Published online Mar 15, 2019. doi: 10.4251/wjgo.v11.i3.250
Peer-review started: November 27, 2018
First decision: January 4, 2019
Revised: January 21, 2019
Accepted: January 29, 2019
Article in press: January 30, 2019
Published online: March 15, 2019
Processing time: 108 Days and 17.1 Hours
After an esophagectomy, the stomach is most commonly used to restore continuity of the upper gastrointestinal tract. These esophago-gastric anastomoses are prone to serious complications such as leakage associated with high morbidity and mortality. Graft perfusion is considered to be an important predictor for anastomotic integrity. Based on the current literature we believe Indocyanine green fluorescence angiography (ICGA) is an easy assessment tool for gastric tube (GT) perfusion, and it might predict anastomotic leakage (AL).
To evaluate feasibility and effectiveness of ICGA in GT perfusion assessment and as a predictor of AL.
This study was designed according to the PRISMA guidelines and registered in the PROSPERO database. PubMed and EMBASE were independently searched by 2 reviewers for studies presenting data on intraoperative ICGA GT perfusion assessment during esophago-gastric reconstruction after esophagectomy. Relevant outcomes such as feasibility, complications, intraoperative surgical changes based on ICGA findings, quantification attempts, anatomical data and the impact of ICGA on postoperative anastomotic complications, were collected by 2 independent researchers. The quality of the included articles was assessed based on the Methodological Index for Non-Randomized Studies. The 19 included studies presented data on 1192 esophagectomy patients, in 758 patients ICGA was used perioperative to guide esophageal reconstruction.
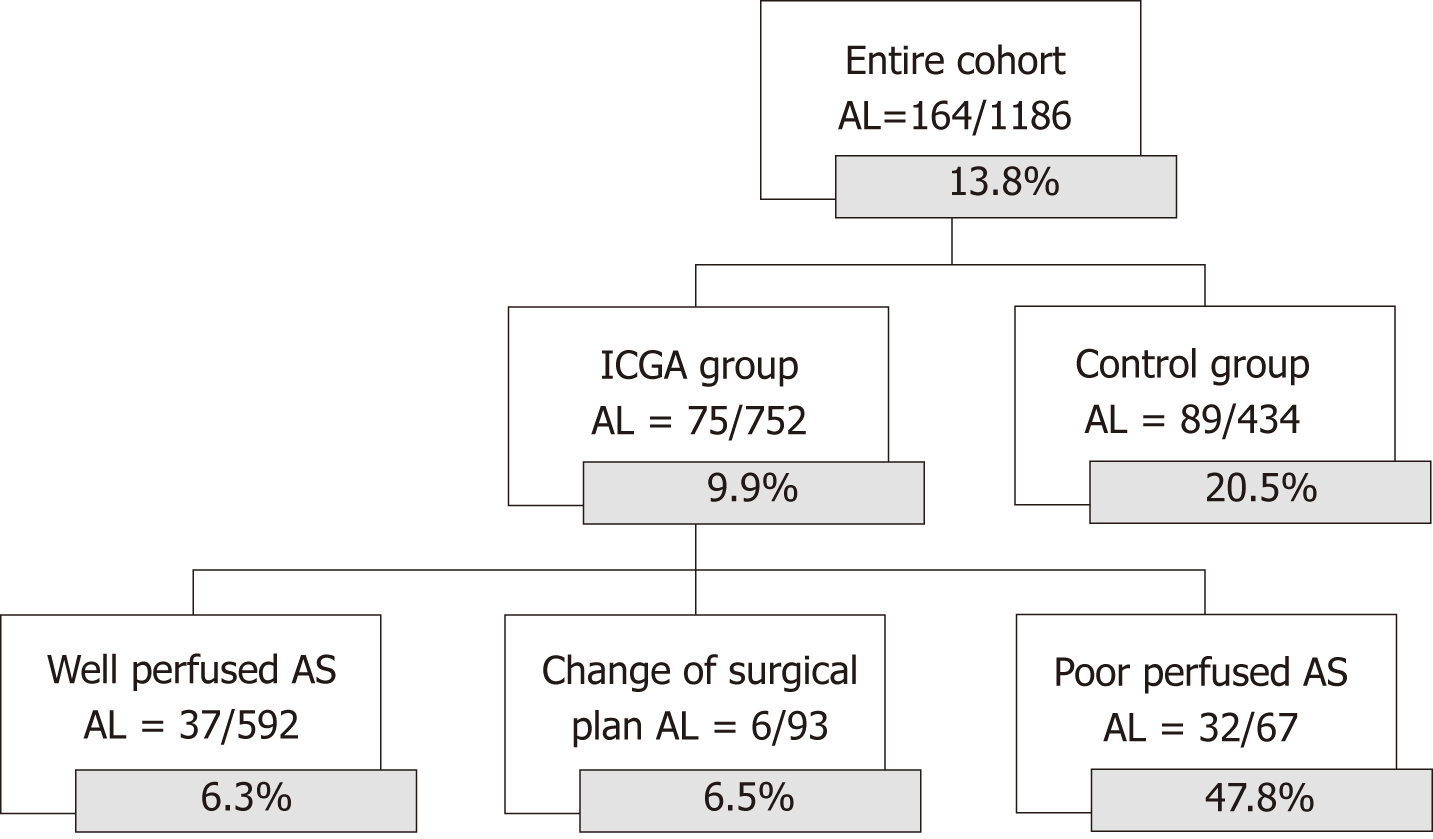
The 19 included studies for qualitative analyses all described ICGA as a safe and easy method to evaluate gastric graft perfusion. AL occurred in 13.8% of the entire cohort, 10% in the ICG guided group and 20.6% in the control group (P < 0.001). When poorly perfused cases are excluded from the analyses, the difference in AL was even larger (AL well-perfused group 6.3% vs control group 20.5%, P < 0.001). The AL rate in the group with an altered surgical plan based on the ICG image was 6.5%, similar to the well perfused group (6.3%) and significantly less than the poorly perfused group (47.8%) (P < 0.001), suggesting that the technique is able to identify and alter a potential bad outcome.
ICGA is a safe, feasible and promising method for perfusion assessment. The lower AL rate in the well perfused group suggest that a good fluorescent signal predicts a good outcome.
Core tip: Esophagectomy is a surgery known for its complexity and potentially high morbidity associated with postoperative anastomotic leakage (AL). This review evaluates Indocyanine green fluorescence angiography (ICGA) as a safe, feasible and promising method to assess graft perfusion during esophageal reconstructive surgery. We discuss the safety, the methodology and the effectiveness of ICGA and its potential to reduce AL rate.
- Citation: Van Daele E, Van Nieuwenhove Y, Ceelen W, Vanhove C, Braeckman BP, Hoorens A, Van Limmen J, Varin O, Van de Putte D, Willaert W, Pattyn P. Near-infrared fluorescence guided esophageal reconstructive surgery: A systematic review. World J Gastrointest Oncol 2019; 11(3): 250-263
- URL: https://www.wjgnet.com/1948-5204/full/v11/i3/250.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i3.250
The incidence of esophageal adenocarcinoma has rapidly increased over the past two decades[1]. For locally and locally advanced esophageal cancer (EC) patients, surgical resection remains the cornerstone of their treatment. The stomach is most commonly used to restore continuity of the upper gastrointestinal tract after an oesophagus resection. However, these esophago-gastric anastomotic sites (AS) are fragile and prone to complications as leakage, fistulas, bleeding, and stricture. Anastomotic leakage (AL) remains the main cause of postoperative morbidity and mortality in digestive reconstructive surgery. After an esophagectomy leakage incidence ranges from 5% to 20%[2-6]. Literature reports leak-associated mortality rates from 18% to 40% compared to an overall in-hospital mortality of 4% to 6%[2,7,8]. Age, male gender, smoking, alcohol abuse, American Society of Anaesthesiologists score, obesity, emergency surgery, prolonged operative time, intraoperative blood loss, diabetes, renal failure, use of corticosteroids and cardiovascular disease are identified as risk factors for AL, potentially through impaired perfusion of the gastric graft[2,4-6,8-11].
Graft perfusion is considered to be an important predictor for anastomotic integrity. Currently, tissue perfusion is assessed using subjective parameters such as tissue colour and vessel pulsations. However, these parameters are known to be of limited predictive value, emphasizing the clear need for a safe, reproducible, and non-invasive method to objectively assess tissue viability and graft perfusion.
Several methods have been tested for intraoperative evaluation of graft perfusion, such as laser speckle contrast imaging, gastric tonometry, Doppler flowmetry, spectroscopy and angiography[12-15]. None of them have been widely accepted due to limited feasibility, reproducibility issues or costs. Near infrared fluorescent (NIRF) imaging requires a fluorescence-imaging agent that can be excited in the tissue at near infrared (NIR) wavelengths by penetrating NIR light. A 2D image of tissue deposition of the NIRF imaging agent can be created based on the fluorescence generated by the excited NIRF agent and captured by adapted cameras. Indocyanine green (ICG) is a clinically approved NIRF agent to determine cardiac output, hepatic function, and ophthalmic angiography. ICG is known for its absorption maximum around 760-780 nm, its immediate binding with plasma proteins resulting in a confinement to the vascular compartment, its low toxicity and its rapid and exclusive biliary excretion. Its excellent safety record has added to the rapid food and drug administration approval for clinical use in 1956[16]. Recent developments of new hardware have made it possible to perform Indocyanine green fluorescence angiography (ICGA) not only in open, but also during minimally invasive surgery, resulting in a general interest of the surgical community in this technique as a method to visualize anastomotic perfusion and its potential to reduce AL risk. At present ICGA is not a standard guideline during esophageal surgery because of the lack of prospective randomized studies, but many expert centers already incorporate perioperative ICGA as a routine step during esophageal reconstruction.
This study aimed to systematically review the literature on feasibility and effectiveness of ICGA use as a method to evaluate graft perfusion and as a predictor of AL after esophageal reconstructive surgery. Additionally, current methods to quantify ICGA in esophageal reconstructive surgery were reviewed.
This study was designed according to the PRISMA guidelines and registered in the PROSPERO 2018 database (http://www.crd.york.ac.uk). The registration identification number is CRD42018100503.
Only English written studies on human subjects were included in the study. Publications were excluded if they reported on a primary intervention other than ICGA or when they did not present original patient data. Conference abstracts, case reports, technical notes, letters, comments, duplications and animal studies were also excluded. Study selection was based on the population, intervention, comparison, outcome principle. The population was defined as patients undergoing esophagectomy for EC with gastric graft reconstruction. The intervention is ICGA for the intraoperative assessment of gastric graft perfusion. Comparison was done with non ICGA guided esophagectomy patients if a control group was present. The primary objective was feasibility of ICGA as a method to evaluate graft perfusion. The secondary objective was the effect of ICGA on alterations of the surgical plan and its ability to predict and avoid AL. The final objective was to evaluate current attempts to quantify ICGA in esophageal reconstructive surgery.
PubMed and EMBASE were independently searched by 2 reviewers for studies presenting data on intraoperative ICGA gastric graft perfusion assessment during esophago-gastric reconstruction after esophagectomy. The search was last updated in October 2018. The following MeSH terms were used in the PubMed database searches: “Esophagectomy”[MeSH] OR “Esophagus surgery” OR “Esophagus cancer” OR “Esophagus” OR “Esophag*” AND “Indocyanine green”[MeSH] OR “angiography”[MeSH] OR “ICG” OR “graft perfusion” OR “near infrared imaging” OR “near infrared fluorescence”. Same Emtree terms were used in the EMBASE database. Titles and abstracts were screened for eligibility. After reading full text articles, duplicates were extracted, and references were screened to identify additional articles. Consensus was reached by the 2 reviewers after re-review in case of discordances in study selection.
The quality of the included articles was assessed based on the Methodological Index for Non-Randomized Studies (MINORS), a validated quality assessment system with 8 items for non-comparative and 12 items for comparative studies. The maximum score for non-comparative studies is 16 and 24 for the comparative studies[17].
Study design, patients’ demographic data, intervention related data and outcomes were extracted from the included studies. Study data were collected by 2 independent researchers in a standard Excel™ data collection sheet which was used to calculate descriptive statistics for all measured parameters. Continuous and categorical variables were summarized as means with standard deviation, and/or median with IQR or ranges. Data were analyzed using SPSS version 22 for windows (SPSS Inc., Chicago, IL). Statistical analysis using a fisher exact- test was performed to compare AL rates between the ICGA guided and non-guided group. A P-value < 0.05 was considered statistically significant.
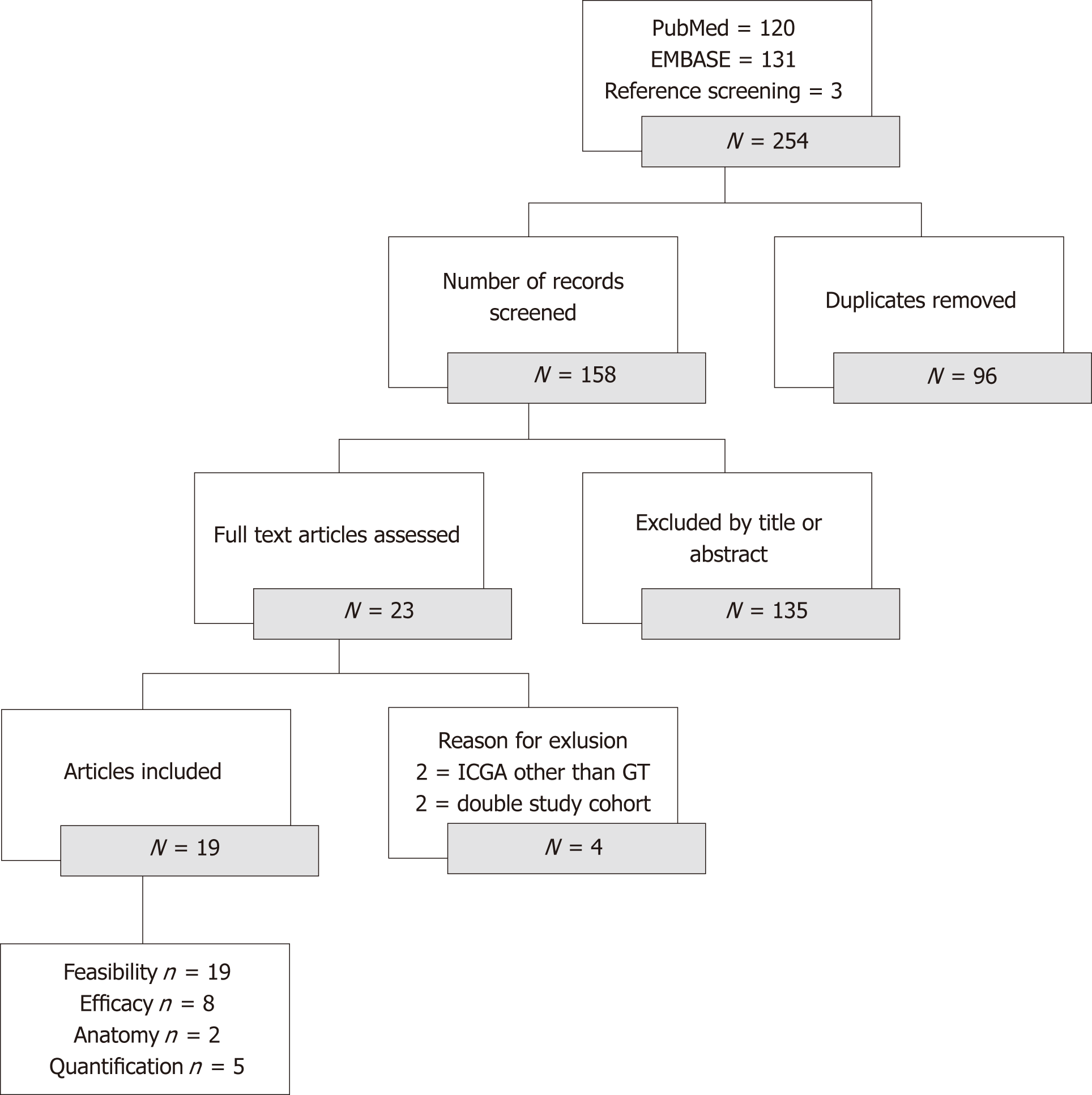
The initial database searches resulted in 251 eligible abstracts and citations and 3 additional records were found through reference checking. After adjusting for duplicates and screening of the abstracts, 23 full text articles remained and were reviewed in detail, resulting in the exclusion of another 4[18-40]. Finally, 19 papers met the inclusion criteria for qualitative analysis[18-23,25-28,30,33-40]. Six articles could be used for separate quantitative analyses as they contained data of a comparison group[25,26,35-38]. The PRISMA flowchart of the search is shown in Figure 1.
Study characteristics of the 19 included studies and demographic data of the included patients are described in Table 1. Ten studies were feasibility studies, 6 studies compared the ICGA study cohort to a historical non ICGA cohort, and 3 studies evaluated outcome of patients with adequately versus poorly ICGA perfused AS. In total, 1192 (82% male) esophagectomy patients were analyzed. In 758 patients, ICGA was used perioperative to guide esophageal reconstruction compared to 434 patients was the anastomotic site was determined based on clinical judgment and/or Doppler ultrasound.
| Ref. | Enrollment | Study design | Non ICG group | No. of patients (M/F) | Median age (range) | Neoadjuvant therapy | Operative technique | Dye and dose | Timing of ICGA | Imaging system | Change of procedure | MINOR score |
| Shimada et al[40] | 2008-2011 (Japan) | Prospective cohort | No | 36 GT (29/7) | 67 (49-81) | 8 CT | Open surgery | 2.5 mg Diagnogreen | After GT creation | PDE | Superdrainage (5) | 6a |
| Feasibility | 1 RT | Cervical AS | ||||||||||
| 1 CRT | ||||||||||||
| Kubota et al[18] | 2010-2011 (Japan) | Prospective cohort | No | 4 GT (3/1) | 69 (64-71) | 4 CT | Open surgery | 0.5 g/kg | After GT creation | HEMS | No | 4a |
| Feasibility | Cervical AS | |||||||||||
| Murawa et al[19] | 2009-2010 (Poland) | Prospective cohort | No | 15 (13/2) | Mean 56 (54-74) | 0 | Not reported | 25 mg | After GT creation | ICView | End-to-end (4) | 6a |
| Feasibility | Cervical AS | |||||||||||
| Transhiatal | ||||||||||||
| Pacheco et al[20] | 2010-2011 (United States) | Retrospective cohort | No | 11 (NR) | Mean 56 (SD ± 9) | 7 CRT | Open surgery | Not reported | After GT creation | SPY | No | 4a |
| Feasibility | - 10 ICGA good AS | Cervical AS | ||||||||||
| - 1 ICGA poor AS | Transhiatal | |||||||||||
| Kumagai et al[21] | 2013 (Japan) | Prospective cohort | No | 20 (16/4) | Mean 68 (50-79) | 0 | Open surgery | 2.5 mg Diagnogreen | After GT creation | PDE | No | 13a |
| Feasibility | Cervical AS | |||||||||||
| Rino et al[22] | 2009-2013 (Japan) | Prospective cohort | No | 33 (29/4) | Mean 68 (NR) | Not reported | Not reported | 2.5 mg Diagnogreen | After GT creation | PDE | No | 4a |
| Feasibility | Thoracic AS | |||||||||||
| Ivor Lewis | ||||||||||||
| Sarkaria et al[23] | 2012-2013 (United States) | Prospective cohort | No | 30 (22/8) | 59 (37-76) | 24 CRT | RAMIE | 10 mg | Before GT creation | FireFly | No | 6a |
| Feasibility | Cervical + thoracic AS | |||||||||||
| Ivor Lewis + McKeown | ||||||||||||
| Hodari et al[25] | 2011-2014 (United States) | Retrospective cohort | Yes | 54 (44/10) | 65 (45-81) | 38 CRT | RAMIE | Not reported | Before anastomosis during anastomosis | FireFly | Guided AS (39) | 6b |
| Historical case control | - 39 ICGA | Thoracic AS | ||||||||||
| - 15 non ICGA | Ivor Lewis | |||||||||||
| Campbell et al[26] | 2007-2013 (United States) | Retrospective cohort | Yes | 90 (74/16) | 62 (22-81) | 6 CT | MIE + Hybrid + Open | 5 mg | After GT creation | SPY | Guided AS (21) | 15b |
| Historical case control | - 21 ICGA | 54 CRT | Thoracic AS | |||||||||
| - 60 non ICGA | Ivor Lewis | |||||||||||
| - 9 Doppler | ||||||||||||
| Yukaya et al[27] | 2013-2014 (Japan) | Prospective cohort | No | 27 (26/1) | 67 (40-86) | Not reported | Not reported | 0,1 mg/kg Diagnogreen | After GT creation | HEMS | No | 11a |
| Feasibility | Cervical AS | |||||||||||
| Zehetner et al[28] | 2008-2011 (United States) | Prospective cohort | No | 150 (125/25) | 67 (IQR 57-74) | 67 CRT | MIE + Open Cervical AS | 2.5 mg | After GT creation | SPY | Guided AS (95) | 13b |
| Case controlled | - 6 delayed AS = excluded | Transhiatal + McKeown | ||||||||||
| - 95 ICGA good AS | ||||||||||||
| - 49 ICGA poor AS | ||||||||||||
| Koyonagi et al[30] | 2014-2015 (Japan) | Prospective cohort | No | 40 (34/6) | 68 (26-82) | 11 CT | Not reported | 2.5 or 1.25 mg Diagnogreen | After GT creation | PDE | No | 17b |
| Case controlled | - 25 ICGA good AS | 4 CRT | Cervical AS | |||||||||
| - 15 ICGA poor AS | ||||||||||||
| Schlottmann et al[33] | NR (United States) | Prospective cohort | No | 5 (3/2) | 59 (56-70) | 1 CRT | Hybrid | 5 mg | Before anastomosis | IMAGE1 | Guided AS (5) | 8a |
| Feasibility | Thoracic AS | ICG-pulsion | - Resection (2) | |||||||||
| Ivor Lewis | ||||||||||||
| Kitagawa et al[29,34] | 2011-2017 (Japan) | Retrospective cohort | No | 72 (57/15) | Mean 66 (SD ±7) | 60 CT | MIE + Hybrid | 5 mg | Before GT creation | HEMS | Guided AS (26) | 18b |
| Case controlled | - 26 ICGA | 2 CRT | Cervical AS | After GT creation | Guided GT (46) | |||||||
| - 46 ICGA-LMM | McKeown | - End-to-end (4) | ||||||||||
| (line marking method) | - Superdrainage (1) | |||||||||||
| Ohi et al[32,35] | 2000-2015 (Japan) | Retrospective cohort | Yes | 120 (101/19) | 68 (IQR 63-74) | 56 Not reported | Open + Hybrid | 2.5 mg Diagnogreen | After GT creation | PDE | Guided AS 60 s (59) | 15b |
| Historical case control | - 59 ICGA | Cervical AS | - End-to-end (3) | |||||||||
| - 61 non ICGA | - Supercharge/drain (1) | |||||||||||
| - Manubriotomy (5) | ||||||||||||
| Karampiris et al[36] | 2010-2016 (Germany) | Retrospective cohort | Yes | 90 (65/23 + 2 NR) | Mean 62 (SD ± 9) | 69 CT | Open + Hybrid | 7.5 mg | After GT creation | PinPoint | Guided AS (33) | 16b |
| Historical case control | - 35 ICGA | 25 RT | cervical + thoracic AS | - Resection (26) | ||||||||
| - 33 ICGA good AS | Thoracophrenico + McKeown | |||||||||||
| - 2 ICGA poor AS | ||||||||||||
| - 55 non ICGA | ||||||||||||
| Noma et al[37] | 2010-2016 (Japan) | Retrospective cohort | Yes | 285 (244/41) | Mean 65 (SD ± 8) | 129 CT | Open+ Hybrid + MIE | 12.5 mg Diagnogreen | After GT creation | PDE | Guided AS 30 s (71) | 17b |
| Historical case control | - 71 ICGA | 21 CRT | Cervical AS | - extended mobilization | ||||||||
| Case matched | - 214 non ICGA | McKeown | -Supercharge/drain (1) | |||||||||
| Dalton et al[38] | 2014-2016 (United States) | Retrospective cohort | Yes | 40 (32/8) | Mean 64 (SD ± 10) | 36 Not reported | MIE | 7.5 mg | After GT creation | PinPoint | Guided AS (20) | 17b |
| Historical case control | - 20 ICGA | Thoracic AS | - Resection (6) | |||||||||
| - 20 non ICGA | Ivor Lewis | |||||||||||
| Kumagai et al[39] | 2014-2017 (Japan) | Prospective cohort | No | 70 (59/11) | 71 (46-82) | Not reported | Not reported | 2.5 mg Diagnogreen | After GT creation | PDE | Guided AS 90 s (70) | 8a |
| Feasibility | Cervical AS | - Resection (35) |
All included studies were feasibility-or non-randomized controlled studies and the risk of bias within the studies was, therefore, analyzed according to the MINORS methodological index[17]. The score varied from 4 to 13 (median 6) for non-comparative studies (max score 16) and from 6 to 18 (median 16) for the comparative studies (max score 24). Most studies had a clear aim and appropriate endpoints, however the follow-up period and loss to follow-up were rarely mentioned. None had unbiased endpoint assessment or power calculations.
All studies reported ICGA to be feasible and easy. Many different imaging systems were described both for open and minimally invasive use, and different dosages of ICG were used (Table 1). Nevertheless, all authors were able to obtain good intraoperative NIRF images and no equipment malfunction was mentioned. The timeframe during which GT perfusion was visualized varied from a few seconds to a few minutes after injection of the dye. Shimada et al[40] described good visualization of the GT vasculature at the greater curvature after 1 min, the mucosal arterial network was nicely visible after 2 min. Kumagai et al[39] reported a median time of 27.9 s. (14.6-141.7) from initial fluorescence of the root of the right gastroepiploic artery to the tip of the GT in his first cohort and 35.3 s (13-204) in the second cohort, comparable to Sarkari’s median 37.5 s (20-105)[21,25,39]. Karampiris et al[36] reported a mean total duration of the ICGA of 173 s (± 74), leading him to conclude that ICGA did not significantly increase operating time (P = 0.8). All authors reported ICGA to be uneventful and well tolerated. None of the authors reported adverse reactions[18-23,25-28,30,33-40]. Murawa et al[19] was the only author who reported a transient but uncomplicated drop in oxygen saturation to 90% immediately after administration of the dye in his study group.
Five studies reported an attempt to objective quantification of the ICGA signal based on time and/or intensity of the fluorescence[21,27,30,37,39]. Kumagai et al[21] first reported a significantly longer time registration from initial fluorescence at the root of right gastroepiploic artery until perfusion of the tip in both AL patients. Yukaya’s study objectively assessed the gastric conduit vascularization based on inflow and outflow delays in perfusion detected on 2 points of the gastric conduit. Patterns of NIRF were categorized into three types of blood flow: Normal flow, delayed inflow and delayed outflow. The study could not show significant differences in AL, but the authors did provide a quantitative evaluation method of the NIRF image based on time intensity curves[27]. Koyanagi et al[30] quantified flow speed of NIRF in the gastric wall. Based on the length of the GT, anatomical connection between the right and left gastroepiploic artery and the speed of the fluorescence, 2 groups of patients were identified. A group with simultaneous NIRF in the gastric conduit wall and in the greater curvature vessels beyond the watershed region and a delayed group with obviously slower NIRF in the gastric conduit wall compared to the greater curvature vessels. Risk assessment identified flow speed of the NIRF as a predictor of AL (P = 0.002). A receiver operating characteristics curve determined a cut-off value of 1.76 cm/s or less as a significant independent AL predictor (P = 0.004)[30]. Noma et al[37] and Kumagai et al[39] published a more simplified quantification method based on time of GT perfusion. Noma’s 30 s rule included an ICGA 20 s-and 30 s demarcation line on the GT. After pulling up the stomach all cervical anastomoses were made proximal from the 30 s border[37]. Recently, Kumagai et al[39] published the 90 s rule where all anastomoses were made in areas where less than 90 s were needed for NIRF enhancement.
The intraoperative consequences of gastric tube (GT) perfusion assessment are summarized in Table 2. Within the entire study cohort, perioperative ICGA resulted in a change of the surgical plan in 93 patients (12.4% of the study group). Different surgical approaches were described to secure sufficient perfusion to the anastomotic site for patients with an impaired perfusion on ICGA. Four authors mentioned relocation of the anastomosis with resection of the poorly perfused tip if the GT was sufficient in length[33,36,38,39]. In three studies, authors chose to switch from an end-to-side anastomosis to an end-to-end anastomosis to reduce the necessary GT length needed for a cervical anastomosis[19,34,35]. Finally, four authors made additional arterial or venous anastomosis between the GT vasculature and carotid artery or jugular vein to increase arterial perfusion or venous drainage of the cervical anastomosis, a technique also known as supercharge or super drainage[34,35,37,40]. Both Ohi et al[35] and Noma et al[37] described a step-up surgical protocol for a well perfused anastomotic site. Changing the surgical plan seemed very efficient, as AL after an altered surgical plan occurred in 6.5% of patients, comparable to the 6.3% AL rate in the well perfused cohort and significantly less common than the 20.5% AL rate in the non ICG cohort (P < 0.001).
| Ref. | Entire cohort n = 1186 | ICGA study group n = 752 | Non ICGA n = 434 | ||
| ICGA good perfused AS, n = 592 | ICGA good perfused altered AS, n = 93 | ICGA poor perfused AS, n = 67 | |||
| Shimada et al[40] | 3/36 | 2/31 | 1/5 Superdrainage | - | - |
| Kubota et al[18] | 0/4 | 0/4 | - | - | - |
| Murawa et al[19] | 1/15 | 1/11 | 0/4 End-to-end | - | - |
| Pacheco et al[20] | 2/11 | 1/10 | - | 1/1 | - |
| Kumagai et al[21] | 2/20 | 2/20 | - | - | - |
| Rino et al[22] | 5/33 | 5/33 | - | - | - |
| Sarkaria et al[23] | 2/30 | 2/30 | - | - | - |
| Hodari et al[25] | 3/54 | 0/39 | - | - | 3/15 |
| Campbell et al[26] | 12/90 | 0/21 | - | - | 12/69 |
| Yukaya et al[27] | 9/27 | 9/27 | - | - | - |
| Zehetner et al[28] | 24/144 | 2/95 | - | 22/49 | - |
| Koyonagi et al[30] | 7/40 | 0/25 | - | 7/15 | - |
| Schlottmann et al[33] | 0/5 | 0/3 | 0/2 resection | - | - |
| Kitagawa et al[29,34] | 7/72 | 3/41 ICG-LMM | 0/5 ICG-LMM | - | - |
| 4/26 ICG | - 0/4 End-to-end | ||||
| - 0/1 Superdrainage | |||||
| Ohi et al[32,35] | 10/120 | 0/50 | 1/9 | - | 9/61 |
| - 0/3 End-to-end | |||||
| - 1/1 Supercharge/drain | |||||
| - 0/5 Manubriotomy | |||||
| Karampiris et al[36] | 13/90 | 0/7 | 1/26 Resection | 2/2 | 10/55 |
| Noma et al[37] | 60/285 | 6/70 | 0/1 Supercharge/drain | - | 54/214 |
| Dalton et al[38] | 3/40 | 0/14 | 2/6 Resection | - | 1/20 |
| Kumagai et al[39] | 1/70 | 0/35 | 1/35 Resection | - | - |
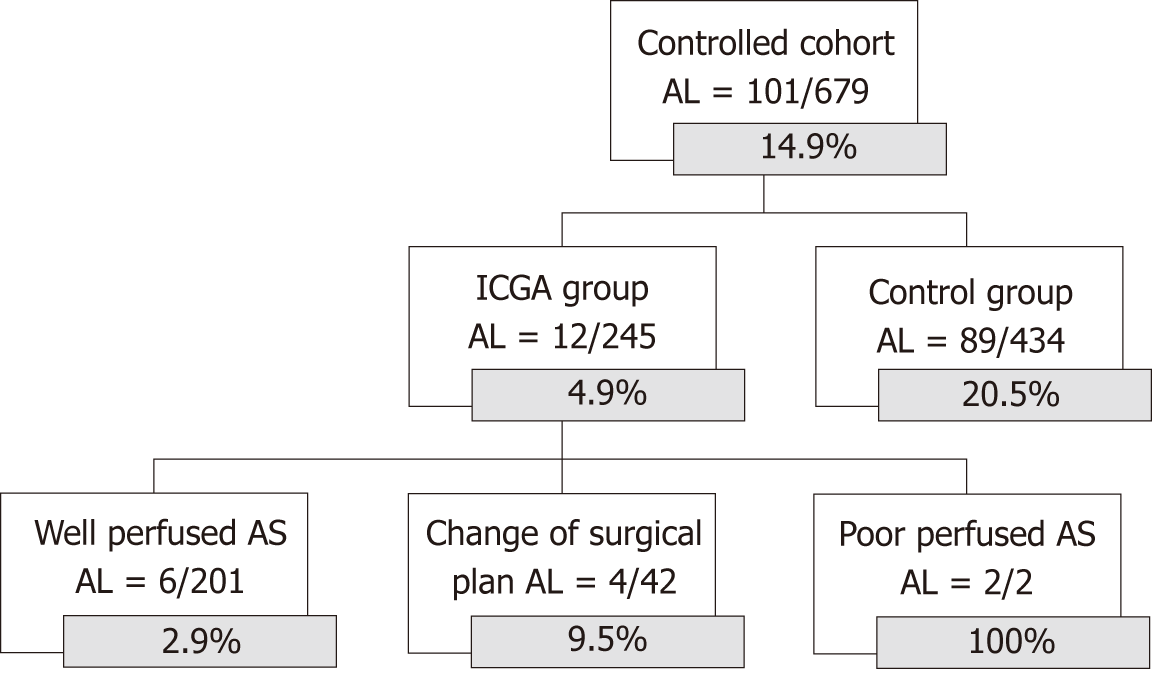
In 1186 patients, a primary esophagogastric anastomosis was made. The 6 patients, in whom Zehetner et al[28] decided to perform an ischemic conditioning with delayed anastomosis were excluded from further analyses. In the entire cohort, 13.8% of patients suffered from AL, 9.9% in the ICGA guided group and 20.5% in the control group (P < 0.001). Within the group of ICGA guided esophagogastric anastomosis, 592 had a good ICGA perfusion, but still resulted in 6.3% AL rate. Ninety-three patients had a low perfusion at the tip of the GT, for which different types of corrections were performed resulting in an adequate tip perfusion and an AL rate of 6.5%, comparable to the AL rate of the well perfused cohort and significantly lower than the 47.8% AL rate in the poorly perfused group (P < 0.001) (Figure 2). The difference in AL rate is even clearer considering only the cohort with a control group (P < 0.001) (Figure 3).
Surgeons lack the ability to predict AL based simply on gross inspection of tissue viability, emphasizing the need for a safe, reproducible, and non-invasive method for perfusion assessment, especially in esophageal reconstructive surgery. We aimed to review the literature on the feasibility and effectiveness of ICGA as a method to evaluate graft perfusion and as a predictor of AL after esophagectomy. In addition, we evaluated published attempts to quantify ICGA based imaging data in esophageal surgery.
Based on the 1192 included patients, we conclude that ICGA is an easy, safe and feasible method for GT perfusion assessment during esophageal reconstructive surgery. There are no reported technical failures of the different ICGA platforms, nor reports of adverse events, except for an uneventful and transient drop in oxygen saturation immediately after admission of the ICG, suggesting that this is a ready to use method with very limited risk and learning curve. The transient drop in percutaneous oxygen saturation measurement via pulse oximetry is a misreading due to the interference of the dye’s absorption spectra used by optical-technology-based monitors who interfere with the oximetry readings.
The time required for assessment of GT perfusion varied from a few seconds to a few minutes after injection of the dye, which does not significantly affect operating time. Different teams used different dosages of ICG varying from 2.5 mg to 25 mg, but none reported serious adverse events, and all described good visualization. Diana[41-43] performed an optimal dosage study in a pig model resulting in a dose of 0.5 mg/kg as optimal dose for a reliable fluorescence that could be converted into a time intensity curve with their VR RENDER perfusion software. At present optimal doses of 0.2-0.5 mg/kg are mentioned in literature, mainly based on the study of Diana, nevertheless many of the included studies have shown that GT perfusion could be visualized using a much lower (and potentially less toxic) dose. A recent practical guide for ICGA guided abdominal surgery also concluded on a bolus dose of 2.5 mg as optimal dose for simple visualization of both esophagogastric and colorectal anastomotic perfusion[44].
Only a few authors have attempted to quantify the method, usually in colorectal surgery, but comparison with a golden standard method is rarely reported. The only quantitative attempt to validate ICGA as a reliable perfusion assessment tool was performed by Diana et al[41-43], who showed that the time intensity curves of the NIRF images correlate with hypo-perfusion and tissue ischemia in an experimental pig model of small bowel ischemia[41-43]. Only five studies reported an attempt to quantify the ICGA signal during esophageal surgery, based on the speed of fluorescent coloring of the GT after injection of the dye[21,27,30,37,39]. Koyanagi et al[30] added the length of the GT and anatomical connection between the right and left gastroepiploic artery. A ROC curve analysis determined a cut-off value of 1.76 cm/s or less as a significant independent AL predictor (P = 0.004)[30]. Yukaya’s study assessed perfusion based on inflow and outflow delays detected on 2 points of the gastric conduit. That study could not show significant differences in AL between flow types, but the authors did provide the first and only quantitative evaluation method based on time intensity curves in esophageal surgery[27].
AL occurred in 13.8% of the entire cohort, which is in line within the normal range according to the literature. At present, there are no randomized trials to support the alteration of the surgical plan based on the ICGA images. Nevertheless, the most promising aspect of ICGA perfusion assessment is probably its ability to change the surgical plan. In this review population of 1186 patients, the surgeon chose to change the surgical plan to increase the blood flow to the anastomotic site, in 93 cases. The chosen technique varied with surgeon preference and location of the anastomosis. The AL rate in this altered anastomotic site group was 6.5%, similar to the well perfused group (6.3%) and significantly less than the poorly perfused group (47.8%) (P < 0.001). These results suggest that the technique is able to predict and remedy a potentially worse outcome, but randomized trials are needed to confirm these retrospective results.
This review is based on 19 studies with considerable inter-study heterogeneity, making comparison between studies difficult. Methods of ICGA interpretation and surgical consequences varied among the different study cohorts. Moreover, the included manuscripts are mainly prospective feasibility studies or retrospective analyses of low-grade evidence and with relatively low MINOR scores. The studies with a control group were all retrospective comparisons to historical control groups. Most studies had incomplete or unmentioned follow-up periods and lacked prospective sample size calculations.
The present review suggests that ICGA is a safe, feasible and promising method to assess graft perfusion that might help reducing AL. At present optimal doses of 0.2-0.5 mg/kg are mentioned in literature but based on this review a bolus dose of 2.5 mg is a sufficient optimal dose for visualization of esophagogastric anastomotic perfusion.
A few authors have attempted to quantify the method, but rarely in oesophageal surgery and without comparison with a golden standard method, stressing the need for objective quantification of the ICGA with validated cutoff levels for sufficient graft perfusion in esophageal surgery. No studies mentioned validation of the method. Objective, in depth assessment of tissue perfusion using NIRF imaging during creation of the esophagogastric anastomosis in EC surgery is lacking. Therefore, we propose a clinical study that uses NIRF dynamic images to calculate physiologically relevant parameters (blood flow, blood volume, vascular leakage) and generate pseudocolor coded parametric maps using advanced curve analysis and compartmental modelling (adiabatic approximation to tissue homogeneity model, AATH)[45,46]. In addition, this study would be the first to validate imaging-based perfusion assessment of the stomach graft using tissue, serum, and cellular hallmarks of hypo-perfusion and hypoxia during esophagectomy[47]. The study is registered in Clinicaltrials.gov as NCT03587532 and is currently recruiting.
The differences in AL rate between the well perfused and poor perfused AS clearly suggest that a good fluorescent signal is a predictor of good outcome. The AL rate in the group with an altered surgical plan based on the ICG image was similar to the well perfused group and significantly less than the poorly perfused group, suggesting that the technique is able to predict and remedy a potentially worse outcome. Reducing AL after oesophageal reconstruction is an ongoing goal of the oesophageal surgical community, this easy and safe new technique of perioperative perfusion assessment has the potential to reduce AL rate and its associated mortality, but randomized trials are needed to confirm these retrospective results. However, a prospective randomized study can only compare ICGA guided surgery versus non ICGA guided surgery and that implies a large sample size demanding a multicentre study. Comparing in a randomized fashion ICGA good perfused anastomoses versus ICGA poor perfused anastomosis would be scientifically perfect but ethically impossible. At present, there are no ongoing randomized trials listed on clinical trial.gov.
After an esophagectomy, the stomach is most commonly used to restore continuity of the upper gastrointestinal tract. These esophago-gastric anastomoses are prone to serious complications such as leakage associated with high morbidity and mortality. The main cause of anastomotic leakage (AL) is tissue hypoxia, which results from impaired perfusion of the pedicled gastric tube (GT). Clinical judgment is unreliable in determining GT perfusion.
An objective, validated, and reproducible method to evaluate tissue perfusion at the anastomotic site is urgently needed. Based on the current literature we believe Indocyanine green fluorescence angiography (ICGA) is an easy and cheap assessment tool for GT perfusion, that might predict and therefore alter a potentially bad outcome.
This study aimed to systematically review the literature on feasibility and effectiveness of ICGA use as a method to evaluate graft perfusion and as a predictor of AL after esophageal reconstructive surgery. Additionally, current methods to quantify ICGA in esophageal reconstructive surgery were reviewed.
This study was designed according to the PRISMA guidelines and registered in the PROSPERO database. Pubmed and Embase were independently searched by 2 reviewers for studies presenting data on intraoperative ICGA GT perfusion assessment during esophago-gastric reconstruction after esophagectomy. Relevant outcomes such as feasibility, complications, intraoperative surgical changes based on ICGA findings, quantification attempts, anatomical data and the impact of ICGA on postoperative anastomotic complications, were collected by 2 independent researchers. The quality of the included articles was assessed based on the MINORS criteria. The 19 included studies presented data on 1192 esophagectomy patients, in 758 patients ICGA was used perioperative to guide esophageal reconstruction.
The 19 included studies for qualitative analyses all described ICGA as a safe and easy. AL occurred in 13.8% of the entire cohort, 10% in the ICG guided group and 20.6% in the control group (P < 0.001). When poorly perfused cases are excluded from the analyses, the difference in AL was even larger (AL well-perfused group 6.3% vs control group 20.5%, P < 0.001). The AL rate in the group with an altered surgical plan based on the ICG image was 6.5%, similar to the well perfused group (6.3%) and significantly less than the poorly perfused group (47.8%) (P < 0.001), suggesting that the technique is able to identify and alter a potential bad outcome.
ICGA is a safe, feasible and promising method for perfusion assessment. Based on this review a bolus dose of 2.5 mg is a sufficient optimal dose for visualization of esophagogastric anastomotic perfusion. The differences in AL rate between the well perfused and poor perfused AS clearly suggest that a good fluorescent signal is a predictor of good outcome. The AL rate in the group with an altered surgical plan based on the ICG image was similar to the well perfused group and significantly less than the poorly perfused group, suggesting that the technique is able to predict and remedy a potentially worse outcome.
A few authors have attempted to quantify the method, but rarely in oesophageal surgery and without comparison with a golden standard method, stressing the need for objective quantification of the ICGA with validated cutoff levels for sufficient graft perfusion in esophageal surgery. No studies mentioned validation of the method. Therefore, we propose a clinical study that validates imaging-based perfusion assessment of the stomach graft using tissue, serum, and cellular hallmarks of hypo-perfusion and hypoxia during esophagectomy. The study is registered in Clinicaltrials.gov as NCT03587532 and is currently recruiting. This easy and safe new technique of perioperative perfusion assessment has the potential to reduce AL rate and its associated mortality, but randomized trials are needed to confirm these retrospective results. At present, there are no ongoing randomized trials listed on clinical trial.gov. Potentially because a prospective randomized study comparing ICGA guided surgery versus non ICGA guided surgery implies a large sample size demanding a large multicentre study.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mastoraki A, Senchukova MA S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | International Agency for Research on Cancer, WHO. Cancer Statistics, GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from: URL: http://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf. |
| 2. | Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 3. | Biere SS, Maas KW, Cuesta MA, van der Peet DL. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg. 2011;28:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Sauvanet A, Mariette C, Thomas P, Lozac'h P, Segol P, Tiret E, Delpero JR, Collet D, Leborgne J, Pradère B, Bourgeon A, Triboulet JP. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Sunpaweravong S, Ruangsin S, Laohawiriyakamol S, Mahattanobon S, Geater A. Prediction of major postoperative complications and survival for locally advanced esophageal carcinoma patients. Asian J Surg. 2012;35:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M. Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg. 2011;35:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012;19:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Van Daele E, Van de Putte D, Ceelen W, Van Nieuwenhove Y, Pattyn P. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy†. Interact Cardiovasc Thorac Surg. 2016;22:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Wright CD, Kucharczuk JC, O'Brien SM, Grab JD, Allen MS; Society of Thoracic Surgeons General Thoracic Surgery Database. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587-595; discussion 596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Junemann-Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg. 2005;27:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Markar SR, Arya S, Karthikesalingam A, Hanna GB. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol. 2013;20:4274-4281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Milstein DM, Ince C, Gisbertz SS, Boateng KB, Geerts BF, Hollmann MW, van Berge Henegouwen MI, Veelo DP. Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine (Baltimore). 2016;95:e3875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Linder G, Hedberg J, Björck M, Sundbom M. Perfusion of the gastric conduit during esophagectomy. Dis Esophagus. 2017;30:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Pham TH, Perry KA, Enestvedt CK, Gareau D, Dolan JP, Sheppard BC, Jacques SL, Hunter JG. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. Ann Thorac Surg. 2011;91:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Tsekov C, Belyaev O, Tcholakov O, Tcherveniakov A. Intraoperative Doppler assessment of gastric tube perfusion in esophagogastroplasty. J Surg Res. 2006;132:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 882] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 17. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5636] [Article Influence: 256.2] [Reference Citation Analysis (0)] |
| 18. | Kubota K, Yoshida M, Kuroda J, Okada A, Ohta K, Kitajima M. Application of the HyperEye Medical System for esophageal cancer surgery: a preliminary report. Surg Today. 2013;43:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Murawa D, Hünerbein M, Spychała A, Nowaczyk P, Połom K, Murawa P. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chir Belg. 2012;112:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Pacheco PE, Hill SM, Henriques SM, Paulsen JK, Anderson RC. The novel use of intraoperative laser-induced fluorescence of indocyanine green tissue angiography for evaluation of the gastric conduit in esophageal reconstructive surgery. Am J Surg. 2013;205:349-352; discussion 352-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Kumagai Y, Ishiguro T, Haga N, Kuwabara K, Kawano T, Ishida H. Hemodynamics of the reconstructed gastric tube during esophagectomy: assessment of outcomes with indocyanine green fluorescence. World J Surg. 2014;38:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Rino Y, Yukawa N, Sato T, Yamamoto N, Tamagawa H, Hasegawa S, Oshima T, Yoshikawa T, Masuda M, Imada T. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med Imaging. 2014;14:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Sarkaria IS, Bains MS, Finley DJ, Adusumilli PS, Huang J, Rusch VW, Jones DR, Rizk NP. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila). 2014;9:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Kamiya K, Unno N, Miyazaki S, Sano M, Kikuchi H, Hiramatsu Y, Ohta M, Yamatodani T, Mineta H, Konno H. Quantitative assessment of the free jejunal graft perfusion. J Surg Res. 2015;194:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Hodari A, Park KU, Lace B, Tsiouris A, Hammoud Z. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg. 2015;100:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Campbell C, Reames MK, Robinson M, Symanowski J, Salo JC. Conduit Vascular Evaluation is Associated with Reduction in Anastomotic Leak After Esophagectomy. J Gastrointest Surg. 2015;19:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Yukaya T, Saeki H, Kasagi Y, Nakashima Y, Ando K, Imamura Y, Ohgaki K, Oki E, Morita M, Maehara Y. Indocyanine Green Fluorescence Angiography for Quantitative Evaluation of Gastric Tube Perfusion in Patients Undergoing Esophagectomy. J Am Coll Surg. 2015;221:e37-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA, DeMeester TR. Intraoperative Assessment of Perfusion of the Gastric Graft and Correlation With Anastomotic Leaks After Esophagectomy. Ann Surg. 2015;262:74-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Kitagawa H, Namikawa T, Munekage M, Akimori T, Kobayashi M, Hanazaki K. Visualization of the Stomach's Arterial Networks During Esophageal Surgery Using the HyperEye Medical System. Anticancer Res. 2015;35:6201-6205. [PubMed] |
| 30. | Koyanagi K, Ozawa S, Oguma J, Kazuno A, Yamazaki Y, Ninomiya Y, Ochiai H, Tachimori Y. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore). 2016;95:e4386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Nakashima Y, Saeki H, Yukaya T, Tsutsumi S, Nakanishi R, Sugiyama M, Ohgaki K, Sonoda H, Oki E, Maehara Y. Blood Flow Assessment with Indocyanine Green Fluorescence Angiography for Pedicled Omental Flap on Cervical Esophagogastric Anastomosis after Esophagectomy. J Am Coll Surg. 2016;222:e67-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Ohi M, Saigusa S, Toiyama Y, Ichikawa T, Shimura T, Yasuda H, Okita Y, Yoshiyama S, Kobayashi M, Araki T, Inoue Y, Mohri Y, Kusunoki M. Evaluation of Blood Flow with Indocyanine Green-Guided Imaging to Determine Optimal Site for Gastric Conduit Anastomosis to Prevent Anastomotic Leak after Esophagectomy. Am Surg. 2017;83:e197-e199. [PubMed] |
| 33. | Schlottmann F, Patti MG. Evaluation of Gastric Conduit Perfusion During Esophagectomy with Indocyanine Green Fluorescence Imaging. J Laparoendosc Adv Surg Tech A. 2017;27:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Kitagawa H, Namikawa T, Iwabu J, Fujisawa K, Uemura S, Tsuda S, Hanazaki K. Assessment of the blood supply using the indocyanine green fluorescence method and postoperative endoscopic evaluation of anastomosis of the gastric tube during esophagectomy. Surg Endosc. 2018;32:1749-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Ohi M, Toiyama Y, Mohri Y, Saigusa S, Ichikawa T, Shimura T, Yasuda H, Okita Y, Yoshiyama S, Kobayashi M, Araki T, Inoue Y, Kusunoki M. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus. 2017;14:351-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Karampinis I, Ronellenfitsch U, Mertens C, Gerken A, Hetjens S, Post S, Kienle P, Nowak K. Indocyanine green tissue angiography affects anastomotic leakage after esophagectomy. A retrospective, case-control study. Int J Surg. 2017;48:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Noma K, Shirakawa Y, Kanaya N, Okada T, Maeda N, Ninomiya T, Tanabe S, Sakurama K, Fujiwara T. Visualized Evaluation of Blood Flow to the Gastric Conduit and Complications in Esophageal Reconstruction. J Am Coll Surg. 2018;226:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Dalton BGA, Ali AA, Crandall M, Awad ZT. Near infrared perfusion assessment of gastric conduit during minimally invasive Ivor Lewis esophagectomy. Am J Surg. 2018;216:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Kumagai Y, Hatano S, Sobajima J, Ishiguro T, Fukuchi M, Ishibashi KI, Mochiki E, Nakajima Y, Ishida H. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Shimada Y, Okumura T, Nagata T, Sawada S, Matsui K, Hori R, Yoshioka I, Yoshida T, Osada R, Tsukada K. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J. Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg. 2014;259:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Diana M, Halvax P, Dallemagne B, Nagao Y, Diemunsch P, Charles AL, Agnus V, Soler L, Demartines N, Lindner V, Geny B, Marescaux J. Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc. 2014;28:3108-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J. Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg. 2015;102:e169-e176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, Mieog JSD. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol. 2018;118:283-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 45. | St Lawrence K, Verdecchia K, Elliott J, Tichauer K, Diop M, Hoffman L, Lee TY. Kinetic model optimization for characterizing tumour physiology by dynamic contrast-enhanced near-infrared spectroscopy. Phys Med Biol. 2013;58:1591-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Milej D, Abdalmalak A, Desjardins L, Ahmed H, Lee TY, Diop M, Lawrence KS. Quantification of blood-brain barrier permeability by dynamic contrast-enhanced NIRS. Sci Rep. 2017;7:1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Van Daele E, Van Nieuwenhove Y, Ceelen W, Vanhove C, Braeckman BP, Hoorens A, Van Limmen J, Varin O, Van de Putte D, Willaert W, Pattyn P. Assessment of graft perfusion and oxygenation for improved outcome in esophageal cancer surgery: Protocol for a single-center prospective observational study. Medicine (Baltimore). 2018;97:e12073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |