Published online Nov 16, 2016. doi: 10.4253/wjge.v8.i19.716
Peer-review started: May 30, 2016
First decision: July 20, 2016
Revised: August 1, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: November 16, 2016
To compare the healing effects of vonoprazan and lansoprazole on gastric ulcers induced by endoscopic submucosal dissection (ESD).
Data were obtained from a total of 26 patients. Fourteen patients were randomized to the vonoprazan group and 12 were randomized to the lansoprazole group. Patients were administered either 20 mg vonoprazan or 30 mg lansoprazole per day after ESD. Endoscopic images just after ESD, on day 8, and on day 28 were used for the evaluation of the shrinking rate of ESD ulcers. The shrinking rates and the incidence of delayed bleeding were compared between the 2 groups.
The shrinking rates of ESD ulcers on day 8 [vonoprazan group: 61.8% (range: 24.0%-91.1%), lansoprazole group: 71.3% (range: 25.2%-88.6%)] and on day 28 [vonoprazan group: 95.3% (range: 76.2%-100%), lansoprazole group: 97.2% (range: 81.1%-99.8%)] were not statistically different between the 2 groups. On day 28, most of the ulcers in both groups healed to more than 90%, whereas 3 of 14 (21.4%) in the vonoprazan group and 1 of 12 (8.3%) in the lansoprazole group had delayed ulcer healing, which was not statistically different (P = 0.356). The frequency of delayed bleeding was 0 in the both groups. Taken together, there were no significant differences between the two drug groups.
Our study indicates that vonoprazan is potent for the management of ESD ulcers although lansoprazole is also sufficient and cost-effective.
Core tip: Our study highlights the comparison of two drugs (vonoprazan and lansoprazole) for the treatment of gastric ulcers induced by endoscopic submucosal dissection (ESD). There were no significant differences between the two drugs with regard to ulcer shrinkage and delayed bleeding. Our study indicated vonoprazan was potent for the management of ESD ulcers although lansoprazole was also sufficient and cost-effective.
- Citation: Takahashi K, Sato Y, Kohisa J, Watanabe J, Sato H, Mizuno K, Hashimoto S, Terai S. Vonoprazan 20 mg vs lansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc 2016; 8(19): 716-722
- URL: https://www.wjgnet.com/1948-5190/full/v8/i19/716.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i19.716
Endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is a significantly less invasive procedure compared with gastrectomy and is a common procedure. The main advantage of ESD is enabling en-bloc resection of the lesion. Consequently, ESD results in precise histopathological assessment and a low local recurrence rate[1,2]. Since en-bloc resection of large lesions is possible with ESD, the iatrogenic ulcers tend to be large and the complications of ESD, including bleeding and perforation, are more frequent than those of endoscopic mucosal resection (EMR)[1]. Therefore, the management of ESD-induced ulcers is important to prevent adverse events such as delayed bleeding or perforation.
Acid inhibitors such as proton pump inhibitors (PPIs) and H2-blockers have been used for the treatment of acid related diseases, including ESD-induced ulcers. PPIs are mainly used for the treatment of ESD-induced ulcers owing to their superiority to H2 blockers[3,4]. Although PPIs have been useful for the management of ESD-induced ulcers, they have several limitations including short plasma half-life, slow onset of effectiveness, and the problem of cytochrome P450 (CYP) 2C19 polymorphism[4-8].
The potassium-competitive acid blocker (P-CAB) is a new class of gastric acid suppressant that inhibits gastric H+, K+-ATPase in a K+-competitive and reversible manner[9,10]. Vonoprazan was the first orally bioavailable P-CAB and it was approved in Japan in 2014 for the treatment and prevention of acid-related diseases[11]. Vonoprazan exhibits rapid, profound, and sustained suppression of gastric acid secretions and is not affected by CYP2C19 polymorphism[10,12]. It has been reported that the acid-inhibitory effect of vonoprazan is more potent than that of PPIs[6], resulting in greater effectiveness for acid-related diseases such as gastroesophageal reflux disease (GERD) or Helicobacter pylori (H. pylori) eradication. Therefore, vonoprazan could be more effective for the management of ESD-induced ulcers compared to PPIs, which are now the gold standard for the management of ESD-induced ulcers. To the best of our knowledge, there have been no reports comparing the healing effect of vonoprazan and PPIs on ESD-induced ulcers. We conducted a prospective randomized controlled study to compare the healing effect of P-CAB (vonoprazan) and PPI (lansoprazole) on ESD-induced ulcers. The primary aim was to evaluate the shrinking rate of ESD-induced ulcers and the secondary aim was to evaluate the preventive effect of vonoprazan on delayed bleeding.
Thirty consecutive patients, who underwent ESD for EGC between August 2015 and March 2016 at Sado General Hospital, were enrolled in this study. Their medical records were checked to verify whether they were administered antiplatelet agents, anticoagulants, and steroids. H. pylori infection status was confirmed by urease test, histopathology, serum antibody, stool antigen, or urinary antibody. The existence of atrophic gastritis was investigated with the endoscopic images at ESD and classified as closed or open type according to the Kimura-Takemoto classification[13]. Before ESD, a chest and abdominal computed tomography scan was performed on all patients. If metastasis or advanced cancer in other organs was detected, the patient was not included in this study. Furthermore, patients who had undergone gastric surgery before ESD were not included in this study. Those who needed any additional anticancer therapy (surgery and/or chemotherapy) after ESD were excluded. Written informed consent was obtained from the patients before enrollment. The study protocol was approved by the Sado General Hospital Institutional Ethics Committee and carried out in accordance with the Declaration of Helsinki. This study was enlisted in UMIN clinical Trials Registry (UMIN000022006).
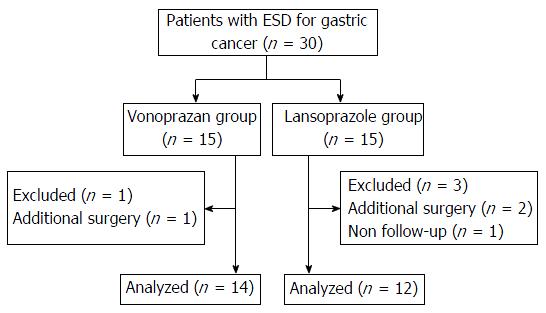
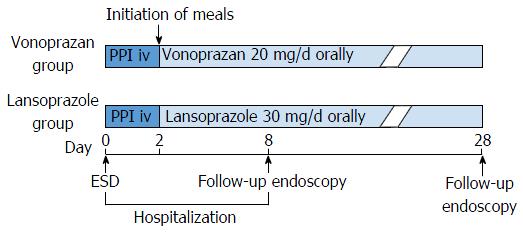
Patients were prospectively and randomly assigned into either the vonoprazan or the lansoprazole group using permuted block randomization (Figure 1). The treatment protocol is shown in Figure 2. Patients were admitted a day before ESD. From the day of ESD, intravenous infusion of PPI (lansoprazole 30 mg) was administered to all patients for 2 d. Two days after ESD, oral intake was initiated and patients in the vonoprazan group were administered vonoprazan (20 mg/d) and patients in the lansoprazole group were administered lansoprazole (30 mg/d) until 28 d after ESD. If the patients were already being administered antiplatelet agents or anticoagulants, these medicines were stopped before ESD and resumed 2 d after ESD. Eight days after the ESD, all patients underwent esophagogastroduodenoscopy (EGD) to evaluate the shrinking rate of ESD ulcers. After EGD on day 8, patients were discharged. Twenty-eight days after ESD, patients underwent follow-up EGD and the shrinking rate of the ulcers on day 28 was evaluated.
ESD procedures were performed using a single channel upper gastrointestinal endoscope (GIF Q260J; Olympus, Tokyo, Japan) with a HookKnife (Olympus, Tokyo, Japan) and a DualKnife (Olympus, Tokyo, Japan). An electrosurgical current was applied using a standard electrosurgical generator (ICC 200; ERBE, Tübingen, Germany). The margin of the lesion was circumferentially dotted using a DualKnife in the forced coagulation mode (30 W). After the application of a 10% glycerin solution containing 0.005 mg/mL of epinephrine into the submucosal layer, a mucosal incision was made using a DualKnife in the endo-cut mode (60 W). Then, the submucosal layer was dissected with a HookKnife in the forced coagulation mode (60 W). Hemostatic forceps (Coagrasper; Olympus, Tokyo, Japan) were used to stop or prevent bleeding in the soft coagulation mode (80 W).
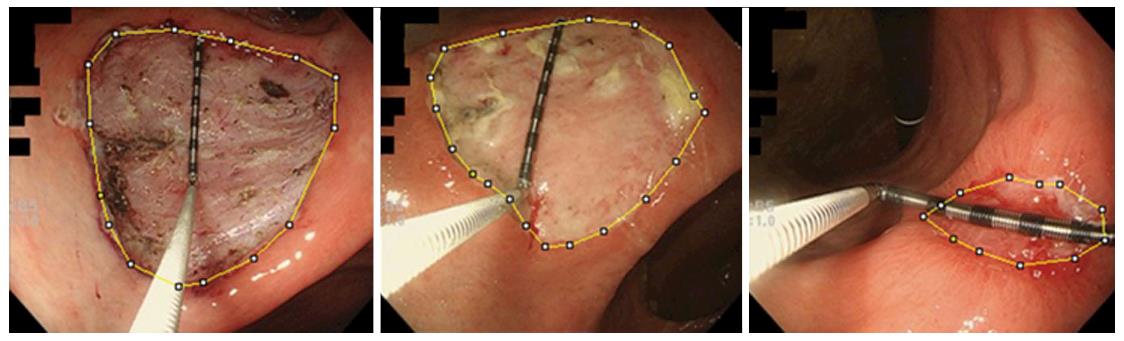
En bloc resection rate, location of the tumors, procedure time, submucosal fibrosis, and histopathology of the tumor were investigated and compared between the two groups. Furthermore, we evaluated the area of ESD ulcer as follows: Endoscopic images were taken just after ESD, on day 8, and on day 28, and image processing software (ImageJ) was used to calculate the area of ESD ulcers (Figure 3). Since this software calculated the area as pixels, measuring forceps were put on the ulcer base and used for the scale, and the area of ESD-induced ulcers was expressed in cubic millimeter. The shrinking rate on day 8 was defined as [1 - (the area of ESD-induced ulcer on day 8)/(the area of ESD-induced ulcer just after ESD)] × 100 (%) and the shrinking rate on day 28 was defined as [1 - (the area of ESD-induced ulcer on day 28)/(the area of ESD-induced ulcer just after ESD)] × 100 (%). Delayed ulcer healing was declared when the shrinking rate on day 28 was less than 90%. The shrinking rates on days 8 and 28 and the frequency of delayed ulcer healing were compared between the 2 groups. The frequency of delayed bleeding was also investigated and compared between the two groups.
Parametric data are expressed as mean ± SD and non-parametric data are expressed as median (range). The χ2 test was used for the categorical data and the Student’s t-test and the Mann-Whitney U test were used for the numerical data. SPSS statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, United States) was used for the statistical analyses. P values of less than 0.05 were considered statistically significant in the χ2 test and the Student’s t-test. Since the critical value of U at P < 0.05 in this study was 45, U values of less than 45 were considered statistically significant in the Mann-Whitney U test.
Thirty patients were enrolled and four of them were excluded because they needed additional surgery or violated the protocol (Figure 1). Data were obtained from a total of 26 patients. Fourteen patients were randomized to the vonoprazan group and the remaining 12 patients were randomized to the lansoprazole group. There were no statistically significant differences between the two groups with regard to backgrounds, including age and sex; use of anticoagulants, antiplatelet agents, and steroids; H. pylori infection state; the degree of endoscopic gastric atrophy; and the location of the tumors (Table 1). Regarding ESD results, en bloc resection rate, procedure time, histopathology of lesions, and the frequency of submucosal fibrosis were not statistically different between the two groups (Table 1). The results of the evaluation of ESD-induced ulcers and delayed bleeding are shown in Table 2. The median areas of ESD-induced ulcers just after ESD in the vonoprazan group and the lansoprazole group were 1446.9 (range: 605-3977.4) mm3 and 1262.6 (range: 597.8-7322.3) mm3, respectively, and were not statistically different. The median shrinking rates of ESD-induced ulcers on day 8 were 61.8% (range: 24.0%-91.1%) in the vonoprazan group and 71.3% (range: 25.2%-88.6%) in the lansoprazole group and those on day 28 were 95.3% (range: 76.2%-100%) in the vonoprazan group and 97.2% (range: 81.1%-99.8%) in the lansoprazole group. The median shrinking rates of ESD-induced ulcers on both days 8 and 28 were not statistically different. On day 28, most of the ulcers in both groups healed to more than 90%, whereas 3 of 14 (21.4%) in the vonoprazan group and 1 of 12 (8.3%) in the lansoprazole group had delayed ulcer healing, which was not statistically different (P = 0.356). The frequency of delayed bleeding was 0 in the both groups. Taken together, there were no significant differences between the two drug groups.
| Vonoprazan (n = 14) | Lansoprazole (n = 12) | P value | |
| Backgrounds | |||
| Age (yr) | 71.9 ± 7.9 | 74.8 ± 8.3 | 0.371 |
| Sex (M/F) | 12/2 | 10/2 | 0.867 |
| Anticoagulants | 1 (7.1) | 1 (8.3) | 0.910 |
| Antiplatelet agents | 3 (21.4) | 3 (21.4) | 0.829 |
| Steroids | 1 (7.1) | 0 (0) | 0.345 |
| Helicobacter pylori infection | 4 (28.6) | 5 (0.417) | 0.484 |
| Atrophic gastritis | |||
| Closed type | 3 (21.4) | 0 (0) | 0.088 |
| Open type | 11 (78.6) | 12 (100.0) | |
| Location | |||
| Upper | 1 | 0 | 0.618 |
| Middle | 5 | 4 | |
| Lower | 8 | 8 | |
| ESD results | |||
| En bloc resection | 14 (100.0) | 12 (100.0) | |
| Procedure time (min) | 88 (36-246) | 51.5 (12-202) | 0.123 |
| Submucosal fibrosis | 3 (25.0) | 1 (8.3) | 0.356 |
| Histopathology | |||
| Tub1 | 12 | 10 | 0.504 |
| Tub1 + tub2 | 1 | 2 | |
| Tub1 + tub2 + por2 | 1 | 0 |
| Vonoprazan | Lansoprazole | P value1 | U value2 | |
| Area of the ulcer just after ESD (mm3) | 1446.9 (605-3977.4) | 1262.6 (597.8-7322.3) | 89 | |
| Results of the follow-up endoscopy | ||||
| Area of the ulcer on day 8 (mm3) | 533.5 (93.6-1735.9) | 459.8 (90.5-5479.5) | 93 | |
| Shrinking rate on day 8 (%) | 61.8 (24.0-91.1) | 71.3 (25.2-88.6) | 70.5 | |
| Area of the ulcer on day 28 (mm3) | 61.6 (0-289.1) | 28.7 (1.1-639.4) | 93 | |
| Shrinking rate on day 28 (%) | 95.3 (76.2-100) | 97.2 (81.1-99.8) | 68 | |
| Delayed ulcer healing n (%) | 3/14 (21.4) | 1/12 (8.3) | 0.356 | |
| Delayed bleeding n (%) | 0/14 (0) | 0/12 (0) | 1 | |
In this study, the shrinking rates of ESD ulcers on days 8 and 28 were not statistically different between the two groups, and all of the patients in both groups were discharged without any severe complications. This suggests that lansoprazole was sufficient for the management of ESD ulcers although vonoprazan is theoretically more potent with regard to acid suppression.
PPIs have been widely used for the treatment of acid-related diseases, including ESD ulcers, and the therapeutic effect of PPIs has been satisfactory. However, there are some inadequacies that should be addressed. First, PPIs have a relatively short plasma half-life (60-90 min)[5,6]. Therefore, taking PPIs twice a day could be insufficient for inhibiting gastric acid at night. Second, PPIs are prodrugs and are activated under acid-secretion conditions. Hence, the effect of PPIs could be affected by food intake[4,6]. Third, since the onset of PPI effect is slow and it takes time to achieve maximum efficacy, rapid effects cannot be achieved[4,6,7]. These limitations could affect the clinical course after ESD. Furthermore, the problem of CYP 2C19 polymorphism could also inhibit the effectiveness of PPIs. With regard to CYP2C19 polymorphism, there are inter-ethnic differences regarding the frequency of extensive and poor metabolizers. In the Japanese population, the frequency of poor metabolizers is reported to be 18.0%-22.5%[8]. Although the frequency of poor metabolizers is relatively high in Japan compared to that in Western countries, the majority of the population still consists of extensive metabolizers. It has been reported that plasma PPI concentrations and intragastric pH are lower in extensive metabolizers compared with those in poor metabolizers, resulting in poor results of acid-suppression therapies in patients with GERD or H. pylori eradication[4,8]. Therefore, PPIs could be insufficient for the management of ESD ulcers, especially in extensive metabolizers.
On the other hand, vonoprazan, which is a novel acid inhibitor and classified as a P-CAB, has a long-lasting and rapid effect on gastric acid inhibition, and it is not affected by the acid secretory state, mealtime, or CYP2C19 polymorphism[5,6,11,12,14]. It has been reported that vonoprazan is more potent regarding acid inhibition and more efficient for acid-related diseases[6,11,14]. Vonoprazan could theoretically be more potent for the management of ESD ulcers. However, in our study, vonoprazan did not show superiority to lansoprazole with regard to ulcer healing after ESD. It has been reported that EMR ulcers heal faster than peptic ulcers because of high blood flow at the margin of EMR ulcers[15-17]. The mechanism of ESD ulcers is similar to that of EMR ulcers and even large ESD ulcers heal within 8 wk after treatment with normal doses of PPIs[18]. In this study, the area of the ESD ulcers reduced to less than 10% on day 28 in most of the cases in both groups, faster than peptic ulcer healing. Therefore, we concluded that vonoprazan was potent and lansoprazole was also effective for healing ESD ulcers. With regard to medical expenses, vonoprazan (20 mg daily) and lansoprazole (30 mg daily) cost 240 JPY (almost $2.4) and 140 JPY (almost $1.4), respectively. In our hospital, we usually use acid suppression medicines for at least 2 mo on the basis of a previous study[18], and the difference in the medical expenses between treatment with vonoprazan and lansoprazole for each patient is up to 5600 JPY (almost $56). Therefore, lansoprazole is more cost-effective although both of them are valid for the management of ESD ulcers.
Our secondary aim was the evaluation of the preventive effect of vonoprazan on delayed bleeding compared to lansoprazole. Clinically, the prevention of delayed bleeding is important after ESD, and the frequency of delayed bleeding has been reported to be approximately 5%[19-24]. Intragastric pH is an important factor for the coagulation system and platelet aggregation, and the activity of fibrinolysis is impaired at pH values above 6[25,26]. The most delayed bleeding occurs within the first 24 to 48 h[19]. Therefore, vonoprazan is theoretically superior for the prevention of delayed bleeding owing to its sustained, rapid, and more potent effect on acid suppression[6,11,12]. In our study, the delayed bleeding rate was 0% in both groups although our sample size was too small for the precise evaluation of the preventive effect on delayed bleeding. There are several reasons for this result. First, the acid suppression of both vonoprazan and lansoprazole was potent enough to prevent delayed bleeding; second, we carefully coagulated thick blood vessels that might bleed afterward. It has been reported that patients with large lesions or those being administered antithrombotic drugs have a high risk for delayed bleeding or perforation[19,21-24]. PPIs are occasionally not adequate to prevent complications in such patients. Vonoprazan is expected to reduce the incident rates of delayed bleeding because of its potent acid suppression.
To the best of our knowledge, this is the first report comparing the healing effects of vonoprazan and lansoprazole on ESD ulcers. However, there are some limitations of this study. First, the sample size was not large enough to obtain conclusive results. However, both vonoprazan and lansoprazole seemed potent enough for the management of ESD ulcers; second, our protocol might not be appropriate because we used intravenous lansoprazole for both groups in the first 2 day after ESD, resulting in underestimation of the healing and preventive effect of vonoprazan; third, we did not investigate the polymorphism of CYP2C19 in this study. This is important for making definitive conclusions. In extensive metabolizers, vonoprazan might prove to be superior to PPIs; fourth, anticoagulant, antiplatelet agent, and steroid users should have been removed from the study to prevent their associated complications from affecting the comparison. However, they were included in the present study.
In summary, our study indicated vonoprazan was potent for the management of ESD ulcers although lansoprazole was also sufficient and cost-effective. Since vonoprazan theoretically has more potent acid-suppression and is not affected by CYP2C19 polymorphism, it could be more effective in the high risk groups or extensive metabolizers. A further prospective study with these patients is needed to make a definitive conclusion.
We would like to thank Editage (http://www.editage.jp) for English language editing.
Proton pump inhibitors (PPIs) have been used for the management of ulcers induced by endoscopic submucosal dissection (ESD). Vonoprazan, an orally bioavailable potassium-competitive acid blocker, is a new class of gastric acid suppressant that inhibits gastric H+, K+-ATPase in a K+-competitive and reversible manner. It was approved in Japan in 2014 for the treatment and prevention of acid-related diseases including ESD-induced ulcers. The aim of this study was to compare the healing effects of vonoprazan and lansoprazole on ESD-induced ulcers.
Vonoprazan exhibits rapid, profound, and sustained suppression of gastric acid secretions and is not affected by CYP2C19 polymorphism. It has been reported that the acid-inhibitory effect of vonoprazan is more potent than that of PPIs, resulting in greater effectiveness for acid-related diseases such as gastroesophageal reflux disease or Helicobacter pylori eradication. Therefore, vonoprazan could be more effective for the management of ESD-induced ulcers compared to PPIs. So far there have been no reports comparing the healing effect of vonoprazan and PPIs on ESD-induced ulcers.
The authors compared shrinking rates of ESD-induced ulcers on days 8 and 28 between the vonoprazan group and the lansoprazole group, and showed that the shrinking rates of ESD ulcers on days 8 and 28 were not statistically different between the two groups.
The result of this study suggested that vonoprazan was potent for the management of ESD ulcers although lansoprazole was also sufficient and cost-effective.
Vonoprazan is a potent option for the management of ESD-induced ulcers.
This article is relatively novel. There is no report on the drug used to treat the post-ESD ulcers. These results are thought to be very useful article from the terms of cost effective.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Matsumoto K, Song WC, Tsuji Y S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Yang Z, Wu Q, Liu Z, Wu K, Fan D. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion. 2011;84:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Sachs G, Shin JM, Munson K, Vagin O, Lambrecht N, Scott DR, Weeks DL, Melchers K. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1383-1401. [PubMed] [Cited in This Article: ] |
| 5. | Shin JM, Inatomi N, Munson K, Strugatsky D, Tokhtaeva E, Vagin O, Sachs G. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339:412-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Sakurai Y, Mori Y, Okamoto H, Nishimura A, Komura E, Araki T, Shiramoto M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol. 2009;2:295-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20:153-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Arikawa Y, Nishida H, Kurasawa O, Hasuoka A, Hirase K, Inatomi N, Hori Y, Matsukawa J, Imanishi A, Kondo M. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem. 2012;55:4446-4456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75:439-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y, Warrington S. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41:636-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 314] [Article Influence: 19.6] [Reference Citation Analysis (3)] |
| 14. | Ashida K, Sakurai Y, Nishimura A, Kudou K, Hiramatsu N, Umegaki E, Iwakiri K, Chiba T. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42:685-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Kobayashi M, Takeuchi M, Hashimoto S, Mizuno K, Sato Y, Narisawa R, Aoyagi Y. Contributing factors to gastric ulcer healing after endoscopic submucosal dissection including the promoting effect of rebamipide. Dig Dis Sci. 2012;57:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Lee SY, Kim JJ, Lee JH, Kim YH, Rhee PL, Paik SW, Rhee JC. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Hashimoto T, Adachi K. Changes in gastric mucosal blood flow during healing of EMR-induced ulcer -Comparison with peptic ulcer. Dig Endosc. 1997;9:127-131. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kakushima N, Yahagi N, Fujishiro M, Iguchi M, Oka M, Kobayashi K, Hashimoto T, Omata M. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327-331. [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Kim SJ, Choi CW, Kang DH, Kim HW, Park SB. Second-look endoscopy and factors associated with delayed bleeding after endoscopic submucosal dissection. World J Gastrointest Endosc. 2016;8:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Choi CW, Kim HW, Kang DH, Hong YM, Kim SJ, Park SB, Cho M, Kim DJ, Hong JB. Clinical outcomes of second-look endoscopy after gastric endoscopic submucosal dissection: predictive factors with high risks of bleeding. Surg Endosc. 2014;28:2213-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Oh JH, Choi MG, Dong MS, Park JM, Paik CN, Cho YK, Jeong JJ, Lee IS, Kim SW, Han SW. Low-dose intravenous pantoprazole for optimal inhibition of gastric acid in Korean patients. J Gastroenterol Hepatol. 2007;22:1429-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Green FW, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38-43. [PubMed] [Cited in This Article: ] |