Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Jul 15, 2025; 17(7): 103337
Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.103337
Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.103337
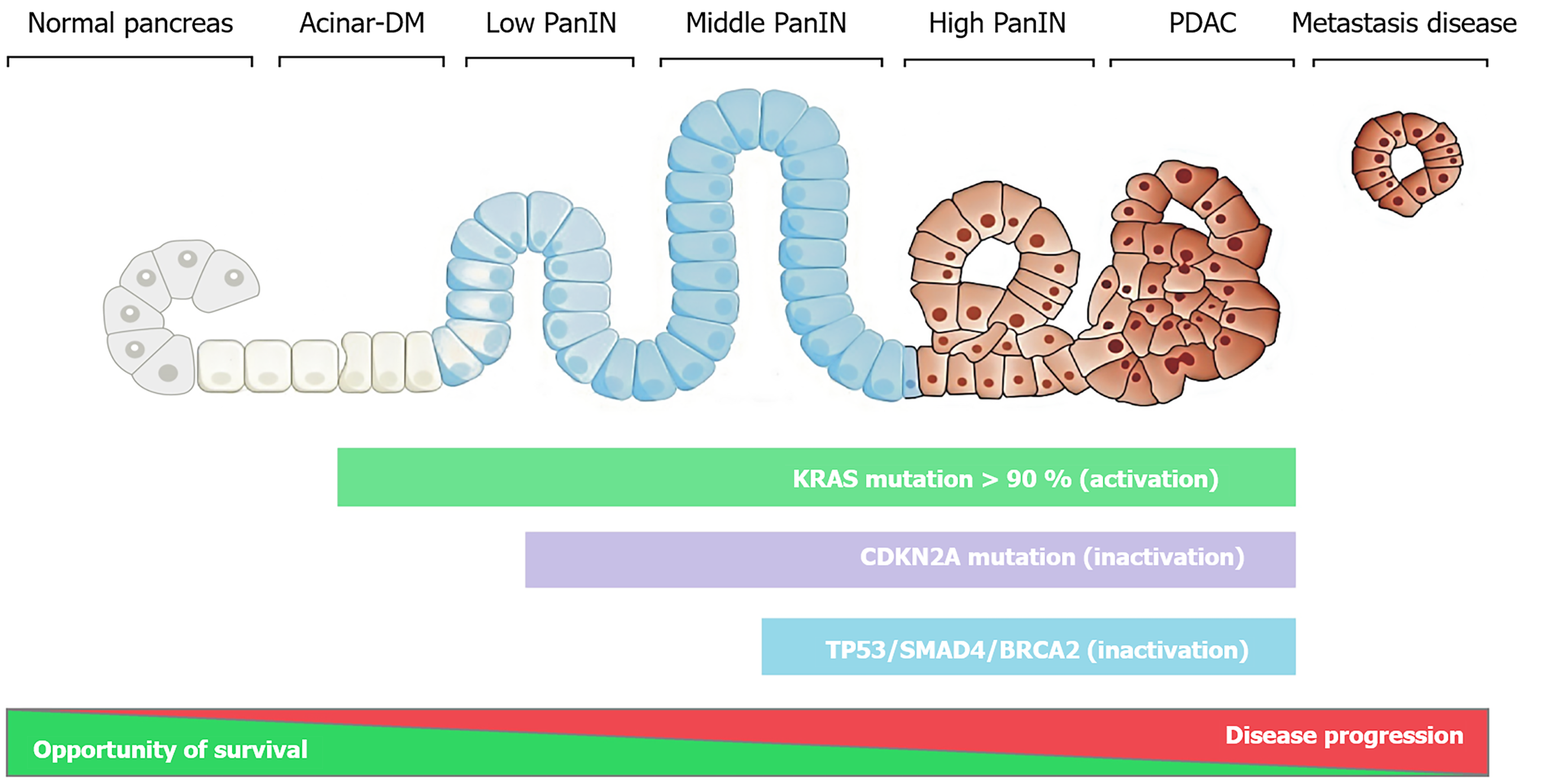
Figure 1 Progression model of pancreatic ductal adenocarcinoma in which normal acinar cells (left) transform into precursor lesions (pancreatic intraepithelial neoplasms) (right).
The degree of histological progression is associated with an accumulation of specific genetic changes. DM: Ductal metaplasia; PanIN: Pancreatic intraepithelial neoplasms; PDAC: Pancreatic ductal adenocarcinoma; KRAS: Kirsten rat sarcoma oncogene; BRCA2: Breast cancer susceptibility gene 2.
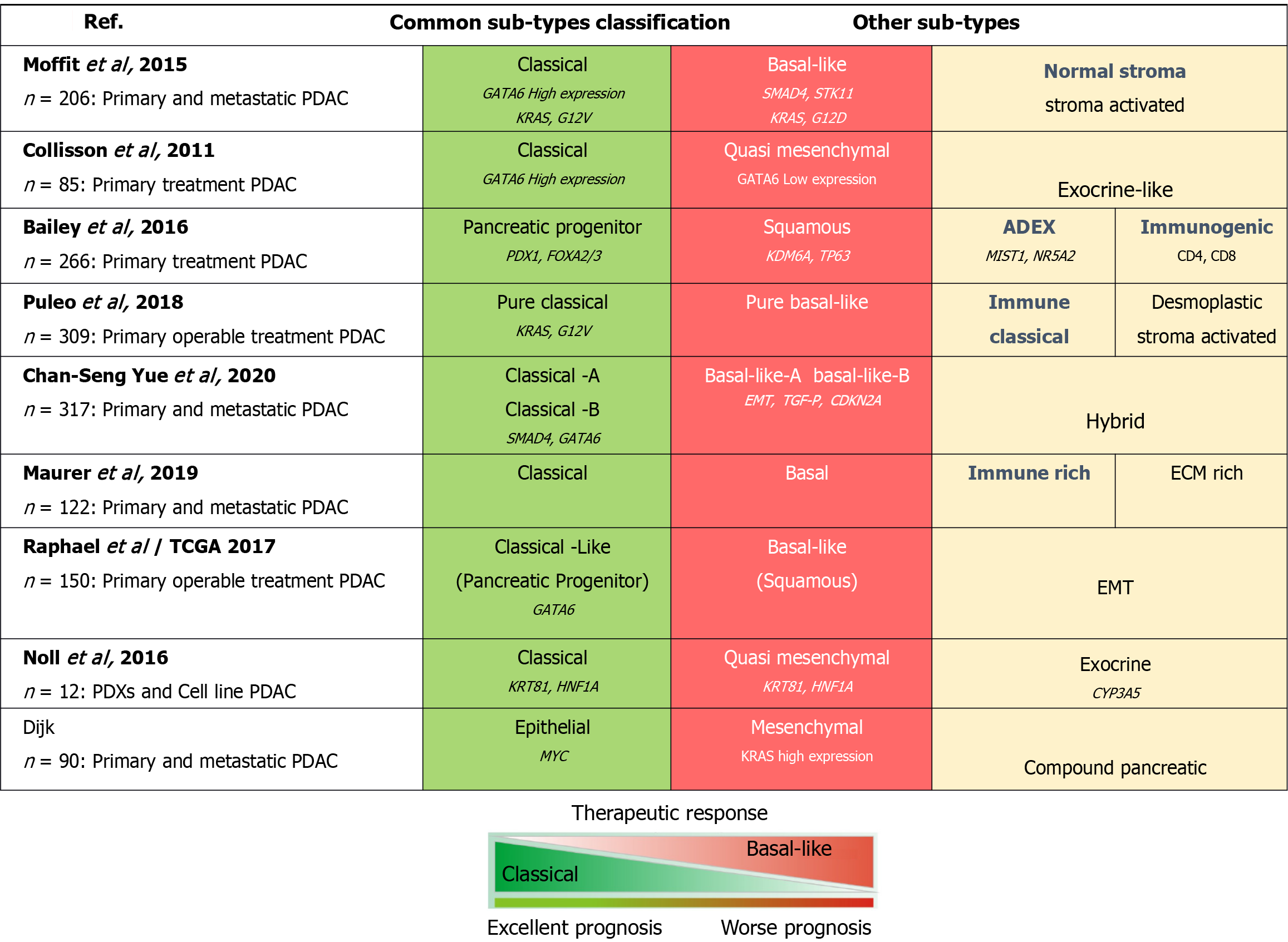
Figure 2 Comparison of different transcription subtype classifications of pancreatic ductal adenocarcinoma tumors.
Subtype classifications of pancreatic ductal adenocarcinoma tumors have been identified and characterized according to transcriptomic signatures and/or protein expression profiles. Findings from previous studies. Classical (excellent prognosis, left) or basal-like (worse prognosis, right) phenotype. The genetic determinants of the proposed classes of subtypes have been proposed to drive transcription phenotypes, including enrichment of GATA binding protein 6 in classical subtype tumors and Kirsten rat sarcoma oncogene in basal-like pancreatic ductal adenocarcinoma tumors. PDAC: Pancreatic ductal adenocarcinoma; GATA6: GATA binding protein 6; KRAS: Kirsten rat sarcoma oncogene; PDX1: Pancreatic duodenal homeobox-1; KDM6A: Lysine-specific demethylase 6A; NR5A2: Nuclear receptor subfamily 5 group A member 2; EMT: Epithelial-mesenchymal transition; TGF: Transforming growth factor; ECM: Extracellular matrix; KRT81: Keratin 81; HNF1A: Hepatocyte nuclear factor 1 alpha.
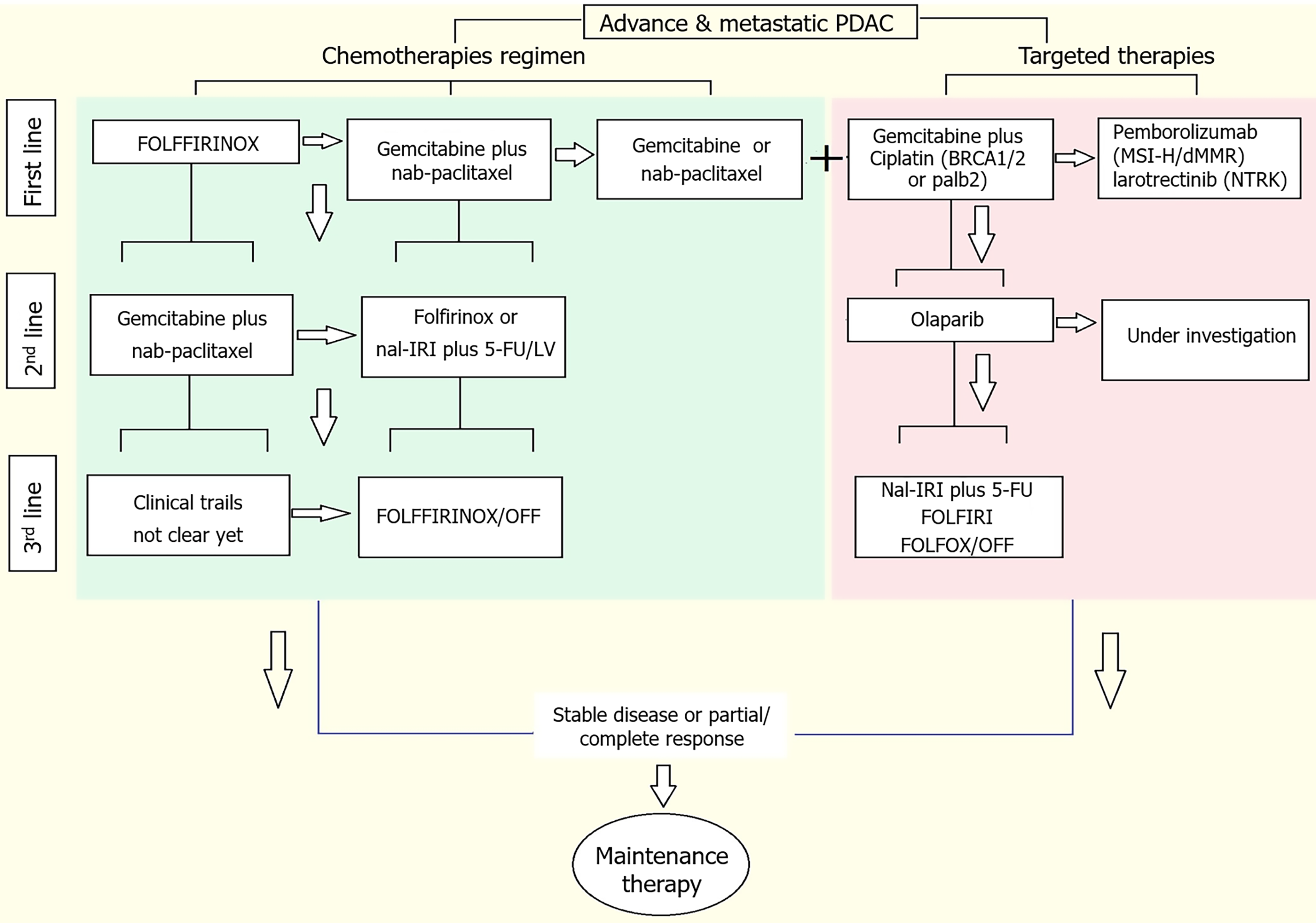
Figure 3 Available targeted therapeutic options for locally advanced pancreatic ductal adenocarcinoma.
PDAC: Pancreatic ductal adenocarcinoma; BRCA1/2: Breast cancer susceptibility gene 1/2; palb2: Partner and localizer of breast cancer 2; MSI-H: Microsatellite instability high; dMMR: Mismatch repair deficient; NTRK: Neurotrophic receptor tyrosine kinase; nal-IRI: Nanoliposomal irinotecan; 5-FU: 5-fluorouracil; LV: Leucovorin.
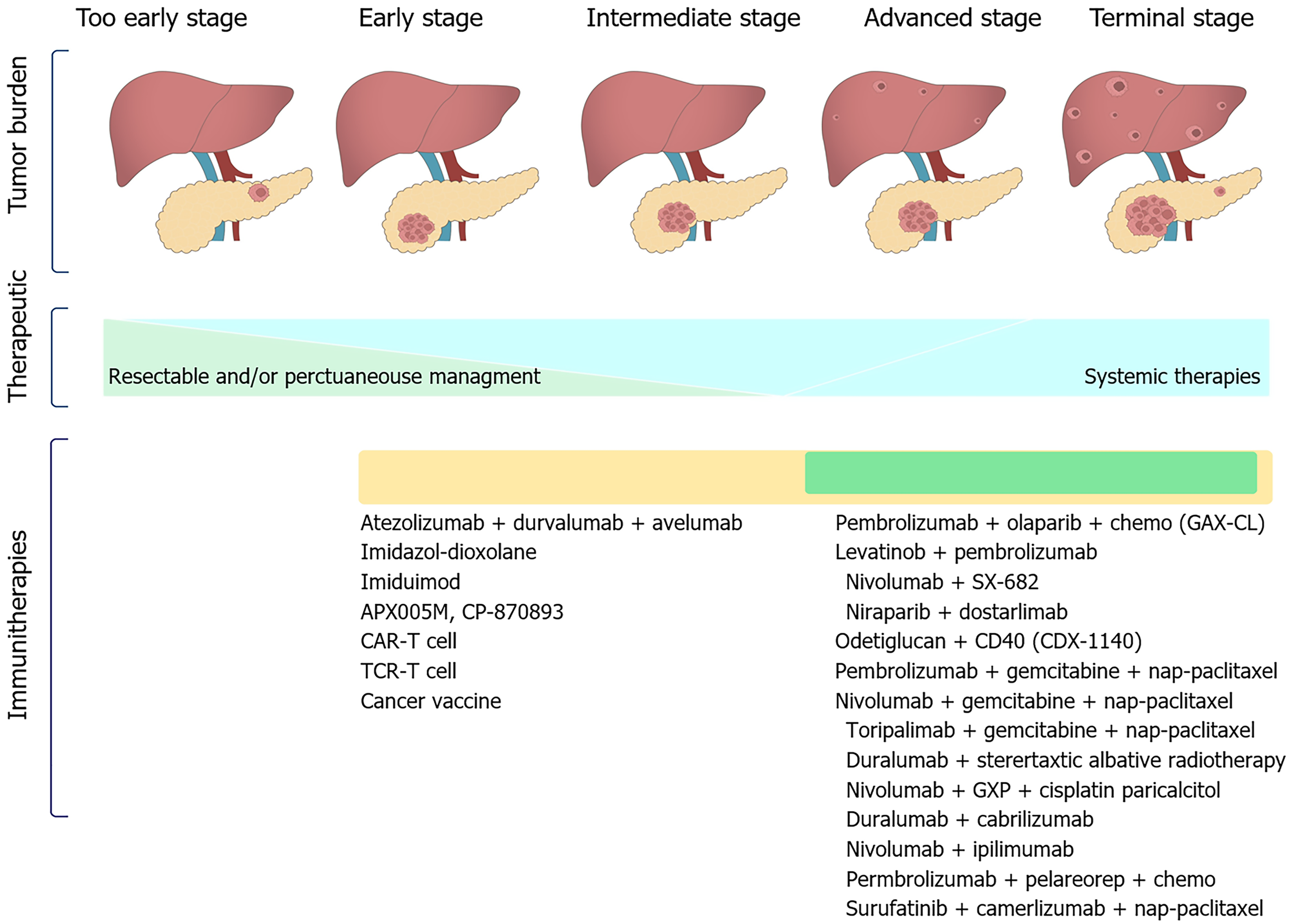
Figure 4 Previously and currently available therapies and future prospects for immunotherapy in the management of pancreatic cancer in 2024.
Single agents and combinations of approved therapies or therapies under investigation in randomized clinical trials across tumor stages are shown. CAR-T: Chimeric antigen receptor-engineered T; TCR: T cell receptor.
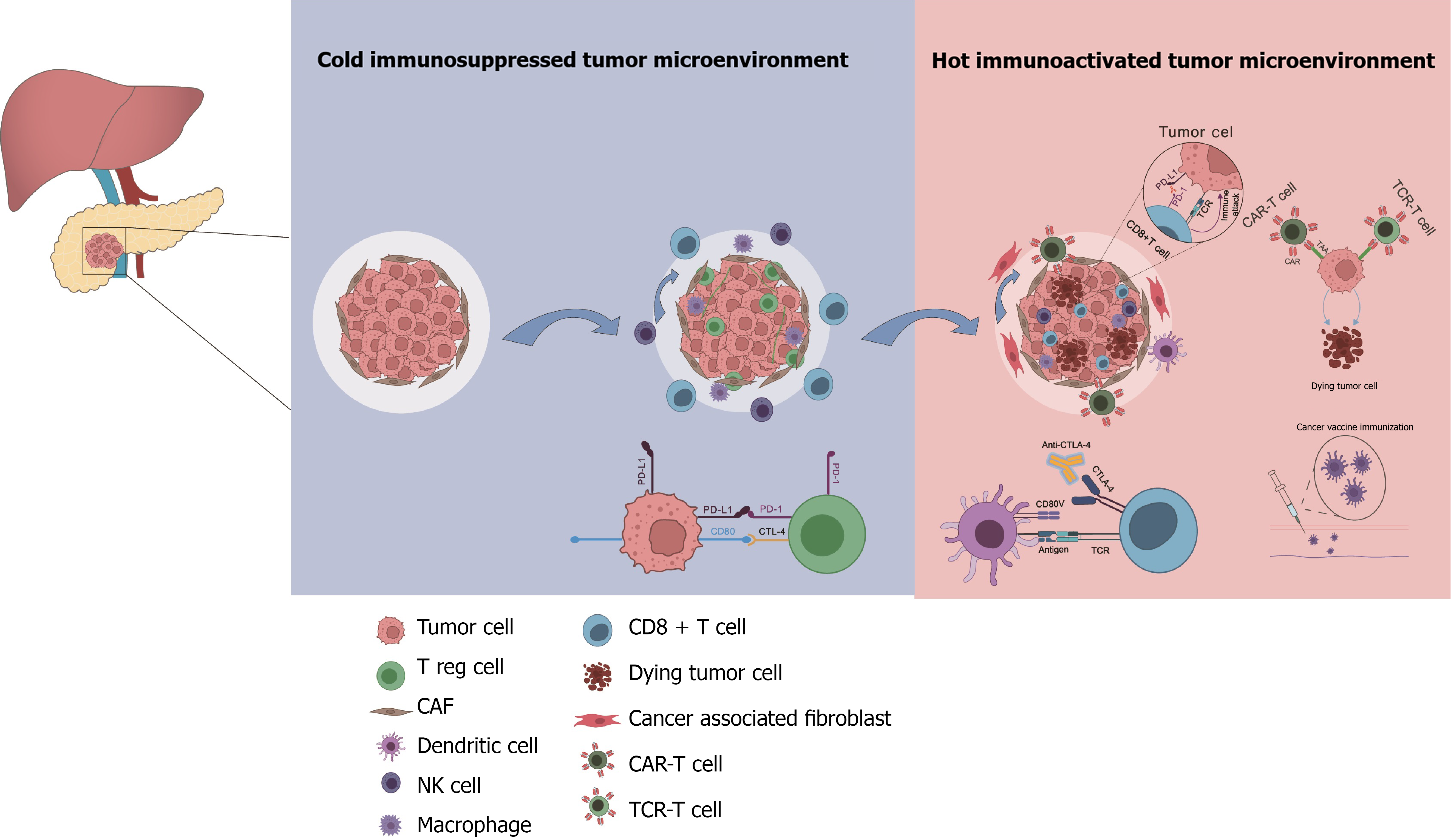
Figure 5 Schematic illustration of the different cell types in the tumor microenvironment.
Some of these cell types can be targeted to turn “cold” pancreatic tumors into “hot” pancreatic tumor. PD-L1: Programmed-death ligand 1; PD-1: Programmed cell death protein 1; CTL-4: Cytotoxic T lymphocyte 4; CAR-T: Chimeric antigen receptor-engineered T; TCR: T cell receptor; CTLA-4: Cytotoxic T lymphocyte associated antigen 4; CAF: Cancer-associated fibroblast; Treg: Regulatory T; NK: Natural killer.
- Citation: Hendi M, Zhang B, Mou YP, Cai XJ. Importance of landscape exploration and progress in molecular therapies and precision medicine for pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2025; 17(7): 103337
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/103337.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.103337