Published online Jun 15, 2019. doi: 10.4251/wjgo.v11.i6.449
Peer-review started: January 4, 2019
First decision: March 14, 2019
Revised: April 17, 2019
Accepted: May 3, 2019
Article in press: May 4, 2019
Published online: June 15, 2019
Processing time: 164 Days and 20.1 Hours
Eukaryotic initiation factor 5A2 (eIF5A2), as one of the two isoforms in the family, is reported to be a novel oncogenic protein that is involved in multiple aspects of many types of human cancer. Overexpression or gene amplification of EIF5A2 has been demonstrated in many cancers. Accumulated evidence shows that eIF5A2 initiates tumor formation, enhances cancer cell growth, increases cancer cell metastasis, and promotes treatment resistance through multiple means, including inducing epithelial–mesenchymal transition, cytoskeletal rearrangement, angiogenesis, and metabolic reprogramming. Expression of eIF5A2 in cancer correlates with poor survival, advanced disease stage, as well as metastasis, suggesting that eIF5A2 function is crucial for tumor development and maintenance but not for normal tissue homeostasis. All these studies suggest that eIF5A2 is a useful biomarker in the prediction of cancer prognosis and serves as an anticancer molecular target. This review focuses on the expression, subcellular localization, post-translational modifications, and regulatory networks of eIF5A2, as well as its biochemical functions and evolving clinical applications in cancer, especially in human digestive system neoplasms.
Core tip: Eukaryotic initiation factor 5A2 (eIF5A2) is one of only two cellular proteins that contain the unusual amino acid hypusine. eIF5A2 initiates tumor formation, enhances cancer cell growth, increases metastasis, and promotes treatment resistance through inducing epithelial–mesenchymal transition, cytoskeletal rearrangement, angiogenesis, and metabolic reprogramming. Isoform eIF5A2 represents a promising target for treatment of human digestive system cancer. Our objective was to consolidate the current literature to better understand the expression, subcellular localization, post-translational modifications, and regulatory networks of eIF5A2, as well as its biochemical functions and evolving clinical applications in human digestive system cancer.
- Citation: Meng QB, Peng JJ, Qu ZW, Zhu XM, Wen Z, Kang WM. Eukaryotic initiation factor 5A2 and human digestive system neoplasms. World J Gastrointest Oncol 2019; 11(6): 449-458
- URL: https://www.wjgnet.com/1948-5204/full/v11/i6/449.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i6.449
In 2000, EIF5A2 was first sequenced and isolated as a novel candidate oncogene from human chromosome 3q26.2[1,2]. Eukaryotic initiation factor 5A2 (eIF5A2) is one of only two eIF5A family members that undergo an unusual post-translational hypusine modification[3]. Unlike isoform eIF5A1, which is ubiquitously expressed, eIF5A2 protein is normally not detected and its mRNA is expressed in a tissue-dependent manner in human tissues[1]. eIF5A2 protein has been shown to be overexpressed in many cancers, including cervical cancer[4,5], ovarian cancer[6-8], colorectal cancer[9,10], gastric cancer[11,12], liver cancer[13,14], melanoma[15,16], lung cancer[17], nasopharyngeal carcinoma[18], bladder cancer[19,20] and esophageal squamous cell carcinoma (ESCC)[21]. Accumulated evidence shows that eIF5A2 plays important roles as a regulatory molecule in many biological processes, including tumor formation, cancer cell growth, metastasis, maintenance of cancer stem cells (CSCs) and treatment resistance through multiple means including epithelial–mesenchymal transition (EMT), cytoskeletal rearrangement, angiogenesis, and metabolic reprogramming.
In this article, we review eIF5A2-related studies, particularly those about the discovery, subcellular location, functions, upstream and downstream regulation, and modification of eIF5A2, as well as its role as a biomarker and its therapeutic potential for human digestive system cancer.
A literature search was conducted using PubMed Library for “eIF5A2”, “eIF-5A2”, “eIF-5A-2”, “eIF5A-2”, “EIF5A2”, “eukaryotic translation initiation factor 5A2”, “eukaryotic initiation 5A2” or “human eukaryotic initiation factor 5A2”.
Human eIF5A2 is a small (approximately 17 kDa) universally conserved acidic protein that contains 153 amino acids and is encoded by EIF5A2 gene, which is located on chromosome 3q26.2; a chromosomal region that is frequently amplified in several human cancers[2,3]. Multiple forms of EIF5A2 mRNA (5.6, 3.8, 1.6 and 0.7 kb, with one at 3.8 kb being the major form) are the products of one gene with various lengths of 3’-untranslated region (UTR), resulting from the use of different polyadenylation (AAUAAA) signals in various human cancer cell lines[22]. In short, for the structure of eIF5A2, the C-terminal domain consists of a three-turn α-helix α2 and five strands of β7-β11 and the N-terminal domain is dominated by β-strands[23].
Unlike EIF5A1, which is ubiquitously expressed, EIF5A2 is normally not detected and its mRNA is expressed in a tissue-dependent and cell-type-specific manner, and is mainly found in testes, parts of adult brain, human cancer tissues (such as primary ovarian cancers) and some cancer cell lines (such as SW480 and UACC-1598)[1,2,24]. Clement et al[3] described the identification of eIF5A2 protein in human colorectal (SW-480) and ovarian (UACC-1598) cancer cell lines, and were first to report that eIF5A2 has an important role in eukaryotic cell survival similar to that of the ubiquitous eIF5A1. Overexpression of EIF5A2 and/or eIF5A2 protein is observed in several human cancer tissues and/or cell lines such as cervical cancer[4,5,25], ovarian cancer[7,8], colorectal cancer[9,10,26-28], gastric cancer[11,12,29,30], ESCC[21,31], liver cancer[13,14,32-35], nasopharyngeal cancer[18], oral squamous cell carcinoma[36,37], pancreatic cancer[38-40], non-small cell lung cancer[17,41-43], melanoma[15,16], bladder cancer[34,44,45], and breast cancer[46,47]. In contrast, eIF5A2 is not generally overexpressed in glioblastoma[48] and chronic myeloid leukemia[49]. These observations suggest that eIF5A2 overexpression is not an invariable hallmark of cancer. Pällmann et al[50] reported high levels of EIF5A2 mRNA in brain, epididymis, lung, prostate and testis tissues of wild-type mice, as assessed by quantitative real-time polymerase chain reaction.
In humans, isoforms eIF5A1 and eIF5A2 are the only two cellular proteins that experience a post-translational hypusination by two essential enzymatic steps involving deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH), which selectively catalyze the polyamine spermidine- to finish eIF5A hypusi-nation[22,51-53]. eIF5A exists mainly as the fully hypusination form in mammalian tissues and cells[54]. First, the 4- aminobutyl moiety of spermidine are transferred to the ϵ-amino group of Lys50 to form a deoxyhypusine-containing intermediate by DHS[3,22,51,55]. Second, DOHH catalyzes the hydroxylation of the deoxyhypusine residue to generate hypusine-containing eIF5A and activates it[22,51]. It has been reported that the endogenous activity of DHS and/or DOHH appears to be insufficient for modification of the excess precursors of mature eIF5A2 and eIF5A1[22], and exogenously expressed eIF5A2 and eIF5A1 is largely unhypusinated, and can be hypusinated only when DHS and DOHH are coexpressed[56,57]. Therefore, transfection studies with eIF5A2 expression vectors, such as our previous study[11] and others[7,9,13,26,27,31], should be re-assessessed by evaluating the real changes in the concentrations of the hypusinated eIF5A2 or its precursor to determine the true cause of the biological effects. Hypusine modification not only activates eIF5A2, but also regulates its subcellular localization. However, in contrast to DHS- and DOHH-mediated hypusination of eIF5A1, which is crucial for embryonic development as well as for viability in adult mice, the cancer-associated isoform eIF5A2 is dispensable for embryonic development and viability in adult organisms[50]. Future work will be needed to determine the contribution of hypusine biosynthetic enzymes of eIF5A2 in tumorigenesis and metastasis.
In addition to unique hypusination, eIF5A2 also undergoes reversible acetylation modification at Lys-47, like eIF5A1 does[56,57]. Histone deacetylase 6 and sirtuin 2 have been identified as the major deacetylases of eIF5A2[56]. Acetylation of eIF5A2 at Lys-47 plays an important role in its subcellular localization. It is also reported that acetylation of the hypusine side chain in the N-terminal domain by a key polyamine catabolic enzyme, spermidine/spermine-N1-acetyltransferase 1 (SSAT1) inactivates eIF5A, which suggests regulation of eIF5A activity by reversible acetylation/ deacetylation at this site though SSAT1 catalysis[58].
eIF5A can be modified by phosphorylation[59,60], ubiquitination[61] and trans-glutaminylation[62], but clear effects on its activity have not been fully detected. eIF5A dephosphorylation is required for translation arrest in stationary phase cells[60]. Shang et al[61] reported that the carboxyl terminus of Hsc70-interacting protein (CHIP) functions as a negative regulator of eIF5A to mediate its ubiquitination for degradation. This was the first report on regulation of eIF5A protein stability via a protein degradation mechanism. It is likely, therefore, that the CHIP–eIF5A2 axis mediates ubiquitination of eIF5A2 for degradation in human cancers. The potential role of eIF5A2 in human cancer development and metastasis has been found in recent years; therefore, the importance of eIF5A2 post-translational modifications in its oncogenic properties should be elucidated in the future.
The nuclear membranes force nucleocytoplasmic exchange to proceed through nuclear pore complexes (NPCs)[63]. The NPC permeability barrier -allows free passage to small molecules, while limiting larger molecules that approach or exceed a limit of > 30 kDa in mass or > 5 nm in diameter[64]. Most evidence demonstrates s that eIF5A2, as a shuttling protein, is responsible for regulating protein translation in the cytoplasm, and only a few studies have shown that it is located and works in the nucleus[15,21,65]. More studies are necessary to address its role in the nucleus. eIF5A2 has an invariably small molecular mass of only 17 kDa and can thus cross the NPC permeability barrier rapidly, even without the help of an importin. The nuclear export of eIF5A may be mediated by the nuclear exporter exportin (XPO)4, which belongs to the importin-β family of nuclear transporters, in a hypusine-dependent manner[66,67]. In addition, the N-terminal 19 amino acids of eIF5A serve as a signal for nuclear localization of eIF5A[68]. Knockdown of XPO4 in murine hepatoma cells leads to nuclear accumulation of eIF5A2 as well as eIF5A1[65].
Post-translational modifications including acetylation at Lys-47 and hypusination at Lys-50 of eIF5A2 direct its subcellular localization[56]. Acetylation acts as a molecular switch for eIF5A2, allowing it to exert distinct functions in the cytoplasm and nucleus. The acetylated form of eIF5A2 is primarily enriched in the nucleus, suggesting that acetylation at Lys-47 induces nuclear accumulation[56]. In addition, the study also showed that unhypusinated eIF5A2 is highly acetylated but is significantly deacetylated upon hypusination, implying crosstalk between acetylation and hypusination[56]. Hypusination can reduce acetylation in eIF5A2, leading to its localization in the cytoplasmic compartment where it is required for protein synthesis. Inhibition of the deacetylases or impaired hypusination increases acetylation of eIF5A2, leading to nuclear accumulation. These findings provide strong evidence that cytoplasmic location of eIF5A2 requires not only hypusination but also hypo-acetylation.
Although the mechanisms of EIF5A2 gene upregulation in tumor cells are not clear yet, most researchers believe that the main reason is genomic instability caused by copy number variation. To date, EIF5A2 has been frequently found, but not always, to be amplified in human cancers and cancer cell lines[2,8,10,17,19,21,69]. Although tumors that exhibit gene amplification typically exhibit high eIF5A2 expression, many have high eIF5A2 levels without gene amplification, and thus other mechanisms, such as transcriptional regulation and/or post-transcriptional regulation, must exist in eIF5A2 upregulation. It has been demonstrated that K-ras activation upregulates eIF5A2 expression as well as hypusination via transcriptional regulation during the early stages of pancreatic ductal adenocarcinoma (PDAC) progression[38]. Another study has reported that hypoxia increases EIF5A2 RNA levels, at least in part via hypoxia-inducible factor (HIF)-1α in ESCC cells[21].
Many studies have demonstrated that miRNAs (miRs) target the 3’-UTR of cytoplasmic mRNA of EIF5A2 to post-transcriptionally regulate mRNA and protein levels[70] (Table 1). EIF5A2 is a putative target for miR-203, miR-30b, miR-9, miR-125b, miR-599 and miR-588, which are predicted by the bioinformatic algorithm TargetScan (http://www.targetscan.org). miR-203 suppresses growth and invasion of colorectal cancer cells (SW620 and LOVO), at least partly, by binding the 3’-UTR of EIF5A2 and repressing EIF5A2 expression at both the mRNA and protein levels[26]. miR-30b[29], miR-599[71] and miR-588[72] suppress gastric cancer cell metastasis via binding to the 3’-UTR of EIF5A2 and repressing eIF5A2 expression. miR-125b inhibits tumorigenic properties of hepatocellular carcinoma (HCC) cells via suppressing eIF5A2 expression, through binding to the 3’-UTR of EIF5A2[73]. miR-9 enhances sensitivity to cetuximab in epithelial phenotype HCC cells through regulation of eIF5A2[74].
| miRs | Ref. | Materials | Function |
| miR-203 | Deng et al[26] | CRC cells (SW620 and LOVO) | Suppressing growth and invasion via miR-203/EIF5A2 axis |
| miR-599 | Wang et al[71] | GC cells (BGC823 and MKN-45) | Inhibiting metastasis and EMT via miR-599/EIF5A2 axis |
| miR-588 | Zhou et al[72] | GC cells (MGC803) | Regulating invasion, migration and EMT via miR-588/EIF5A2 axis |
| miR-30b | Tian et al[29] | GC cells (AGS and MGC803) | Downregulation of EIF5A2 by miR-30b inhibits EMT |
| miR-9 | Xue et al [74] | HCC cells (Hep3B and Huh7) | Enhancing sensitivity to cetuximab via miR-9/EIF5A2 axis |
| miR-125b | Tsang et al[73] | HCC tissue and cells | Inhibiting tumorigenic properties via miR-125b/EIF5A2 axis |
Zender et al[65] has reported that eIF5A2 is a key downstream effector of XPO4 in tumor inhibition, and XPO4 is a negative regulator of eIF5A2, which may play a role in inhibiting cell proliferation in the nucleus. In murine hepatoma cells, knockdown of XPO4 leads to accumulation of eIF5A1 and eIF5A2 in the nucleus[65]. The sonic hedgehog-GLI family zinc finger 1 signaling pathway upregulates eIF5A2 in pancreatic cancer cells[28]. Moreover, hypoxia can induce eIF5A2 upregulation and promote eIF5A2 translocation from the cytoplasm to the nucleus in ESCC cell lines (KYSE140, KYSE180, KYSE410, KYSE510 and EC109)[21].
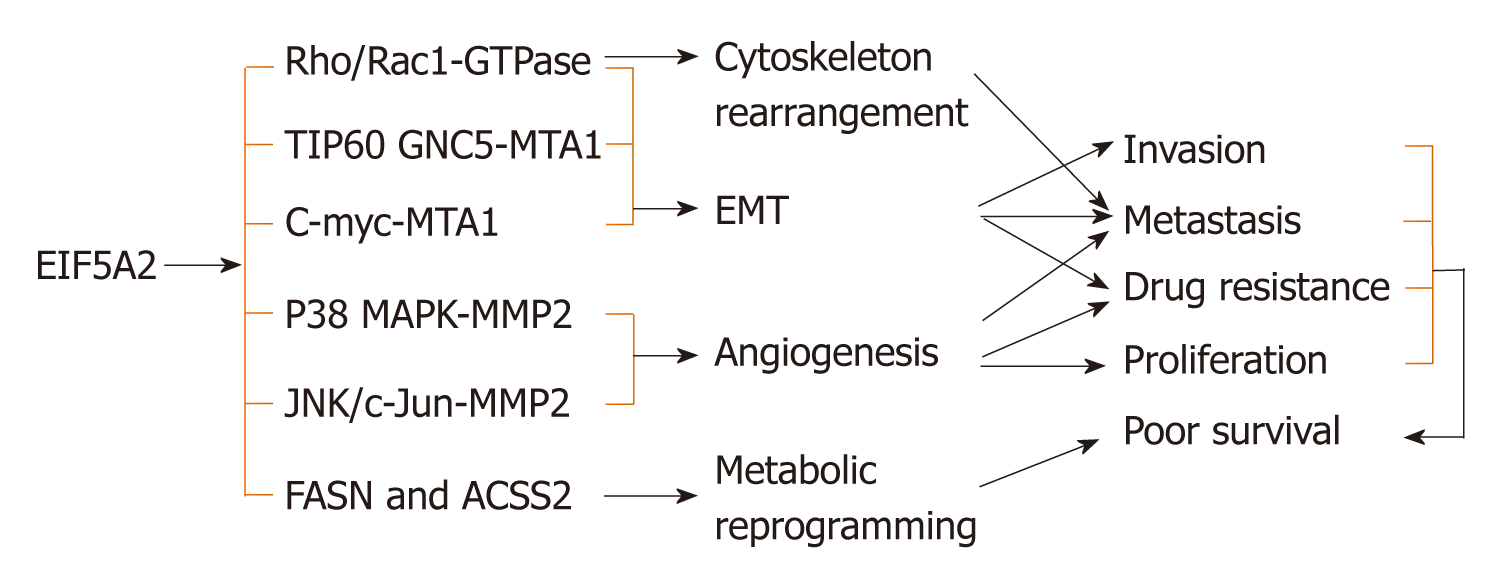
The cancer-associated isoform eIF5A2 is not essential for normal development and viability, which has been confirmed in vivo[50]. Accumulating evidence shows that eIF5A2 plays important roles in tumor proliferation[11], metastasis[13], EMT[9,11,13,28-29,35,75,76], cytoskeletal rearrangement[13], angiogenesis[21], metabolic reprogramming[14], maintenance of CSCs[31,77] and drug resistance[33,38,74,75,78-80] via its subsequent signaling pathways. Additionally, eIF5A2 is associated with survival of many digestive cancer patients[9,11,12,14,21,32] (Figure 1).
Over the past 10 years, many studies have evaluated the role of eIF5A2 in activating EMT in human cancer cells. Tang et al[13] first reported that eIF5A2 induces EMT; an important event in tumor invasion and metastasis that is chiefly characterized by upregulation of mesenchymal markers (Vimentin, fibronectin, E-cadherin and α-smooth muscle actin) and downregulation of epithelial markers (E-cadherin and β-catenin) in HCC. Shek et al[35] and Lou et al[75] confirmed that eIF5A2 enhances the aggressiveness of HCC cells by inducing EMT. Zhu et al[9] found that overexpression of eIF5A2 also promotes colorectal carcinoma cell aggressiveness by upregulating Metastasis-associated protein 1 through C-myc to induce EMT[76]. In addition, eIF5A2 induces EMT of other human digestive system neoplasms such as gastric cancer[11,29] and pancreatic cancer[28].
In HCC, eIF5A2 stimulates rearrangement of the cytoskeleton through activation of the RhoA/Rac1 GTPase signaling pathway[13]. That study showed that overexpression of eIF5A2 in human liver LO2 cells provokes the formation of stress fibers and lamellipodia, without affecting expression level of Rho/Rac GTPase in the cells[13]. However, the precise mechanism underlying EIF5A2-mediated Rho-GTPase activation requires further investigation.
Increased expression of eIF5A2, via hypoxia or gene amplification, contributes to angiogenesis in ESCC via the HIF-1α-mediated signaling pathway[21]. In vitro and in vivo assays have both indicated that eIF5A2 increases angiogenesis by enhancing matrix metalloproteinase 2 activity via activation of the p38 mitogen-activated protein kinase pathway, and eIF5A2 silencing increases tumor vessel wall continuity, increases blood perfusion, and improves tumor oxygenation in HCC[33].
A recent study reported that eIF5A2 triggers cellular metabolic reprogramming, including glucose metabolism, by promoting aerobic glycolysis and fatty acid biosynthesis via upregulation of FASN and ACSS2 in human liver cancer cells[14].
CSCs are suggested to be responsible for driving resistance to conventional therapies and for cancer metastasis and/or recurrence. It has been reported that eIF5A2 overexpression increases the stemness of ESCC cells (KYSE510)[31]. A recent study showed that eIF5A2 also contributes to the maintenance of HCC CSCs (CD133+ HCC cells) via the c-Myc/miR-29b axis[77].
Overexpression of cytoplasmic eIF5A2 detected by immunohistochemistry is correlated with poor survival of patients with digestive system malignancies, including colorectal cancer[9], ESCC[21], gastric cancer[11,12] and liver cancer[14,32]. All these studies suggest that a high level of eIF5A2 expression in the cytoplasm is a potential prognostic indicator in many human cancers. However, a recent study demonstrated that nuclear eIF5A2 expression is also an independent prognostic marker in human melanoma[15]. Therefore, nuclear eIF5A2 may have the potential to serve as a therapeutic marker for some human cancers, and further study is needed to establish the subcellular localization of eIF5A2.
Primary or secondary anticancer drug resistance is a clinical problem shared by both chemotherapy and targeted therapy. The development of resistance may be predicted from pre-existing genomic and proteomic profiles in patients[78]. eIF5A2 can be used as a biomarker for predicting drug resistance. N1-guanyl-1,7-diaminoheptane (GC7), an inhibitor of DHS, enhances the therapeutic efficacy of doxorubicin in epithelial HCC cells (Huh7, Hep3B and HepG2)[75,79] by preventing the doxorubicin-induced EMT through inhibition of eIF5A2 activation. GC7 can also enhance the sensitivity of oral cancer cells to cisplatin[37]. eIF5A2 promotes resistance to doxorubicin via regulation of EMT in colon cancer cells[27]. Downregulation of eIF5A2 increases tumor perfusion and reduces tumor hypoxia, thus increasing the chemosensitivity of HCC cells to 5-fluorouracil by remodeling tumor vessels[33]. eIF5A2 is significantly related to gemcitabine sensitivity in PDAC cells[38]. Recently, Xue et al[74] reported that eIF5A2 is associated with cytotoxicity of cetuximab in epithelial HCC cells[80]. A high level of eIF5A2 expression is related to drug resistance in many human digestive system cancers. However, other studies have shown no significant relationship between EIF5A2 expression and effects of preoperative radiotherapy in human rectal cancer[81].
Basic research and clinical evidence show that EIF5A2 is a candidate oncogene and may be a key biomarker for the prognosis of various human digestive system cancers. There is growing evidence that inhibition of hypusination of eIF5A2 inhibits tumorigenesis. Hypusine modification of eIF5A by DHPS and DOHH forms an attractive platform for therapeutic intervention. Many studies have shown that GC7, as an inhibitor of DHS, enhances the sensitivity of drugs through inhibition of eIF5A2 activation in many kinds of human cancer cells[27,37,39,42,47,75,79,80,82,83]. However, hypusination takes place in all eukaryotic cells and has been shown to be necessary for proliferation of mammalian cell lines[52] and crucial for embryonic development as well as viability in adult mice[50]. So, important questions remain regarding how to selectively target tumors and reduce adverse effects.
In contrast to EIF5A1, the of EIF5A2 is limited to tissue such as testes and a few parts of the adult brain, but it is abundant in many human cancers. The eIF5A2 protein is associated with cancer metastasis by influencing the processes of EMT, angiogenesis, cytoskeletal rearrangement, and metabolic reprogramming. Thus, the isoform eIF5A2 represents a promising target for the treatment of malignant tumors. Moreover, in contrast to DHS or DOHH, the eIF5A2 isoform is not essential for embryonic development or for viability in an adult organism. So, we speculated whether eIF5A2, which is only expressed in a few tissues in the normal human body, but abundant in various tumor cells, might represent a better target for therapy. Therefore, we propose that specific inhibitors of eIF5A2 will exhibit selective toxicity toward eIF5A2-dependent cancer cells. Better understanding of the physiological and pathophysiological functions of eIF5A2 may lead to more effective management of many human digestive system cancers with high expression of EIF5A2, via early detection, precise prognostication, and molecular targeted treatment. A recent study demonstrated that Mg(II)-catechin nanocomposite particles (Mg(II)-Cat NPs) delivering siEIF5A2 inhibited bladder cancer cell growth in vitro and in vivo[45,84]. These results provide preclinical evidence for use of Mg(II)-Cat/siEIF5A2 combined therapeutic methods in cancer.
However, it is also clear that more researches are needed to clarify the underlying mechanisms that regulate eIF5A2 expression, for example, how does noncoding RNA regulate the UTR of EIF5A2 and how is its promoter epigenetically modified. With regard to the downstream pathway, the exact mechanism of eIF5A2 in regulating its target and whether it can act as a transcriptional factor have not been elucidated.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arville B, Gordon LG S-Editor: Ji FF L-Editor: A E-Editor: Xing YX
| 1. | Jenkins ZA, Hååg PG, Johansson HE. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics. 2001;71:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61:3806-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, Park MH, Johansson HE. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254-4263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Yang SS, Gao Y, Wang DY, Xia BR, Liu YD, Qin Y, Ning XM, Li GY, Hao LX, Xiao M, Zhang YY. Overexpression of eukaryotic initiation factor 5A2 (EIF5A2) is associated with cancer progression and poor prognosis in patients with early-stage cervical cancer. Histopathology. 2016;69:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Liu X, Chen D, Liu J, Chu Z, Liu D. Blocking Modification of Eukaryotic Initiation 5A2 Antagonizes Cervical Carcinoma via Inhibition of RhoA/ROCK Signal Transduction Pathway. Technol Cancer Res Treat. 2017;16:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Quanico J, Franck J, Cardon T, Leblanc E, Wisztorski M, Salzet M, Fournier I. NanoLC-MS coupling of liquid microjunction microextraction for on-tissue proteomic analysis. Biochim Biophys Acta Proteins Proteom. 2017;1865:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, Sham JS. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua WF, Wu HM, Kung HF, Zeng YX, Guan XY. Expression and amplification of eIF-5A2 in human epithelial ovarian tumors and overexpression of EIF-5A2 is a new independent predictor of outcome in patients with ovarian carcinoma. Gynecol Oncol. 2009;112:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, Guan XY, Xie D. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymaltransition. Gut. 2012;61:562-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, Kung HF, Guan XY. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Meng QB, Kang WM, Yu JC, Liu YQ, Ma ZQ, Zhou L, Cui QC, Zhou WX. Overexpression of eukaryotic translation initiation factor 5A2 (EIF5A2) correlates with cell aggressiveness and poor survival in gastric cancer. PLoS One. 2015;10:e0119229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Yang Q, Ye Z, Zhang Q, Zhao Z, Yuan H. Expression of eukaryotic translation initiation factor 5A-2 (eIF5A-2) associated with poor survival in gastric cancer. Tumour Biol. 2016;37:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Cao TT, Lin SH, Fu L, Tang Z, Che CM, Zhang LY, Ming XY, Liu TF, Tang XM, Tan BB, Xiang D, Li F, Chan OY, Xie D, Cai Z, Guan XY. Eukaryotic translation initiation factor 5A2 promotes metabolic reprogramming in hepatocellular carcinoma cells. Carcinogenesis. 2017;38:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Khosravi S, Martinka M, Zhou Y, Ong CJ. Prognostic significance of the expression of nuclear eukaryotic translation initiation factor 5A2 in human melanoma. Oncol Lett. 2016;12:3089-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Khosravi S, Wong RP, Ardekani GS, Zhang G, Martinka M, Ong CJ, Li G. Role of EIF5A2, a downstream target of Akt, in promoting melanoma cell invasion. Br J Cancer. 2014;110:399-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY, Bian XW, Zeng YX, Xie D. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Huang PY, Zeng TT, Ban X, Li MQ, Zhang BZ, Zhu YH, Hua WF, Mai HQ, Zhang L, Guan XY, Li Y. Expression of EIF5A2 associates with poor survival of nasopharyngeal carcinoma patients treated with induction chemotherapy. BMC Cancer. 2016;16:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Luo JH, Hua WF, Rao HL, Liao YJ, Kung HF, Zeng YX, Guan XY, Chen W, Xie D. Overexpression of EIF-5A2 predicts tumor recurrence and progression in pTa/pT1 urothelial carcinoma of the bladder. Cancer Sci. 2009;100:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Chen W, Luo JH, Hua WF, Zhou FJ, Lin MC, Kung HF, Zeng YX, Guan XY, Xie D. Overexpression of EIF-5A2 is an independent predictor of outcome in patients of urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Epidemiol Biomarkers Prev. 2009;18:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, Qian C, Cai Z, Xie D, Huang P, Guan XY. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701-1713.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Clement PM, Johansson HE, Wolff EC, Park MH. Differential expression of eIF5A-1 and eIF5A-2 in human cancer cells. FEBS J. 2006;273:1102-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Tong Y, Park I, Hong BS, Nedyalkova L, Tempel W, Park HW. Crystal structure of human eIF5A1: insight into functional similarity of human eIF5A1 and eIF5A2. Proteins. 2009;75:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Caraglia M, Park MH, Wolff EC, Marra M, Abbruzzese A. eIF5A isoforms and cancer: two brothers for two functions? Amino Acids. 2013;44:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Liu J, Chen D, Liu X, Liu Z. Cyclosporine A attenuates cardiac dysfunction induced by sepsis via inhibiting calcineurin and activating AMPK signaling. Mol Med Rep. 2017;15:3739-3746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin Q, Zhou L, Sun X. MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci Rep. 2016;6:28301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Bao Y, Lu Y, Wang X, Feng W, Sun X, Guo H, Tang C, Zhang X, Shi Q, Yu H. Eukaryotic translation initiation factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer through epithelial mesenchymal transition. Cancer Cell Int. 2015;15:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Xu X, Liu H, Zhang H, Dai W, Guo C, Xie C, Wei S, He S, Xu X. Sonic Hedgehog-GLI Family Zinc Finger 1 Signaling Pathway Promotes the Growth and Migration of Pancreatic Cancer Cells by Regulating the Transcription of Eukaryotic Translation Initiation Factor 5A2. Pancreas. 2015;44:1252-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Tian SB, Yu JC, Liu YQ, Kang WM, Ma ZQ, Ye X, Yan C. MiR-30b suppresses tumor migration and invasion by targeting EIF5A2 in gastric cancer. World J Gastroenterol. 2015;21:9337-9347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Marchet A, Mocellin S, Belluco C, Ambrosi A, DeMarchi F, Mammano E, Digito M, Leon A, D'Arrigo A, Lise M, Nitti D. Gene expression profile of primary gastric cancer: towards the prediction of lymph node status. Ann Surg Oncol. 2007;14:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Yang H, Li XD, Zhou Y, Ban X, Zeng TT, Li L, Zhang BZ, Yun J, Xie D, Guan XY, Li Y. Stemness and chemotherapeutic drug resistance induced by EIF5A2 overexpression in esophageal squamous cell carcinoma. Oncotarget. 2015;6:26079-26089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Liu RR, Lv YS, Tang YX, Wang YF, Chen XL, Zheng XX, Xie SZ, Cai Y, Yu J, Zhang XN. Eukaryotic translation initiation factor 5A2 regulates the migration and invasion of hepatocellular carcinoma cells via pathways involving reactive oxygen species. Oncotarget. 2016;7:24348-24360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Wang FW, Cai MY, Mai SJ, Chen JW, Bai HY, Li Y, Liao YJ, Li CP, Tian XP, Kung HF, Guan XY, Xie D. Ablation of EIF5A2 induces tumor vasculature remodeling and improves tumor response to chemotherapy via regulation of matrix metalloproteinase 2 expression. Oncotarget. 2014;5:6716-6733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Yang J, Yu H, Shen M, Wei W, Xia L, Zhao P. N1-guanyl-1,7-diaminoheptane sensitizes bladder cancer cells to doxorubicin by preventing epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2 activation. Cancer Sci. 2014;105:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Shek FH, Fatima S, Lee NP. Implications of the Use of Eukaryotic Translation Initiation Factor 5A (eIF5A) for Prognosis and Treatment of Hepatocellular Carcinoma. Int J Hepatol. 2012;2012:760928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Bhosale PG, Cristea S, Ambatipudi S, Desai RS, Kumar R, Patil A, Kane S, Borges AM, Schäffer AA, Beerenwinkel N, Mahimkar MB. Chromosomal Alterations and Gene Expression Changes Associated with the Progression of Leukoplakia to Advanced Gingivobuccal Cancer. Transl Oncol. 2017;10:396-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Fang L, Gao L, Xie L, Xiao G. GC7 enhances cisplatin sensitivity via STAT3 signaling pathway inhibition and eIF5A2 inactivation in mesenchymal phenotype oral cancer cells. Oncol Rep. 2018;39:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Fujimura K, Wright T, Strnadel J, Kaushal S, Metildi C, Lowy AM, Bouvet M, Kelber JA, Klemke RL. A hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014;74:6671-6681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Yao M, Hong Y, Liu Y, Chen W, Wang W. N1-guanyl-1, 7-diaminoheptane enhances the sensitivity of pancreatic ductal adenocarcinoma cells to gemcitabine via the inhibition of eukaryotic translation initiation factor 5A2. Exp Ther Med. 2017;14:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Cao D, Hustinx SR, Sui G, Bala P, Sato N, Martin S, Maitra A, Murphy KM, Cameron JL, Yeo CJ, Kern SE, Goggins M, Pandey A, Hruban RH. Identification of novel highly expressed genes in pancreatic ductal adenocarcinomas through a bioinformatics analysis of expressed sequence tags. Cancer Biol Ther. 2004;3:1081-1089; discussion 1090-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Xu G, Shao G, Pan Q, Sun L, Zheng D, Li M, Li N, Shi H, Ni Y. MicroRNA-9 regulates non-small cell lung cancer cell invasion and migration by targeting eukaryotic translation initiation factor 5A2. Am J Transl Res. 2017;9:478-488. [PubMed] |
| 42. | Wang X, Jiang R, Cui EH, Feng WM, Guo HH, Gu DH, Tang CW, Xue T, Bao Y. N1-guanyl-1,7-diaminoheptane enhances the chemosensitivity of NSCLC cells to cetuximab through inhibition of eukaryotic translation initiation factor 5A2 activation. Eur Rev Med Pharmacol Sci. 2016;20:1244-1250. [PubMed] |
| 43. | Chen C, Zhang B, Wu S, Song Y, Li J. Knockdown of EIF5A2 inhibits the malignant potential of non-small cell lung cancer cells. Oncol Lett. 2018;15:4541-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM, Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, Liang CZ, Liao YB, Pang J, Wu RH, Fang Y, Chen ZH, Li B, Xie D, Chen W, Luo JH. EIF5A2 predicts outcome in localised invasive bladder cancer and promotes bladder cancer cell aggressiveness in vitro and in vivo. Br J Cancer. 2014;110:1767-1777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Chen Z, Yu T, Zhou B, Wei J, Fang Y, Lu J, Guo L, Chen W, Liu ZP, Luo J. Mg(II)-Catechin nanoparticles delivering siRNA targeting EIF5A2 inhibit bladder cancer cell growth in vitro and in vivo. Biomaterials. 2016;81:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Liu Y, Du F, Chen W, Yao M, Lv K, Fu P. EIF5A2 is a novel chemoresistance gene in breast cancer. Breast Cancer. 2015;22:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Liu Y, Liu R, Fu P, Du F, Hong Y, Yao M, Zhang X, Zheng S. N1-Guanyl-1,7-Diaminoheptane Sensitizes Estrogen Receptor Negative Breast Cancer Cells to Doxorubicin by Preventing Epithelial-Mesenchymal Transition through Inhibition of Eukaryotic Translation Initiation Factor 5A2 Activation. Cell Physiol Biochem. 2015;36:2494-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Preukschas M, Hagel C, Schulte A, Weber K, Lamszus K, Sievert H, Pällmann N, Bokemeyer C, Hauber J, Braig M, Balabanov S. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: implications for new targeted therapies. PLoS One. 2012;7:e43468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Ziegler P, Chahoud T, Wilhelm T, Pällman N, Braig M, Wiehle V, Ziegler S, Schröder M, Meier C, Kolodzik A, Rarey M, Panse J, Hauber J, Balabanov S, Brümmendorf TH. Evaluation of deoxyhypusine synthase inhibitors targeting BCR-ABL positive leukemias. Invest New Drugs. 2012;30:2274-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Pällmann N, Braig M, Sievert H, Preukschas M, Hermans-Borgmeyer I, Schweizer M, Nagel CH, Neumann M, Wild P, Haralambieva E, Hagel C, Bokemeyer C, Hauber J, Balabanov S. Biological Relevance and Therapeutic Potential of the Hypusine Modification System. J Biol Chem. 2015;290:18343-18360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 52. | Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem. 2006;139:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Park MH, Lee YB, Joe YA. Hypusine is essential for eukaryotic cell proliferation. Biol Signals. 1997;6:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Klier H, Csonga R, Joäo HC, Eckerskorn C, Auer M, Lottspeich F, Eder J. Isolation and structural characterization of different isoforms of the hypusine-containing protein eIF-5A from HeLa cells. Biochemistry. 1995;34:14693-14702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Park MH, Wolff EC, Folk JE. Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors. 1993;4:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Ishfaq M, Maeta K, Maeda S, Natsume T, Ito A, Yoshida M. The role of acetylation in the subcellular localization of an oncogenic isoform of translation factor eIF5A. Biosci Biotechnol Biochem. 2012;76:2165-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Ishfaq M, Maeta K, Maeda S, Natsume T, Ito A, Yoshida M. Acetylation regulates subcellular localization of eukaryotic translation initiation factor 5A (eIF5A). FEBS Lett. 2012;586:3236-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Lee SB, Park JH, Folk JE, Deck JA, Pegg AE, Sokabe M, Fraser CS, Park MH. Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1). Biochem J. 2011;433:205-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Kang HA, Schwelberger HG, Hershey JW. Translation initiation factor eIF-5A, the hypusine-containing protein, is phosphorylated on serine in Saccharomyces cerevisiae. J Biol Chem. 1993;268:14750-14756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Chung J, Rocha AA, Tonelli RR, Castilho BA, Schenkman S. Eukaryotic initiation factor 5A dephosphorylation is required for translational arrest in stationary phase cells. Biochem J. 2013;451:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Shang Y, Zhao X, Tian B, Wang Y, Ren F, Jia B, Zhai Y, Chen W, He D, Chang Z. CHIP/Stub1 interacts with eIF5A and mediates its degradation. Cell Signal. 2014;26:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Beninati S, Nicolini L, Jakus J, Passeggio A, Abbruzzese A. Identification of a substrate site for transglutaminases on the human protein synthesis initiation factor 5A. Biochem J. 1995;305:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Kabachinski G, Schwartz TU. The nuclear pore complex--structure and function at a glance. J Cell Sci. 2015;128:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 64. | Schmidt HB, Görlich D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem Sci. 2016;41:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 65. | Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, McCombie WR, Wigler M, Hicks J, Hannon GJ, Powers S, Lowe SW. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 66. | Aksu M, Trakhanov S, Görlich D. Structure of the exportin Xpo4 in complex with RanGTP and the hypusine-containing translation factor eIF5A. Nat Commun. 2016;7:11952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, Kutay U, Görlich D. Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19:4362-4371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Parreiras-E-Silva LT, Gomes MD, Oliveira EB, Costa-Neto CM. The N-terminal region of eukaryotic translation initiation factor 5A signals to nuclear localization of the protein. Biochem Biophys Res Commun. 2007;362:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Wang FW, Guan XY, Xie D. Roles of eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci. 2013;9:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Rasko JE, Wong JJ. Nuclear microRNAs in normal hemopoiesis and cancer. J Hematol Oncol. 2017;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Wang X, Jin Y, Zhang H, Huang X, Zhang Y, Zhu J. MicroRNA-599 inhibits metastasis and epithelial-mesenchymal transition via targeting EIF5A2 in gastric cancer. Biomed Pharmacother. 2018;97:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Zhou X, Xu M, Guo Y, Ye L, Long L, Wang H, Tan P, Xu M. MicroRNA-588 regulates invasion, migration and epithelial-mesenchymal transition via targeting EIF5A2 pathway in gastric cancer. Cancer Manag Res. 2018;10:5187-5197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Tsang FH, Au V, Lu WJ, Shek FH, Liu AM, Luk JM, Fan ST, Poon RT, Lee NP. Prognostic marker microRNA-125b inhibits tumorigenic properties of hepatocellular carcinoma cells via suppressing tumorigenic molecule eIF5A2. Dig Dis Sci. 2014;59:2477-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Xue F, Liang Y, Li Z, Liu Y, Zhang H, Wen Y, Yan L, Tang Q, Xiao E, Zhang D. MicroRNA-9 enhances sensitivity to cetuximab in epithelial phenotype hepatocellular carcinoma cells through regulation of the eukaryotic translation initiation factor 5A-2. Oncol Lett. 2018;15:813-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Lou B, Fan J, Wang K, Chen W, Zhou X, Zhang J, Lin S, Lv F, Chen Y. N1-guanyl-1,7-diaminoheptane (GC7) enhances the therapeutic efficacy of doxorubicin by inhibiting activation of eukaryotic translation initiation factor 5A2 (eIF5A2) and preventing the epithelial-mesenchymal transition in hepatocellular carcinoma cells. Exp Cell Res. 2013;319:2708-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Kolligs FT. An alternative way for epithelial-to-mesenchymal transition in colorectal cancer via EIF5A2? Gut. 2012;61:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Bai HY, Liao YJ, Cai MY, Ma NF, Zhang Q, Chen JW, Zhang JX, Wang FW, Wang CY, Chen WH, Jin XH, Xu RH, Guan XY, Xie D. Eukaryotic Initiation Factor 5A2 Contributes to the Maintenance of CD133(+) Hepatocellular Carcinoma Cells via the c-Myc/microRNA-29b Axis. Stem Cells. 2018;36:180-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Cree IA, Charlton P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer. 2017;17:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 79. | Zhou QY, Tu CY, Shao CX, Wang WK, Zhu JD, Cai Y, Mao JY, Chen W. GC7 blocks epithelial-mesenchymal transition and reverses hypoxia-induced chemotherapy resistance in hepatocellular carcinoma cells. Am J Transl Res. 2017;9:2608-2617. [PubMed] |
| 80. | Xue F, Liu Y, Chu H, Wen Y, Yan L, Tang Q, Xiao E, Zhang D, Zhang H. eIF5A2 is an alternative pathway for cell proliferation in cetuximab-treated epithelial hepatocellular carcinoma. Am J Transl Res. 2016;8:4670-4681. [PubMed] |
| 81. | Ojima E, Inoue Y, Miki C, Mori M, Kusunoki M. Effectiveness of gene expression profiling for response prediction of rectal cancer to preoperative radiotherapy. J Gastroenterol. 2007;42:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Liu Y, Xue F, Zhang Y, Lei P, Wang Z, Zhu Z, Sun K. N1-guanyl-1,7-diaminoheptane enhances the chemosensitivity of acute lymphoblastic leukemia cells to vincristine through inhibition of eif5a-2 activation. Anticancer Drugs. 2017;28:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Xu G, Yu H, Shi X, Sun L, Zhou Q, Zheng D, Shi H, Li N, Zhang X, Shao G. Cisplatin sensitivity is enhanced in non-small cell lung cancer cells by regulating epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2. BMC Pulm Med. 2014;14:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Atala A. Re: Mg(II)-Catechin Nanoparticles Delivering siRNA Targeting EIF5A2 Inhibit Bladder Cancer Cell Growth In Vitro and In Vivo. J Urol. 2017;198:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |