatocellular carcinoma (SHC) is a rare subtype of hepatocellular carcinoma (HCC), with a high recurrence rate after surgery. In addition to limited effective treatment for the advanced stage of SHC, the prognosis of patients with this malignancy is worse than that of patients with conventional HCC.
We present the case of a 54-year-old man with SHC who underwent radical segmental hepatectomy, which relapsed 4 mo after surgery due to lymphatic metastasis in the porta hepatis. Although a second surgery was performed, new metastasis developed in the mediastinal lymph nodes. Therefore, sorafenib and lenvatinib were sequentially administered as first- and second-line systemic therapies, respectively. However, progressive disease was confirmed based on a recurrent hepatic lesion and new metastatic lesion in the abdominal cavity. Percutaneous transhepatic cholangial drainage was performed to alleviate the biliary obstruction. Because the tumor was strongly positive for programmed death-ligand 1, the patient was started on nivolumab. Imaging studies revealed that after two cycles of immunotherapy, the metastatic lesions decreased to undetectable levels.
The patient experienced continuous complete remission for 8 mo. Immune checkpoint inhibitors are useful for the treatment of advanced SHC.
Core Tip: Here, we report a rare case of advanced sarcomatoid hepatocellular carcinoma (SHC), which was successfully treated with the anti-programmed cell death 1 antibody. To the best of our knowledge, this is the first case report on the successful treatment of advanced SHC using immunotherapy.
- Citation: Zhu SG, Li HB, Yuan ZN, Liu W, Yang Q, Cheng Y, Wang WJ, Wang GY, Li H. Achievement of complete response to nivolumab in a patient with advanced sarcomatoid hepatocellular carcinoma: A case report. World J Gastrointest Oncol 2020; 12(10): 1209-1215
- URL: https://www.wjgnet.com/1948-5204/full/v12/i10/1209.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i10.1209
Sarcomatoid hepatocellular carcinoma (SHC) is a rare subtype of hepatocellular carcinoma (HCC) characterized by features of mixed epithelial and mesenchymal tumors[1]. Surgical resection is the primary treatment for SHC; however, this malignancy has a high recurrence rate. Unfortunately, an effective treatment for advanced-stage SHC is very limited. In addition, the prognosis for SHC is worse than that of conventional HCC[2,3]. Immune checkpoint inhibitors (ICIs) that target programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) have resulted in a significant breakthrough in the treatment of multiple solid cancers, such as melanoma, lymphoma, and lung cancer. However, their effectiveness in treating SHC has not been sufficiently demonstrated. Here, we report the case of a patient with advanced SHC, which was successfully treated with an anti-PD-1 antibody.
A 54-year-old man was referred to our hospital because of 10-d discomfort in the hepatic region.
The patient had a tumor in the segments 4/8 of the liver, along with hepatitis B cirrhosis, portal hypertension, and type 2 diabetes.
Approximately 15 years earlier, he was diagnosed with hepatitis B, and has since suffered from recurrent anorexia and jaundice. Five years earlier, he began treatment with entecavir. In the past 9 mo, he experienced unexplained weight loss (approximately 10 kg).
His physical examination did not reveal any abnormalities, except for liver palmar and spider nevus.
Plasma tumor marker levels, including alpha-fetoprotein (AFP), and liver function appeared normal.
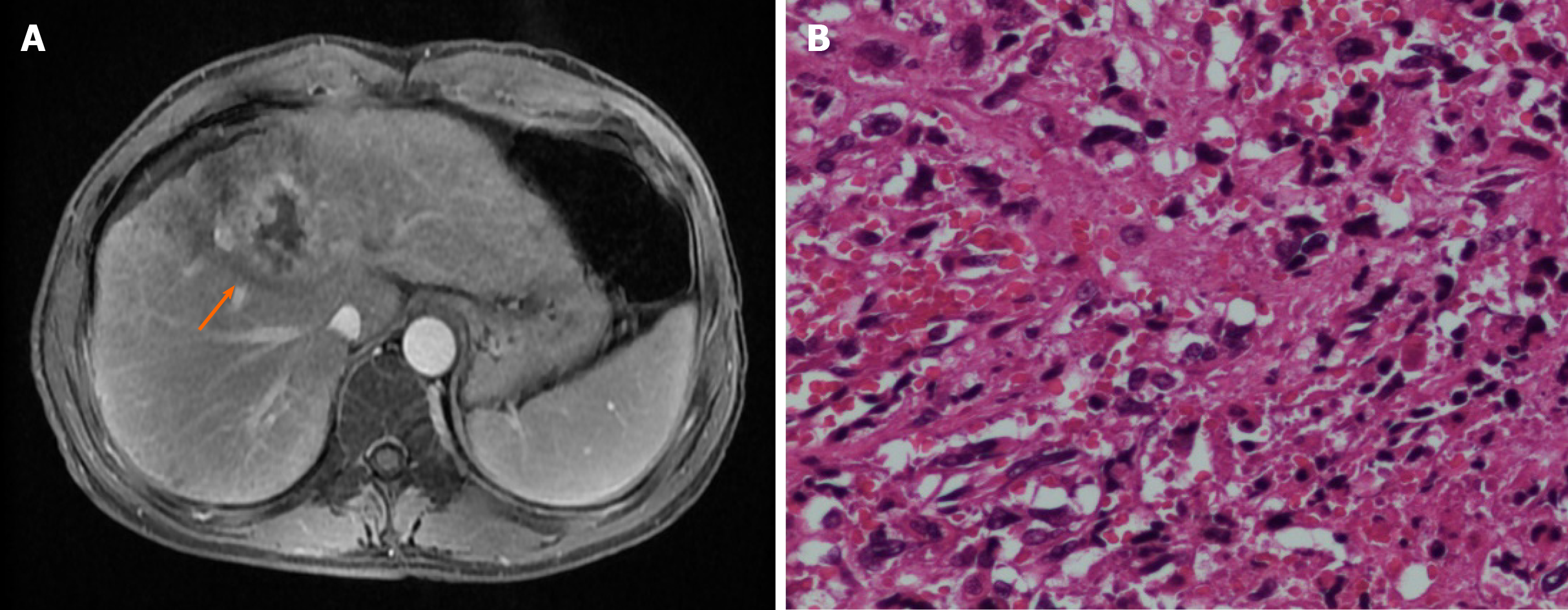
Abdominal ultrasound revealed a solid tumor, which was considered to be an HCC (38 mm × 35 mm), occupying the segments 4/8 of the liver. Abdominal dynamic contrast-enhanced magnetic resonance imaging (MRI) revealed a lesion in the segments 4/5/8 (45 mm × 41 mm) of the liver, with multiple satellite nodules. The lesions invaded the middle hepatic vein (Figure 1A). MRI also revealed liver cirrhosis and portal hypertension. No extrahepatic metastasis was observed on positron emission tomography-computed tomography (PET-CT).
The patient had a history of smoking for over 20 years.
Following a preoperative examination, the patient underwent resection of the liver segments 4/5/8. Postoperative pathological results revealed an SHC with satellite nodules, neurologic invasion, and no definite intravascular thrombus formation (Figure 1B). Immunohistochemical (IHC) results revealed the following: Cytokeratin (CK, +), Vimentin (+), Hepatocyte antigen (-), Arginase-1 (-), Cytokeratin 7 (+), Cytokeratin19 (CK19, partial +), Cytokeratin18 (CK18, +), Cytokeratin 8 (CK8, +), Glypican-3 (-), CD34 (vascular +), hepatitis B surface antigen (-), polyclone carcinoembryonic antigen (-), glutamine synthetase (partially +), and Ki-67 (30%). The patient recovered well after surgery.
Four months after the surgery, a follow-up CT scan revealed tumor recurrence at the hilar lymph nodes. A lymph node dissection was performed to resect the enlarged hilar lymph nodes, and the pathology revealed SHC metastasis (Supplementary Figure 1).
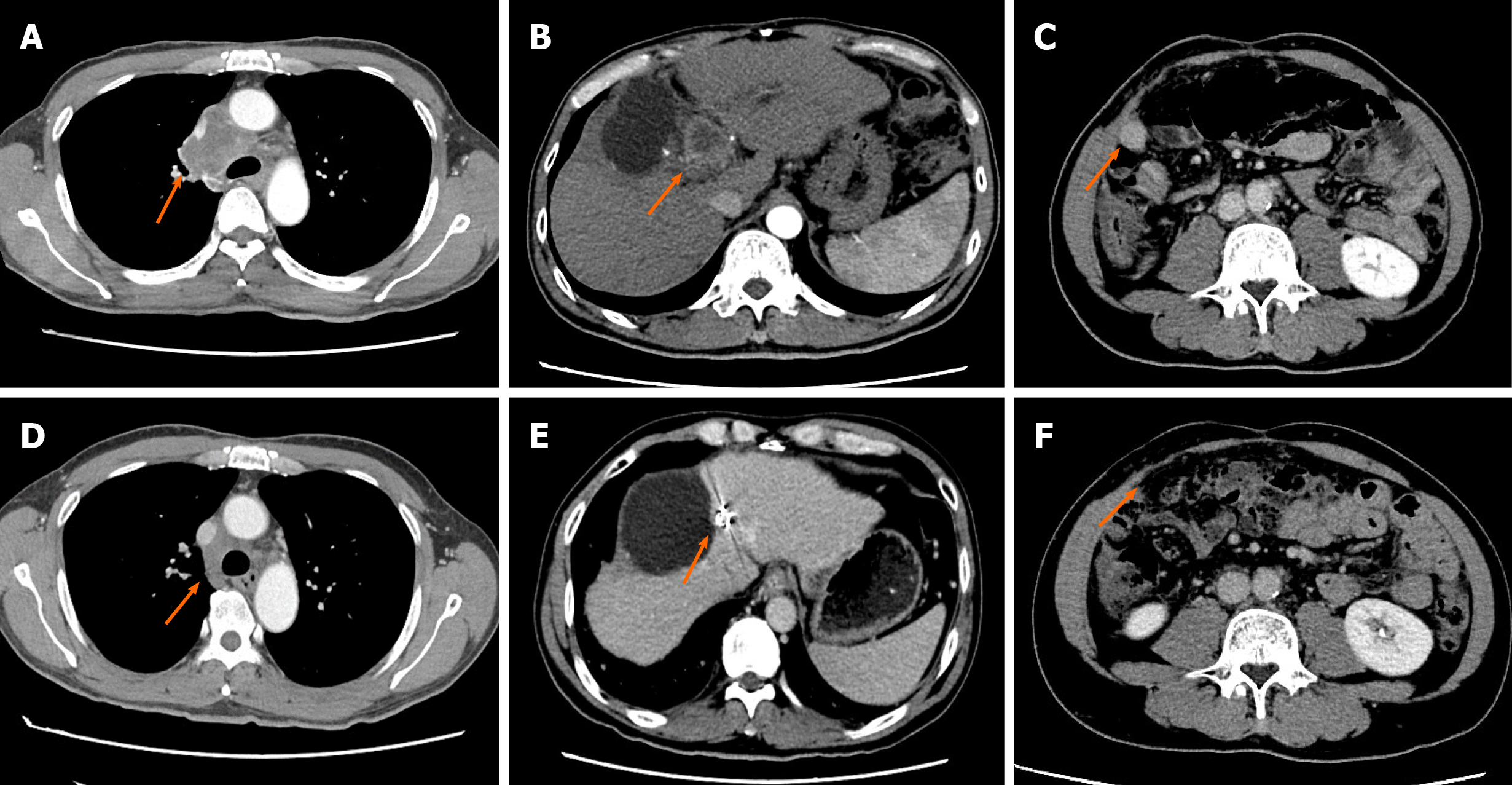
One month after the second surgery, a CT scan revealed new metastasis in the mediastinal lymph nodes (Supplementary Figure 1). Because the patient was diagnosed with an advanced disease, oral targeted treatment with sorafenib (0.4 g, bid) was initiated. Metastasis in the mediastinal lymph nodes slowly enlarged. In addition, the patient developed obvious chest distress owing to the compression of the enlarged mediastinal lymph nodes 12 mo after the second surgery (Figure 2). The patient was subsequently switched to lenvatinib as second-line treatment. However, after 2 mo, the patient developed severe jaundice, and the CT scan revealed recurrence of a hepatic lesion, which compressed the bile duct (Figure 2). New metastasis in the abdominal cavity was also found. Laboratory blood tests revealed increased serum levels of alanine aminotransferase (ALT; 126 U/L, normal range 0-40 U/L), aspartate aminotransferase (AST; 89 U/L, normal range 0-40 U/L), and total bilirubin (142.8 µmol/L, normal range 0-17.1 µmol/L). Percutaneous transhepatic cholangial drainage was performed to treat jaundice. The patient had an Eastern Cooperation Oncology Group (ECOG) score of 2.
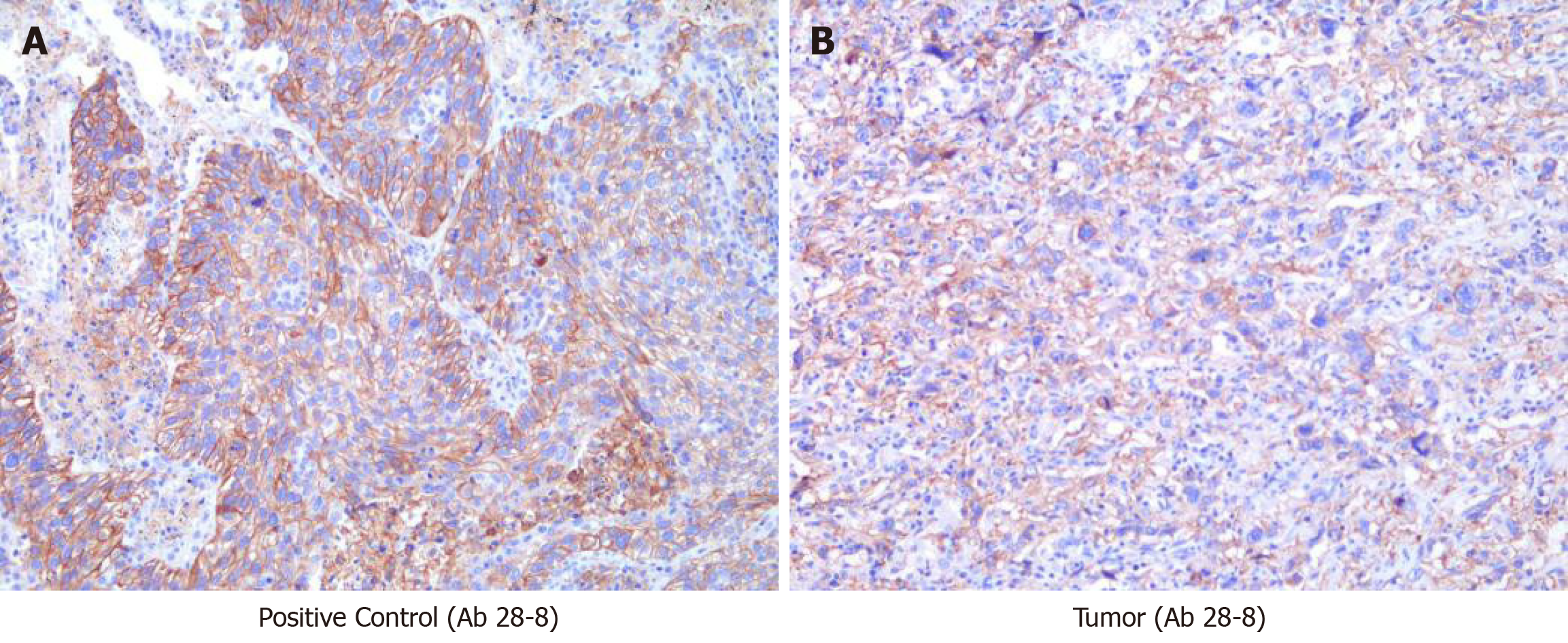
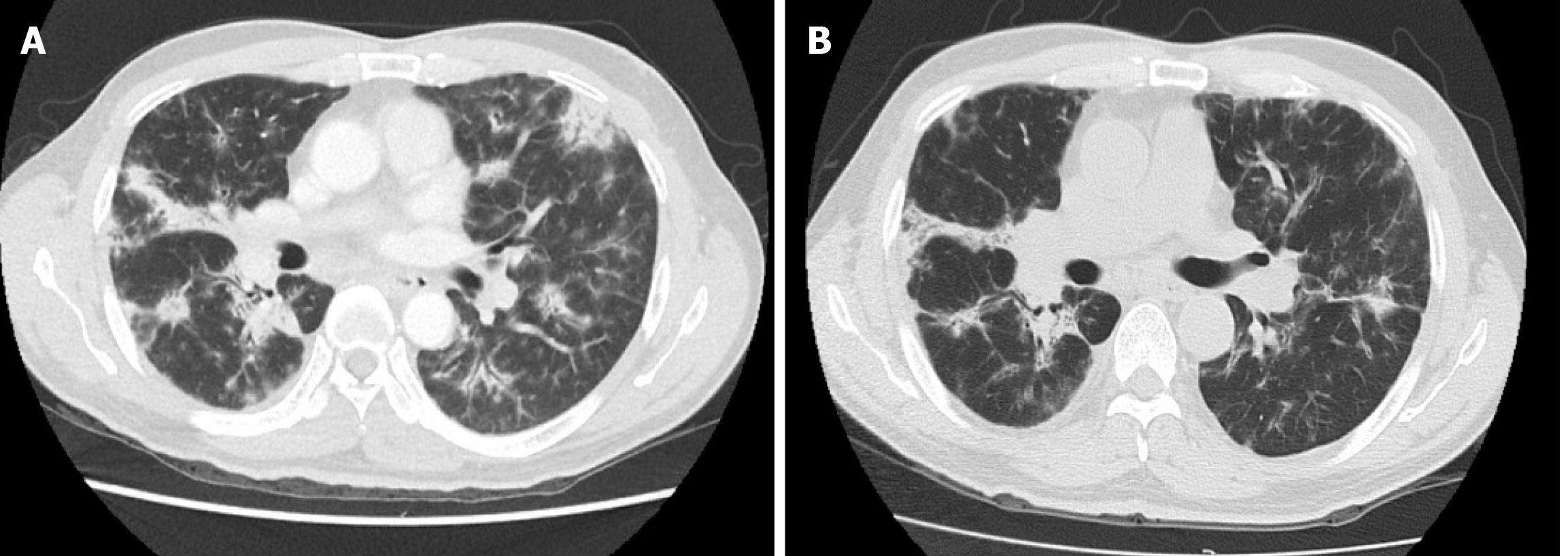
The tissue sample collected during surgery was submitted for next-generation sequencing on a 450-gene panel to determine whether precision therapy was an option for SHC treatment. Genomic testing revealed adenomatous polyposis coli, P53, RNA binding motif protein 10, RELB proto-oncogene/NF-kB subunit, and succinate dehydrogenase complex flavoprotein subunit A mutations and DNA polymerase beta amplification. Tumor mutational burden was 2.3 Muts/Mb, and the microsatellite state was stable. The IHC results revealed that the PD-L1 tumor proportion score (TPS) of the tissue sample was 60%, indicating high expression of PD-L1 (Figure 3). Based on these findings, immunotherapy was administered with the patient’s consent. First, the patient received nivolumab (3 mg/kg, Q3W). After two cycles of immunotherapy, a follow-up CT scan was performed, which revealed a recurrent lesion in the liver, metastasis in the abdominal cavity, and shrinkage of the mediastinal lymph nodes to undetectable levels (Figure 2). After the 6th cycle of nivolumab treatment, the patient was again referred to the hospital due to severe cough. A CT scan confirmed that the patient had developed interstitial pneumonia owing to the immunotherapy (Figure 4).
Owing to the development of interstitial pneumonia, nivolumab treatment was discontinued, and the patient achieved relief after treatment with corticosteroids (pulsed methylprednisolone). The patient showed continuous complete remission for 8 mo up until the latest follow-up.
SHC is a rare malignant tumor that accounts for 2%–5% of all HCC cases[3,4]. SHC is characterized by a high degree of malignancy, rapid progression, and poor prognosis. The 5-year overall survival (OS) of patients with SHC was found to be worse than that of patients with conventional HCC (5.7% vs 30.1%)[5]. A lack of specificity is associated with clinical manifestation and etiology of SHC[3]. Pathological and IHC staining are the gold standards for diagnosing this malignancy[4,6]. The main pathological features of SCH include the following: (1) Coexistence of cancer and sarcoma with a certain migration existing between them; and (2) positive epithelial and mesenchymal tumor markers on IHC staining. Intercytokeratins CAM5.2, CK8, CK18, and CK19 could be positively expressed in SHC[7]. The imaging manifestations of SHC must be differentiated from those of typical cholangiocarcinoma, inflammatory mass, and HCC[8]. Currently, preoperative diagnosis is difficult, and final diagnosis still requires pathological confirmation for this tumor. Early surgical excision is the first choice for radical treatment but recurrence easily occurs following the surgery. Currently, there is no effective treatment for recurrence[9], and the efficacy of other therapies such as radiotherapy, chemotherapy, and targeted therapy has been rarely reported for SHC.
Use of ICIs is a rapidly developing immunotherapy method in recent years[10]. ICIs can kill tumors by primarily restoring the suppressed immune function of the body. Tumors that have high expression of PD-L1 on the surface of T cells can be mistaken for autologous cells by binding to PD-1 on the surface of T cells. Ultimately, this would inhibit the killing effect of the immune system on tumor cells[11]. ICIs are effective for solid tumors. In China, melanoma, lung cancer, Hodgkin's lymphoma, and head and neck squamous cell carcinomas are considered for immunotherapy. A previous study revealed that the objective response rate (ORR) of PD-1 inhibitor monotherapy is approximately 20% for advanced HCC[12]. As a first-line treatment for advanced HCC, compared with sorafenib, nivolumab caused clinically meaningful improvements in ORR and OS. In addition, patients with positive PD-L1 expression showed an increased response rate in the nivolumab arm, suggesting that PD-L1 expression could be a potential predictive biomarker for HCC.
In the current report, we have presented the case of a patient with advanced and heavily treated SHC. Following treatment, we found that the tumor tissue had positive PD-L1 expression (antibody 28-8, TPS = 60%). These findings suggest that immunotherapy with PD-1/PD-L1 inhibitors may be effective in treating SHC patients. Furthermore, the patient achieved a complete response to nivolumab and was followed for 8 mo. Although the use of nivolumab as the first-line treatment for HCC was demonstrated to significantly prolong patient survival, its efficacy against the SHC subtype has never been reported, in the form of case reports or cohort studies. Thus, to our knowledge, this is the first case report of advanced SHC, which was successfully treated via immunotherapy. We speculated that the effectiveness of immunotherapy in this patient may be related to the high expression of PD-L1. Furthermore, a recent study suggested that patients who respond to an anti-PD-1/L1 antibody are more likely to report immune-mediated adverse events[13]. In the present case, the patient developed interstitial pneumonia after the sixth cycle of immunotherapy. Therefore, systemic corticosteroid treatment was required. This result may serve as an important sign of good response to immunotherapy. Hyper-progression, which refers to a paradoxical acceleration in tumor progression after PD-1/PD-L1 inhibitor monotherapy, has been commonly reported[14]. Several biomarkers, such as MDM2/MDM4 amplification, EGFR alteration, and 11q13 amplification, are suggested to be related to hyper-progression[15]. In the present case, the patient did not harbor any hyper-progression biomarkers related to immunotherapy.
In this study, anti-PD-1 antibody was used for the treatment of advanced SHC, with the patient experiencing continuous complete remission for 8 mo until the recent follow-up. Effective and predictive biomarkers should thus be confirmed through further clinical studies. We propose that immunotherapy should be considered as a viable treatment option for advanced SHC.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dourakis SP, Hann HW, Kai K S-Editor: Wang DM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Seo N, Kim MJ, Rhee H. Hepatic sarcomatoid carcinoma: magnetic resonance imaging evaluation by using the liver imaging reporting and data system. Eur Radiol. 2019;29:3761-3771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Zhu CC, Li MR, Lin TL, Zhao G. Sarcomatoid carcinoma of the stomach: A case report and literature review. Oncol Lett. 2015;10:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Liao SH, Su TH, Jeng YM, Liang PC, Chen DS, Chen CH, Kao JH. Clinical Manifestations and Outcomes of Patients with Sarcomatoid Hepatocellular Carcinoma. Hepatology. 2019;69:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Shafizadeh N, Kakar S. Hepatocellular Carcinoma: Histologic Subtypes. Surg Pathol Clin. 2013;6:367-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Wu L, Tsilimigras DI, Farooq A, Hyer JM, Merath K, Paredes AZ, Mehta R, Sahara K, Shen F, Pawlik TM. Management and outcomes among patients with sarcomatoid hepatocellular carcinoma: A population-based analysis. Cancer. 2019;125:3767-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Gu Q, Yu X, Chen H, Chen G. Clinicopathological features of combined hepatocellular-cholangiocarcinoma with sarcomatous change: Case report and literature review. Medicine (Baltimore). 2018;97:e9640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Cho MS, Lee SN, Sung SH, Han WS. Sarcomatoid hepatocellular carcinoma with hepatoblastoma-like features in an adult. Pathol Int. 2004;54:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Torbenson MS. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2017;46:365-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 9. | Yoshida N, Midorikawa Y, Kajiwara T, Yoshida N, Nakayama H, Sugitani M, Takayama T. Hepatocellular Carcinoma with Sarcomatoid Change without Anticancer Therapies. Case Rep Gastroenterol. 2013;7:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Greally M, Chou JF, Chatila WK, Margolis M, Capanu M, Hechtman JF, Tuvy Y, Kundra R, Daian F, Ladanyi M, Kelsen DP, Ilson DH, Berger MF, Tang LH, Solit DB, Diaz LA, Schultz N, Janjigian YY, Ku GY. Clinical and Molecular Predictors of Response to Immune Checkpoint Inhibitors in Patients with Advanced Esophagogastric Cancer. Clin Cancer Res. 2019;25:6160-6169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Berraondo P, Ochoa MC, Olivera I, Melero I. Immune Desertic Landscapes in Hepatocellular Carcinoma Shaped by β-Catenin Activation. Cancer Discov. 2019;9:1003-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 13. | Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning YM, Singh H, Suzman D, Xu J, Goldberg KB, Sridhara R, Ibrahim A, Theoret M, Beaver JA, Pazdur R. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol. 2019;37:2730-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 14. | Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, Ferté C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Fuentes-Antrás J, Provencio M, Díaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev. 2018;70:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |