Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.424
Peer-review started: February 14, 2019
First decision: March 14, 2019
Revised: March 26, 2019
Accepted: April 8, 2019
Article in press: April 8, 2019
Published online: May 15, 2019
Processing time: 106 Days and 4.8 Hours
Many advanced hepatocellular carcinoma (HCC) patients are receiving sorafenib treatment. Sorafenib reportedly improves overall survival (OS) significantly in patients with HCC. Prediction of sorafenib response and prognosis in patients with HCC receiving sorafenib treatment are important due to the potentially serious side effects of sorafenib. A disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS13) and von Willebrand factor (VWF) are associated with the pathophysiology of liver cirrhosis and HCC through their roles in hypercoagulability; they are also associated with angiogenesis via vascular endothelial growth factor (VEGF). The imbalance between ADAMTS13 and VWF was associated with prognosis of various cancers in patients undergoing chemotherapy.
To investigate ADAMTS13 and VWF as potential biomarkers for sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
Forty-one patients with HCC receiving sorafenib treatment were included in this study. The initial daily sorafenib dose was 400 mg in all patients. ADAMTS13 activity (ADAMTS13:AC), VWF antigen (VWF:Ag), VEGF levels were determined by enzyme-linked immunosorbent assay. Univariate and multivariate analyses were used to determine predictive factors for sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
ADAMTS13:AC was significantly higher in patients with stable disease (SD), partial response (PR), and complete response (CR) than in those with progressive disease (PD) (P < 0.05). In contrast, VWF:Ag and the VWF:Ag/ADAMTS13:AC ratio were significantly lower in patients with SD, PR, and CR than in those with PD (P < 0.05 for both). Multivariate analysis showed that the VWF:Ag/ADAMTS13:AC ratio was the only predictive factor for sorafenib response and ADAMTS13:AC was the only prognostic factor in patients with HCC receiving sorafenib treatment. The patients with a low ADAMTS13:AC (< 78.0) had significantly higher VEGF levels than those with a high ADAMTS13:AC (≥ 78.0) (P < 0.05).
The VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC are potentially useful biomarkers for sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment.
Core tip: There is an urgent clinical need to prediction of sorafenib response and prognosis in patients with hepatocellular carcinoma (HCC) receiving sorafenib treatment due to the potentially serious side effects of sorafenib in these patients. Multivariate analysis showed that the von Willebrand factor (VWF) antigen (VWF:Ag)/a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS13) activity (ADAMTS13:AC) ratio was the only predictive factor for sorafenib response and ADAMTS13:AC was the only prognostic factor in patients with HCC receiving sorafenib treatment. The VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC are potentially useful biomarkers for sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment.
- Citation: Takaya H, Namisaki T, Shimozato N, Kaji K, Kitade M, Moriya K, Sato S, Kawaratani H, Akahane T, Matsumoto M, Yoshiji H. ADAMTS13 and von Willebrand factor are useful biomarkers for sorafenib treatment efficiency in patients with hepatocellular carcinoma. World J Gastrointest Oncol 2019; 11(5): 424-435
- URL: https://www.wjgnet.com/1948-5204/full/v11/i5/424.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i5.424
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the second most common cause of cancer-related deaths worldwide[1,2]. In Japan, medical treatment policies for HCC are based on the consensus-based clinical practice guidelines for HCC management set by the Japan Society of Hepatology (JSH)[3], which recommend that patients with HCC who cannot undergo liver resection, radiofrequency ablation, or transcatheter arterial chemoembolization (TACE) should be considered to receive molecularly targeted drugs including sorafenib[3]. Sorafenib is a small inhibitor of several tyrosine protein kinases, including vascular endothelial growth factor (VEGF) receptor, platelet derived growth factor (PDGF) receptor, and Raf family kinases[4]. Sorafenib was shown to significantly improve overall survival (OS) in patients with HCC[4]. It is important to predict response to sorafenib and prognosis of HCC patients treated with sorafenib to avoid ineffective treatments because sorafenib has various side effects including hand-foot syndrome.
A disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS13) is a metalloproteinase that specifically cleaves multimeric von Willebrand factor (VWF) between Tyr1605 and Met1606 residues in the A2 domain[5-8]. ADAMTS13 is produced exclusively in hepatic stellate cells adjacent to endothelial cells[9]. VWF is synthesized in vascular endothelial cells and released into plasma as unusually large VWF multimers[10]. In the presence of an imbalance between ADAMTS13 and VWF, VWF multimers are cleaved improperly, leading to their accumulation and induction of platelet thrombus formation under high-shear stress in microvessels[11].
We previously reported that the imbalance of ADAMTS13 and VWF was associated with the pathophysiology of liver cirrhosis (LC) and HCC[8,12,13], suggesting that LC and HCC might be related to hypercoagulability[8,12,13]. Previous studies have reported that the imbalance of ADAMTS13 and VWF is associated with angiogenesis through VEGF[14-17], which in turn is associated with LC and HCC development[18-20]. In addition, the imbalance of ADAMTS13 and VWF might be associated with sorafenib treatment efficiency because VEGF is inhibited by sorafenib[4]. Furthermore, blood coagulation cascade was demonstrated to be related to cancer development[21,22], indicating that the imbalance of ADAMTS13 and VWF is associated with hypercoagulability as well as cancer development[23]. Recent studies have also reported that the imbalance between ADAMTS13 and VWF was associated with prognosis of various cancers in patients undergoing chemotherapy[24].
In the current study, we investigated the relationship between plasma ADAMTS13 and VWF levels in patients with HCC receiving sorafenib treatment and determined whether plasma ADAMTS13 and VWF levels were useful biomarkers for prediction of sorafenib response and prognosis of HCC in patients with HCC receiving sorafenib treatment.
There were 44 patients with HCC who were initiated on sorafenib treatment from December 2012 to November 2017 at our hospital. After excluding three patients who discontinued sorafenib treatment in the first month, 41 patients were included in this study. The initial daily sorafenib dose was 400 mg in all patients, and sorafenib therapy are based on the JSH consensus-based clinical practice guidelines for the management of HCC[3]. All patients underwent dynamic computed tomographic scanning or dynamic magnetic resonance imaging before sorafenib treatment, at 1 mo after starting sorafenib treatment, and every 3 mo thereafter. Radiologic response to therapy was evaluated according to modified response evaluation criteria in solid tumors[25]. Tumor-node-metastasis (TNM) stage was evaluated according to the TNM classification of the Union for the International Cancer Control of malignant tumors. No patient had infection, uncontrolled ascites, uncontrolled hepatic encephalopathy, or uncontrolled gastroesophageal varices. This study was approved by the local ethics committee of Nara Medical University and performed in accordance with the ethical standards stated in the Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Blood samples were collected from all patients at the time of admission, during their hospital stay or during regular outpatient treatment within 1 mo before sorafenib treatment initiation. The samples were stored in plastic tubes containing 0.38% v/v sodium citrate. Platelet-poor plasma, which was prepared by centrifuging the samples at 3000 g at 4 °C for 15 min, was stored as aliquots at −80 °C until analysis. Plasma ADAMTS13 activity (ADAMTS13:AC) was determined by a sensitive chromogenic enzyme-linked immunosorbent assay (Kainos Laboratories, Tokyo, Japan)[26]. Mean normal ADAMTS13:AC level was 99% ± 22%. Plasma VWF antigen (VWF:Ag) was measured by sandwich enzyme-linked immunosorbent assay using a rabbit anti-human VWF polyclonal antiserum (Dako, Glostrup, Denmark). Mean normal VWF:Ag level was 102% ± 33%[27].
VEGF and VEGF receptor 2 (VEGFR-2) levels were determined by commercially available immunoassay kits (RayBiotech, United States, and R and D Systems, United States, respectively). The detection limits for VEGF and VEGFR-2 were 10 and 11.4 pg/mL, respectively.
Differences between groups were analyzed using the Mann–Whitney U-test, and correlations were calculated with Spearman’s rank test. Categorical data were analyzed using Fisher’s exact test. Univariate and multivariate analyses were per-formed for predictive and prognostic factors for sorafenib treatment in HCC. Logistic regression analysis with stepwise selection of variables was performed to determine independent predictive factors of sorafenib treatment for HCC, and the Cox proportional hazards regression analysis with stepwise selection of variables was conducted to determine independent prognostic factors of sorafenib treatment for HCC. Progression-free survival (PFS) and OS curves were calculated using the Kaplan–Meier method, and differences between groups were assessed using the log-rank test. Data were expressed as medians with interquartile ranges. A two-tailed P value of less than 0.05 was considered statistically significant. Analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). Specifically, EZR is a modified version of R commander (version 1.6-3) that includes statistical functions that are frequently used in biostatistics[28].
The patient characteristics are shown in Table 1. The median age of patients with HCC was 74.0 (69.0–81.0) years. The study population comprised 38 males and three females. Among these, 7, 20, 3, and 11 patients had hepatitis B virus, hepatitis C virus, non-alcoholic steatohepatitis, and alcohol abuse, respectively. The median maximum tumor size was 3.3 (2.5–7.7) cm. In this cohort, 3, 2, 1, and 33 patients had 1, 2, 3, and ≥ 4 tumors, respectively, whereas two patients had only distant metastases. Portal vein tumor thrombosis and distant metastasis were present in 7 and 17 patients, respec-tively. Serum levels of alpha-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP), lens culinaris agglutinin-reactive fraction of AFP (AFP-L3%), VEGF, and VEGFR-2 were 121.8 (11.3–2611.0) ng/mL, 359.5 (58.0–5277.5) mAU/mL, 13.2 (1.7–42.4)%, 25.8 (14.1–40.1) pg/mL, and 6500 (5750–7400) pg/mL, respectively. DCP was directly correlated with VEGF (r = 0.503, P < 0.05). However, DCP was not correlated with VEGFR-2, and AFP or AFP-L3% was not correlated with VEGF or VEGFR-2. In the current study cohort, there were no differences in the characteristics of patients with stable disease (SD), partial response (PR), and complete response (CR) compared with those with progressive disease (PD), except the DCP levels and observation (survival) period.
| Variable | Total(n = 41) | SD + PR + CR(n = 17) | PD(n = 24) | P value |
| Age (yr) | 74.0 (69.0-81.0) | 74.0 (71.0-78.0) | 74.0 (63.5-70.0) | 0.400 |
| Sex (male/female) | 38/3 | 17/0 | 21/3 | 0.254 |
| Etiology (HBV/HCV/NASH/alcohol) | 7/20/3/11 | 0/13/1/3 | 7/7/2/8 | 0.0707 |
| Albumin (g/dL) | 3.6 (3.3-3.9) | 3.7 (3.5-4.0) | 3.5 (3.3-3.7) | 0.0827 |
| Prothrombin time (%) | 82.0 (77.0-89.0) | 83.0 (78.0-89.0) | 82.0 (78.5-91.0) | 0.751 |
| Total bilirubin (mg/dL) | 0.8 (0.6-0.9) | 0.7 (0.5-0.9) | 0.8 (0.6-0.9) | 0.397 |
| Platelet count (× 104/mm3) | 14.1 (11.1-17.3) | 16.1 (9.4-18.3) | 14.1 (11.6-16.9) | 0.568 |
| AFP (ng/mL) | 121.8 (11.3-2611.0) | 31.8 (6.7-161.0) | 286.0 (41.6-5193.5) | 0.103 |
| DCP (mAU/mL) | 359.5 (58.0-5277.5) | 192 (24.5-5177) | 5177 (183-17214) | 0.012 |
| AFP-L3% (%) | 13.2 (1.7-42.4) | 12.3 (1.45-34.3) | 21.8 (1.85-47.7) | 0.529 |
| VEGF (pg/mL) | 25.8 (14.1-40.1) | 18.5 (10.0-35.1) | 28.2 (22.0-50.7) | 0.106 |
| VEGFR-2 (pg/mL) | 6500 (5750-7400) | 6400 (5200-7100) | 6800 (6350-7600) | 0.211 |
| Maximum tumor size (cm) | 3.3 (2.5-7.7) | 3.2 (2.0-6.0) | 4.3 (2.7-10.8) | 0.220 |
| Tumor number (1/2/3/4 or more/only distant metastasis) | 3/2/1/33/2 | 0/2/1/13/1 | 3/0/0/20/1 | 0.131 |
| PVTT (presence/absence) | 7/34 | 2/15 | 5/19 | 0.679 |
| Distant metastasis (presence/absence) | 17/24 | 10/7 | 7/17 | 0.107 |
| Child-pugh score | 5 (5-6) | 5 (5-6) | 5 (5-6) | 0.469 |
| UICC TNM stage (2/3/4) | 5/16/20 | 1/6/10 | 4/10/10 | 0.455 |
| Observation (survival) period (d) | 328 (156-530) | 564 (405-880) | 162 (116-319) | 0.000322 |
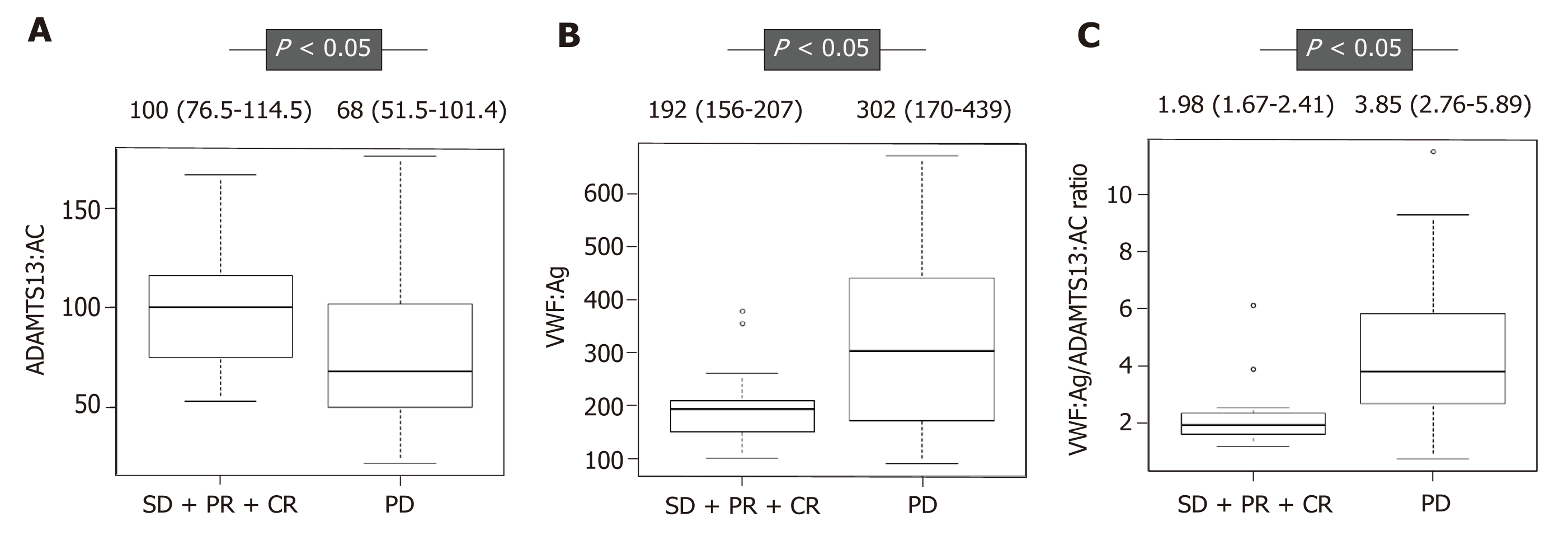
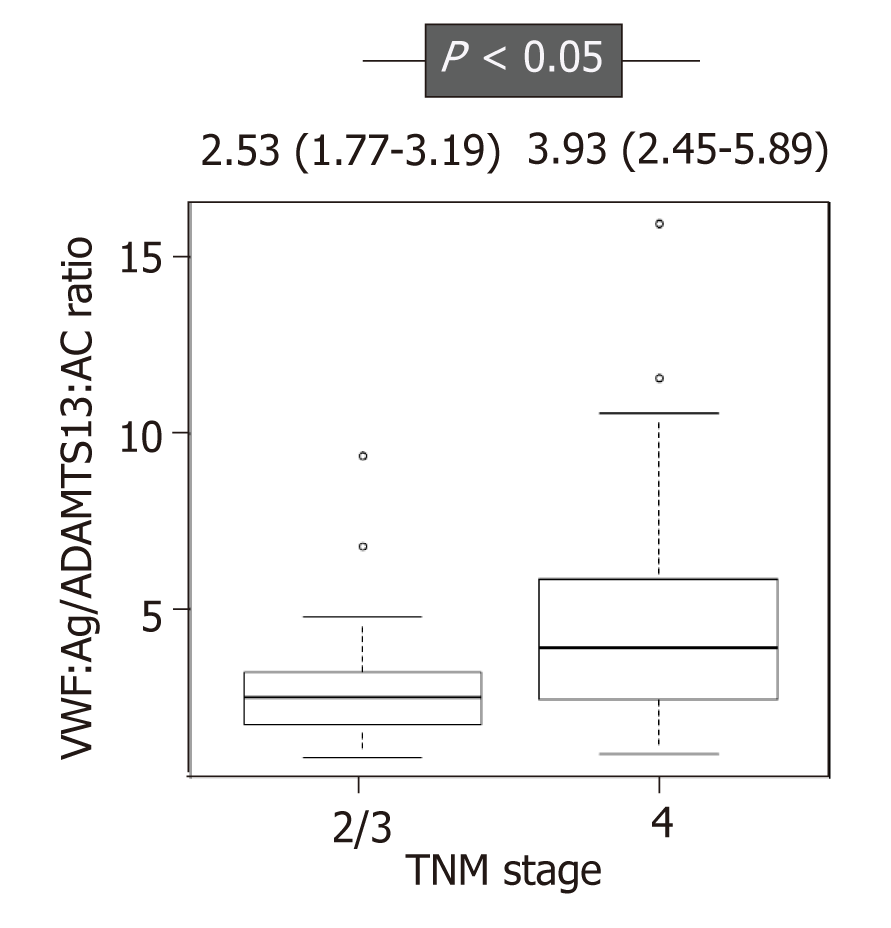
ADAMTS13:AC level was significantly higher in patients with HCC who had SD, PR, and CR than those with PD (P < 0.05) (Figure 1A). In contrast, VWF:Ag and the VWF:Ag/ADAMTS13:AC ratio levels were significantly lower in those with SD, PR, and CR than those with PD (P < 0.05 for both) (Figure 1B and C). ADAMTS13:AC level was directly correlated with albumin (r = 0.457, P < 0.05), and VWF:Ag and the VWF:Ag/ADAMTS13:AC ratio levels were directly correlated with total bilirubin(r = 0.329, P < 0.05 and r = 0.316, P < 0.05, respectively). In addition, the patients were categorized into two, according to the TNM stage (TNM stage 2 and 3 is 21 patients, and TNM stage 4 is 20 patients). The VWF:Ag/ADAMTS13:AC ratio levels was higher in patients with TNM stage 4 HCC than those with TNM stage 2 and 3 HCC (Figure 2).
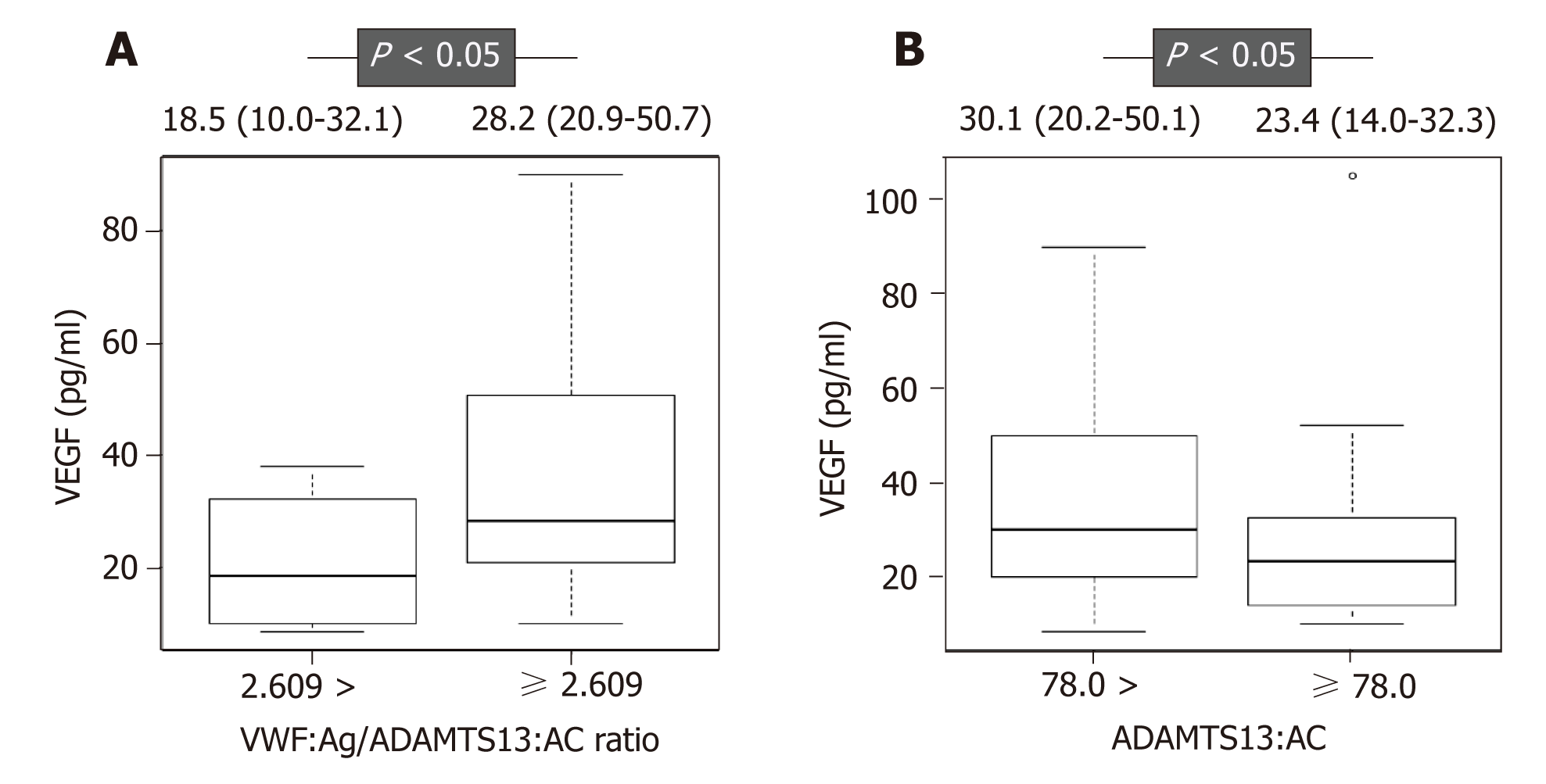
ADAMTS13:AC and the VWF:Ag/ADAMTS13:AC ratio were associated with sora-fenib response in univariate analysis (Table 2). To determine predictive factors for sorafenib response, we performed multivariate analysis using albumin, DCP, VEGF, maximum tumor size, distant metastasis, ADAMTS13:AC, VWF:Ag, and the VWF:Ag/ADAMTS13:AC ratio, which had P-values of < 0.2 in the univariate analysis. The VWF:Ag/ADAMTS13:AC ratio was significantly associated with sorafenib response in multivariate analysis (Table 2). The receiver operating characteristic (ROC) analysis revealed that a cutoff VWF:Ag/ADAMTS13:AC ratio of 2.609 had a specificity of 84.2% and a sensitivity of 88.2%, and the area under the ROC curve (AUC) was 0.836 (Figure 3). Next, the study patients were categorized into two groups according to the ROC cutoff VWF:Ag/ADAMTS13:AC ratio: Low (VWF:Ag/ADAMTS13:AC ratio < 2.609) and high (VWF:Ag/ADAMTS13:AC ratio ≥ 2.609). The patients with a high VWF:Ag/ADAMTS13:AC ratio had significantly higher VEGF levels than those with a low VWF:Ag/ADAMTS13:AC ratio (Figure 4A), indicating that the VWF:Ag/ADAMTS13:AC ratio might be associated with VEGF in patients with HCC. However, the patients with VEGFR-2, AFP, DCP and AFP-L3% levels were not different between the low and high VWF:Ag/ADAMTS13:AC ratio groups.
| Univariate analysis | OR (95%CI) | P value |
| Age (per 1 yr increase) | 1.05 (0.967-1.13) | 0.2660 |
| Sex (male vs female) | 0.556 (0.0464-6.66) | 0.9950 |
| Viral hepatitis (presence vs absence) | 0.527(0.123-2.27) | 0.6430 |
| Albumin (per 1 g/dL decrease) | 6.59 (0.827-52.6) | 0.0750 |
| Prothrombin time (per 1% decrease) | 0.977 (0.928-1.03) | 0.3910 |
| Total bilirubin (per 1 mg/dL increase) | 0.376 (0.0378-3.74) | 0.4040 |
| Platelet count (per 104/µL decrease) | 1.040 (0.937-1.14) | 0.4970 |
| AFP (per 1 ng/mL increase) | 1.000 (1.000-1.00) | 0.5330 |
| DCP (per 1 mAU/mL increase) | 1.00 (0.999-1.00) | 0.1100 |
| AFP-L3% (per 1% increase) | 1.00 (0.996-1.01) | 0.4420 |
| VEGF (per 1 pg/mL increase) | 0.98 (0.949-1.01) | 0.1950 |
| VEGFR-2 (per 1 pg/mL increase) | 1.00 (0.999-1.000) | 0.2210 |
| Maximum tumor size (per 1 cm increase) | 0.892 (0.752-1.06) | 0.1900 |
| Tumor number (per 1 increase) | 1.11 (0.646-1.90) | 0.7070 |
| PVTT (presence vs absence) | 0.835 (0.455-1.53) | 0.5590 |
| Distant metastasis (presence vs absence) | 2.450 (0.64-9.37) | 0.1910 |
| ADAMTS13:AC (per 1% increase) | 1.020 (1.0001-1.050) | 0.0039 |
| VWF:Ag (per 1% increase) | 0.996 (0.991-1.000) | 0.0740 |
| VWF:Ag/ADAMTS13:AC (per 1 increase) | 0.465 (0.265-0.817) | 0.0077 |
| Multivariate analysis | ||
| VWF:Ag/ADAMTS13:AC (per 1 increase) | 0.495 (0.281-0.870) | 0.0147 |
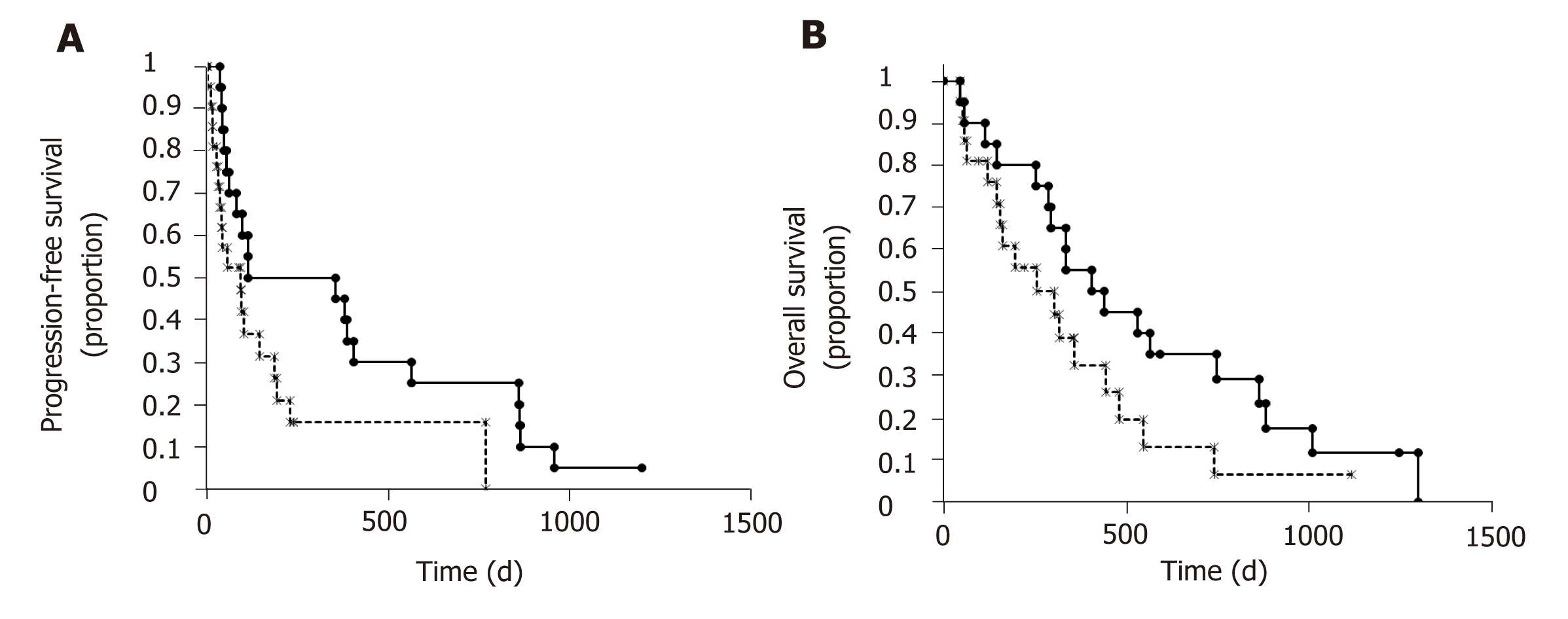
In univariate analysis, age, DCP, and ADAMTS13:AC were associated with prognosis in patients with HCC receiving sorafenib treatment (Table 3). To determine prognostic factors in patients with HCC receiving sorafenib treatment, we performed mul-tivariate analysis using age, sex, DCP, tumor number, ADAMTS13:AC, and the VWF:Ag/ADAMTS13:AC ratio, which had P-values < 0.2 in the univariate analysis. ADAMTS13:AC was associated significantly with prognosis in multivariate analysis (Table 3). Therefore, the patients were categorized into two groups according to the median cutoff ADAMTS13:AC: Low (ADAMTS13:AC < 78.0) and high (ADAMTS13:AC ≥ 78.0). The patients with a high ADAMTS13:AC had significantly longer PFS and OS than those with a low ADAMTS13:AC (Figure 5). The patients with a low ADAMTS13:AC had significantly higher VEGF levels than those with a high ADAMTS13:AC (Figure 4B). These results indicated that ADAMTS13:AC might be associated with VEGF in patients with HCC. However, the patients with VEGFR-2, AFP, DCP, and AFP-L3% levels were not different between the low and high ADAMTS13:AC groups.
| Univariate analysis | HR (95%CI) | P value |
| Age (per 1 yr increase) | 1.16 (1.032-1.304) | 0.0127 |
| Sex (male vs female) | 0.062 (0.000382-1.008) | 0.0561 |
| Viral hepatitis (presence vs absence) | 1.416(0.405-4.95) | 0.5862 |
| Albumin (per 1 g/dL decrease) | 0.427 (0.0750-2.44) | 0.3388 |
| Prothrombin time (per 1% decrease) | 1.008 (0.969-1.048) | 0.3910 |
| Total bilirubin (per 1 mg/dL increase) | 4.203 (0.417-42.30) | 0.2230 |
| Platelet count (per 104/µL decrease) | 0.906 (0.807-1.018) | 0.4970 |
| AFP (per 1 ng/mL increase) | 1.000 (1.000-1.00) | 0.3334 |
| DCP (per 1 mAU/mL increase) | 1.000 (1.000-1.00) | 0.0136 |
| AFP-L3% (per 1% increase) | 0.999 (0.969-1.031) | 0.9484 |
| VEGF (per 1 pg/mL increase) | 0.982 (0.952-1.01) | 0.2426 |
| VEGFR-2 (per 1 pg/mL increase) | 1.00 (0.999-1.000) | 0.8947 |
| Maximum tumor size (per 1 cm increase) | 1.026 (0.868-1.214) | 0.7622 |
| Tumor number (per 1 increase) | 0.751 (0.5-1.129) | 0.1690 |
| PVTT (presence vs absence) | 0.347 (0.0384-3.141) | 0.3464 |
| Distant metastasis (presence vs absence) | 0.489 (0.129-1.854) | 0.2936 |
| ADAMTS13:AC (per 1% increase) | 0.936 (0.895-0.978) | 0.0035 |
| VWF:Ag (per 1% increase) | 0.996 (0.991-1.000) | 0.9227 |
| VWF:Ag/ADAMTS13:AC (per 1 increase) | 1.33 (0.998-1.772) | 0.0520 |
| Multivariate analysis | ||
| ADAMTS13:AC (per 1% increase) | 0.937 (0.895-0.980) | 0.0045 |
The results of the present study suggest that the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC are potential biomarkers for sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment. Previous studies reported that Child-Pugh score A patients with HCC receiving sorafenib treatment had longer OS and PFS than those with Child-Pugh score B patients[29] and that VWF:Ag was associated with prognosis in cirrhotic patients[30]. Furthermore, we previously reported that ADAMTS13:AC and VWF:Ag were associated with functional liver capacity[12,13] and that ADAMTS13:AC was associated with prognosis in cirrhotic patients[6]. In other words, ADAMTS13:AC and VWF:Ag are useful biomarkers to evaluate functional liver capacity in detail for cirrhotic patients[6,30]. As the result, the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC might be associated with sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment.
A recent study reported that HCC patients with Barcelona Clinic Liver Cancer (BCLC) stage B receiving sorafenib treatment had longer OS than those with BCLC stage C[29]. The current study revealed that the VWF:Ag/ADAMTS13:AC ratio was associated with TNM stage in HCC. Furthermore, several studies reported that the VWF:Ag/ADAMTS13:AC ratio was associated with TNM stage and prognosis in various cancers[24,31,32]. The association of the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC with sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment reflects that the ADAMTS13–VWF imbalance might be associated with tumor stage. Furthermore, this may be caused by blood coagulation cascade associated with cancer development[21,22] because the ADAMTS13–VWF imbalance associated with blood coagulation cascade[11].
In addition, angiogenesis plays an important role in LC and HCC development, which are related to VEGF, as VEGF levels are increased in patients with LC and HCC[18-20]. Recent studies reported that VWF reduced VEGF-depend angiogenesis via multiple intracellular and extracellular pathways involving integrin avβ3 and angiopoietin-2[14-16] and that ADAMTS13 induced angiogenesis by ADAMTS13-mediated cleavage of VWF and ADAMTS13-mediated VEGFR-2 phosphorylation, which lead to enhanced VEGF expression[17]. Tabernero et al[16] revealed the critical role of the balance between ADAMTS13 and VWF in regulating blood vessel formation. Furthermore, we observed that VWF:Ag was a predictive factor[8] and that the VWF:Ag/ADAMTS13:AC ratio was a diagnostic factor (unpublished observations) for HCC in cirrhotic patients. As sorafenib inhibits VEGF and the change in VEGF during sorafenib treatment is associated with the prognosis of patients with HCC receiving sorafenib treatment[4], the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC might be associated sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment, through angiogenesis. In fact, in the present study, we also found that the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC were associated with VEGF levels.
Furthermore, antiplatelet therapy inhibits PDGF, which induces HCC develop-ment[33]. Recent studies reported that antiplatelet therapy prevented HCC development in cirrhotic patients[34] and improved survival in a mouse model of chronic hepatitis B[33]. An imbalance between ADAMTS13 and VWF induces platelet thrombus formation[11] and thereby is possibly associated with PDGF. The association of the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC with sorafenib response and prognosis in patients with HCC receiving sorafenib treatment might be occurring via PDGF, which is inhibited by sorafenib and VEGF. The relationship of the ADAMTS13-VWF imbalance with angiogenic factors requires further investigation.
There are several promising candidate biomarkers for predicting sorafenib response and prognosis in patients with HCC receiving sorafenib treatment, including VEGF-A, angiopoietin-2, insulin-like growth factor-1, and neutrophil/lymphocyte ratio[29,35], most of which are not identified as biomarkers for these patients due to high costs and limited practicality in a clinical setting[29,35]. In addition, while these biomarkers were reported as prognostic factors for OS in patients with HCC receiving sorafenib treatment, their relationship with PFS was not determined. Importantly, OS is affected by after-treatment. In fact, in the present study, several patients underwent TACE and/or hepatic arterial infusion chemotherapy using cisplatin. Therefore, we propose that PFS might be more important than OS for evaluating sorafenib treatment efficiency in patients with HCC in clinical setting. Because ADAMTS13:AC is a prognostic factor for OS and PFS in patients with HCC receiving sorafenib treatment, we believe that ADAMTS13:AC is a more useful biomarker than other biomarkers.
The present study has several limitations, including a short observation period and the small sample size. Cirrhotic patients with HCC occasionally develop thrombosis or inflammation, including portal thrombosis and bacterial overgrowth and translocation, which may impact the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC and impact their value as biomarkers. In addition, only 7.3% of patients were female (male: 38, female: 3). We believe that the difference in gender had no effects in our study because a previous study has reported that the relation-ships between ADAMTS13:AC and other parameters (e.g., albumin, total bilirubin, aspartate aminotransferase, alkaline phosphatase, and creatinine) are not associated with gender bias[36].
In summary, the VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC, which were associated with VEGF, were independent predictive factors for sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment. To our knowledge, this is the first report the ADAMTS13-VWF imbalance in association with sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
Sorafenib reportedly improves overall survival (OS) significantly in patients with HCC. Prediction of sorafenib response and prognosis in patients with HCC receiving sorafenib treatment are important due to the potentially serious side effects of sorafenib.
A disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS13) and von Willebrand factor (VWF) are associated with the pathophysiology of liver cirrhosis and HCC through their roles in hypercoagulability; they are also associated with angiogenesis via vascular endothelial growth factor (VEGF). The imbalance between ADAMTS13 and VWF was associated with prognosis of various cancers in patients undergoing chemotherapy.
To investigate ADAMTS13 and VWF as potential biomarkers for sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
Forty-one patients with HCC receiving sorafenib treatment were included in this study. The initial daily sorafenib dose was 400 mg in all patients. ADAMTS13 activity (ADAMTS13:AC), VWF antigen (VWF:Ag), VEGF levels were determined by enzyme-linked immunosorbent assay. Univariate and multivariate analyses were used to determine predictive factors for sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
Multivariate analysis showed that the VWF:Ag/ADAMTS13:AC ratio was the only predictive factor for sorafenib response and ADAMTS13:AC was the only prognostic factor in patients with HCC receiving sorafenib treatment. The patients with a low ADAMTS13:AC (< 78.0) had significantly higher VEGF levels than those with a high ADAMTS13:AC (≥ 78.0) (P < 0.05).
The VWF:Ag/ADAMTS13:AC ratio and ADAMTS13:AC are potentially useful biomarkers for sorafenib response and prognosis, respectively, in patients with HCC receiving sorafenib treatment.
This is the first report the ADAMTS13-VWF imbalance in association with sorafenib response and prognosis in patients with HCC receiving sorafenib treatment.
This work was completed with the help of Ms. Yoshie Nakai, Professor Hiroshi Fukui, and Professor Masahito Uemura.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu X, Raffaniello R, Saglam S S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1865] [Article Influence: 207.2] [Reference Citation Analysis (4)] |
| 2. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 3. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, Suzuki Y, Tamaki N, Yasui Y, Suzuki S, Hosokawa T, Nishimura T, Ueda K, Kuzuya T, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Enomoto N, Izumi N. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer. 2014;120:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Takaya H, Kawaratani H, Kubo T, Seki K, Sawada Y, Kaji K, Okura Y, Takeda K, Kitade M, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. Platelet hyperaggregability is associated with decreased ADAMTS13 activity and enhanced endotoxemia in patients with acute cholangitis. Hepatol Res. 2018;48:E52-E60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Takaya H, Uemura M, Fujimura Y, Matsumoto M, Matsuyama T, Kato S, Morioka C, Ishizashi H, Hori Y, Fujimoto M, Tsujimoto T, Kawaratani H, Toyohara M, Kurumatani N, Fukui H. ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child-Turcotte-Pugh score and the Model for End-Stage Liver Disease score. Hepatol Res. 2012;42:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Takaya H, Yoshiji H, Kawaratani H, Sakai K, Matsumoto M, Fujimura Y, Fukui H. Decreased activity of plasma ADAMTS13 are related to enhanced cytokinemia and endotoxemia in patients with acute liver failure. Biomed Rep. 2017;7:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Takaya H, Kawaratani H, Tsuji Y, Nakanishi K, Saikawa S, Sato S, Sawada Y, Kaji K, Okura Y, Shimozato N, Kitade M, Akahane T, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. von Willebrand factor is a useful biomarker for liver fibrosis and prediction of hepatocellular carcinoma development in patients with hepatitis B and C. United European Gastroenterol J. 2018;6:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 422] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lämmle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1203] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 12. | Uemura M, Fujimura Y, Ko S, Matsumoto M, Nakajima Y, Fukui H. Determination of ADAMTS13 and Its Clinical Significance for ADAMTS13 Supplementation Therapy to Improve the Survival of Patients with Decompensated Liver Cirrhosis. Int J Hepatol. 2011;2011:759047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Uemura M, Fujimura Y, Matsumoto M, Ishizashi H, Kato S, Matsuyama T, Isonishi A, Ishikawa M, Yagita M, Morioka C, Yoshiji H, Tsujimoto T, Kurumatani N, Fukui H. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, Foschi FG, De Matteis S, Ercolani G, Valgiusti M, Frassineti GL, Scartozzi M, Casadei Gardini A. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J Gastroenterol. 2018;24:4152-4163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 138] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (2)] |
| 15. | Kalambokis GN, Oikonomou A, Christou L, Kolaitis NI, Tsianos EV, Christodoulou D, Baltayiannis G. von Willebrand factor and procoagulant imbalance predict outcome in patients with cirrhosis and thrombocytopenia. J Hepatol. 2016;65:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Yoshino T, Goldberg RM, Sargent DJ, Wagner A, Laurent D, Teufel M, Jeffers M, Grothey A, Van Cutsem E. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 17. | Salum GM, Bader El Din NG, Ibrahim MK, Anany MA, Dawood RM, Khairy A, El Awady MK. Vascular Endothelial Growth Factor Expression in Hepatitis C Virus-Induced Liver Fibrosis: A Potential Biomarker. J Interferon Cytokine Res. 2017;37:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 394] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 19. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Tsujinoue H, Masaki T, Fukui H. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF, Laffan MA, Randi AM. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Randi AM. Angiogenesis and the ADAMTS13-VWF balance. Blood. 2017;130:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci. 2015;72:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV, Iannacone M, Guidotti LG. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109:E2165-E2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 25. | Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Matsumoto M, Kawaguchi S, Ishizashi H, Yagi H, Iida J, Sakaki T, Fujimura Y. Platelets treated with ticlopidine are less reactive to unusually large von Willebrand factor multimers than are those treated with aspirin under high shear stress. Pathophysiol Haemost Thromb. 2005;34:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13209] [Article Influence: 1100.8] [Reference Citation Analysis (0)] |
| 28. | Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Snyder KM, Kessler CM. The pivotal role of thrombin in cancer biology and tumorigenesis. Semin Thromb Hemost. 2008;34:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Guo R, Yang J, Liu X, Wu J, Chen Y. Increased von Willebrand factor over decreased ADAMTS-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer. J Clin Lab Anal. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3289] [Article Influence: 219.3] [Reference Citation Analysis (36)] |
| 32. | Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, Cho H, Ahn H, Cho YY, Yoo JJ, Cho Y, Lee DH, Cho EJ, Yu SJ, Lee DH, Lee JM, Kim YJ, Yoon JH. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Kanthaje S, Makol A, Chakraborti A. Sorafenib response in hepatocellular carcinoma: MicroRNAs as tuning forks. Hepatol Res. 2018;48:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Pépin M, Kleinjan A, Hajage D, Büller HR, Di Nisio M, Kamphuisen PW, Salomon L, Veyradier A, Stepanian A, Mahé I. ADAMTS-13 and von Willebrand factor predict venous thromboembolism in patients with cancer. J Thromb Haemost. 2016;14:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Yang X, Sun HJ, Li ZR, Zhang H, Yang WJ, Ni B, Wu YZ. Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC Cancer. 2015;15:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Enooku K, Kato R, Ikeda H, Kurano M, Kume Y, Yoshida H, Ono T, Aizawa K, Suzuki T, Yamazaki T, Yatomi Y. Inverse correlations between serum ADAMTS13 levels and systolic blood pressure, pulse pressure, and serum C-reactive protein levels observed at a general health examination in a Japanese population: a cross-sectional study. Clin Chim Acta. 2013;421:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |