Published online Sep 15, 2019. doi: 10.4251/wjgo.v11.i9.750
Peer-review started: February 15, 2019
First decision: April 15, 2019
Revised: May 5, 2019
Accepted: August 18, 2019
Article in press: August 19, 2019
Published online: September 15, 2019
Processing time: 213 Days and 8.8 Hours
Gastrointestinal schwannomas are slow-growing benign mesenchymal neoplasms that originate from Schwann cells of the nerve sheath of Auerbach´s plexus or less frequently from Meissner´s plexus. The main differential diagnosis of gastric schwannomas are the gastrointestinal stromal tumors (GISTs), which are classified by their immunohistochemistry. The treatment of choice for gastric schwannomas is surgery where laparoscopy plays an important role. Wedge resection, subtotal or total gastrectomy can be done. In its counterpart, esophageal schwannomas are benign tumors of the esophagus that are very uncommon since they comprise less than 2% of all esophageal tumors. The main differential diagnosis is the leiomyoma which corresponds to the most common benign esophageal tumor, followed by GIST. The treatment consists on tumoral enucleation or esophagectomy.
To review the available literature about gastrointestinal schwannomas; especially lesions from de stomach and esophagus, including diagnosis, treatment, and follow up, as well as, reporting our institutional experience.
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes guidelines. The following databases were used for reviewing process: PubMed, Ovid, MEDLINE, and Scopus. Only English language manuscripts were included. All gastrointestinal schwannomas specifically located in the esophagus and stomach were included. Cases that did not report long-term follow-up were excluded.
Gastric localization showed a higher prevalence in both, the literature review and our institution: 94.95% (n = 317) and 83% (n = 5) respectively. With a follow-up with disease-free survival greater than 36 mo in most cases: 62.01% (n = 80) vs 66.66% (n = 4). In both groups, the median size was > 4.1 cm. Surgical treatment is curative in most cases
Schwannoma must be taken into account in the differential diagnosis of gastrointestinal mesenchymal tumors. It has a good prognosis, and most are benign. A disease-free survival of more than 36 mo can be achieved by surgery.
Core tips: We performed a systematic review of the literature searching two types of rare tumors; esophageal and gastric schwannomas. We review its form of presentation, diagnosis, differential diagnosis and treatment. We also performed a systematic review trying to gather all case reports and case series in a single paper. We have found 16 cases of esophageal schwannomas and 301 cases of gastric schwannomas in all literature. We also reviewed our institutional experience with the report of 6 cases of gastrointestinal schwannomas, 1 is esophageal and 5 are gastric.
- Citation: Morales-Maza J, Pastor-Sifuentes FU, Sánchez-Morales GE, Ramos ESG, Santes O, Clemente-Gutiérrez U, Pimienta-Ibarra AS, Medina-Franco H. Clinical characteristics and surgical treatment of schwannomas of the esophagus and stomach: A case series and systematic review. World J Gastrointest Oncol 2019; 11(9): 750-760
- URL: https://www.wjgnet.com/1948-5204/full/v11/i9/750.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i9.750
Gastrointestinal schwannomas are slow-growing benign mesenchymal neoplasms that originate from Schwann cells. The most frequent locations are the stomach and colon, with the esophagus being the least frequent site.
Despite being tumors widely known by clinicians, gastrointestinal schwannomas are rare. It is accepted that the treatment of choice is laparoscopic or open resection, where in wedge, subtotal or total gastrectomy can be performed.
We present an institutional case series, and a systematic review of esophagic and gastric schwannomas. The aim of this study is to review the available literature about gastrointestinal schwannomas; especially lesions from de stomach and esophagus, including diagnosis, treatment, and follow up, as well as, reporting our institutional experience.
Gastric schwannomas: Gastrointestinal schwannomas are slow-growing benign mesenchymal neoplasms that originate from Schwann cells of the nerve sheath of Auerbach´s plexus or less frequently from Meissner´s plexus[1]. They were first described by Daimaru et al[2] since 1988 to date, only 300 cases approximately have been reported in the literature[3]. It has been suggested that gastric schwannomas play a different genetic mechanism compared to soft tissue schwannomas by not expressing monosomy on chromosome 22 and very rarely mutations in NF2[4]. Mesenchymal tumors include leiomyomas, gastrointestinal stromal tumors (GIST), and schwannomas, with the latter being very rare since it represents 0.2% of all gastric tumors, 6.3% of gastric mesenchymal tumors, and 4% of all benign gastric tumors[5-7]. Gastric schwannomas tend to appear as single lesions in the stomach with the following subdivisions: the body (59.3%), antrum (26.7%) fundus (12%) and cardia (2%), followed by colon as submucosal tumors[1,3]. They have an average reported diameter of 4.69 cm ranging from 0.8 to 15.5 cm. Female predominance has been observed with a male to female ratio of 1: 2.64 and can occur at any age, although predominantly between the fifth and eighth decades of life, with 86.43% diagnosed in people over 40 years of age, with an average age of 56.82 years[1-4,6].
The main differential diagnosis of gastric schwannomas are GISTs which careful differentiation of these two entities should be done, since clinical, histological and demographic presentation are very similar, but the treatment and prognosis of each one is very different[1,4]. It is estimated that for every 45 cases of gastric GISTs there is gastric schwannoma[4]. Gastric schwannomas have an excellent prognosis after surgical resection, while GIST can recur, have malignant potential ranging from 10% to 30% and in certain cases can be treated with imatinib achieving great response[1].
Gastric schwannomas can rarely progress into malignant tumors of the peripheral nerve sheath (MTPNS), known as malignant schwannomas, mainly when they have a mitotic index greater than 10/50 HPF[1,4]. Only 10 cases (4.5%) of all gastric schwannomas reported in the literature have been described as MTPNS wich are characterized by a greater number of mitotic index, presence of necrosis and nuclear atypia[1,3]. However, this idea of malignant transformation has been questioned in recent research, since most of the malignant transformation were reported prior to modern immunohistochemistry, which they probably corresponded to GISTs instead of schwannomas[4].
Patients with gastric schwannomas are usually asymptomatic with incidental findings in 43.3% during endoscopies for unrelated conditions[1,3,6]. Symptomatic patients typically present with abdominal pain or discomfort followed by upper gastrointestinal bleeding and less frequently with a palpable abdominal mass (3.05%), poor appetite (3.05%), dyspepsia (1.82%), weight loss (1.21%), nausea or vomiting (0.6%)[1,3,6]. There’s only one case of gastroduodenal intussusception due to gastric schwannoma reported in the literature[8].
Preoperative diagnosis is a challenge for the surgeon because of the difficulty to differentiate gastric schwannomas from GISTs. In upper gastrointestinal endoscopy, gastric schwannomas are frequently seen as firm, protruding submucosal masses and in patients with active bleeding the ulcerated mucosa is observed[1,6]. Endoscopic biopsies have limited usefulness since false negatives can occur when the mucosa of the lesion is intact[1]. Fine needle aspiration biopsy (FNA) guided by endoscopic ultrasound (EUS) is a reliable diagnostic method although with a reported diagnostic accuracy of 50 to 85.2% for mesenchymal gastric tumors, which have a hypoechoic appearance in the EUS[6,9].
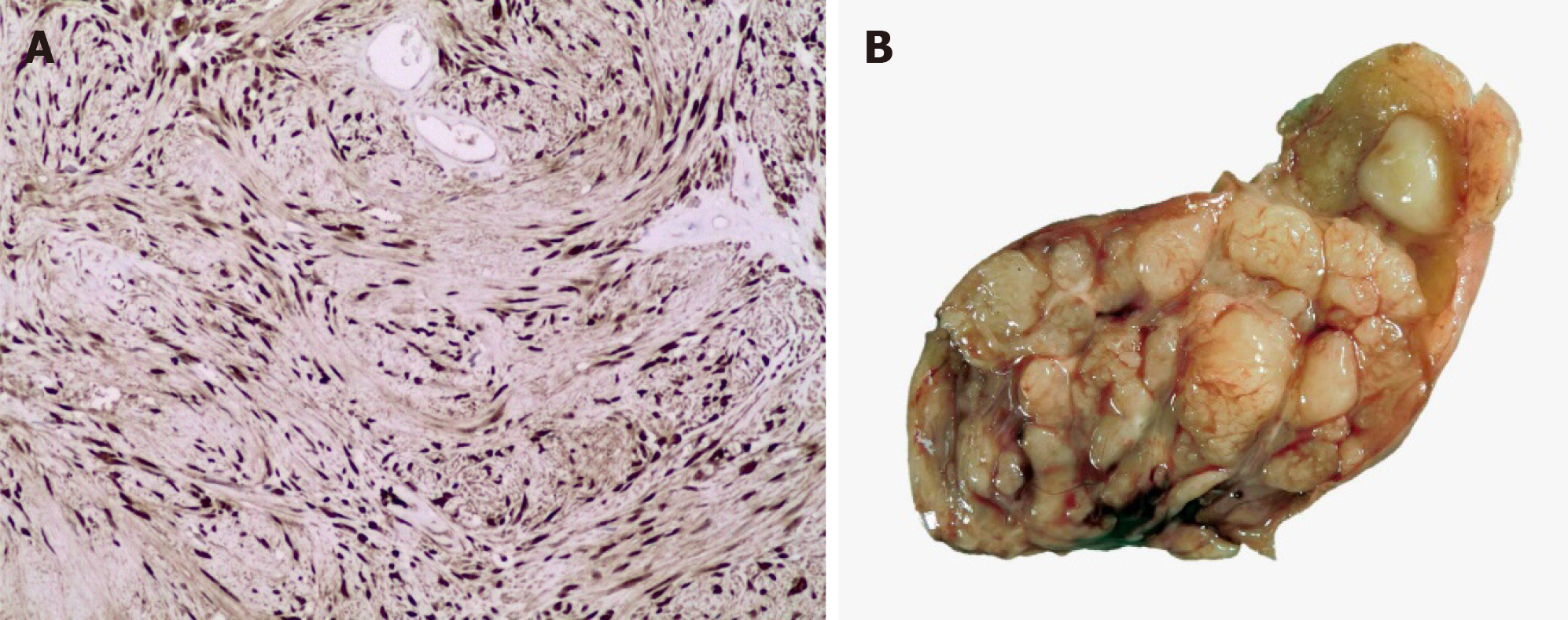
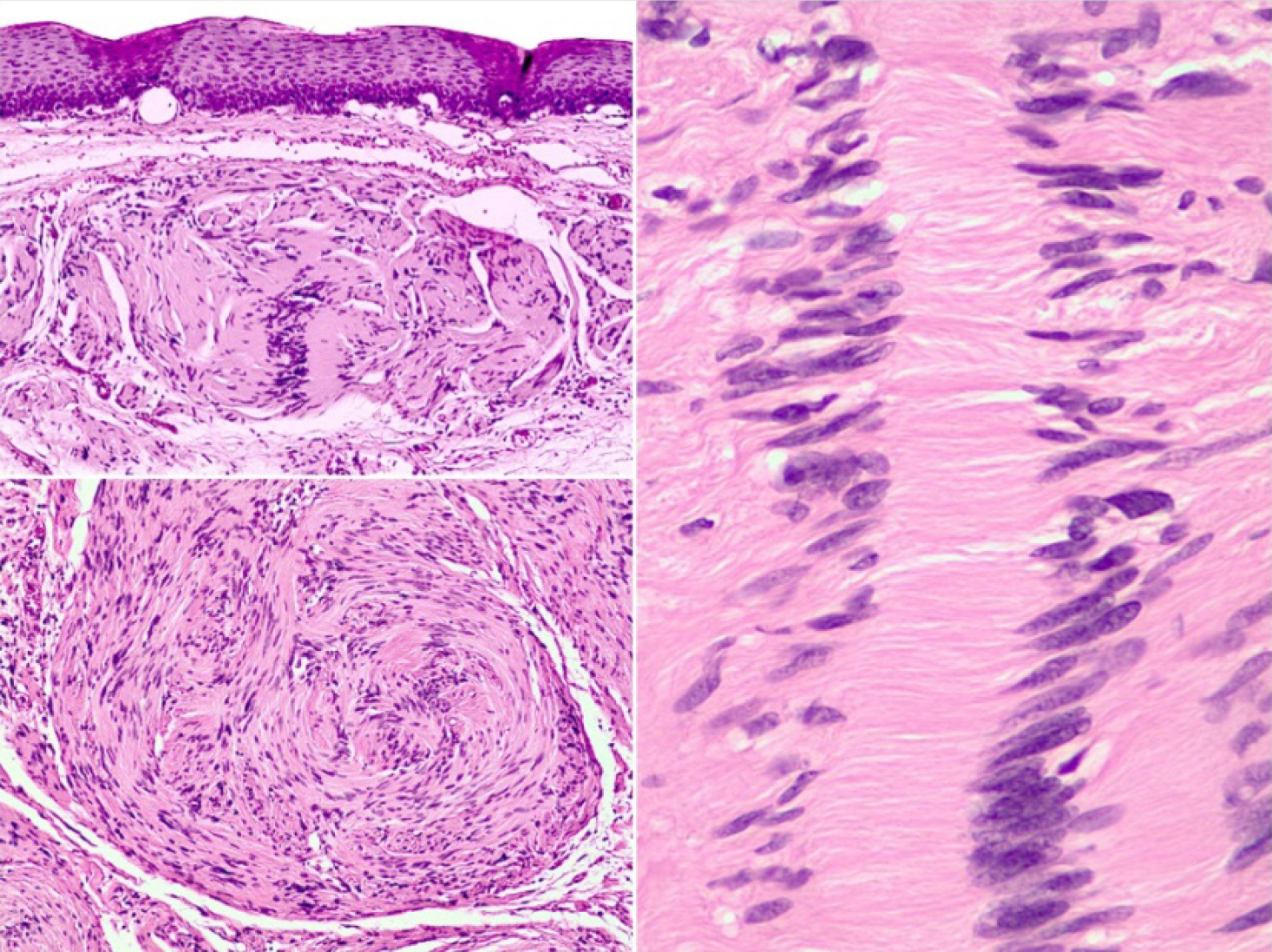
In computed tomography (CT), gastric schwannomas are distinguished by having homogeneous density, leiomyomas often show calcifications and leiomyosarcomas are usually more heterogeneous[10]. However, the radiological findings in both CT and magnetic resonance imaging (MRI) of gastric schwannomas are not specific and can be confused with GISTs even with positron emission tomography with 18-fluorodeoxyglucose (PET-FDG), since they present as hypermetabolic[6,11]. The diagnosis is based on histology and confirmed with the immunohistochemical report which is the gold standard. Histopathology of these tumors shows a fascicular arrangement with spindle-shaped nuclei. Immunohistochemistry is positive for S-100 protein(Figure 1), vimentin and glial fibrillary acidic protein (Figure 2), CD34 positive or negative, and negative for smooth muscle actin, desmin, DOG-1 and c-Kit (CD117); the latter positive in GIST[12,13]. It is important to distinguish gastric schwannomas from malignant tumors with positivity for S-100 such as clear cell gastrointestinal sarcoma and metastatic melanoma[4].
The treatment of choice for gastric schwannomas is surgery where laparoscopy plays an important role. Wedge resection, subtotal or total gastrectomy can be done since they have low malignant potential[1,3,4]. Lymphadenectomy is not usually performed unless enlarged lymph nodes are seen, since gastric schwannoma rarely metastasizes to lymph nodes. Only minimally invasive endoscopic approaches should be performed with or without laparoscopic approach, when the diagnosis of gastric schwannoma is definitively confirmed[3]. Given its benignity, the recurrence of gastric schwannoma is only associated with positive surgical margins. Frequent CT follow-up is not recommended[1,14].
Schwannomas of the esophagus: These benign esophageal tumors are very rare since they comprise less than 2% of all esophageal tumors[15-17]. The most common submucosal esophageal tumor is leiomyoma, diagnosed in 50% of every benign case, followed by GISTs, which have malignant potential[18-20]. Other less frequent benign submucosal esophageal tumors are lipomas, granular cell tumors and schwannomas[21]. Gastrointestinal schwannomas comprise between 0.4 and 1% of all submucosal tumors of the gastrointestinal tract, and most of them are found in the stomach, making them extremely rare in the esophagus[15,21,22].
Schwannomas are the most common neurogenic tumor, which are derived from Schwann cells hence its name[15]. Schwannomas typically originate in the posterior mediastinum, followed by the chest wall and lung parenchyma, and extremely rare from the esophagus[15,20]. Esophageal schwannomas are usually found in the upper thoracic segment[17,23]. An average size of 5.6cm has been reported, with a range of 0.5 to 10 cm[24]. They are predominant in the female gender with a female to male ratio of 19: 8 and a mean age at diagnosis of 50 years[25,26].
Most patients are asymptomatic and are diagnosed incidentally[17]. The most common symptom is moderate to severe dysphagia followed by dyspnea[15,17,20]. Symptoms usually correlate with tumor size due to the effect of mass on neighboring structures which can also result in chest pain, pneumonia or hemoptysis[17]. There are two reported cases of benign schwannoma in the upper esophagus with compression to the trachea[23,25].
The preoperative diagnosis of esophageal schwannoma is a challenge for the surgeon since there are no special characteristics on imaging studies and even endoscopic biopsies can be useless because schwannomas are found in the submucosal tissue[15,21]. A successful preoperative diagnosis can lead to a less invasive surgical treatment[26]. CT and MRI have limited diagnostic utility; however, its use in conjunction with PET-FDG has been reported to be useful in the diagnosis of esophageal tumors [27].
On CT, esophageal schwannoma tends to show a homogeneous density while leiomyomas often show calcifications; on the other hand, leiomyosarcomas tend to be more heterogeneous[10]. Currently, fine FNA biopsy guided by EUS is used to diagnose submucosal tumors because it has a diagnostic accuracy of 85.2%, allowing more adequate samples to be obtained than upper gastrointestinal endoscopy biopsy[10]. Some unlikely risks of EUS-guided FNAB are bleeding, infection, trachea perforation; however, it is considered a safe, reliable and accurate method[9,17,21]. Through PET, schwannomas prove to be hypermetabolic; however, this has no clinical correlation with malignancy since these tumors are derived from nerve cells that express the type 3 glucose transporter which increases FDG uptake[28]. When the preoperative diagnosis is not reliable, a transoperative pathological diagnosis may be useful[17]. Schwannomas are usually benign and can show one or two histological patterns: Antoni A and B. The Antoni A pattern shows compact areas with palisaded spindle cells, while the Antoni B pattern consists of poorly organized tissue with variable cystic changes and hemorrhage. MTPNS are the counterpart of benign schwannomas. These tumors are very rare and their histopathological report is characterized by greater mitotic cells, necrosis and atypia[15]. Positive immunohistochemistry for S-100 protein and negative for smooth muscle markers such as actin and desmin support the diagnosis of MTPNS[11]. Immunohistochemistry is essential to differentiate a GIST from a schwannoma[20,25].
The treatment of esophageal schwannoma is tumoral enucleation or esophagectomy[17,25]. Local resection versus esophagectomy is preferred since the former has lower morbidity and is curative for benign schwannomas while the latter has a higher rate of postoperative complications such as recurrent laryngeal nerve palsy, pulmonary involvement or chylothorax[29-31]. Tumor enucleation is usually viable because esophageal schwannoma is usually limited to the submucosa; however, this technique is not recommended for very large tumors due to an increased risk of esophageal stenosis[31,32].
Surgery is indicated for symptomatic lesions, lesions with a diameter greater than 4 to 5 cm, suspicion of malignancy and lesions that have increased in size during follow up[15]. The surgical approach should be considered based on the tumor size, the location of the lesion and the patient's condition[17].
When there is suspicion of malignancy, such as tumor larger than 10 cm, biopsy with mitotic figures, esophageal muscle invasion or cellular atypia; the indication is esophagectomy with negative margins and lymph node dissection to prevent recurrences or lymph node metastases[33,34]. For sporadic tumors in the upper thoracic segment, a right thoracotomy is preferred, and less often a cervical approach[15,23,33]. There are case reports of successful resections of schwannomas by minimally invasive thoracic surgery (VATS) with less pain, fewer postoperative complications such as pneumonia and shorter recovery time compared to standard thoracotomy[15,18,35]. However, VATS is not recommended for large submucosal tumors due to an increased risk of mucosal damage when performing extensive dissections[36].
In general, esophageal schwannomas have a good prognosis after surgical resection and a long term with CT and esophagoscopy is recommended[35].
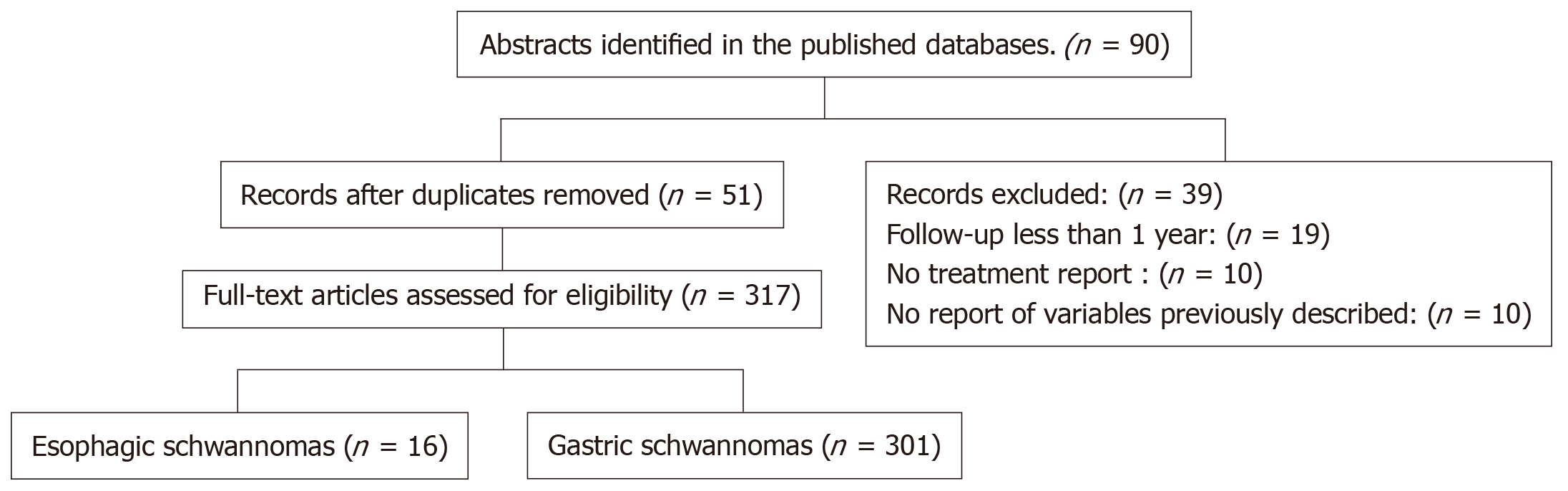
A comprehensive literature search was conducted using controlled vocabulary and key words in the following databases: PubMed, MEDLINE, Cochrane and Ovid. The following MeSH terms were used "schwannoma" and "gastrointestinal schwannoma". The detailed search strategy is shown in the study flow diagram (Figure 3). The references of identified studies were also searched to identify additional studies for inclusion. Only English language manuscripts were included. All Gastrointestinal schwannomas specifically located in the esophagus and stomach were included. Cases that did not report long-term follow-up were excluded. Only one researcher carried out the review of the cases reported in the literature. The following variables were extracted for each case report: age, sex, race of the patient, tumor site, tumor size, surgical procedure, long-term follow-up, and disease-free time.
Initially, 90 case reports and series of cases were found in the literature, of which 39 publications were excluded due to lack of complete data according to the methodology previously described, and finally 51 articles were included. We extracted a total of 317 cases of esophageal and gastric schwannomas, with a total of 16 and 301 cases respectively[2-4,8,12,34,37-75]. The clinical characteristics of these 317 cases [including the documented cases of our center (INCMNSZ)] are summarized in Table 1.
| Characteristics | Institutional experience (n = 6) | Literature review1 (n = 317) |
| Location | ||
| Stomach | 5 (83.3) | 301 (94.9) |
| Esophagus | 1 (16.6) | 16 (5) |
| Size | ||
| 1-2 cm | Stomach: 2 (33) | Stomach: 20 |
| 2.1 – 4 cm | Stomach: 1 (16.6) | Stomach: 66; Esophagus: 1 |
| > 4.1 cm | Stomach: 2 (33); Esophagus: 1 (16.6) | Stomach: 88; Esophagus: 3 |
| Follow-up with disease- free survival in months | ||
| < 12 | Stomach: 1 (16.6); Esophagus: 1 (16.6) | Stomach: 18 |
| 43823 | 0 | Stomach: 21; Esophagus: 2 |
| 25 – 36 | 0 | Stomach: 8 |
| >36 | Stomach: 4 (66.6) | Stomach: 78; Esophagus: 2 |
| Age | ||
| < 30 yr | Stomach: 1 (16.6) | Stomach: 6 (2.47); Esophagus: 3 (18.75) |
| 30-40 yr | Stomach: 1 (16.6) | Stomach: 18 (7.43); Esophagus: 1 (6.25) |
| 41-50 yr | Esophagus: 1 (16.6) | Stomach: 49 (20.24); Esophagus: 3 (18.75) |
| 51-60 yr | Stomach: 2 (33.3) | Stomach: 72 (29.75); Esophagus: 4 (25) |
| >60 yr | Stomach: 1 (16.6) | Stomach: 97 (40.08); Esophagus 5 (31.25) |
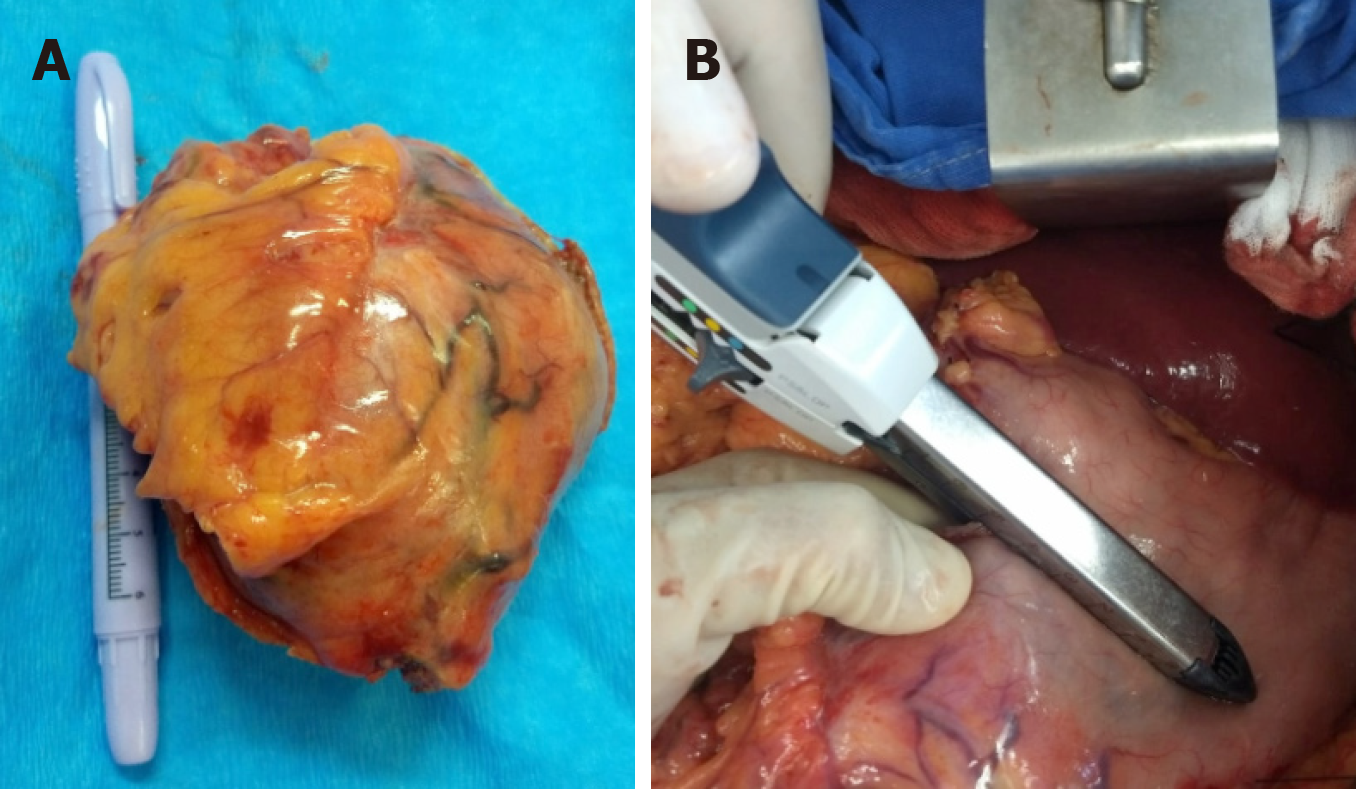
We included a total of 6 upper gastrointestinal schwannomas (esophagus and stomach) (Figure 4). Only one case was at the esophageal level and the remaining 5 at gastric level. The clinical and pathological characteristics of these patients are shown in Table 2. In all 6 cases, complete surgical resection was attempted, with only one case reporting positive surgical margins in the histopathologic report.
| Case | Sex | Age, yr | Tumor | Size | Location | Margins | IHQ |
| 1 | F | 41 | Schwannoma | 7.5 cm x4.5 cm x1.9 cm | Esophagus | Positive | S100+, GFAP-, CD117-, COD1-, KI67 <1% |
| 2 | F | 37 | Schwannoma | 4.2 cm x3.1 cm | Stomach | Negative | S100+, CD117-, DOG1-, CD34-, Actina-, Desmina-, CD56-. |
| 3 | F | 29 | Schwannoma | 2.3 cm x2.3 cm | Stomach | Negative | S100+, CD56+, DOG1-, CD117 ,CD34-, Desmina-, Actina-. |
| 4 | F | 67 | Schwannoma | 1.9 cm x1.5 cm | Stomach | Negative | DOG1-, CD117-, CD34-, Actina-, Calponina-, S-100+, CD56-. |
| 5 | F | 54 | Schwannoma | 4.5 cm x4.3 cm x4 cm | Stomach | Negative | S100+, CD34+, CD117-, Actina-. |
| 6 | F | 55 | Schwannoma | 8 cm x5 cm | Stomach | Negative | DOG1-, CD117-, S100+, KI671%. |
Schwannomas or neurinomas are benign mesenchymal neoplasms that originate from Schwann cells located at the sheath nerve of the Auerbach’s plexus or less frequently at the Meissner plexus. Its presentation as gastrointestinal tumors are uncommon; being the most frequent location at the level of the stomach, representing 4% of benign tumors at this level, and 2% of all esophageal tumors; a female predominance has been reported, and tend to appear in the 6th decade of life. Most of them are asymptomatic[3,6].
Among the most important differential diagnoses to be ruled out is GIST, which often has a malignant behavior (10%-30%) with different prognosis and treatment. The definitive diagnosis is made through immunohistochemistry which plays a fundamental role, since it allows distinguishing them from other tumors such as GIST and leiomyoma[8,9,10].
Our research tend to review the existing literature in a systematic way and report the clinical experience of INCMNSZ. A total of 317 cases were reported in the main databases while we present a total of 6 cases. Gastric localization showed a higher prevalence in both, the literature review and our institution: 94.95% (n = 317) and 83% (n = 5) respectively. With a follow-up with disease-free survival greater than 36 months in most cases: 62.01% (n = 80) vs 66.66% (n = 4). In both groups, the median size was > 4.1 cm. However, there was a slight discrepancy regarding the age of presentation, which was manifested earlier in the patients of our center with a predominance of 51-60 years of age.
Surgical treatment is curative in most cases and usually a wedge resection, subtotal or total gastrectomy without lymphadenectomy is enough to achieve negative margins with a low recurrence rate.
In conclusion, schwannoma is a clinical entity that must be taken into account in the differential diagnosis of gastrointestinal mesenchymal tumors. It has a good prognosis, and most are benign.
The clinical characteristics reported in the literature are very similar to our series of cases, gastric localization is more prevalent than esophageal location. A disease-free survival of more than 36 mo can be achieved by surgery.
Schwannomas are benign neoplasms originated from Schwann cells. Schwannomas are more commonly locate in the stomach. Schwannomas are usually asymptomatic lesions.
We have reviewed the available literature about gastrointestinal schwannomas, especially lesions from de stomach and esophagus (diagnosis, treatment, and follow up).
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes guidelines, a systematic review was conducted. The PubMed, Ovid, MEDLINE, and Scopus databases were used for reviewing process. Only English language manuscripts were included. All gastrointestinal schwannomas specifically located in the esophagus and stomach were included, and cases that did not report long-term follow-up were excluded.
Gastric localization showed a higher prevalence in both, the literature review and our institution. With a follow-up with disease-free survival greater than 36 mo in most cases. In both groups, the median size was > 4.1 cm. Surgical treatment is curative in most cases.
Schwannoma must be taken into account in the differential diagnosis of gastrointestinal mesenchymal tumors. Schwannoma has a good prognosis, and most are benign.A disease-free survival of more than 36 mo can be achieved by surgery.
PRISMA 2009 Checkliststatement: The PRISMA 2009 has been adopted.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer:Ciocalteu A, Jeong KY, Losanoff JES-Editor: Wang JL L-Editor: AE-Editor:Qi LL
| 1. | Singh A, Mittal A, Garg B, Sood N. Schwannoma of the stomach: a case report. J Med Case Rep. 2016;10:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 210] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Hu BG, Wu FJ, Zhu J, Li XM, Li YM, Feng Y, Li HS. Gastric Schwannoma: A Tumor Must Be Included in Differential Diagnoses of Gastric Submucosal Tumors. Case Rep Gastrointest Med. 2017;2017:9615359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Sreevathsa MR, Pipara G. Gastric Schwannoma: A Case Report and Review of Literature. Indian J SurgOncol. 2015;6:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Park SH, Kim GH, Park DY, Shin NR, Cheong JH, Moon JY, Lee BE, Song GA, Seo HI, Jeon TY. Endosonographic findings of gastric ectopic pancreas: a single center experience. J GastroenterolHepatol. 2011;26:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Yang JH, Zhang M, Zhao ZH, Shu Y, Hong J, Cao YJ. Gastroduodenal intussusception due to gastric schwannoma treated by Billroth II distal gastrectomy: one case report. World J Gastroenterol. 2015;21:2225-2228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012;24:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Vinhais SN, Cabrera RA, Nobre-Leitão C, Cunha TM. Schwannoma of the esophagus: computed tomography and endosonographic findings of a special type of schwannoma. ActaRadiol. 2004;45:718-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Beaulieu S, Rubin B, Djang D, Conrad E, Turcotte E, Eary JF. Positron emission tomography of schwannomas: emphasizing its potential in preoperative planning. AJR Am J Roentgenol. 2004;182:971-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Shah AS, Rathi PM, Somani VS, Mulani AM. Gastric Schwannoma: A Benign Tumor Often Misdiagnosed as Gastrointestinal Stromal Tumor. ClinPract. 2015;5:775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hong X, Wu W, Wang M, Liao Q, Zhao Y. Benign gastric schwannoma: how long should we follow up to monitor the recurrence? A case report and comprehensive review of literature of 137 cases. IntSurg. 2015;100:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Jeon HW, Kim KS, Hyun KY, Park JK. Enucleation of giant esophageal schwannoma of the upper thoracic esophagus: reports of two cases. World J SurgOncol. 2014;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. SeminThoracCardiovascSurg. 2003;15:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Moro K, Nagahashi M, Hirashima K, Kosugi SI, Hanyu T, Ichikawa H, Ishikawa T, Watanabe G, Gabriel E, Kawaguchi T, Takabe K, Wakai T. Benign esophageal schwannoma: a brief overview and our experience with this rare tumor. Surg Case Rep. 2017;3:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Seremetis MG, Lyons WS, deGuzman VC, Peabody JW. Leiomyomata of the esophagus.An analysis of 838 cases. Cancer. 1976;38:2166-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Riku Y, Ito M, Atsuta N, Watanabe H, Momota H, Sobue G. [Intracranial germinoma masquerading as a granulomatous inflammation, diagnostic failure after brain biopsy]. RinshoShinkeigaku. 2013;53:835-838. [PubMed] |
| 20. | Tokunaga T, Takeda S, Sumimura J, Maeda H. Esophageal schwannoma: report of a case. Surg Today. 2007;37:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Watanabe T, Miyazaki T, Saito H, Yoshida T, Kumakura Y, Honjyo H, Yokobori T, Sakai M, Sohda M, Kuwano H. Resection of an esophageal schwannoma with thoracoscopic surgery: a case report. Surg Case Rep. 2016;2:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kitada M, Matsuda Y, Hayashi S, Ishibashi K, Oikawa K, Miyokawa N. Esophageal schwannoma: a case report. World J SurgOncol. 2013;11:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Mizuguchi S, Inoue K, Imagawa A, Kitano Y, Kameyama M, Ueda H, Inoue Y. Benign esophageal schwannoma compressing the trachea in pregnancy. Ann ThoracSurg. 2008;85:660-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Sato K, Maekawa T, Maekawa H, Ouchi K, Sakurada M, Kushida T, Sato S, Nasu M, Tsurumaru M. A case of esophageal schwannoma and literature analysis of 18 cases. Esophagus. 2005;2:145-149. [DOI] [Full Text] |
| 25. | Chen HC, Huang HJ, Wu CY, Lin TS, Fang HY. Esophageal schwannoma with tracheal compression. ThoracCardiovascSurg. 2006;54:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kassis ES, Bansal S, Perrino C, Walker JP, Hitchcock C, Ross P, Daniel VC. Giant asymptomatic primary esophageal schwannoma. Ann ThoracSurg. 2012;93:e81-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Toyama E, Nagai Y, Baba Y, Yoshida N, Hayashi N, Miyanari N, Baba H. A case of thoracoscopically resected benign esophageal schwannoma with high uptake on FDG-PET. Esophagus. 2008;5:167-170. [DOI] [Full Text] |
| 28. | Klapman J, Chang KJ, Wiersema M, Murata Y, Vilmann P. Endoscopic ultrasound-guided fine-needle aspiration biopsy in esophageal cancer. Endoscopy. 2005;37:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Akaogi E, Mitsui K, Sohara Y, Endo S, Ishikawa S, Hori M. Treatment of postoperative chylothorax with intrapleural fibrin glue. Ann ThoracSurg. 1989;48:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J SurgOncol. 2016;14:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 31. | Asteriou C, Konstantinou D, Lalountas M, Kleontas A, Setzis K, Zafiriou G, Barbetakis N. Nine years experience in surgical approach of leiomyomatosis of esophagus. World J SurgOncol. 2009;7:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Shien K, Nozaki I, Kobatake T, Ohta K, Kubo Y, Tanada M, Kurita A. Two Case Reports of Esophageal schwannoma and Literature Review of Case Reports. Japanese J GastroenterolSurg. 2010;43:1106-1011. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | von Rahden BH, Stein HJ, Feussner H, Siewert JR. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. SurgEndosc. 2004;18:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Murase K, Hino A, Ozeki Y, Karagiri Y, Onitsuka A, Sugie S. Malignant schwannoma of the esophagus with lymph node metastasis: literature review of schwannoma of the esophagus. J Gastroenterol. 2001;36:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Iwata H, Kataoka M, Yamakawa Y, Kuwabara Y, Kureyama Y, Masaoka A. Esophageal schwannoma. Ann ThoracSurg. 1993;56:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Manger T, Pross M, Haeckel C, Lippert H. Malignant peripheral nerve sheath tumor of the esophagus. Dig Surg. 2000;17:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Shelat VG, Li K, Naik S, Ng CY, Rao N, Rao J, Koura A. Abdominal schwannomas: case report with literature review. IntSurg. 2013;98:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Tomono A, Nakamura T, Otowa Y, Imanishi T, Tanaka Y, Maniwa Y, Kakeji Y. A Case of Benign Esophageal Schwannoma Causing Life-threatening Tracheal Obstruction. Ann ThoracCardiovascSurg. 2015;21:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Kanneganti K, Patel H, Niazi M, Kumbum K, Balar B. Cecal schwannoma: a rare cause of gastrointestinal bleeding in a young woman with review of literature. Gastroenterol Res Pract. 2011;2011:142781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Wang G, Chen P, Zong L, Shi L, Zhao W. Cellular schwannoma arising from the gastric wall misdiagnosed as a gastric stromal tumor: A case report. Oncol Lett. 2014;7:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Tao K, Chang W, Zhao E, Deng R, Gao J, Cai K, Wang G, Zhang P. Clinicopathologic Features of Gastric Schwannoma: 8-Year Experience at a Single Institution in China. Medicine (Baltimore). 2015;94:e1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Zheng L, Wu X, Kreis ME, Yu Z, Feng L, Chen C, Xu B, Bu Z, Li Z, Ji J. Clinicopathological and immunohistochemicalcharacterisation of gastric schwannomas in 29 cases. Gastroenterol Res Pract. 2014;2014:202960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Rodriguez E, Tellschow S, Steinberg DM, Montgomery E. Cytologic findings of gastric schwannoma: a case report. DiagnCytopathol. 2014;42:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Takasumi M, Hikichi T, Takagi T, Suzuki R, Watanabe K, Nakamura J, Sugimoto M, Kikuchi H, Konno N, Waragai Y, Asama H, Obara K, Ohira H. Efficacy of endoscopic ultrasound-guided fine-needle aspiration for schwannoma: six cases of a retrospective study. Fukushima J Med Sci. 2017;63:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Park HC, Son DJ, Oh HH, Oak CY, Kim MY, Chung CY, Myung DS, Kim JS, Cho SB, Lee WS, Joo YE. Endoscopic ultrasonographic characteristics of gastric schwannoma distinguished from gastrointestinal stromal tumor. Korean J Gastroenterol. 2015;65:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Mohanty SK, Jena K, Mahapatra T, Dash JR, Meher D, John A, Nayak M, Bano S. Gastric GIST or gastric schwannoma-A diagnostic dilemma in a young female. Int J Surg Case Rep. 2016;28:60-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Yoon HY, Kim CB, Lee YH, Kim HG. Gastric schwannoma. Yonsei Med J. 2008;49:1052-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Williamson JM, Wadley MS, Shepherd NA, Dwerryhouse S. Gastric schwannoma: a benign tumour often mistaken clinically, radiologically and histopathologically for a gastrointestinal stromal tumour--a case series. Ann R CollSurgEngl. 2012;94:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Romdhane H, Cheikh M, Mzoughi Z, Slama SB, Ennaifer R, Belhadj N. Gastric Schwannoma: A Case Report. ClinPract. 2016;6:849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Sanei B, Kefayat A, Samadi M, Goli P, Sanei MH, Khodadustan M. Gastric Schwannoma: A Case Report and Review of the Literature for Gastric Submucosal Masses Distinction. Case Rep Med. 2018;2018:1230285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Sarlomo-Rikala M, Miettinen M. Gastric schwannoma--a clinicopathological analysis of six cases. Histopathology. 1995;27:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Sunkara T, Then EO, Reddy M, Gaduputi V. Gastric schwannoma-a rare benign mimic of gastrointestinal stromal tumor. Oxf Med Case Reports. 2018;2018:omy002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Yoon W, Paulson K, Mazzara P, Nagori S, Barawi M, Berri R. Gastric schwannoma: a rare but important differential diagnosis of a gastric submucosal mass. Case Rep Surg. 2012;2012:280982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Manji M, Ismail A, Komba E. Gastric Schwannoma: Case report from Tanzania and brief review of literature. Clin Case Rep. 2015;3:562-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Hong SW, Cho WY, Kim JO, Chun CG, Shim KY, Bok GH, Um WH, Lee JE. Gastric schwannoma diagnosed by endoscopic ultrasonography-guided trucut biopsy. ClinEndosc. 2013;46:284-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Komatsu D, Koide N, Hiraga R, Furuya N, Akamatsu T, Uehara T, Miyagawa S. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer. 2009;12:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Tahir TM, Anwar S, Naseem N, Mansoor-Ul-Haq H, Saqib M. Gastric schwannoma in a female patient with pulmonary tuberculosis - a clinicopathological assessment and diagnosis. Malays J Med Sci. 2010;17:45-50. [PubMed] |
| 58. | Oh SJ, Suh BJ, Park JK. Gastric Schwannoma Mimicking Malignant Gastrointestinal Stromal Tumor Exhibiting Increased Fluorodeoxyglucose Uptake. Case Rep Oncol. 2016;9:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Tatangelo F, Cantile M, Collina F, Belli A, DE Franciscis S, Bianco F, Botti G. Gastric schwannoma misdiagnosed as GIST: A case report with immunohistochemical and molecular study. Oncol Lett. 2016;11:2497-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Guthrie G, Mullen R, Moses A. Gastric Schwannoma or GIST: accuracy of preoperative diagnosis? Scott Med J. 2011;56:236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Raber MH, Ziedses des Plantes CM, Vink R, Klaase JM. Gastric Schwannoma Presenting as an Incidentaloma on CT-Scan and MRI. Gastroenterology Res. 2010;3:276-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Lyros O, Schickel S, Schierle K, Hoffmeister A, Gockel I. Schwannom des Magens: selteneDifferenzialdiagnoseeinerakutenoberengastrointestinalenBlutung. Z Gastroenterol. 2017;55:761-765. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Shimizu S, Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Fujiwara Y. Gastric Schwannoma with Enlargement of the Regional Lymph Nodes Resected Using Laparoscopic Distal Gastrectomy: Report of a Patient. YonagoActa Med. 2017;60:59-63. [PubMed] |
| 64. | Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Gastric schwannomas: endosonographic characteristics. Abdom Imaging. 2008;33:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Fujiwara S, Nakajima K, Nishida T, Takahashi T, Kurokawa Y, Yamasaki M, Miyata H, Takiguchi S, Mori M, Doki Y. Gastric schwannomas revisited: has precise preoperative diagnosis become feasible? Gastric Cancer. 2013;16:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Ohno T, Ogata K, Kogure N, Ando H, Aihara R, Mochiki E, Zai H, Sano A, Kato T, Sakurai S, Oyama T, Asao T, Kuwano H. Gastric schwannomas show an obviously increased fluorodeoxyglucose uptake in positron emission tomography: report of two cases. Surg Today. 2011;41:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Mekras A, Krenn V, Perrakis A, Croner RS, Kalles V, Atamer C, Grützmann R, Vassos N. Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract. BMC Surg. 2018;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 68. | Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005;184:797-802. [PubMed] [DOI] [Full Text] |
| 69. | Watanabe A, Ojima H, Suzuki S, Mochida Y, Hirayama I, Hosouchi Y, Nishida Y, Kashiwabara K, Ohno T, Mochiki E, Kuwano H. An individual with gastric schwannoma with pathologically malignant potential surviving two years after laparoscopy-assisted partial gastrectomy. Case Rep Gastroenterol. 2011;5:502-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Vargas Flores E, Bevia Pérez F, Ramirez Mendoza P, Velázquez García JA, Ortega Román OA. Laparoscopic resection of a gastric schwannoma: A case report. Int J Surg Case Rep. 2016;28:335-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Iwata Y, Tanaka C, Komori S, Nagao N, Kawai M, Yoshida K, Kunieda K. Lobulated esophageal schwannoma resected with concurrent approach from the thorax and cervix. World J SurgOncol. 2018;16:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Mishra B, Madhusudhan KS, Kilambi R, Das P, Pal S, Srivastava DN. Malignant Schwannoma of the Esophagus: A Rare Case Report. Korean J ThoracCardiovascSurg. 2016;49:63-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Enshaei A, Hajipour B, Abbasi F, Doost PR, Rezaei S. Schwannoma of stomach. J Pak Med Assoc. 2015;65:672-674. [PubMed] |
| 74. | Matsuki A, Kosugi S, Kanda T, Komukai S, Ohashi M, Umezu H, Mashima Y, Suzuki T, Hatakeyama K. Schwannoma of the esophagus: a case exhibiting high 18F-fluorodeoxyglucose uptake in positron emission tomography imaging. Dis Esophagus. 2009;22:E6-E10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, Wang J, Zhu XZ. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |