Published online Jan 25, 2016. doi: 10.4253/wjge.v8.i2.67
Peer-review started: July 7, 2015
First decision: August 5, 2015
Revised: October 30, 2015
Accepted: November 17, 2015
Article in press: November 25, 2015
Published online: January 25, 2016
Processing time: 201 Days and 11.9 Hours
Endoscopic ultrasound (EUS) has become an important component in the diagnosis and treatment of carcinoma pancreas. With the advent of advanced imaging techniques and tissue acquisition methods the role of EUS is becoming increasingly important. Small pancreatic tumors can be reliably diagnosed with EUS. EUS guided fine needle aspiration establishes diagnosis in some cases. EUS plays an important role in staging of carcinoma pancreas and in some important therapeutic methods that include celiac plexus neurolysis, EUS guided biliary drainage and drug delivery. In this review we attempt to review the role of EUS in diagnosis and management of carcinoma pancreas.
Core tip: Endoscopic ultrasound (EUS) is becoming increasingly important in the diagnosis and management of carcinoma pancreas. It helps in identification of small tumors, histological diagnosis by fine needle aspiration, staging of the disease and its treatment. Palliation of pain with celiac plexus neurolysis and palliation of jaundice by biliary drainage can be achieved with EUS guided techniques. In this review we attempt to review the role of EUS in different aspects of diagnosis and treatment of carcinoma pancreas.
- Citation: Puri R, Manrai M, Thandassery RB, Alfadda AA. Endoscopic ultrasound in the diagnosis and management of carcinoma pancreas. World J Gastrointest Endosc 2016; 8(2): 67-76
- URL: https://www.wjgnet.com/1948-5190/full/v8/i2/67.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i2.67
Pancreatic cancer, according to SEER database in the United States, constitutes 3% of all new cancer cases. The number of new cases of pancreas cancer was 12.4 per 100000 men and women per year and the number of deaths were 10.9 per 100000 men and women per year based on 2008-2012 cases. It is more common with increasing age and slightly more common in men than women. The median age of diagnosis was 71 years, the median age of death being 73 years. It is estimated that there will be 48960 new cases of pancreas cancer and an estimated 40560 people will die of this disease in 2015. Using statistical models for analysis, rates for new pancreas cancer cases have been rising on average 0.8% each year over the last 10 years but the death rates have been stable, the 5 year survival being a dismal 5%-7.2%[1,2]. This spells out the magnitude of the problem with this disease.
The role of endoscopic ultrasound (EUS) evaluation of pancreatic cancer was suggested as an independent predictor of survival and improvement in patients with loco regional pancreatic cancer in a recent study[3]. We will highlight the various aspects of the role of EUS in the setting of pancreatic cancer.
Nattermann et al[4] and Catalano et al[5] described the pancreatic parenchyma as a homogeneous fine granular, reticulated pancreas with smooth margins without evidence of side-branch ectasia. The pancreatic duct diameter in the body was 1.7 to 1.9 mm on average (range, 1-3 mm), a ventral anlage (echogenic difference between the ventral and dorsal pancreas) was seen in up to 68% of controls. These data from control populations and healthy volunteers provide important standards for the normal endosonographic appearance of the pancreas but are limited by their small numbers and potential biases in control populations.
On the other hand, neoplastic masses may obscure the normal parenchymal and ductal features. They are generally more homogeneous; hypoechoic compared to surrounding tissue and are rarely calcified. In a calcified pancreas, neoplastic lesions frequently push the calcified parenchyma towards the periphery. In addition signs of vascular invasion are highly suggestive of malignancy[6].
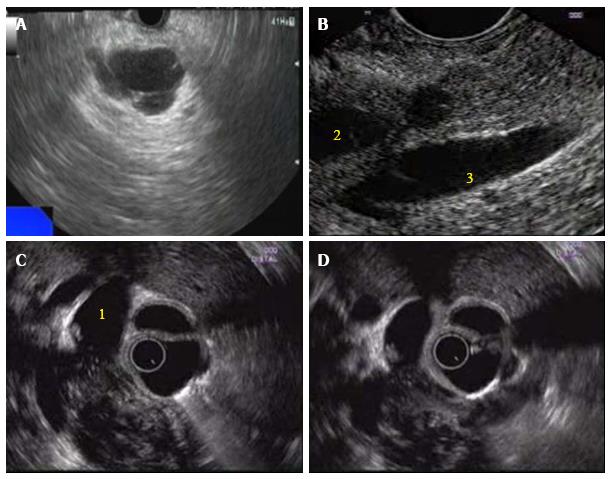
EUS has high sensitivity for detecting pancreatic neoplasms and further provides the ability to obtain samples from suspected lesions by fine needle aspiration (FNA) contributing to its accuracy in the diagnosis of pancreatic cancer. It has been considered one of the most precise methods for the detection of pancreatic focal lesions, especially in patients with small tumors of 3 cm or less[7,8] (Figure 1). The reported sensitivity and accuracy of combined EUS-FNA for detecting pancreatic malignancy usually exceeds 90%[9-14]. A recent meta-analysis mentioned the pooled sensitivity and specificity of EUS FNA ranging between 87% and 96%, respectively, for diagnosing a solid pancreatic mass lesion[15]. The sensitivity and accuracy of EUS are slightly higher than the sensitivity and accuracy of computed tomography (CT) and Magnetic resonance imaging (MRI) in detecting small pancreatic lesions[16-19].
EUS can be used to assess TNM staging of pancreatic tumors. T1 lesions are smaller than 2 cm, T2 are lesions larger than 2 cm, tumor extending beyond the pancreas is either a T3 (portal vein, duodenum, or ampulla of Vater) or T4 lesions (extending to the celiac artery or superior mesenteric artery; being unresectable). Malignant nodes around the pancreas are N1 lesions and rarely distant metastasis may be seen (M1 lesion). The accuracy of CT, MRI, and EUS in assessing TNM staging of pancreatic cancer was compared by Soriano et al[20] wherein EUS had the highest accuracy for N-staging (65%) although CT was more accurate in assessing vascular invasion and T-staging. However in a retrospective study from Russia by Egorov et al[21], arterial encasement on CT did not necessarily indicate arterial invasion and in unresectable pancreatic cancers (on CT), EUS data for peripancreatic involvement might suggest possible radical resection, providing survival benefits. It has also been used as a screening tool for individuals at a high risk for pancreatic cancer with incidence of clinically relevant findings at first screening being 7% with asymptomatic cancer and 16% premalignant IPMN-like lesions in a study by Poley et al[22].
The diagnostic reliability of EUS-FNA in the evaluation of pancreatic lesions is predictably affected by operator expertise, cytopathologic interpretation, and other variables including the presence of inflammatory changes[9,23]. A definite diagnosis cannot be ascertained in a significant minority of EUS-FNA samples alone, resulting in a cytological diagnosis of suspicious or indeterminate for neoplasm which is seen in approximately 8% to 10% of EUS-FNA samples, representing a challenging diagnostic dilemma[12,23,24]. In addition, presence of chronic pancreatitis may decrease the sensitivity of EUS-FNA as noted by Varadarajulu et al[25] where in the sensitivity was ranging from 73% to 91%, being lower in patients with chronic pancreatitis; and the No Endosonographic Detection of Tumor study[26] had revealed 60% patients with co-existing chronic pancreatitis and 15% patients with a diffuse malignancy which was not detected earlier. Furthermore Siddiqui et al[27] in their retrospective cohort trial found a false positive rate for EUS-FNA of solid pancreatic lesions of 1.1% as a result of cytologic misinterpretation in the setting of chronic pancreatitis.
Few basic remedial factors to improve the yield of EUS FNA were the use of 25 gauge needle as less blood is aspirated instead of conventional 22 gauge needle[28-30], combining cytologic and histologic analyses of the specimen to decrease the number of passes to 2[31] from 4 to 7 passes[32] (higher in pancreatic cancer than in other lesions), to cater for rapid on-site cytological evaluation[33-35], the use of serum CA19-9[36] and fluid CEA and CA19-9 for increasing the ability to diagnose malignancy especially in suspicious cases[37].
Developments have taken place to further refine the ability to differentiate a malignant lesion from a benign one with a reasonable certainty and overcome other limitations. There have been improvements in the imaging techniques with EUS as well as advances in cytopathology analysis. Among the newer technologies there are EUS elastography, contrast enhanced EUS and use of chromosomal detection techniques in FNA specimen.
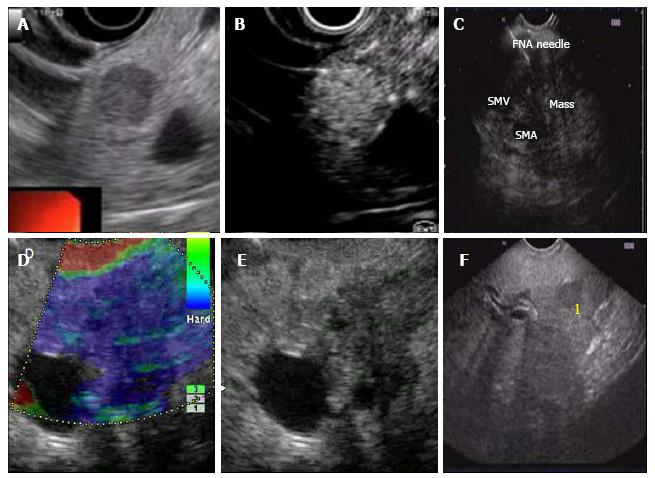
EUS elastography is a noninvasive technique that measures elasticity in real time by registration of differences in distortion of the EUS image after application of slight pressure by the EUS probe (Figures 2 and 3). Tissue elasticity may be altered by inflammation, fibrosis and cancer resulting in distinct elastographic appearance. Initial studies were based on qualitative elastography evaluation, using a hue-color scale representing different degrees of tissue elasticity. Giovannini et al[38] had sensitivity and a specificity of 100% and 67% respectively while analyzing pancreatic masses using a scoring system based on different color patterns to differentiate between benign and malignant pancreatic masses. In a subsequent multicenter study[39], the sensitivity and specificity of EUS elastography to differentiate benign from malignant pancreatic lesions were 92% and 80.0%, respectively, compared to 92% and 69%, respectively, for the conventional B-mode images. In another paper by Iglesias-Garcia et al[40], malignancy could be diagnosed by qualitative EUS-elastography using color patterns with a sensitivity, specificity and overall accuracy of 100%, 85.5% and 94%, respectively. Recently quantitative EUS elastography has been developed in an attempt to make the elastography interpretation less subjective. Quantitative elastography gives a numeric result, either as mean value of hues in a selected area (mean hue histogram) or as a ratio of elasticity in the target area over soft reference tissue (strain ratio). Iglesias-Garcia et al[41], have evaluated strain ratio in 86 consecutive patients with solid pancreatic masses and found the strain ratio was significantly higher among patients with malignant pancreatic tumors compared to those with inflammatory masses (Normal pancreatic tissue: 1.68; inflammatory masses: 3.28; pancreatic adenocarcinoma: 18.12; and the highest strain ratio was found among endocrine tumors). The sensitivity and specificity of the strain ratio for detecting pancreatic malignancies using a cutoff value of 6.04 were 100% and 92.9%, respectively, exceeding the accuracy obtained with qualitative elastography. Săftoiu et al[42] evaluated the usefulness of the hue-histograms in a multicenter study wherein a sensitivity of 93.4%, a specificity of 66.0%, a positive predictive value of 92.5% and an overall accuracy of 85.4% for the mean hue-histogram in the detection of malignancy were observed. In a further development, Schrader et al[43] had 100% sensitivity and specificity in differentiating benign from malignant lesions in tissues with blue color (hard tissue), on histogram with less discrimination on evaluating areas with red or green colors representing softer tissue. The role of this modality is still evolving to reduce the various biases of calculation of strain.
Contrast-enhanced (CE)-EUS consists of administration of contrast agents through the blood stream. The contrast agent contains microbubbles that can be detected by EUS in the small, low-velocity vasculature of pancreatic tumors on real-time evaluation. Initial studies using Levovist®, Albunex and FS 069 Optison as contrast agents demonstrated that the hyper vascular aspect of neuroendocrine tumors and the hypo vascular aspect of pancreatic adenocarcinoma[44-48]. Modern contrast enhanced EUS relies on a dedicated contrast harmonic echo-EUS (CHE-EUS) technique that detects signals from micro bubbles delivered by new contrast agents like Sonovue® in vessels with very slow flow as they have longer perfusion time and stronger backscatter without the burden of Doppler-related artifacts. Fusaroli et al[49] investigated 90 patients with solid pancreatic lesions by CEH-EUS, using Sonovue® as contrast agent. The finding of a hypo-enhancing mass with an inhomogeneous pattern diagnosed pancreatic adenocarcinoma with a sensitivity of 96% and an accuracy of 82%. The study also indicated that this CEH-EUS pattern diagnosed malignancy more accurately than the finding of a hypoechoic mass on standard EUS. Hyper-enhancement specifically excluded adenocarcinoma (98%), although sensitivity was low (39%). In a study by Napoleon et al[50], the finding of a hypo-enhanced lesion was able to detect malignancy with a sensitivity, specificity and accuracy of 89%, 88%, and 88.5%, respectively. Seicean et al[51] investigated the possibility to use quantitative CEH-EUS data in the differential diagnosis between pancreatic cancer and chronic pancreatitis. A hypo-enhanced pattern was the most common finding both in pancreatic adenocarcinoma and in mass forming chronic pancreatitis. However, an index of contrast uptake ratio was calculated and this was significantly lower in adenocarcinoma compared to cases with mass-forming chronic with a sensitivity of 80% and a specificity of 91.7%. A recent prospective study by Kitano et al[52] showed that when CH-EUS was combined with EUS-FNA, the sensitivity of EUS-FNA increased from 92.2% to 100%. Data from South Korea showed a sensitivity and diagnostic accuracy of 93% and 92%, respectively for the diagnosis of pancreatic cancer[53]. In a recent retrospective study by Park et al[54] pancreatic adenocarcinomas showed a hypoenhanced pattern on CH-EUS with a sensitivity of 92%, the specificity of 68% and the accuracy approximately 82%.
In a recent review, Kitano et al[55] have mentioned that CH-EUS identifies pancreatic adenocarcinomas as solid lesions exhibiting hypo-enhancement with a sensitivity and specificity of 88%-96% and 88%-94%, respectively. In particular, 80%-100% of false-negative cases in EUS-FNA are correctly classified by CH-EUS, suggesting its complementary role. In addition, it improves depiction of some subtle lesions in conventional EUS, thus facilitating EUS-FNA. For quantitative perfusion analysis, a time-intensity curve (TIC) for the region of interest can be generated during CH-EUS. The maximum intensity gain and the echo intensity reduction rate from the peak at 1 min obtained by TIC can be used for differentiation of pancreatic adenocarcinoma from other tumors. CH-EUS is also useful for differentiation of invasive intraductal papillary mucinous neoplasms (IPMN) from non-invasive IPMN[55]. Thus, CH-EUS technology is very promising and is likely to play a role in the precise diagnosis of malignant pancreatic lesions.
The detection of various chromosomal abnormalities in FNA aspirates is a field which is rapidly evolving. It is useful in cases with indeterminate results and might help in confirming the diagnosis of a malignancy. Among the earlier studies, telomerase activity was studied by Mishra et al[56] which on combination with cytology results increased the sensitivity from 85% to 98% with 100% specificity. The use of fluorescence in situ hybridization (FISH) analysis by Kubiliun et al[57] on FNA specimens with inconclusive results revealed a sensitivity of 74% for detecting pancreatic cancer which increased to 85% on combining with cytology. Reicher et al[58] from US demonstrated the use of detecting K-ras mutation in addition to FISH analysis in precisely identifying 60% of atypical FNAs with final malignant diagnosis yielding 88% sensitivity and 94% specificity with 90% accuracy. The pooled sensitivity of EUS-FNA for the differential diagnosis of pancreatic adenocarcinoma was 80.6%, specificity was 97% and probable sensitivity and specificity were 76.8% and 93.3% for K-ras gene analysis, respectively. For combined EUS-FNA plus K-ras mutation analysis it was 88.7% and 92%, in a meta-analysis by Fuccio et al[59]. Overall, K-ras mutation testing applied to inconclusive cases by EUS-FNA reduced the false-negative rate by 55.6% albeit with a false-positive rate of 10.7%. Layfield et al[60] in their guidelines mention that many gene mutations (KRAS, GNAS, VHL, RNF43, and CTNNB1) may be of aid in the diagnosis of cystic neoplasms. The shortcoming of detecting chromosomal abnormalities in FNA specimens is that pancreatic cancers may express multiple mutations, detecting more might increase the sensitivity but with doubtful cost effectiveness.
The increasing use of EUS as a diagnostic modality has also led to its importance as an interventional tool in the management of pancreatic cancer. It ranges from assisting in radiotherapy, delivery of chemotherapeutic agents to palliation by celiac plexus neurolysis and biliary drainage wherever ERCP fails.
EUS delivery of antitumor agents is largely investigational and is still in experimental stage. The requirement to develop this option is due to pancreatic carcinoma having a poor response to chemotherapeutic agents and radiation; and neoadjuvant chemotherapy can lead to a desmoplastic reaction further impairing drug delivery. Chang et al[61] used cytoimplant (Allogenic mixed lymphocyte culture) advanced pancreatic cancer with partial response noted in two patients. TNFerade biologic is a replication-deficient adenoviral vector that expresses tumor necrosis factor-α (TNF-α), regulated by a radiation inducible promoter; inducible by chemotherapy and radiation has been used by various authors. Hecht et al[62] had shown one complete response, 3 partial responses, and 12 patients with stable disease, overall 3 survived > 24 mo. Subsequently Herman et al[63], reported in the randomized phase III trial among patients with locally advanced pancreatic cancer (LAPC) that though it is safe in combination with chemotherapy, it does not increase survival. ONYX-015, an adenovirus which preferentially replicates and kills malignant cells was studied by Hecht et al[64] wherein 2 patients had partial regression of the injected tumor, 2 had minor responses, 6 had stable disease, and 11 had progressive disease with 2 patients each having sepsis and duodenal perforation. The injection of immature dendritic cells, which induce T-cell immune response against malignant cells, was used by Irisawa et al[65] successfully into the tumors of 7 patients with unresectable pancreatic cancer, with a cohort median survival of 9.9 mo. Thereafter, Hirooka et al[66] using the same therapy demonstrated effective responses in three of five patients; 1 had partial remission and 2 had long stable disease of more than 6 mo. This combined therapy was synergistically effective. Despite these studies, much more large prospective studies are required before these techniques are translated into clinical practice.
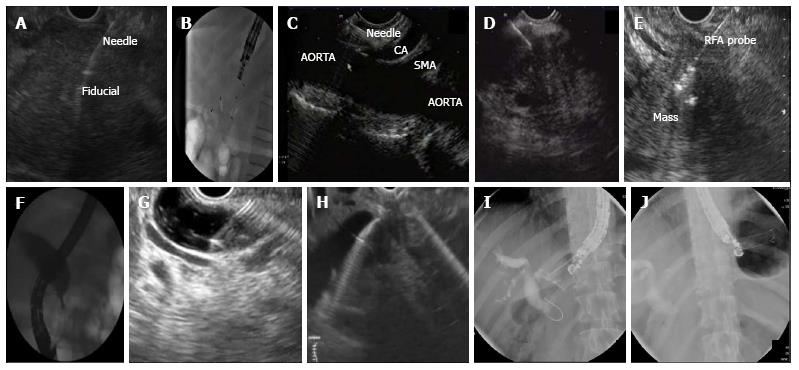
EUS guided brachytherapy has been carried out with radioactive seeds being placed into the tumour with the help of linear echoendoscope. The most popular radioactive seeds are Iodine 125, palladium 103 and iridium 192; iodine being the preferred radioactive material due to its long half life of 60 d in pancreatic cancers with rapidly dividing cells. Jin et al[67] in their experience achieved partial remission in three cases, estimated median survival time of nine months with improvement in pain but no survival benefit.
EUS guided fiducial insertion is being done in pancreatic malignancy to place markers inside the tumor for guiding stereotactic body radiotherapy. These markers can be radioactive spheres, coils or seeds. Its feasibility was shown by Pishvaian et al[68] wherein he reported a technical success of 85%. Subsequently in a prospective study by Park et al[69] fiducial insertion was successful in 88% of the 57 patients, Sanders et al[70] had a success rate of 90% for EUS fiducial insertion in a prospective study of 51 patients while DiMaio et al[71] achieved a success rate of 97% with a 22-gauge needle. Law et al[72] found this technique safe and feasible to assist intraoperative localization of small pancreatic neuroendocrine tumors. The 2 types of fiducials were compared by Khashab et al[73] in 39 patients with advanced pancreatic cancer. Traditional fiducials of 5 mm length had better visibility scores with similar migration rates as compared to viscoil fiducials of 10 mm length.
EUS-guided cryothermal ablation has been studied by Arcidiacono et al[74] in 22 patients with unresectable stage III pancreatic adenocarcinoma with a feasibility of 73% with insignificant tumor size reduction. Further studies are required to demonstrate progression-free survival and local effects. Recently Pai et al[75] used radiofrequency ablation (RF) which was applied with a monopolar RF probe (1.2 mm Habib EUS-RFA catheter) placed through a 19 or 22 gauge FNA needle after FNA was performed in patients with a tumor in the head of the pancreas with a 100% success rate. The response ranged from complete resolution to a 50% reduction in size. Oh et al[76,77] used EUS-guided ethanol lavage with paclitaxel injection (EUS-EP) for cystic tumors of the pancreas in two studies and found a 62%-99% resolution rate with adequate safety and feasibility. These data indicate the need for further large prospective studies to ascertain their roles in the management of pancreatic cancer.
EUS guided celiac plexus neurolysis (CPN) provides pain relief, palliation and reduces narcotic use in patients with unresectable pancreatic cancer[78]. The injection of a neurolytic drug into the celiac plexus disrupts the signal transmission to spinal cord and central nervous system. Due to the anatomical location of the celiac plexus around the origin of the celiac trunk and the superior-mesenteric artery, EUS- CPN provides real-time visualization for a safe approach.
EUS-CPN was demonstrated to be safe and effective in alleviating refractory pain due to pancreatic cancer in a meta-analysis of 8 studies by Puli et al[79]. Alcohol-based EUS-CPN was found safe and effective in this setting providing pain relief to 73% patients[80]. A recent RCT by Wyse et al[81] in 96 patients demonstrated greater pain relief in the early EUS-CPN group at three months than in conventional management group. As compared to opioids, EUS-CPN reduced pain at four and eight weeks and significantly reduced opioid consumption[82]. In addition a single central injection was found to be as effective as bilateral or multiple injections[83,84]. In another comparison between EUS-CPN and EUS-celiac ganglia neurolysis (CGN), Doi et al[85] observed higher treatment response rate and complete response rate in the EUS-CGN group compared to the EUS-CPN group.
EUS guided biliary drainage is another important area where therapeutic EUS is helpful. With failed ERCP, biliary drainage can be established by 3 endoscopic methods (1, transluminal biliary drainage with hepaticogastrostomy or choledochoduodenomstomy, 2, EUS antegrade drainage and 3, EUS rendezvous drainage)[86]. In 7% to 13% of patients with pancreatic head malignancy have duodenal stenosis, making ERCP technically challenging or impossible[87].
The role EUS guided biliary drainage in pancreatic cancer in failed ERCP has been recently demonstrated by Weilert[88] in 21 patients, 52% patients with pancreatic cancer wherein he achieved technical success in 20/21 (95.2%) and clinical success 19/21 (90.4%). He noted that EUS-guided anterograde biliary drainage using the intra-hepatic access route had high technical and clinical success with low adverse rate. In a recent study of 208 patients with malignant distal CBD obstruction requiring SEMS placement, authors compared the short-term outcome of single session EUS guided biliary drainage with ERCP[89]. SEMS placement was successful in 97 and 98 patients in the respective groups (93.26% vs 94.23%, P = 1.00). The incidence of pancreatitis was higher with ERCP, and EUS group had superior treatment success rates in patients with duodenal stenosis.
EUS is rapidly becoming a sensitive and specific modality for diagnosing pancreatic cancer especially on combining with EUS-FNA albeit with difficulty in the presence of chronic pancreatitis. With the advent of newer technology in the form of EUS elastography, CE-EUS, and gene mutations detection in FNA specimens the diagnostic dilemma is better resolved. The availability of interventional EUS has allowed gastroenterologists to make significant difference in management of pancreatic cancer by its various therapeutic options including areas which have been traditionally dealt by surgeons and interventional radiologists. It is likely to become an important modality in the multidisciplinary management of pancreatic cancer.
P- Reviewer: Klinge U, Yoshida H S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Surveillance Research Program. SEER Stat Fact Sheets: Pancreas Cancer. Available from: http: //seer.cancer.gov/statfacts/html/pancreas.html. |
| 3. | Ngamruengphong S, Li F, Zhou Y, Chak A, Cooper GS, Das A. EUS and survival in patients with pancreatic cancer: a population-based study. Gastrointest Endosc. 2010;72:78-83, 83.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis--a comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 208] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Holt BA, Varadarajulu S. Features of Chronic Pancreatitis and Associated Masses: A Focus on Endosonography. VJGIEN. 2014;2:50-54. |
| 7. | Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 266] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, Modena JL. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112-3116. [PubMed] |
| 10. | Faigel DO, Ginsberg GG, Bentz JS, Gupta PK, Smith DB, Kochman ML. Endoscopic ultrasound-guided real-time fine-needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol. 1997;15:1439-1443. [PubMed] |
| 11. | Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 13. | Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, van Velse A, Osborne JF, Hoffman BJ. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 16. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, Broemeling LD, Cleary KR, Charnsangavej C. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Koelblinger C, Ba-Ssalamah A, Goetzinger P, Puchner S, Weber M, Sahora K, Scharitzer M, Plank C, Schima W. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology. 2011;259:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Arslan A, Buanes T, Geitung JT. Pancreatic carcinoma: MR, MR angiography and dynamic helical CT in the evaluation of vascular invasion. Eur J Radiol. 2001;38:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Egorov VI, Petrov RV, Solodinina EN, Karmazanovsky GG, Starostina NS, Kuruschkina NA. Computed tomography-based diagnostics might be insufficient in the determination of pancreatic cancer unresectability. World J Gastrointest Surg. 2013;5:83-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-736; quiz 751, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Bhutani MS, Gress FG, Giovannini M, Erickson RA, Catalano MF, Chak A, Deprez PH, Faigel DO, Nguyen CC. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Siddiqui AA, Kowalski TE, Shahid H, O’Donnell S, Tolin J, Loren DE, Infantolino A, Hong SK, Eloubeidi MA. False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc. 2011;74:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Othman MO, Raimondo M. Endoscopic ultrasound fine needle aspiration of pancreatic lesions: is a smaller needle safer and better? Dig Liver Dis. 2011;43:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Baccarini P, Collina G, Fornelli A, Macchia S, Zanini N, Jovine E. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Möller K, Papanikolaou IS, Toermer T, Delicha EM, Sarbia M, Schenck U, Koch M, Al-Abadi H, Meining A, Schmidt H. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Jhala NC, Eltoum IA, Eloubeidi MA, Meara R, Chhieng DC, Crowe DR, Jhala D. Providing on-site diagnosis of malignancy on endoscopic-ultrasound-guided fine-needle aspirates: should it be done? Ann Diagn Pathol. 2007;11:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Yang D, MoezArdalan K, Collins DP, Chauhan SS, Draganov PV, Forsmark CE, Wagh MS. Predictors of malignancy in patients with suspicious or indeterminate cytology on pancreatic endoscopic ultrasound-guided fine-needle aspiration: a multivariate model. Pancreas. 2014;43:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Aljebreen AM, Romagnuolo J, Perini R, Sutherland F. Utility of endoscopic ultrasound, cytology and fluid carcinoembryonic antigen and CA 19-9 levels in pancreatic cystic lesions. World J Gastroenterol. 2007;13:3962-3966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 42. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M, Dietrich C, Havre R, Gheorghe C, McKay C, Gheonea DI, Ciurea T; European EUS Elastography Multicentric Study Group. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Schrader H, Wiese M, Ellrichmann M, Belyaev O, Uhl W, Tannapfel A, Schmidt W, Meier J. Diagnostic value of quantitative EUS elastography for malignant pancreatic tumors: relationship with pancreatic fibrosis. Ultraschall Med. 2012;33:E196-E201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Bhutani MS, Hoffman BJ, van Velse A, Hawes RH. Contrast-enhanced endoscopic ultrasonography with galactose microparticles: SHU508 A (Levovist). Endoscopy. 1997;29:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Hirooka Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hayakawa T, Naitoh Y. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: a preliminary study. Am J Gastroenterol. 1998;93:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Becker D, Strobel D, Bernatik T, Hahn EG. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Giovannini M. Endosonography: new developments in 2006. ScientificWorldJournal. 2007;7:341-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246-250. [PubMed] |
| 49. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 50. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 53. | Lee TY, Cheon YK, Shim CS. Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating solid lesions of the pancreas: a single-center experience in Korea. Gut Liver. 2013;7:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Park JS, Kim HK, Bang BW, Kim SG, Jeong S, Lee DH. Effectiveness of contrast-enhanced harmonic endoscopic ultrasound for the evaluation of solid pancreatic masses. World J Gastroenterol. 2014;20:518-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Kitano M, Kamata K, Imai H, Miyata T, Yasukawa S, Yanagisawa A, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27 Suppl 1:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Mishra G, Zhao Y, Sweeney J, Pineau BC, Case D, Ho C, Blackstock AW, Geisinger K, Howerton R, Levine E. Determination of qualitative telomerase activity as an adjunct to the diagnosis of pancreatic adenocarcinoma by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2006;63:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Kubiliun N, Ribeiro A, Fan YS, Rocha-Lima CM, Sleeman D, Merchan J, Barkin J, Levi J. EUS-FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on-site cytopathology results. Gastrointest Endosc. 2011;74:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Reicher S, Boyar FZ, Albitar M, Sulcova V, Agersborg S, Nga V, Zhou Y, Li G, Venegas R, French SW. Fluorescence in situ hybridization and K-ras analyses improve diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses. Pancreas. 2011;40:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Fuccio L, Hassan C, Laterza L, Correale L, Pagano N, Bocus P, Fabbri C, Maimone A, Cennamo V, Repici A. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | Layfield LJ, Ehya H, Filie AC, Hruban RH, Jhala N, Joseph L, Vielh P, Pitman MB. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: the Papanicolaou Society of Cytopathology guidelines for pancreatobiliary cytology. Diagn Cytopathol. 2014;42:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Hecht JR, Farrell JJ, Senzer N, Nemunaitis J, Rosemurgy A, Chung T, Hanna N, Chang KJ, Javle M, Posner M. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 64. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. [PubMed] |
| 65. | Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, Ohto H, Ohira H. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 71. | DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010;71:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Law JK, Singh VK, Khashab MA, Hruban RH, Canto MI, Shin EJ, Saxena P, Weiss MJ, Pawlik TM, Wolfgang CL. Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg Endosc. 2013;27:3921-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 75. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 76. | Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Oh HC, Seo DW, Song TJ, Moon SH, Park do H, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 78. | Penman ID, Rösch T. EUS 2008 Working Group document: evaluation of EUS-guided celiac plexus neurolysis/block (with video). Gastrointest Endosc. 2009;69:S28-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 81. | Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 82. | Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel Jde J, Ramírez-Luna MA. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound. 2013;2:153-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 85. | Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 86. | Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94-102. [PubMed] |
| 87. | Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. [PubMed] |
| 88. | Weilert F. Prospective evaluation of simplified algorithm for EUS-guided intra-hepatic biliary access and anterograde interventions for failed ERCP. Surg Endosc. 2014;28:3193-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Dhir V, Itoi T, Khashab MA, Park do H, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [PubMed] |