Published online Nov 16, 2017. doi: 10.4253/wjge.v9.i11.540
Peer-review started: June 12, 2017
First decision: July 13, 2017
Revised: July 20, 2017
Accepted: August 15, 2017
Article in press: August 16, 2017
Published online: November 16, 2017
Processing time: 158 Days and 7.1 Hours
To investigate changes in polyp detection throughout fellowship training, and estimate colonoscopy volume required to achieve the adenoma detection rate (ADRs) and polyp detection rate (PDRs) of attending gastroenterologists.
We reviewed colonoscopies from July 1, 2009 to June 30, 2014. Fellows’ procedural logs were used to retrieve colonoscopy procedural volumes, and these were treated as the time variable. Findings from screening colonoscopies were used to calculate colonoscopy outcomes for each fellow for the prior 50 colonoscopies at each time point. ADR and PDR were plotted against colonoscopy procedural volumes to produce individual longitudinal graphs. Repeated measures linear mixed effects models were used to study the change of ADR and PDR with increasing procedural volume.
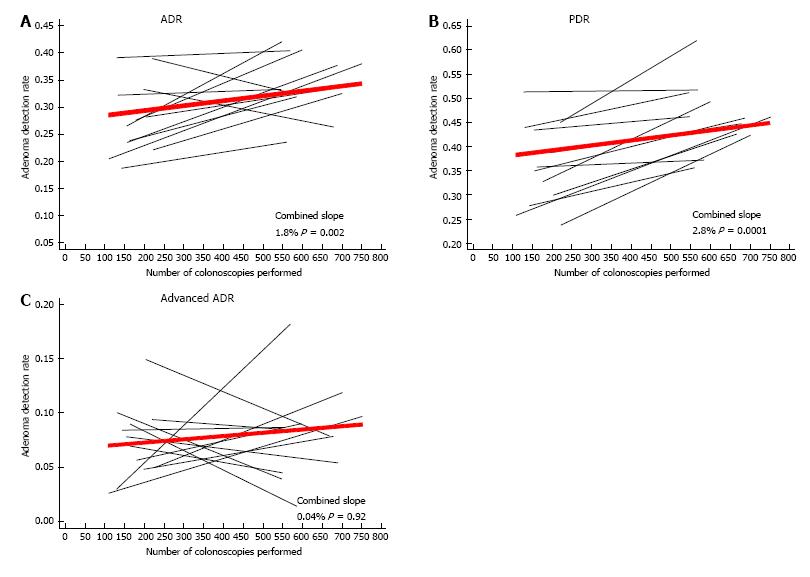
During the study period, 12 fellows completed full three years of training and were included in the analysis. The average ADR and PDR were, respectively, 31.5% and 41.9% for all fellows, and 28.9% and 38.2% for attendings alone. There was a statistically significant increase in ADR with increasing procedural volume (1.8%/100 colonoscopies, P = 0.002). Similarly, PDR increased 2.8%/100 colonoscopies (P = 0.0001), while there was no significant change in advanced ADR (0.04%/100 colonoscopies, P = 0.92). The ADR increase was limited to the right side of the colon, while the PDR increased in both the right and left colon. The adenoma per colon and polyp per colon also increased throughout training. Fellows reached the attendings’ ADR and PDR after 265 and 292 colonoscopies, respectively.
We found that the ADR and PDR increase with increasing colonoscopy volume throughout fellowship. Our findings support recent recommendations of ≥ 275 colonoscopies for colonoscopy credentialing.
Core tip: Adenoma and polyp detection rates are important colonoscopy quality indicators. Competence in colonoscopy is measured by motor skills and not adenoma detection rate (ADR) and polyp detection rate (PDR). Recent guidelines recommend at least 275 colonoscopies to achieve competence. In this study, we found that ADR, PDR, adenoma per colon, and polyp per colon significantly increase throughout fellowship training. Fellows achieve the ADR and PDR of attendings after 262 and 292 colonoscopies. The variability of polyp detection among fellows suggests that ADR and PDR could be used during fellowship as part of periodic feedback.
- Citation: Qayed E, Vora R, Levy S, Bostick RM. Colonoscopy procedural volume increases adenoma and polyp detection rates in gastroenterologytrainees. World J Gastrointest Endosc 2017; 9(11): 540-551
- URL: https://www.wjgnet.com/1948-5190/full/v9/i11/540.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i11.540
Colorectal cancer (CRC) is the third most common cancer and second leading cause of cancer deaths in the United States[1]. Colonoscopy is the preferred modality for screening for colon cancer[2], and an essential follow-up procedure when other screening tests are positive. Several studies found that colonoscopy and polypectomy decrease colon cancer-specific mortality[3-5]. However, this is dependent on the quality of colonoscopy, and the ability of the endoscopist to detect and remove precancerous polyps. The adenoma detection rate (ADR), the most important quality indicator of colonoscopy, was found to be inversely associated with risk for interval colon cancer[6,7]. Most interval cancers are related to missed lesions during a screening colonoscopy[8]. Current practice guidelines include a recommendation for a minimum ADR of 20% in women and 30% in men to ensure adequate colonoscopy quality[9]. During gastroenterology training, competency in colonoscopy has been traditionally measured by the ability of the trainee to achieve cecal intubation in a timely manner (< 15 min) and resect polyps independently. The current Accreditation Council for Graduate Medical Education (ACGME) guidelines include a recommendation that fellows perform at least 140 colonoscopies during training to achieve competence. However, previous studies found that the number of procedures needed to achieve competence is much higher (275-500)[10-13]. Furthermore, polyp and adenoma detection, which is an essential goal of colonoscopy, is not part of competency assessment. Therefore, it is important to examine the change in adenoma and polyp detection as fellows increase their colonoscopy volume, and determine the number of colonoscopies that allows fellows to achieve an adequate polyp and adenoma detection rate.
In a retrospective study, it was found that the ADR was higher among third year fellows than among first and second year fellows[14]. Other studies found no change in adenoma and polyp detection with increasing fellowship training level[15,16]. In a prospective tandem colonoscopy study it was found that fellows with a higher colonoscopy volume had lower adenoma miss rates (AMR), and it was estimated that 450 colonoscopies would be required to achieve an AMR of < 25%[17]. In a retrospective study in which trainees were followed throughout their fellowship training, it was found that fellows’ ADRs and polyp detection rates (PDR) improved when the fellows had conducted > 140 colonoscopies[18]. There are several limitations to these studies, including the small number of procedures performed by the fellows, inclusion of non-screening colonoscopies in calculating the ADR, and including fellows from various stages of training during only a limited part of their fellowship. In addition, none of these studies examined the change in individual fellow’s ADRs and other colonoscopy metrics throughout fellowship training.
Our primary aim for the present study was to evaluate changes in the ADR, PDR, and advanced ADR with increasing colonoscopy procedural volume among gastroenterology trainees. Our secondary aims were to investigate changes in other colonoscopy metrics, such as adenoma per colon, polyp per colon, and left vs right-sided detection rates. We also aimed to estimate the number of procedures required for fellows to achieve the ADRs and PDRs of attending gastroenterologists. This was done by examining a large sample of screening colonoscopies performed by 12 gastroenterology trainees throughout their complete three-year fellowship, using a longitudinal analysis method that accounts for the individual and combined trajectories of change in outcome with increasing procedural volume.
For this retrospective study, which was approved by the Emory University Institutional Review Board, we used the endoscopic procedure database at Grady Memorial Hospital in Atlanta, Georgia. Informed consent was waived by the Institutional Review Board due to the large sample size, retrospective study design, and the fact that this study does not affect the welfare of the patients. Information about all endoscopic procedures performed in the gastroenterology endoscopy unit is prospectively collected and entered into the database, and includes variables such as procedure type, patient’s medical record number, age, race, procedure indication, endoscopist, and fellow participation in the procedure. We reviewed screening colonoscopies for patients aged 40-85 performed by gastroenterology fellows who completed their entire gastroenterology training between July 1, 2009 and June 30, 2014. Gastroenterology trainees in the training program rotate through three different sites: Grady Memorial Hospital, Veterans Affairs Medical Center, and Emory University Hospital. However, all screening colonoscopies are performed at Grady Memorial hospital. For each training fellow we created a separate Microsoft Excel dataset that included all of his or her screening colonoscopies performed at Grady throughout their fellowship training. This included the patient’s age, race (black or non-black), and sex; colon preparation (“prep”) quality (good, fair-adequate, fair-inadequate, poor) and success at cecal intubation; and polyp size (1-5 mm, 6-9 mm, ≥ 9 mm), number, location, and histology. Procedures with unsuccessful cecal intubation, fair-inadequate prep or poor prep were considered “inadequate” procedures, while those with successful cecal intubation in addition to fair-adequate or good prep were considered “adequate” procedures. Polyp location was categorized as right colon (cecum, ascending colon, hepatic flexure, and transverse colon) and left colon (splenic flexure, descending colon, sigmoid, and rectum). Adenomatous polyps were categorized as advanced and non-advanced adenomas. Advanced adenomas included adenomas larger than 9 mm in size and those that had histologic features of tubulovillous/villous structure, high-grade dysplasia, or adenocarcinoma.
We then sorted the colonoscopies in ascending temporal order, starting with the first day a screening colonoscopy was performed and continuing until the last screening colonoscopy was performed during fellowship. Next, we reviewed the fellow’s procedure logs that contained the total number of colonoscopies (for all indications) performed at all training locations. Using this information, we assigned a procedure number that reflected the “rank” of each screening colonoscopy for that fellow. In assigning the rank, all colonoscopies performed by fellows for screening, polyp surveillance, and diagnostic indications at all locations contributed to the procedural volume. However, only screening colonoscopies were included in the analysis to calculate procedural outcomes. Patients with a personal history of colon cancer or prior colonic surgery were excluded from analysis. Procedural outcomes were defined as follows: Adenoma detection rate (ADR) - the percentage of screening colonoscopies with at least one histologically proven adenoma; polyp detection rate (PDR) - the percentage of screening colonoscopies with at least one polyp removed during the colonoscopy; and advanced ADR - the percentage of screening colonoscopies with at least one advanced adenoma (see above). The mean number of adenoma per colon (APC) was calculated by dividing the total number of adenomas by the number of screening colonoscopies performed. The mean number of polyps per colon (PPC) was calculated by dividing the total number of polyps by the number of screening colonoscopies performed.
Starting at the 50th screening colonoscopy, we calculated procedural outcomes for the current colonoscopy and the previous 49 colonoscopies (50 procedures for each outcome measurement). We also calculated the mean age and the percentage of patients in this block of 50 procedures who were male, black, and had an adequate exam. With each additional screening colonoscopy, these outcomes and control variables were recalculated until the last screening colonoscopy in the dataset was reached. The final dataset contained observations organized in ascending order by colonoscopy procedure rank number. Each ranked observation, starting at the 50th screening colonoscopy, contained colonoscopy outcome measures and time varying percentages as mentioned above. This process was conducted for each of the 12 fellows. Finally, we merged the 12 individual spreadsheets into one longitudinal dataset that contained the fellows’ ID code, the procedural rank variable, time varying outcomes (ADR, PDR, APC, PPC), and time varying control variables (percentage of male patients, black patients, procedures with inadequate prep, and mean age).
To obtain a reference standard to which to compare the fellow’s performance, we reviewed all screening colonoscopies performed by the attending physicians alone without the involvement of fellows at Grady Memorial Hospital. We used the same inclusion and exclusion criteria mentioned above for the fellows’ procedures. The demographic characteristics of the patients who underwent screening colonoscopies were similar to those of the patients included in the calculation of outcomes for the fellows’ procedures. We calculated the ADR, PDR, APC, and PPC for the attending-alone group. These values were used as target levels to estimate the number of procedures it takes for fellows to achieve attendings’ level of polyp detection.
At our hospital, patients are referred for screening colonoscopy by their primary care physician or their gastroenterologist. For bowel preparation, patients received 4 L of polyethylene glycol solution as a single dose regimen the evening before the procedure. All procedures were performed with moderate sedation. During the study period, there were 8 attendings who supervised the 12 fellows who performed the colonoscopies. The fellow began the procedure and attempted insertion of the colonoscope to the cecum. The attending physician assisted when there was difficulty passing an area of the colon. The attending usually returned the scope to the fellow once the problematic area of the colon was traversed, though this varied per procedure, attending, and fellow level of training. The attending physicians were present and monitored fellows throughout the duration of the procedure. In the attending-alone group, the attending started and completed the procedure with no fellow involvement.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States) statistical software. Descriptive statistics, including means, ranges, and frequencies, were used to characterize the study population. For each fellow, from their measurement time points, we calculated ranges and mean values for each predictor and outcome. We constructed individual and combined graphs to illustrate the change in colonoscopy outcome with increasing colonoscopy procedural volume, which was used as a proxy measure of the time variable. To examine the individual and combined trajectories of all fellows, defined as change in colonoscopy outcome with increasing colonoscopy volume, we used repeated measures linear mixed effects longitudinal models. The unconditional growth model to investigate the individual fellows’ trajectories included the outcome and procedural volume (main exposure), and accounted for the random effect of the intercept with an unstructured covariance matrix. For the combined trajectories, the models included the outcome and procedural volume (main exposure), and mean age, percentages of black patients, sex, and inadequate prep as time-varying predictors, and accounted for the random effect of the intercept and procedural volume with an unstructured covariance matrix. The time varying predictors were centered to their mean, and the procedural volume was centered to procedure n = 50 to ease the interpretation of the initial status. The unconditional means model was used to calculate the mean outcome for the entire cohort. This included the outcome in the model statement and accounted for the variable effect of the intercept. The results are reported as the estimated means and 95%CIs. A P-value ≤ 0.05 (two-sided) was used to assess statistical significance. We used the results of the longitudinal growth model (initial status and rate of change) to estimate the number of colonoscopies required to achieve the attending-alone group mean ADR, PDR, APC, and PPC.
Between July 1, 2009 and June 30, 2014, 12 fellows completed their full three-year clinical fellowship training. A total of 3123 screening colonoscopies performed by these fellows were included in the analysis. The attending physicians performed 2174 procedures without fellow involvement. The characteristics of the screening colonoscopies performed by the fellows and the attendings alone are summarized in Table 1. The overall mean ADR, PDR, and advanced ADR for all fellows were 31.5%, 41.9%, and 7.8%, respectively. There was substantial inter- and intra-individual variation in the ADR, PDR, and advanced ADR. The mean ADR ranged from 21.6% to 39.8%, and ADR values ranged from 8% to 52% throughout all measurement time points. The mean PDR ranged from 32.5% to 53.8%, while PDR values ranged from 14% to 70%. The overall ADR and PDR of the attending-alone group during the study period were 28.9% and 38.2%, respectively.
| Fellow | Number of screening colonoscopies | Total colonoscopy procedure volume | Patient’s mean age (yr) | Male patients (%) | Black patients (%) | Adequate exam (%) | Mean ADR (%) | Mean PDR (%) | Mean advanced ADR (%) |
| A | 326 | 751 | 58.5 | 39.4 | 86.3 | 90.5 | 31.0 | 38.1 | 6.9 |
| B | 277 | 702 | 58.2 | 36.3 | 83.1 | 91.9 | 28.4 | 34.9 | 9.1 |
| C | 282 | 680 | 57.8 | 39.1 | 88.7 | 90.8 | 28.8 | 42.4 | 6.7 |
| D | 328 | 668 | 58.4 | 35.4 | 87.1 | 92.8 | 31.2 | 37.5 | 10.7 |
| E | 214 | 566 | 57.8 | 35.4 | 89.2 | 92.6 | 35.7 | 53.8 | 8.9 |
| F | 275 | 546 | 57.9 | 37.7 | 86.1 | 93.1 | 32.7 | 47.8 | 6.7 |
| G | 254 | 561 | 58.4 | 41.2 | 87.7 | 85.3 | 21.6 | 32.5 | 8.6 |
| H | 226 | 689 | 58.0 | 35.9 | 90.4 | 81.1 | 31.5 | 41.2 | 6.5 |
| I | 229 | 600 | 57.0 | 37.9 | 85.2 | 89.4 | 34.6 | 41.8 | 7.5 |
| J | 206 | 586 | 58.0 | 35.2 | 90.8 | 91.7 | 28.2 | 36.5 | 4.8 |
| K | 244 | 549 | 58.2 | 36.5 | 84.0 | 91.8 | 34.5 | 44.8 | 5.7 |
| L | 261 | 569 | 58.6 | 35.7 | 90.5 | 91.7 | 39.8 | 51.5 | 12.0 |
| All fellows | 3123 | 7467 | 58.1 | 37.2 | 87.3 | 89.8 | 31.5 | 41.9 | 7.8 |
| Attendings alone | 2174 | 57.9 | 36.3 | 89.3 | 90.1 | 28.9 | 38.2 | 8.5 |
Plots of individual and combined fellows’ ADR, PDR, and advanced ADR are shown in Figure 1. There was a statistically significant increase in the ADR among all fellows (1.8% per 100 colonoscopies, P = 0.002) (Figure 1A). Similarly, there was a statistically significant increase in the PDR among all fellows (2.8% per 100 colonoscopies, P = 0.0001) (Figure 1B). Overall, there was no substantial or statistically significant change in the advanced ADR with increasing procedural volume (0.04% per 100 colonoscopies, P = 0.92) (Figure 1C).
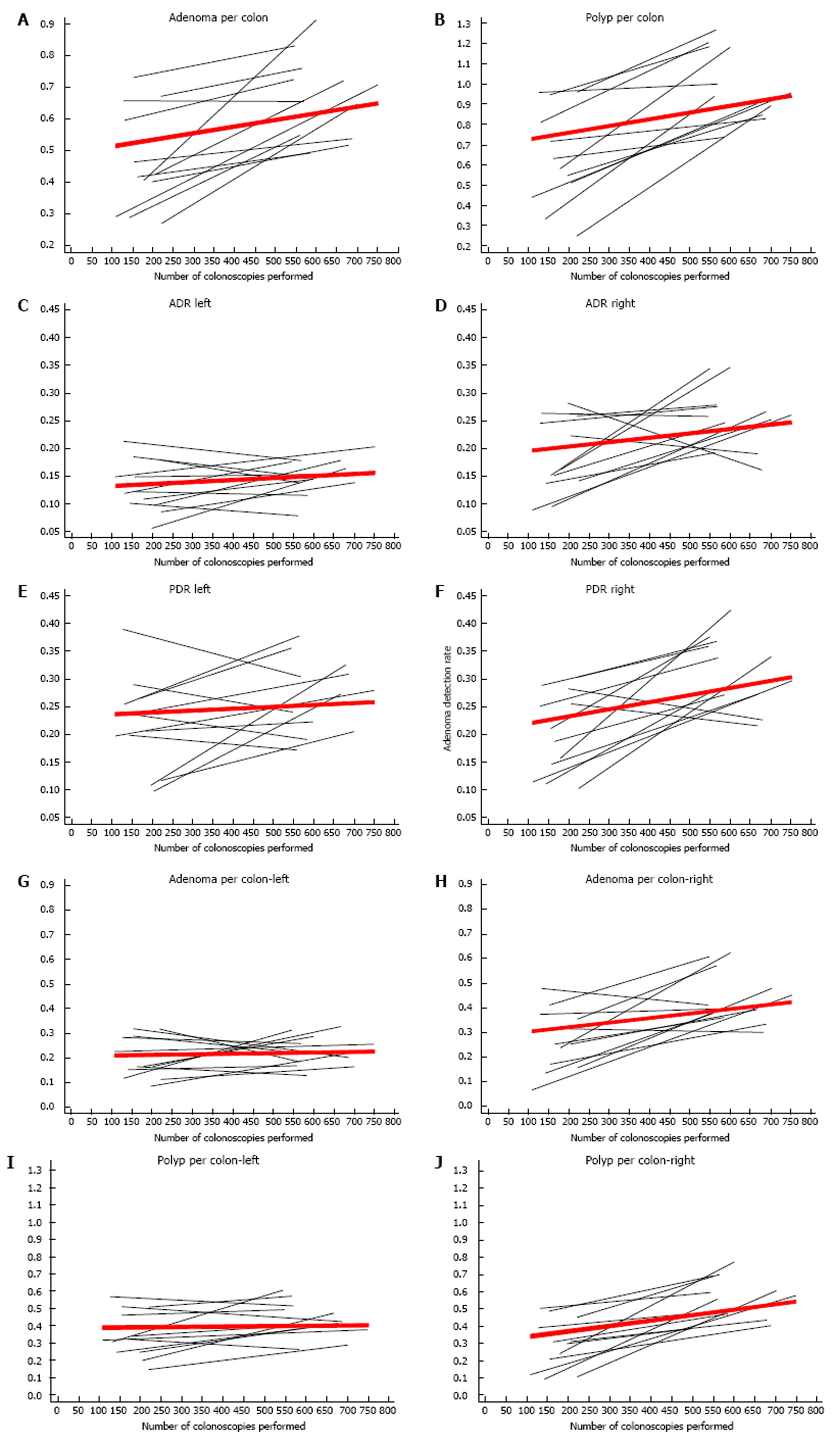
In addition to increasing ADR and PDR, the adenoma per colon (APC) and polyp per colon (PPC) also increased with increasing procedural volume (Figure 2A and B, and Table 2). The mean APC for the entire cohort was 0.58, and it increased by 0.05 per 100 colonoscopies, (P = 0.0001). The mean PPC was 0.84, and there was positive trend of 0.09 per 100 colonoscopies for this metric (P < 0.0001). However, there was a difference in the trends for detecting polyps in the right vs the left colon. The right-side ADR (ADR-right) increased with increasing procedural volume (1.9% per 100 colonoscopies, P = 0.006), while the left-side ADR (ADR-left) increased slightly (0.6% per 100 colonoscopies, P = 0.05) (Figure 2C and D). This was also observed for the APC, for which where APC-right increased by 0.04 per 100 colonoscopies, P = 0.001; while the estimated increase in APC-left was only 0.01 per 100 colonoscopies and not statistically significant (P = 0.24) (Figure 2G and 2H). The PDR and PPC for both sides of the colon increased with increasing procedural volume; however, the increase in PDR-right was higher than PDR-left (2.9%, P = 0.0001 vs 1.6%, P = 0.02, respectively), and the increase in PPC-right was higher than PPC left (0.06, P < 0.0001 vs 0.03, P = 0.01) (Figure 2E, F, I, J). In the attending-alone group, the overall APC and PPC were 0.58 and 0.8, respectively.
| Outcome1 | Overall mean2 (95%CI) | Mean after first 50 colonoscopies (95%CI) | Change in outcome per 100 colonoscopies (95%CI) | P value3 | Number of procedures to achieve mean attending value4 |
| ADR (%) | 31.5 (28.7-34.3) | 25.1 (21.1-29.2) | 1.8 (0.8-2.7) | 0.002 | 265 |
| ADR-right (%) | 22.3 (20.1-24.4) | 15.3 (10.6-20.0) | 1.9 (0.7-3.2) | 0.01 | |
| ADR-left (%) | 14.2 (12.4-16.0) | 11.6 (8.3-14.9) | 0.6 (0.001-1.3) | 0.05 | |
| PDR (%) | 41.9 (37.9-45.9) | 31.4 (26.7-36.0) | 2.8 (1.7-3.9) | 0.0001 | 292 |
| PDR-right (%) | 26.5 (23.8-29.2) | 16.0 (10.6-21.4) | 2.9 (1.5-4.3) | 0.001 | |
| PDR-left (%) | 24.9 (21.5-28.3) | 18.7 (13.1-24.3) | 1.6 (0.3-2.9) | 0.02 | |
| AADR (%) | 7.8 (6.6-9.1) | 7.4 (4.6-10.2) | 0.04 (-0.80-0.90) | 0.92 | |
| APC | 0.58 (0.52-0.65) | 0.39 (0.28-0.49) | 0.05 (0.03-0.07) | 0.0001 | 399 |
| APC-right | 0.37 (0.32-0.42) | 0.20 (0.12-0.28) | 0.04 (0.02-0.06) | 0.001 | |
| APC-left | 0.22 (0.19-0.25) | 0.18 (0.11-0.26) | 0.01 (-0.01-0.02) | 0.24 | |
| PPC | 0.84 (0.74-0.94) | 0.51(0.36-0.66) | 0.09 (0.06-0.12) | < 0.0001 | 375 |
| PPC-right | 0.45 (0.39-0.50) | 0.21 (0.11-0.31) | 0.06 (0.04-0.09) | < 0.0001 | |
| PPC-left | 0.40 (0.34-0.46) | 0.30 (0.21-0.39) | 0.03 (0.01-0.05) | 0.01 |
The numbers of colonoscopies required to achieve the outcomes (ADR, PDR, APC, and PPC) of those of attendings estimated using the results of longitudinal analysis are shown in Table 2. Overall, on average, fellows achieved the attendings’ level of ADR and PDR after 265 and 292 colonoscopies, respectively. The corresponding numbers for the APC and PPC were 399 and 375.
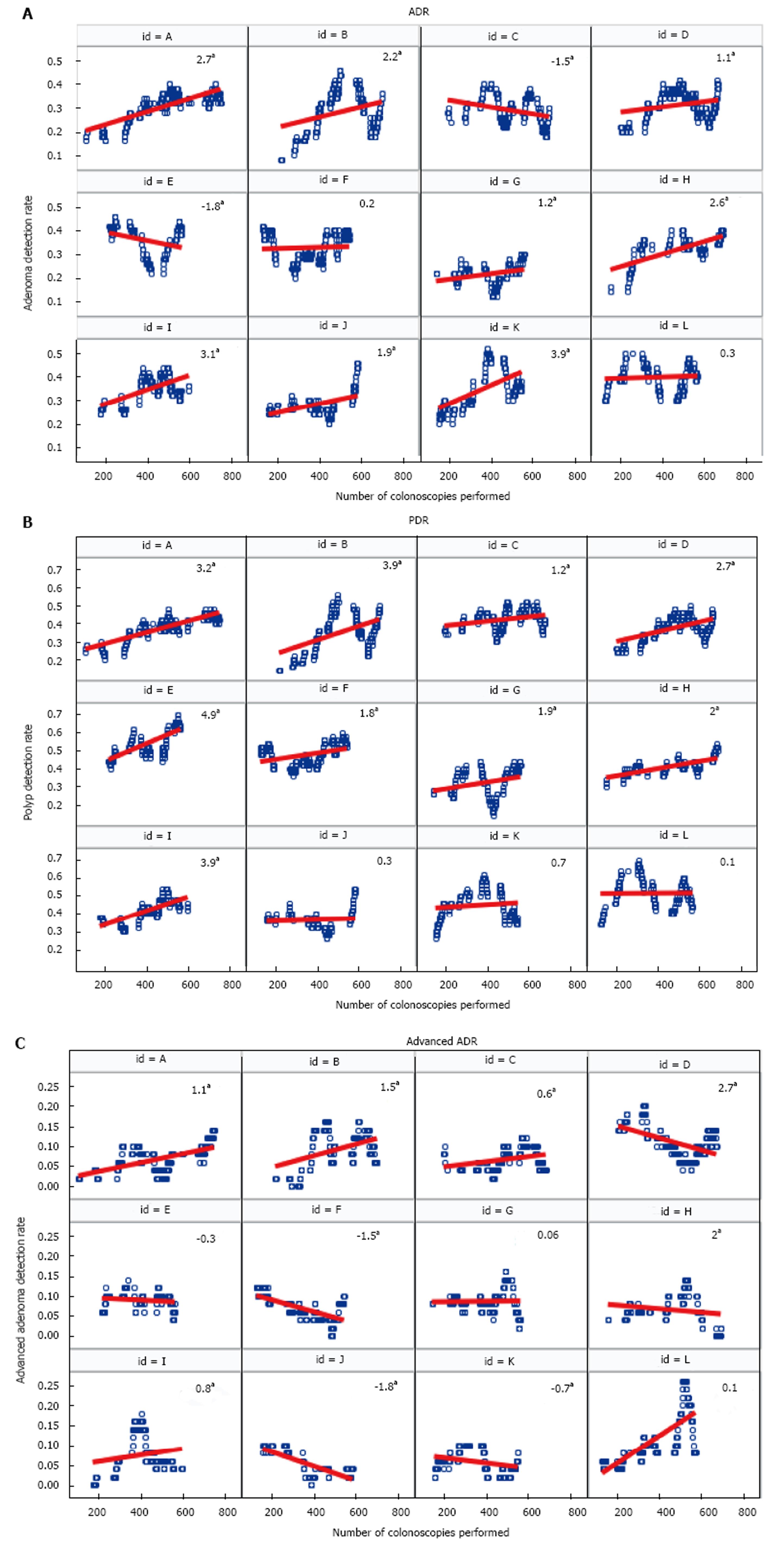
Changes in the trajectories of the ADR, PDR, and advanced ADR for individual fellows throughout their fellowship training are shown in Figure 3. The ADR for most fellows statistically significantly increased with increasing procedural volume. The ADR for eight fellows increased, while for two fellows it remained the same, and for two it decreased (Figure 3A). Similarly, the PDR for nine fellows statistically significantly increased, whereas for three fellows it remained relatively stable (Figure 3B). The trends for change in the advanced ADR were variable among fellows; some fellows had increasing rates, some had decreasing rates, and others remained stable (Figure 3C).
Our results indicate that there are clinically important increases in the ADR, PDR, APC, and PPC as gastroenterology fellows increase their colonoscopy procedural volume. This strongly suggests that polyp detection is a learned skill that improves as fellows perform more procedures. This is highly plausible because polyp detection requires skill in colon distension, residual stool cleanup, and deliberate and systematic examination of each colon fold. The improvement in adenoma detection (as measured by the ADR and APC) was mainly observed in the right colon. The reason behind this finding is unclear. In our study, all patients received a single dose colon preparation the night prior to the colonoscopy. This may have led to the presence of residual stool preferentially in the right colon[20], which needs to be cleaned adequately to improve polyp detection. Previous studies found that cleaning the colon of residual stool by using air, water, and suction is an important motor skill in colonoscopy that improves with increasing procedural volume[13]. Therefore, it is possible that as this skill improved in our fellows, ADR and PDR increased in the right colon.
Traditional ways of assessing competence in colonoscopy have not included polyp detection, but rather focused on other metrics such as cecal intubation rate (> 90%), cecal intubation times, rate of ileocecal valve intubation, patient comfort level, and number of biopsy forceps passes for removal of small polyps. More recently, a dedicated colonoscopy skill assessment tool was developed [Assessment of Competency in Endoscopy (ACE) tool] that incorporates several motor and cognitive skills, in addition to polyp detection. In a multicenter prospective assessment of the ACE tool that included gastroenterology fellows at various stages of training over a one-year period, there was a gradual increase in the PDR from 24% early in training to 65% by the end of training[13]. The ACE tool does not include the ADR or other metrics (APC, PPC). In our study, we found a similar overall upward trend in the ADR and PDR throughout fellowship training. This suggests that measurement of the PDR for competency assessment, while not ideal, could be sufficient for assessing fellows’ polyp detection skills. However, it is important to mention that the PDR is a “corruptible” measure of quality, with potential for the endoscopists (including fellows) to artificially inflate their PDR by removing insignificant diminutive polyps. The ADR remains the most objective and validated quality measure of colonoscopy.
The number of colonoscopies needed to achieve competence is a matter of continuous debate. It has been consistently found in retrospective and prospective studies that the previously recommended number of 140 colonoscopies is inadequate for achieving competence[10,11,13,17,21]. Furthermore, there is a general shift towards performance-based assessment of competency, and away from merely documenting the number of procedures performed[21]. Nevertheless, our findings support the need for a higher number of colonoscopies. Using the ADR and APC of attendings as a reference standard, we found that it requires 265 and 400 procedures to achieve the reference ADR and APC, respectively. This is in accordance with the most recent literature and guidelines for privileging and credentialing, which recommend a minimum of 275 colonoscopies before assessment of competence and seeking of privileges[22]. It is noteworthy that we did not use the recommended minimal quality metrics (ADR of 25%) in calculating the number of required procedures because the average initial ADR for the fellows in the study was already 25.1% at the first measurement occasion.
Our study has several strengths. To our knowledge, we included the largest number of fellows to be followed longitudinally throughout their fellowship training. Our unique method of analysis allowed us to evaluate individual as well as combined trajectories of change in polyp detection. Previous studies used a linear regression analysis method to examine the change in the ADR with procedural volume[23]. This method of analysis is suboptimal because the observations are not independent, but are interrelated and performed by the same gastroenterology fellows over time. A longitudinal analysis method considers the individual and combined change trajectories, examines the change in outcome with time, and allows for estimation of outcome at different time points by using the initial ADR and other detection rates and their rates of change. Furthermore, this method allows comparison of different trends even if values are not available for all fellows at all time points. When calculating the ADR and other outcomes, we only included screening colonoscopies and excluded colonoscopies performed for polyp surveillance and for diagnostic indications; nevertheless, we included all colonoscopies in the calculation of procedural volume. We believe that this approach provides a valid estimation of the ADR and other outcomes, while still incorporating an accurate measure of procedural experience. Previous studies examined differences in polyp detection among fellows according to their year of training. However, using procedural volume is likely a better approach because fellows perform a variable number of procedures during their years of training. The ADR and other polyp detection outcomes were measured using a fixed number of colonoscopies at each time point (50 procedures) which eliminated the variability in these values that can occur if a different number of procedures is used at each time point. We also adjusted for important time-varying predictors of polyp detection in the combined model to account for the varying contribution of these factors on colonoscopy outcomes. Our study extends the traditional analysis of polyp detection beyond the ADR and PDR to include other outcomes such as the advanced ADR, APC, and PPC, and the right- vs left-side detection rates, thereby providing more insight into changes in these outcomes with increasing procedural volume and skill in colonoscopy.
The study also has several limitations. We did not evaluate the exact involvement of fellows in the procedure. Part of the withdrawal could have been performed by the attendings, especially for first year fellows. We did not evaluate other features of fellows’ performance such as independent cecal intubation rates, insertion, and withdrawal times. This would have given more insight into the learning curves of the fellows in respect to motor skills in addition to polyp detection, and would have helped evaluate whether withdrawal times are linked to higher polyp detection by fellows. Our study was limited to one gastroenterology training program with a small number of supervising attendings, and our results may not be generalizable to other gastroenterology programs. This study focused on procedural volume as a determinant of improvement in polyp detection. However, the quality of the endoscopic and didactic training of fellows is also important when considering improvement in their polyp detection skills.
Measurement of the ADR is an essential component of continuous quality improvement in colonoscopy, and is an important metric for all practicing gastroenterologists. Yet there are no requirements for measuring the ADR or PDR during fellowship training, and there seems to be a gap in trainee knowledge when it comes to quality in colonoscopy. In a survey of gastroenterology trainees, less than 50% of respondents correctly identified the recommended national benchmarks for ADR[24]. The inclusion of the ADR (or the less preferred PDR) as a component of the colonoscopy assessment tool is a critical step towards a more objective measure of trainee performance, and provides the needed emphasis on quality of colonoscopy during training. It is likely that fellows achieve a 90% cecal intubation rate long before they acquire the necessary skills to improve their polyp detection skills. Therefore, we recommend establishing a separate category of “colonoscopy quality” for assessing colonoscopy skills. To do this, fellows can be evaluated using objective colonoscopy assessment tools (e.g., the ACE tool) and periodically given an overall motor skill score, cognitive skill score, and quality score (ADR or PDR). Despite the overall increase in the ADR and PDR, we found that fellows vary substantially in their individual polyp detection rates (Table 1 and Figure 1). In addition, there are intra-individual variations and fluctuations in ADR, PDR and AADR throughout fellowship training (Figure 3), which are likely substantially related to variations in the characteristics of patients undergoing colonoscopy (e.g., true numbers of polyps/adenomas, age, prep quality). Therefore, it is important to measure these metrics at multiple intervals throughout fellowship in order to evaluate trends rather than inappropriately weighing a single value. A few fellows had a relatively low ADR and PDR even in their later stages of training. Such trainees could benefit from targeted feedback and training to improve their polyp detection. Some studies found that providing a quality report card to gastroenterologists results in an improved ADR[25,26]. It is unclear whether this would have a similar effect on trainees during fellowship. Nevertheless, continuous measurement of the ADR during fellowship could instill the habit of quality monitoring, provide opportunities for self-improvement, and prepare fellows for similar activities when they start practicing as independent gastroenterologists.
In summary, we found that the ADR, PDR, and other indicators of polyp detection increase with increasing colonoscopy volume during training, and that it requires between 265-400 colonoscopies for fellows to reach the adenoma detection level of attendings. We recommend increased focus on colonoscopy quality during fellowship training, with establishment of a separate colonoscopy quality score for each fellow to be incorporated in periodic trainee feedback and evaluations.
Adenoma and polyp detection rates (ADR and PDR) are important quality metrics for colonoscopy. Several studies found that participation of gastroenterology fellows in screening colonoscopies is associated with increased ADR and PDR. During gastroenterology training, competency in colonoscopy is measured by the ability of the trainee to achieve cecal intubation in a timely manner (< 15 min) and resect polyps independently.
In addition to traditional milestones of competence in colonoscopy, it is important to examine the effect of procedural volume on the quality of colonoscopy performed by fellows under the supervision of attendings. The aim of this study was to investigate changes in polyp detection throughout fellowship training, and estimate the colonoscopy volume required to achieve the ADRs and PDRs of attending gastroenterologists.
The authors performed a retrospective cohort study of 12 fellows who completed three full years of training. The authors examined the change in ADR, PDR, and advanced ADR for each individual fellow and as a group using longitudinal modelling. The majority of fellows increased their ADR and PDR throughout their fellowship training as they performed more colonoscopies. The ADR increase was limited to the right side of the colon, while the PDR increased for both the right and left colon. The adenoma per colon and polyp per colon also increased throughout training, providing further evidence that polyp detection is a skill that continues to improve throughout fellowship. Fellows reached ADR and PDR levels similar to those of the attendings’ average values after 265 and 292 colonoscopies, respectively.
This study provides important insight into the progression of polyp detection skills of trainees throughout fellowship. It also supports the recent recommendations of ≥ 275 colonoscopies for colonoscopy credentialing. Quality metrics during fellowship training could complement other evaluation tools for colonoscopy training. Fellows should monitor their own ADR throughout fellowship and strive for continued improvement.
The manuscript written by Qayed et al analyzed the relation between adenoma or polyp detection rates and colonoscopy volume. They found that ADR and PDR increase with increasing colonoscopy volume throughout fellowship. The data are well analyzed and important.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shimizu Y, Trifan A S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12982] [Article Influence: 1442.4] [Reference Citation Analysis (2)] |
| 2. | Dominic OG, McGarrity T, Dignan M, Lengerich EJ. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104:2626-2627; author reply 2628-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2276] [Article Influence: 175.1] [Reference Citation Analysis (1)] |
| 4. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1151] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 5. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1462] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 7. | Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [PubMed] |
| 8. | Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 10. | Spier BJ, Benson M, Pfau PR, Nelligan G, Lucey MR, Gaumnitz EA. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010;71:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Sedlack RE. Training to competency in colonoscopy: assessing and defining competency standards. Gastrointest Endosc. 2011;74:355-366.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Patwardhan VR, Feuerstein JD, Sengupta N, Lewandowski JJ, Tsao R, Kothari D, Anastopoulos HT, Doyle RB, Leffler DA, Sheth SG. Fellowship Colonoscopy Training and Preparedness for Independent Gastroenterology Practice. J Clin Gastroenterol. 2016;50:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Sedlack RE, Coyle WJ; ACE Research Group. Assessment of competency in endoscopy: establishing and validating generalizable competency benchmarks for colonoscopy. Gastrointest Endosc. 2016;83:516-523.e1. [PubMed] [DOI] [Full Text] |
| 14. | Peters SL, Hasan AG, Jacobson NB, Austin GL. Level of fellowship training increases adenoma detection rates. Clin Gastroenterol Hepatol. 2010;8:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Lee SH, Chung IK, Kim SJ, Kim JO, Ko BM, Hwangbo Y, Kim WH, Park DH, Lee SK, Park CH. An adequate level of training for technical competence in screening and diagnostic colonoscopy: a prospective multicenter evaluation of the learning curve. Gastrointest Endosc. 2008;67:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Buchner AM, Shahid MW, Heckman MG, Diehl NN, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc. 2011;73:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Munroe CA, Lee P, Copland A, Wu KK, Kaltenbach T, Soetikno RM, Friedland S. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc. 2012;75:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Gianotti RJ, Oza SS, Tapper EB, Kothari D, Sheth SG. A Longitudinal Study of Adenoma Detection Rate in Gastroenterology Fellowship Training. Dig Dis Sci. 2016;61:2831-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, Bain AS, Mackintosh EE, Paek AM, Crissien AM. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc. 2011;74:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Rex DK. Split dosing for bowel preparation. Gastroenterol Hepatol (N Y). 2012;8:535-537. [PubMed] |
| 21. | ASGE Training Committee. Sedlack RE, Coyle WJ, Obstein KL, Al-Haddad MA, Bakis G, Christie JA, Davila RE, DeGregorio B, DiMaio CJ, Enestvedt BK, Jorgensen J, Mullady DK, Rajan L. ASGE’s assessment of competency in endoscopy evaluation tools for colonoscopy and EGD. Gastrointest Endosc. 2014;79:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | ASGE Standards of Practice Committee. Faulx AL, Lightdale JR, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Kelsey L, Khashab MA, Kothari S, Muthusamy VR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Guidelines for privileging, credentialing, and proctoring to perform GI endoscopy. Gastrointest Endosc. 2017;85:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 23. | Jung DK, Kim TO, Kang MS, Kim MS, Kim MS, Moon YS. The Colonoscopist’s Expertise Affects the Characteristics of Detected Polyps. Clin Endosc. 2016;49:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Thompson JS, Lebwohl B, Syngal S, Kastrinos F. Knowledge of quality performance measures associated with endoscopy among gastroenterology trainees and the impact of a web-based intervention. Gastrointest Endosc. 2012;76:100-106.e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kahi CJ, Ballard D, Shah AS, Mears R, Johnson CS. Impact of a quarterly report card on colonoscopy quality measures. Gastrointest Endosc. 2013;77:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Keswani RN, Yadlapati R, Gleason KM, Ciolino JD, Manka M, O’Leary KJ, Barnard C, Pandolfino JE. Physician report cards and implementing standards of practice are both significantly associated with improved screening colonoscopy quality. Am J Gastroenterol. 2015;110:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |