Published online Nov 15, 2019. doi: 10.4251/wjgo.v11.i11.946
Peer-review started: February 22, 2019
First decision: July 31, 2019
Revised: August 18, 2019
Accepted: September 26, 2019
Article in press: September 26, 2019
Published online: November 15, 2019
Processing time: 267 Days and 21.5 Hours
The dependence of tumor growth on neovascularization has become an important aspect of cancer biology. Tumor angiogenesis is one of the key mechanisms of tumorigenesis, growth and metastasis. The key events involved in this process are endothelial cell proliferation, migration, and vascular formation. Recent studies have revealed the importance of tumor-associated endothelial cells (TECs) in the development and progression of colorectal cancer (CRC), including epithelial proliferation, stem cell maintenance, angiogenesis, and immune remodeling. Decades of research have identified that the molecular basis of tumor angiogenesis includes vascular endothelial growth factors (VEGFs) and their receptor family, which are the main targets of antiangiogenesis therapy. VEGFs and their receptors play key roles in the pathology of angiogenesis, and their overexpression indicates poor prognosis in CRC. This article reviews the characteristics of the tumor vasculature and the role of TECs in different stages of CRC and immune remodeling. We also discuss the biological effects of VEGFs and their receptor family as angiogenesis regulators and emphasize the clinical implications of TECs in clinical treatment.
Core tip: In 1971, Folkman highlighted the importance of angiogenesis in tumor growth. The key events involved in this process are endothelial cell proliferation, migration, and vascular formation. Recent studies have revealed the importance of tumor-associated endothelial cells (TECs) in the development and progression of colorectal cancer (CRC). However, few systematically reviewed the role of TECs in CRC. Our objective is to compare the characteristics of normal endothelial cells and TECs, review the role of TECs in the stages of CRC, and discuss the possibility of TECs to serve as a biomarker to predict the prognosis and a potential therapeutic target.
- Citation: Chen WZ, Jiang JX, Yu XY, Xia WJ, Yu PX, Wang K, Zhao ZY, Chen ZG. Endothelial cells in colorectal cancer. World J Gastrointest Oncol 2019; 11(11): 946-956
- URL: https://www.wjgnet.com/1948-5204/full/v11/i11/946.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i11.946
Colorectal cancer (CRC) is the leading cause of cancer death worldwide and is the second most common cancer in women and the third most common cancer in men[1,2]. A considerable number of patients have died from metastatic diseases, even after systemic therapy[3,4]. Although new drugs and treatments have emerged in recent years, the median overall survival of metastatic CRC remains less than 2 years[5].
In 1971, Folkman[6] highlighted the importance of angiogenesis in tumor growth. Targeting tumor blood vessels has been recognized as a promising strategy for cancer therapy. The tumor blood vessel unit includes endothelial cells, pericytes, endothelial progenitor cells, smooth muscle cells and extracellular matrix. Tumor blood vessels provide a vector to transport nutrition and oxygen and clear metabolite waste in tumor tissues. They also serve as gatekeepers to facilitate the metastasis of tumor cells[7]. The angiogenesis process is highly complex and dynamic and includes recruiting germinated blood vessels from existing blood vessels and incorporating endothelial progenitor cells into the growing vascular bed[8]. The main pathway involved in this process includes vascular endothelial growth factor (VEGF) and its receptor family (VEGF receptor, VEGFR)[9,10]. Studies have shown that the VEGF family is upregulated in the tumor vasculature and can activate downstream Akt signaling[10-12]. More importantly, the VEGF family promotes the transduction of tumor cells into endothelial cells[13]. Tumor-associated endothelial cells (TECs) could cause local immune remodeling. TECs promote tumor evasion by suppressing the response to inflammatory activation and downregulating leukocyte adhesion molecules[14]. VEGF overexpression has been proved to be associated with tumor progression and poor prognosis in CRC[15].
In this article, we compare the characteristics of normal endothelial cells (NECs) and TECs, review the role of TECs in the stages of carcinogenesis development and metastasis in CRC and highlight their effects on immune remodeling. We also discuss the biological effects of the VEGF/VEGFR family as angiogenesis regulators and emphasize the clinical implications of TECs in CRC treatment.
Endothelial cells line the innermost layer of the blood and lymphatic vessels and form a selectively permeable exchange barrier between the blood and tissue[16]. In normal tissues, endothelial cells arise from blast-like bipotential cells[17]. They form a uniform and continuous cell monolayer with few cytoplasmic processes. Endothelial cells are involved in a number of physiological processes, including the regulation of vasomotor tension, permeability, vascular tone and blood vessel growth, the transport of cells and nutrients, the maintenance of blood flow[18] and the quiescence of the inflammatory response[19]. These physiological functions of the endothelium are indispensable for body homeostasis. The dysfunction and activation of the endothelial cell response could cause many diseases, including peripheral vascular disease, stroke, heart disease, diabetes, insulin resistance, chronic kidney failure, tumor growth, metastasis, and venous thrombosis[20,21].
In contrast to NECs, TECs originate not only from endothelial progenitor cells but also from the differentiation of cancer stem cells (CSCs). It has been reported that poorly differentiated HCT116 CRC cells can be converted into TECs under specific hypoxia conditions via VEGFR-2[22]. In addition, similar somatic mutations are found in both ECs and tumor cells. This evidence indicates that TECs may be derived from tumor cells. Moreover, it has been demonstrated that CSCs may also generate pericytes to support vessel function and tumor growth[23]. Compared to NECs, TECs have abnormal structural and functional characteristics. In tumor tissues, tumor vessels often have a tortuous appearance, with uneven blood vessel diameters due to immature vessel wall compression by tumor cells[24]. Abnormalities in tumor blood vessels are attributed to paramorphic TECs and unbalanced expression of angiogenic factors and inhibitors. Irregular TECs with fragile cytoplasmic protrusions could penetrate the vessel lumen and create openings on the vessel wall[24]. Therefore, TECs do not form a regular single layer and have normal barrier function[25]. Pericytes are also present on TECs, which physically surround the blood vessels, establish tight junctions with ECs and participate in the regulation of ECs survival. However, tumor polarized pericytes form impaired electronic coupling and abnormally loose associations with TECs[26]. Basement membrane of tumor vessels also has a loose association with ECs, and consists of abnormal focal holes. Owing to these abnormal structure of tumor vascular vessels, tumor cells could infiltrate through the blood vessels, which is the first step in metastasis[27]. Due to the extravasation of intravascular fluids and plasma proteins, a remarkable increase occurs in the interstitial tissue pressure[28]. High interstitial fluid pressure impair endothelial monolayer function, causes the blood vessels to collapse and reduces blood flow, resulting in the irregular blood flow or even no perfusion in tumor vessels[29]. In addition, tumor blood vessels are unevenly distributed in tissues, and there are many areas where blood vessels are insufficiently supplied, resulting in a local hypoxic environment[30]. Hypoxia then promotes angiogenesis by supporting the expression of multiple angiogenic factors via hypoxia inducible factor (HIF) activation, which could contribute to tumor growth and metastasis[31,32]. The angiogenic growth factors triggered by insufficient local perfusion and chronic hypoxia in tumor tissue can also result in reduced leukocytes recruitment, a lack of immune activation and resistance to chemotherapy and radiotherapy, which is called “endothelial cell energy”[14]. In a mouse model of human LS174T colon carcinoma, researchers found that leukocyte adhesion was diminished in vessels inside the tumor, which was related to lower intercellular adhesion molecule 1 (ICAM1) expression on TECs, while anti-VEGF antibody prevented rescued this result[33].
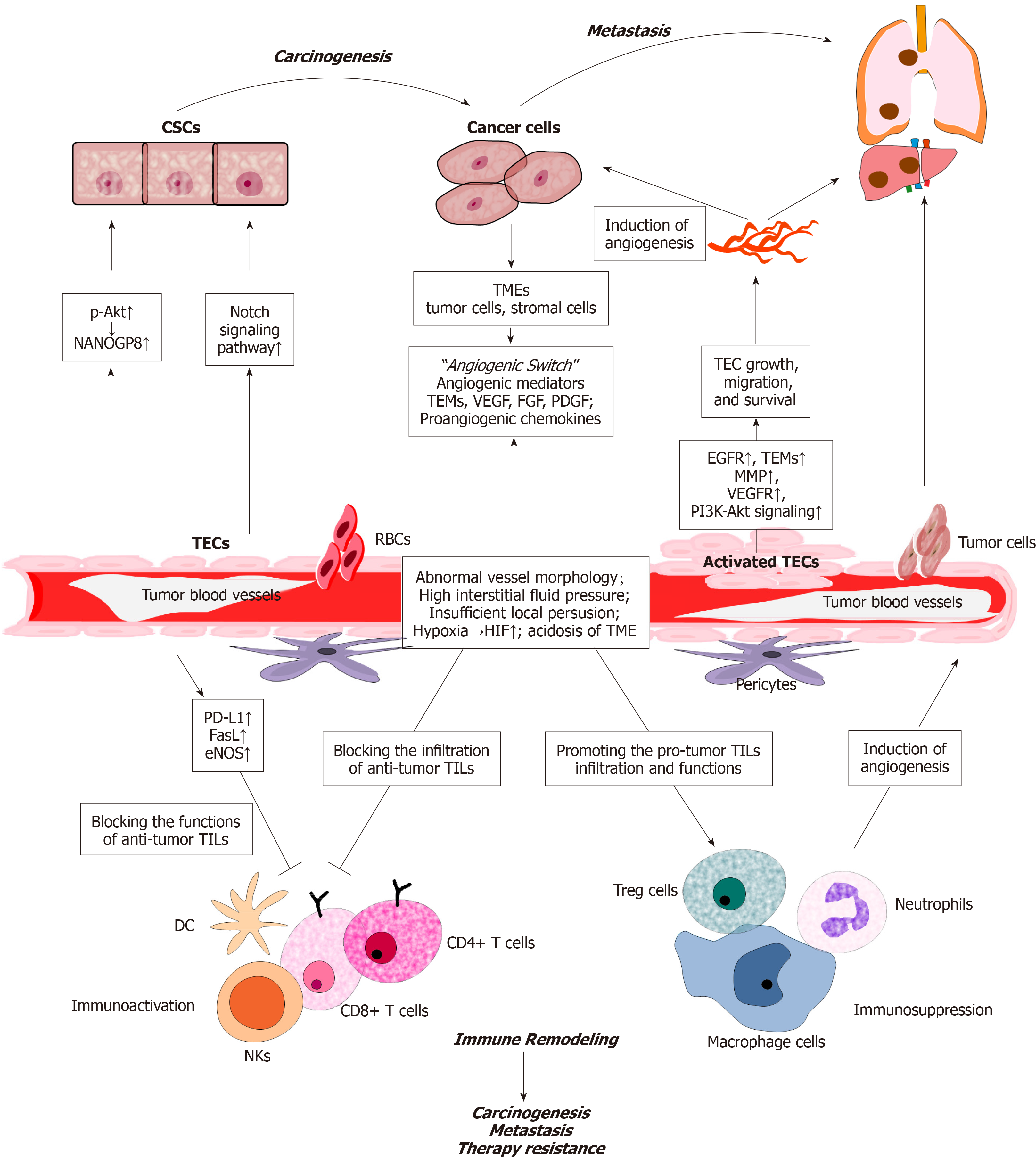
Heterogeneity can be found in the cell morphology, function and gene expression of TECs. A study from Japan suggested that phenotypic heterogeneity may be induced by different tumor microenvironment (TMEs). The researchers isolated two types of TECs from high-metastatic (HM) and low-metastatic (LM) tumors, and compared their characteristics. The HM-TECs were more proliferative, motile, sensitive to VEGF, and invasive to the ECM than the LM-TECs. In addition, HM-TECs showed upregulation of the angiogenesis-related genes VEGFR-1, VEGFR-2 and VEGF. Akt phosphorylation levels were higher in HM-TECs than in LMTECs. The researchers also reported that a Stem-like phenotype existed in HM-TECs[34]. In addition, a recent study of human patients with CRC investigated the impact on TEC heterogeneity in different TME and discovered that TEC heterogeneity is regulated by SPARCL1. SPARCL1 in TECs promotes cell quiescence and vessel homeostasis, thus contributing to a favorable prognosis[35]. Recent studies have revealed the importance of TECs in the different stages of CRC, including carcinogenesis, development, metastasis, and immune remodeling (Figure 1).
CRC carcinogenesis is affected by many genetic and environmental factors[36]. Many studies have shown that CSCs exist in CRC. Although they account for a small proportion, CSCs play an important role in carcinogenesis, development and metastasis. These cells not only initiate and maintain tumor growth but also mediate chemical resistance and promote CRC recurrence[37,38]. TECs can release soluble factors by paracrine action to increase the CSC ratio, clonal sphere formation, tumorigenicity, and chemical resistance. These effects are caused by the activation of Notch signaling in CRC cells and changes in the CSC phenotype[39]. In addition, endothelial cells can regulate the CSC phenotype and chemoresistance via the Akt-mediated induction of the NANOGP8 pathway in a paracrine fashion[40].
Angiogenesis, a process guided by proliferation of TECs, is also a key factor in carcinogenesis. For one thing, abundant blood vessels can provide nutrients and oxygen for tumor cells and remove toxic metabolic products. Besides, proliferative factors such as RAS signaling also participate in the initiation of neovascularization as well as TME[41]. A significant increase in VEGF and microvessel density was found in the colon pre-malignant lesions such as adenoma with dysplasia[42,43]. The VEGF-related gene family of angiogenic and lymphangiogenic growth factors comprises six secreted glycoproteins called VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and placenta growth factors (PlGF)-1 and PlGF-2[44-46]. VEGFs mediate multiple functions via the stimulation of cognate receptors on TECs. TECs were found to overexpress specific genes, such as VEGFR, tumor endothelium markers (TEMs) and EGFR, thereby promoting angiogenesis and carcinogenesis. TEMs are specific genes of TECs that are involved in tumor-specific angiogenesis[47], and they include TEM1, TEM5, TEM7, TEM7R and TEM8[48]. Moreover, in several experimental models, the expression of VEGF and VEGFR were associated with the progression of adenoma, indicating that both of them could be targeted to reduce the risk of carcinogenesis[49,50].
The development of neo-vasculature promoting tumor growth is now a well-known aspect of cancer biology. The vital regulator of this process is VEGF, which is overexpressed in approximate 40% to 60% of CRC cases and correlated with disease progression. As we have described above, hypoxia is developed in the solid tumor and further induce the activation of HIF1, which increases the expression of pro-angiogenic factors such as VEGF-A, angiopoietin2 and CXCL12[51]. Activation of the VEGF receptor and CXCL12-CXCR4 pathway polarizes the TME towards more pro-angiogenic state[52]. Besides immunosuppressive cells such as M2-macrophages, myeloid derived suppressor cells, granulocytes, cancer-associated fibroblasts and pericytes are polarized as well and play crucial role in angiogenesis and tumor development. Moreover, VEGFs play an important role in the mobilization of endothelial progenitor cells from the bone marrow to distant neovascularization sites. VEGFs could also enhance vascular permeability and is involved in the angiogenetic function of platelet-derived growth factor (PDGF) family in several model systems[53].
In addition to angiogenesis, TECs have been demonstrated to promote cancer cell growth and chemo-resistance by releasing soluble factors via a paracrine way in multiple solid tumors such as lung cancer and gastric cancer, which is referred as “angiocrine”[54,55]. Angiocrine factors mainly consist of growing factors such as VEGF-A, PGF, PDGF, Jagged 1/2 and NO, and adhesion molecules such as E-selectins, ICAM1 and vascular cell adhesion molecule 1 (VCAM-1). A recent study used a transgenic mouse model (EndoIRKO-Min) to detect the role of insulin in TECs during tumor development[56]. The authors suggested that insulin decreased the expression of VCAM-1 and leukocyte adhesion in TECs with intact insulin receptors. Knocking out insulin receptors in endothelial cells promotes intestinal tumor formation. Human hepatic sinusoidal endothelial cells could secrete macrophage migration inhibitory factor, to promote the chemotaxis and outgrowth of CRC in liver pro-metastasis.
The formation of remote CRC metastasis is the main cause of death in patients. A great of researches have shown that TECs play critical role in CRC metastasis. TECs in metastatic tumors express higher levels of vascular secretion factors than TECs in non-metastatic tumors, suggesting that TECs may affect the behavior of tumor cells[57]. This phenomenon is further confirmed by the result that TEC apoptosis could inhibit liver metastasis and improve the survival rate of a liver metastasis CRC animal model[58]. TEC proliferation, migration and differentiation are the key steps during the neovascularization and spreading of CRC cells promoted by tumor-derived interleukin-33 (IL-33) in the liver[59,60]. Blocking IL-33 signaling could inhibit angiogenesis and reduce liver metastasis[59,60]. TECs could also promote the formation of CRC-CSCs by secreting Jagged-1, which activates NOTCH signaling in tumor cells and serves as an important factor in CRC metastasis[39,61-63]. It has also been reported that miR-497 can inhibit CRC metastasis and invasion in vitro and in vivo by mediating the VEGF-A/ERK/MMP-9 signaling pathway[64]. In addition, VEGF-C signaling via VEGFR3 promotes lymphangiogenesis and metastasis by orthotopic colorectal tumors in mice and reduces lymphatic endothelial barrier integrity[65].
TECs could promote the transendothelial migration of CRC cells by expressing E-selectin. Studies have shown that in mouse models with E-selectin overexpression in the liver, more metastases are observed in the liver than at other sites, suggesting that E-selectin present on activated TECs promotes tumor cell metastasis[66,67]. The binding of death receptor-3 (DR-3) to E-selectin could activate the mitogen-activated protein kinases p38 and ERK, thus inducing signaling in endothelial cells and increasing endothelial permeability, which allows the trans-endothelial migration of cancer cells[68,69]. The binding of human CRC cells to E-selectin-expressing TECs or a recombinant E-selectin/Fc chimera leads to the activation of SAPK2/p38, and blocking the activation of SAPK2/p38 in these CRC cells inhibits their transendothelial migration[70]. In addition, the selectin-mediated interaction between circulating tumor cells with blood components such as platelets and leukocytes causes endothelial cell activation, induces C-C chemokine ligand 5 (CCL5) production and promotes metastasis[71,72]. A study found that an anti-P-selectin monoclonal antibody inhibited the metastasis of gastric cancer in mouse models without adversely affecting immune function[73]. The use of glycometabolic inhibitors to inhibit the O-glycosylation of mucin and fucosyltransferase, which indirectly reduced the production of selectin ligands, suggests that cancer cell metastasis is attenuated after treatment with glycometabolic inhibitors[74-76].
TECs can remodel the local immune system and help tumor cells escape immunity in many ways. NECs can express adhesion molecules to promote peripheral leukocyte capture and release chemokines to facilitate leukocyte migration into tissues[77]. Integrins bind to immunoglobulin superfamily members on endothelial cells, resulting in the adhesion of leukocytes to endothelial cells[78]. TECs lack a response to inflammatory activation (endothelial anergy) and downregulate leukocyte adhesion molecules. TECs can also inhibit the chemotaxis and activation of immune cells and regulate the expression of immune co-stimulatory and inhibitory molecules to promote immune tolerance[6,79]. TECs also show increased expression of PD-L1, which in turn can bind to PD-1 in activated lymphocytes to inhibit T cell activation[80-82]. In addition, FasL expression in TECs promotes their ability to kill effector CD8+ T cells, causing TEC-associated immune cell death and enhanced tumor escape[83,84]. Activated or damaged endothelial cells could release E-selectin, which is chemotactic to neutrophils and contributes to the migration of neutrophils[85,86]. Leukocytes then migrate out of the blood vessels and into the tissue through the endothelial cell layer and underlying basement membrane. The maturation and normalization of tumor blood vessels restore the potential of tumor endothelial cells to recruit and direct circulating immune cells into tumor tissues[87,88].
Researches have proved that angiogenesis inhibitors can promote tumor vascular stability and vascular bed normalization, as well as restore immune surveillance[89,90]. Endothelial cell activation can usually be induced by a variety of factors, including circulating inflammatory cytokines, such as tumor necrosis factor and IL, and reactive oxygen species[87,91,92]. Antiangiogenic therapy corrects structural abnormalities and dysfunction in tumor blood vessels[24,93,94], promotes lymphocyte infiltration and enhanced cytotoxic T cell activity, thus reflecting the successful activation of anti-tumor T cell immunity[95,96]. In addition, antiangiogenic therapy enhanced the efficacy of anti-PD-L1 therapy in preclinical studies using mouse breast and pancreatic neuroendocrine tumor models, and anti-PD-L1 therapy sensitized tumors to antiangiogenic therapy and prolonged their efficacy as well[95].
Tumor cells produce several angiogenic factors that promote neovascularization, including VEGF, basic fibroblast growth factor, angiopoietins, hepatocyte growth factor, chemokines, and PDGF[97]. As we have reviewed above, VEGF is the most important identified regulator of angiogenesis, and its expression is significantly increased in metastatic colorectal tumors[18]. The VEGF-VEGFR axis consists of multiple ligands and receptors with different and overlapping ligand-receptor binding specificities. The VEGF family is consisting of VEGFA, VEGFB, VEGFC, VEGFD and PGF, among which VEGFA is studied more than other members and is usually simplified as VEGF. VEGFRs are ligands binding to the tyrosine kinases (RTKs) of these VEGFs, such as VEGFR1, VEGFR2 and VEGFR3. VEGF-VEGFR activation can trigger a network of signaling processes that promote endothelial cell survival, growth and migration from the pre-existing vasculature. In addition, VEGFs expression are related to increased vascular density, mediate vascular permeability and are associated with malignant exudation[98,99]. Thus, the role of VEGF in promoting tumor pathogenesis and angiogenesis has led to the development of agents that selectively target the VEGF-VEGFR axis. Studies of various anti-VEGF/VEGFR therapies have shown that these drugs can effectively inhibit angiogenesis and tumor growth in preclinical models. Recently, several anticancer therapies, such as neutralizing antibodies to VEGF, low molecular weight VEGFR tyrosine kinase inhibitors, and soluble VEGF constructs (VEGF-Trap), have been developed to block this factor[100,101]. Bevacizumab, a recombinant humanized monoclonal IgG1 antibody that neutralizes VEGF-A, has been shown to increase survival in stage IV CRC patients in combination with chemotherapy[36,102,103]. Aflibercept is a fully humanized recombinant fusion protein consisting of a VEGF-binding segment (VEGFR-1 and VEGFR-2) fused to the Fc segment of human immunoglobulin G1. This fusion protein has been associated with survival benefits in metastatic CRC patients when combined with FOLFIRI chemotherapy[104]. Regorafenib, an orally active inhibitor of angiogenic tyrosine kinases (VEGFR-1 and VEGFR-3), appears to be active in patients with metastatic CRC who experience disease progression after standard treatment[63,105].
Progressive survival and overall survival could be marginally improved when metastatic CRC patients receive an antiangiogenic mediator combined with chemotherapy[106]. There are no clear clinical or biological tools available to select patients who may benefit from VEGF pathway inhibitors or to exclude those who may experience specific adverse events[107,108]. Current evidence suggests that some specific markers in the peripheral blood have some predictive value, such as increased VEGF expression, decreased circulating endothelial cells, decreased VEGFR-2 expression, KRAS and BRAF mutations, polymorphisms in VEGF pathway components, and microvascular density[62]. Circulating endothelial cells (CECs) have been reported to serve as a potential surrogate biomarker for angiogenesis. A study including 140 patients with metastatic CRC suggested that CECs were independent predictors of poor survival (HR = 1.81; P = 0.03)[109]. The clinical value of CECs and their subpopulations [total CECs (tCECs) and resting CECs (rCECs)] as biomarkers in antiangiogenic therapy has been introduced[101]. Patients who achieved a radiological response showed a significant decrease in rCECs and a decreasing trend for tCECs in comparison with patients not achieving a response[110]. However, the vascular structures of specific cancers at different clinical stages and under different treatment regimens are still unknown[62,111].
Despite the limitations of our review, TECs have been shown to have positive effects on the development of tumor cells and play a key role in cancer progression. TECs play an important role in the carcinogenesis, development and metastasis of CRC and participate in TME immune remodeling. Studies on the molecular events and animal models of TECs have increased our understanding of TEC-related signaling pathways and cellular biology. Clinically, antiangiogenic (anti-VEGF) therapy for metastatic CRC has become the standard therapy. Despite the increasing evidence that has been reported in this field, antiangiogenic therapy resistance is still a challenging issue. Several mechanisms can account for this therapeutic failure, such as the heterogeneity of TECs. Understanding the interaction between TECs and the TME would help to improve existing treatment options and discover new molecular targets. How to identify TEC-specific biological targets for anti-angiogenesis therapy in clinical decision-making remains a challenge in the near future. In addition, it is necessary to further understand the VEGF-VEGFR family and its role in tumor angiogenesis. For example, is there a certain VEGF-VEGFR pair more important than others in tumor angiogenesis? Which VEGF-VEGFRs are the key for targeting TECs in tumor angiogenesis? What is the role of VEGF-VEGFRs in tumor cell growth and survival? We await in-depth studies to answer these questions. A next step would be to design biomarker-based anti-VEGF trials for identifying patients who could benefit from this therapy and to develop new treatment strategies to overcome anti-VEGF resistance. A comprehensive and continuous understanding of the biological effects of TECs and VEGF-VEGFRs in angiogenesis remains challenging, but there is potential for promising breakthroughs.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jimenez Rodriguez RM, Vynios D S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Stewart BW, Wild CP. World Cancer Report 2014. World Health Organization. 2014; Available from: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1codcol=76codcch=31. |
| 3. | Cancer Facts and figures 2013. American Cancer Society Atlanta 2010: 1-34 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2013.html. |
| 4. | Grothey A. EGFR antibodies in colorectal cancer: where do they belong? J Clin Oncol. 2010;28:4668-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Davies JM, Goldberg RM. First-line therapeutic strategies in metastatic colorectal cancer. Oncology (Williston Park). 2008;22:1470-1479. [PubMed] |
| 6. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5899] [Article Influence: 109.2] [Reference Citation Analysis (1)] |
| 7. | Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1632] [Cited by in RCA: 1660] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 8. | Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 488] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Diaz LA, Coughlin CM, Weil SC, Fishel J, Gounder MM, Lawrence S, Azad N, O'Shannessy DJ, Grasso L, Wustner J, Ebel W, Carvajal RD. A first-in-human phase I study of MORAb-004, a monoclonal antibody to endosialin in patients with advanced solid tumors. Clin Cancer Res. 2015;21:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Kim DH, Sung B, Kang YJ, Hwang SY, Kim MJ, Yoon JH, Im E, Kim ND. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells. Int J Oncol. 2015;47:2226-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, St Croix B, Kinzler KW, Huso DL. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci U S A. 2006;103:3351-3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Wang R, Bhattacharya R, Ye X, Fan F, Boulbes DR, Ellis LM. Endothelial Cells Promote Colorectal Cancer Cell Survival by Activating the HER3-AKT Pathway in a Paracrine Fashion. Mol Cancer Res. 2019;17:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Giatromanolaki A, Sivridis E, Koukourakis MI. Angiogenesis in colorectal cancer: prognostic and therapeutic implications. Am J Clin Oncol. 2006;29:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Klein D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front Oncol. 2018;8:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Hamdollah Zadeh MA, Amin EM, Hoareau-Aveilla C, Domingo E, Symonds KE, Ye X, Heesom KJ, Salmon A, D'Silva O, Betteridge KB, Williams AC, Kerr DJ, Salmon AH, Oltean S, Midgley RS, Ladomery MR, Harper SJ, Varey AH, Bates DO. Alternative splicing of TIA-1 in human colon cancer regulates VEGF isoform expression, angiogenesis, tumour growth and bevacizumab resistance. Mol Oncol. 2015;9:167-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 701] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 17. | Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725-732. [PubMed] |
| 18. | Kawczyk-Krupka A, Kwiatek B, Czuba ZP, Mertas A, Latos W, Verwanger T, Krammer B, Sieroń A. Secretion of the angiogenic factor VEGF after photodynamic therapy with ALA under hypoxia-like conditions in colon cancer cells. Photodiagnosis Photodyn Ther. 2018;21:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Steyers CM, Miller FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324-11349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 20. | Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 352] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1007] [Cited by in RCA: 1034] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 22. | Liu Z, Qi L, Li Y, Zhao X, Sun B. VEGFR2 regulates endothelial differentiation of colon cancer cells. BMC Cancer. 2017;17:593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 683] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 24. | Hida K, Maishi N, Torii C, Hida Y. Tumor angiogenesis--characteristics of tumor endothelial cells. Int J Clin Oncol. 2016;21:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1170] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 26. | Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 545] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608-14613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 461] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1204] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 29. | McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381-5385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4319] [Cited by in RCA: 3960] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 31. | Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89:3362-3366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 33. | Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322-2329. [PubMed] |
| 34. | Hida K, Maishi N, Annan DA, Hida Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 35. | Naschberger E, Liebl A, Schellerer VS, Schütz M, Britzen-Laurent N, Kölbel P, Schaal U, Haep L, Regensburger D, Wittmann T, Klein-Hitpass L, Rau TT, Dietel B, Méniel VS, Clarke AR, Merkel S, Croner RS, Hohenberger W, Stürzl M. Matricellular protein SPARCL1 regulates tumor microenvironment-dependent endothelial cell heterogeneity in colorectal carcinoma. J Clin Invest. 2016;126:4187-4204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Mathonnet M, Perraud A, Christou N, Akil H, Melin C, Battu S, Jauberteau MO, Denizot Y. Hallmarks in colorectal cancer: angiogenesis and cancer stem-like cells. World J Gastroenterol. 2014;20:4189-4196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 817] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 38. | Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 39. | Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, Hawke DH, Rak J, Mani SA, Zweidler-McKay P, Ellis LM. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 40. | Kong DH, Kim MR, Jang JH, Na HJ, Lee S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 653] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 42. | Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050-6054. [PubMed] |
| 43. | Staton CA, Chetwood AS, Cameron IC, Cross SS, Brown NJ, Reed MW. The angiogenic switch occurs at the adenoma stage of the adenoma carcinoma sequence in colorectal cancer. Gut. 2007;56:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6948] [Article Influence: 315.8] [Reference Citation Analysis (0)] |
| 45. | Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 893] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 46. | Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947-11954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1378] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 48. | Mehran R, Nilsson M, Khajavi M, Du Z, Cascone T, Wu HK, Cortes A, Xu L, Zurita A, Schier R, Riedel B, El-Zein R, Heymach JV. Tumor endothelial markers define novel subsets of cancer-specific circulating endothelial cells associated with antitumor efficacy. Cancer Res. 2014;74:2731-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Korsisaari N, Kasman IM, Forrest WF, Pal N, Bai W, Fuh G, Peale FV, Smits R, Ferrara N. Inhibition of VEGF-A prevents the angiogenic switch and results in increased survival of Apc+/min mice. Proc Natl Acad Sci U S A. 2007;104:10625-10630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Goodlad RA, Ryan AJ, Wedge SR, Pyrah IT, Alferez D, Poulsom R, Smith NR, Mandir N, Watkins AJ, Wilkinson RW. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumor burden in the ApcMin/+ mouse model of early intestinal cancer. Carcinogenesis. 2006;27:2133-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 52. | Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16:35-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 53. | Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Park HJ, Kim BG, Lee SJ, Heo SH, Kim JY, Kwon TH, Lee EB, Ryoo HM, Cho JY. Proteomic profiling of endothelial cells in human lung cancer. J Proteome Res. 2008;7:1138-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Tokumoto MW, Tanaka H, Tauchi Y, Kasashima H, Kurata K, Yashiro M, Sakurai K, Toyokawa T, Kubo N, Amano R, Kimura K, Muguruma K, Maeda K, Ohira M, Hirakawa K. Identification of tumour-reactive lymphatic endothelial cells capable of inducing progression of gastric cancer. Br J Cancer. 2015;113:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Wang X, Häring MF, Rathjen T, Lockhart SM, Sørensen D, Ussar S, Rasmussen LM, Bertagnolli MM, Kahn CR, Rask-Madsen C. Insulin resistance in vascular endothelial cells promotes intestinal tumour formation. Oncogene. 2017;36:4987-4996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Ohga N, Ishikawa S, Maishi N, Akiyama K, Hida Y, Kawamoto T, Sadamoto Y, Osawa T, Yamamoto K, Kondoh M, Ohmura H, Shinohara N, Nonomura K, Shindoh M, Hida K. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am J Pathol. 2012;180:1294-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Ahn JH, Yu HK, Lee HJ, Hong SW, Kim SJ, Kim JS. Suppression of colorectal cancer liver metastasis by apolipoprotein(a) kringle V in a nude mouse model through the induction of apoptosis in tumor-associated endothelial cells. PLoS One. 2014;9:e93794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, Peña MM. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog. 2017;56:272-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Zumwalt TJ, Goel A. Immunotherapy of Metastatic Colorectal Cancer: Prevailing Challenges and New Perspectives. Curr Colorectal Cancer Rep. 2015;11:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123-10128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 765] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 62. | Custodio A, Barriuso J, de Castro J, Martínez-Marín V, Moreno V, Rodríguez-Salas N, Feliu J. Molecular markers to predict outcome to antiangiogenic therapies in colorectal cancer: current evidence and future perspectives. Cancer Treat Rev. 2013;39:908-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2114] [Article Influence: 176.2] [Reference Citation Analysis (0)] |
| 64. | Qiu Y, Yu H, Shi X, Xu K, Tang Q, Liang B, Hu S, Bao Y, Xu J, Cai J, Peng W, Cao Q, Yin P. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016;49:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Steinskog ES, Sagstad SJ, Wagner M, Karlsen TV, Yang N, Markhus CE, Yndestad S, Wiig H, Eikesdal HP. Impaired lymphatic function accelerates cancer growth. Oncotarget. 2016;7:45789-45802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Nübel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Gout S, Morin C, Houle F, Huot J. Death receptor-3, a new E-Selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Res. 2006;66:9117-9124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene. 2006;25:6563-6573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Laferriere J, Houle F, Taher MM, Valerie K, Huot J. Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. J Biol Chem. 2001;276:33762-33772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Läubli H, Spanaus KS, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114:4583-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 72. | Tözeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW. E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995;60:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Chen JL, Chen WX, Zhu JS, Chen NW, Zhou T, Yao M, Zhang DQ, Wu YL. Effect of P-selectin monoclonal antibody on metastasis of gastric cancer and immune function. World J Gastroenterol. 2003;9:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 75. | Brown JR, Fuster MM, Li R, Varki N, Glass CA, Esko JD. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin Cancer Res. 2006;12:2894-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Fuster MM, Brown JR, Wang L, Esko JD. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Res. 2003;63:2775-2781. [PubMed] |
| 77. | Tilki D, Kilic N, Sevinc S, Zywietz F, Stief CG, Ergun S. Zone-specific remodeling of tumor blood vessels affects tumor growth. Cancer. 2007;110:2347-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2044] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 79. | Ager A, Watson HA, Wehenkel SC, Mohammed RN. Homing to solid cancers: a vascular checkpoint in adoptive cell therapy using CAR T-cells. Biochem Soc Trans. 2016;44:377-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Bichsel CA, Wang L, Froment L, Berezowska S, Müller S, Dorn P, Marti TM, Peng RW, Geiser T, Schmid RA, Guenat OT, Hall SRR. Increased PD-L1 expression and IL-6 secretion characterize human lung tumor-derived perivascular-like cells that promote vascular leakage in a perfusable microvasculature model. Sci Rep. 2017;7:10636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Bagaria SP, Gatalica Z, Maney T, Serie D, Parasramka M, Attia S, Krishna M, Joseph RW. Association Between Programmed Death-Ligand 1 Expression and the Vascular Endothelial Growth Factor Pathway in Angiosarcoma. Front Oncol. 2018;8:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Jennewein L, Bartsch G, Gust K, Kvasnicka HM, Haferkamp A, Blaheta R, Mittelbronn M, Harter PN, Mani J. Increased tumor vascularization is associated with the amount of immune competent PD-1 positive cells in testicular germ cell tumors. Oncol Lett. 2018;15:9852-9860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 780] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 84. | Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 616] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 85. | Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543-549. [PubMed] |
| 86. | Pigott R, Dillon LP, Hemingway IH, Gearing AJ. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 463] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 87. | Weisshardt P, Trarbach T, Dürig J, Paul A, Reis H, Tilki D, Miroschnik I, Ergün S, Klein D. Tumor vessel stabilization and remodeling by anti-angiogenic therapy with bevacizumab. Histochem Cell Biol. 2012;137:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Tilki D, Seitz M, Singer BB, Irmak S, Stief CG, Reich O, Ergün S. Molecular imaging of tumor blood vessels in prostate cancer. Anticancer Res. 2009;29:1823-1829. [PubMed] |
| 89. | Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, Chung E, Clark JW, Willett CG, Luster AD, Yun SH, Langer R, Anderson DG, Padera TP, Jain RK, Fukumura D. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J Clin Invest. 2017;127:3039-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 90. | Willms-Kretschmer K, Flax MH, Cotran RS. The fine structure of the vascular response in hapten-specific delayed hypersensitivity and contact dermatitis. Lab Invest. 1967;17:334-349. [PubMed] |
| 91. | Ribatti D, Nico B, Crivellato E, Vacca A. The structure of the vascular network of tumors. Cancer Lett. 2007;248:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Klein D. Vascular Wall-Resident Multipotent Stem Cells of Mesenchymal Nature within the Process of Vascular Remodeling: Cellular Basis, Clinical Relevance, and Implications for Stem Cell Therapy. Stem Cells Int. 2016;2016:1905846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Yang Z, Yao H, Fei F, Li Y, Qu J, Li C, Zhang S. Generation of erythroid cells from polyploid giant cancer cells: re-thinking about tumor blood supply. J Cancer Res Clin Oncol. 2018;144:617-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Haase K, Kamm RD. Advances in on-chip vascularization. Regen Med. 2017;12:285-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 95. | Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 597] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 96. | Tang DG, Conti CJ. Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin Thromb Hemost. 2004;30:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2465] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 98. | Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 1153] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 99. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2187] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 100. | Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1352] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 101. | Manzoni M, Comolli G, Torchio M, Mazzini G, Danova M. Circulating endothelial cells and their subpopulations: role as predictive biomarkers in antiangiogenic therapy for colorectal cancer. Clin Colorectal Cancer. 2015;14:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 735] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 103. | van Dijk E, Biesma HD, Cordes M, Smeets D, Neerincx M, Das S, Eijk PP, Murphy V, Barat A, Bacon O, Prehn JHM, Betge J, Gaiser T, Fender B, Meijer GA, McNamara DA, Klinger R, Koopman M, Ebert MPA, Kay EW, Hennessey BT, Verheul HMW, Gallagher WM, O'Connor DP, Punt CJA, Loupakis F, Lambrechts D, Byrne AT, van Grieken NCT, Ylstra B. Loss of Chromosome 18q11.2-q12.1 Is Predictive for Survival in Patients With Metastatic Colorectal Cancer Treated With Bevacizumab. J Clin Oncol. 2018;36:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Ruff P, Van Cutsem E, Lakomy R, Prausova J, van Hazel GA, Moiseyenko VM, Soussan-Lazard K, Dochy E, Magherini E, Macarulla T, Papamichael D. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol. 2018;9:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Büchert M, Christensen O, Jeffers M, Heinig R, Boix O, Mross K. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 106. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2269] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 107. | Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 108. | Wilson PM, LaBonte MJ, Lenz HJ. Assessing the in vivo efficacy of biologic antiangiogenic therapies. Cancer Chemother Pharmacol. 2013;71:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Rahbari NN, Schölch S, Bork U, Kahlert C, Schneider M, Rahbari M, Büchler MW, Weitz J, Reissfelder C. Prognostic value of circulating endothelial cells in metastatic colorectal cancer. Oncotarget. 2017;8:37491-37501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Ronzoni M, Manzoni M, Mariucci S, Loupakis F, Brugnatelli S, Bencardino K, Rovati B, Tinelli C, Falcone A, Villa E, Danova M. Circulating endothelial cells and endothelial progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann Oncol. 2010;21:2382-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 111. | Bertolini F, Mancuso P, Braidotti P, Shaked Y, Kerbel RS. The multiple personality disorder phenotype(s) of circulating endothelial cells in cancer. Biochim Biophys Acta. 2009;1796:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |