Published online Nov 16, 2016. doi: 10.4253/wjge.v8.i19.690
Peer-review started: May 5, 2015
First decision: October 26, 2015
Revised: October 10, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 16, 2016
Processing time: 565 Days and 17.7 Hours
Advanced therapeutic endoscopy, in particular endoscopic mucosal resection, endoscopic submucosal dissection, per-oral endoscopic myotomy, submucosal endoscopic tumor resection opened a new era where direct esophageal visualization is possible. Combining these information with advanced diagnostic endoscopy, the esophagus is organized, from the luminal side to outside, into five layers (epithelium, lamina propria with lamina muscularis mucosa, submucosa, muscle layer, adventitia). A specific vascular system belonging to each layer is thus visible: Mucosa with the intra papillary capillary loop in the epithelium and the sub-epithelial capillary network in the lamina propria and, at the lower esophageal sphincter (LES) level with the palisade vessels; submucosa with the drainage vessels and the spindle veins at LES level; muscle layer with the perforating vessels; peri-esophageal veins in adventitia. These structures are particularly important to define endoscopic landmark for the gastro-esophageal junction, helpful in performing submucosal therapeutic endoscopy.
Core tip: In the last years advanced endoscopic technology and techniques allowed the possibility to in vivo evaluate the esophageal vasculature. We aimed to review the endoscopic endoluminal and transluminal appearance of the esophageal vascular structures. This paper will allow the reader to deeply understand mucosal, submucosal and muscular layer vessels by a direct endoscopic visualization. The authors’ knowledge of the characteristic changes in health and disease, as well as descriptions of anatomical landmarks, will serve to inform the practice of endoscopic surgery in the future.
- Citation: Maselli R, Inoue H, Ikeda H, Onimaru M, Yoshida A, Santi EG, Sato H, Hayee B, Kudo SE. Microvasculature of the esophagus and gastroesophageal junction: Lesson learned from submucosal endoscopy. World J Gastrointest Endosc 2016; 8(19): 690-696
- URL: https://www.wjgnet.com/1948-5190/full/v8/i19/690.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i19.690
Flexible endoscopes were first introduced in 1950s and since that time physicians have been able visualize the gastrointestinal tract. In the past 10 years endoscopy benefited from several technologies such as high-definition television, high-resolution endoscopy, magnification and narrow band imaging (NBI)[1]. Results from the anatomical ex-vivo studies have informed the approach to endoscopic examination, but these technologies herald a new era of observation, where the direct visualization of living tissue can confirm, and add to, the observations of the past.
In this article we aimed to review the endoscopic endoluminal and transluminal appearance of the esophageal vascular structures, with particular attention to the state-of-the-art endoscopic equipment and techniques now available.
Magnification endoscopy up to 80x (GIF-H260, Olympus Medical Systems Co. Tokyo, Japan) is an excellent tool for the visualization of the normal esophageal mucosa and in the diagnosis of early esophageal cancer[2]. Using magnification, one can begin to visualize the esophageal microvasculature, with the surface capillaries displaying a looped configuration[3]: The intra-papillary capillary loops (IPCLs).
NBI is a relatively recent modality employing narrow-bandwidth filters [red-green-blue (R/G/B) sequential system][4], to increase the contrast between the mucosal surface and the underlying vascular pattern[5]. The depth of penetration, and thus the color seen in the screen, depends on the wavelength used: It is superficial for the blue band, deep for the red band and intermediate for the green band. The blue filter in particular has been designed to be similar to the peak absorption of hemoglobin, in order to emphasize capillary vessels at the mucosal surface[6]. Magnification endoscopy with NBI (M-NBI), therefore, has been developed for two distinct applications: The analysis of the architecture of the epithelium (or microsurface) and analysis of the microvasculature[7].
New optical imaging modalities to enable in vivo characterization of suspicious lesions involves both endogenous optical contrast as well as the use of contrast agents targeted against biomarkers that are associated with early and superficial neoplasias[8].
Recently the confocal laser endomicroscopy (CLE) has been studied in the evaluation of the gastrointestinal (GI) tract. Fluorescence diagnosis can be achieved by measuring the tissue fluorescence following administration of an agent (usually fluorescine).
The typical resolution achievable with CLE is on the order of 1-2 μm with a field of view of approximately 500-700 μm2. It allows for the immediate evaluation of the superficial GI layers and can be used for morphological diagnosis because of the recognition of morphological changes in cells and nuclei[9].
Several studies have compared the performance of confocal microendoscopy to white light endoscopy examination and NBI in the esophagus and colon. In particular in the esophageal field, most of these papers were focused on Barrett Esophagus changes.
More recently the endocytoscopy was introduced. A prototype gastroscope (Olympus Medical Systems Corp., Tokyo, Japan) with a high-power magnifying endocytoscope (450 × magnification) was used to compare the size and appearance of nuclei and cytoplasm ratio, without the need of a contrast agent. In the esophagus the endocytoscopic images has been classified into five grades of endocytoscopic atypia (ECA) from healthy squamous epithelium (ECA 1) to lesion recognized as malignant (ECA 5)[10].
Advanced operative endoscopy, ranging from endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), submucosal endoscopic tumor resection (SET), has open the door to the direct view of the submucosal virtual space and its anatomy. If with diagnostic endoscopy the interest was related only in understanding “superficial” findings and in wondering the submucosal subsequent meanings, the current procedures let the physician to watch from “inside” with his/her eyes a real, true anatomy of submucosal space, until now only imagined by both diagnostic endoscopists and surgeons. EMR and ESD are performed as indicated by local clinical guidelines for early esophageal cancers, POEM for esophageal achalasia[11-15] and SET for subepithelial tumors[16]. These procedures enable clear and direct visualization of the layers of the esophageal wall, as therapy progresses.
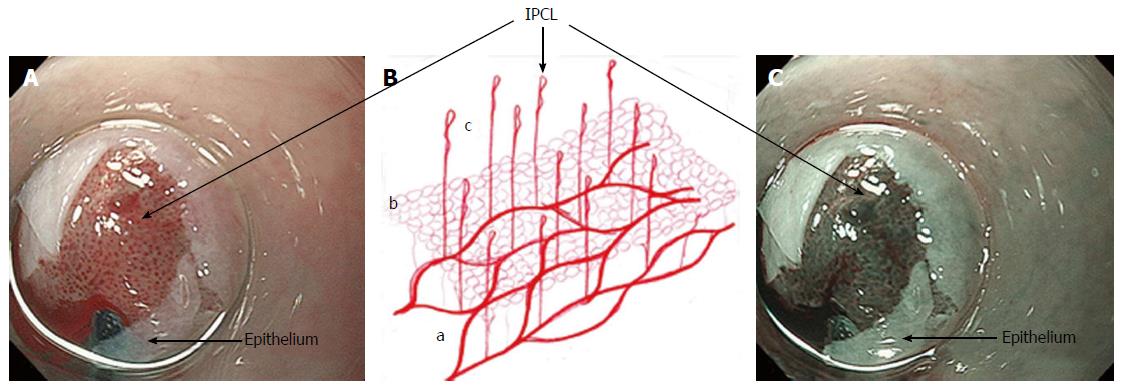
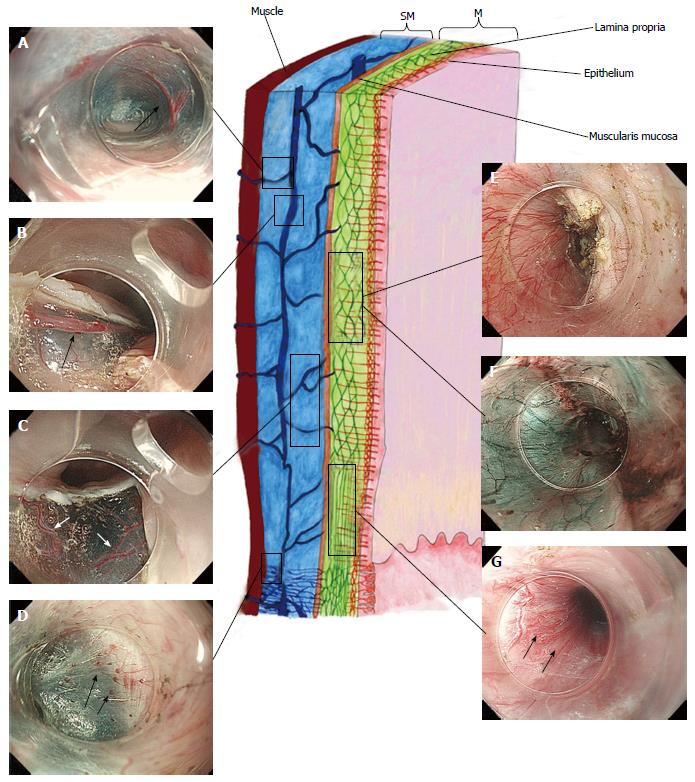
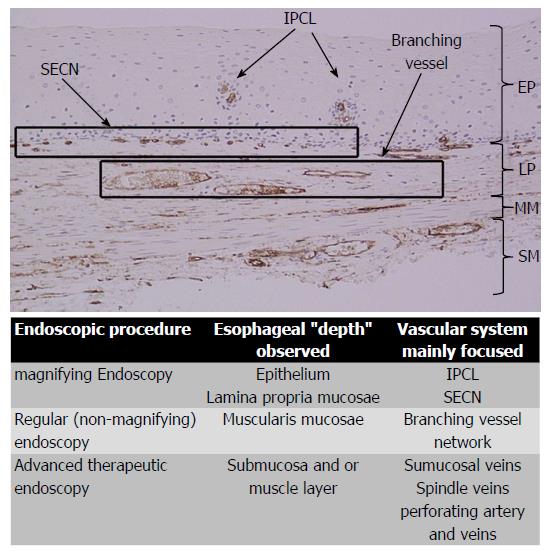
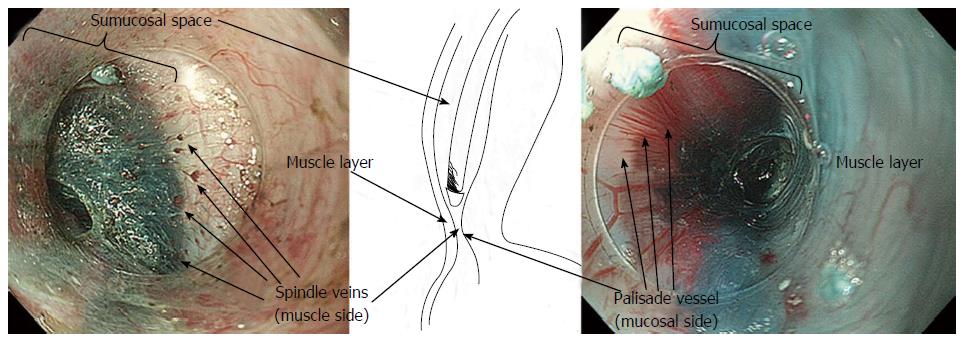
Combining the information gained from the therapeutic endoscopy, the esophageal wall is organized, from the lumen to outside, in five different layers: Epithelium, lamina propria with lamina muscularis mucosa, submucosa, muscle layer, adventitia. Different vascular system are recognize, belonging to each layer and connecting each other: In the mucosal layer we can find IPCL in the epithelium and sub-epithelial capillary network (SECN) in the lamina propria (Figure 1); at the lower esophageal sphincter (LES) level, we can recognize palisade vessels running in this layer; in the submucosa we find drainage vessels and the spindle veins just under the LES; in the muscle layer are present perforating vessels and peri-esophageal veins in Adventitia. In particular, considering the vasculature by each layer we can find the following structures (Figure 2)[16].
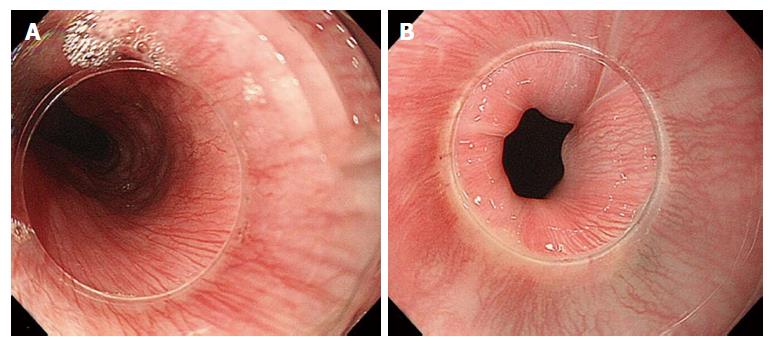
Mucosa: IPCLs and the SECN can be visualized laying all along the esophagus, from the upper esophageal sphincter (UES) to the LES (Figure 3). IPCLs are terminal vessels laying in the epithelial papilla and they drain into the branching vessels located within the lamina propria; they can be clearly demonstrated with M-NBI, although they are visible even with magnification alone. The branching vessels finally drain into the submucosal drainage vessels.
Submucosa: It is a connective “space” between the mucosa and the muscle layer. In this layer drainage vessels can be found running in the entire esophageal length; at the esophagogastric junction (GEJ) level the drainage veins become elongated.
Muscle layer: It is a double layer composed by muscular fibers running circularly in the inner layer and longitudinally in outer part. It is crossed by a venous network running in the intramuscular space. The muscle layer is also crossed by additional perforating vessels, large veins connecting the submucosal drainage veins/arteries with the main longitudinal arteries and veins of the adventitia, the outer esophageal layer.
Adventitia: It is the outermost connective tissue layer, enclosing the esophagus in all its length. The peri-esophageal vessels are clearly demonstrated during submucosal endoscopy for POEM, after the myotomy.
From the early 90s several studies focused on IPCL changes relevant to malignant tumors[2]. These studies led to the development of the IPCL classification[1]: IPCLs show characteristic changes in carcinoma in situ (irregular caliber, weaving, dilatation and different shape of IPCL). Analyzing grades of IPCL changes, the mucosa can be differentiated from normal (Type I) to carcinoma (Type V). By this classification, infiltration depth of the esophageal lesion can also be evaluated.
The immunohistochemical analysis on non-pathological esophageal specimens using CD34, specific for the vascular endothelium, and D2-40, specific for lymphatics, shows a high expression of CD34 in the areas corresponding to the IPCLs, SECN and branching vessels (Figure 4). IPCLs and SECN stained with CD34, but they are negative for D2-40 staining.
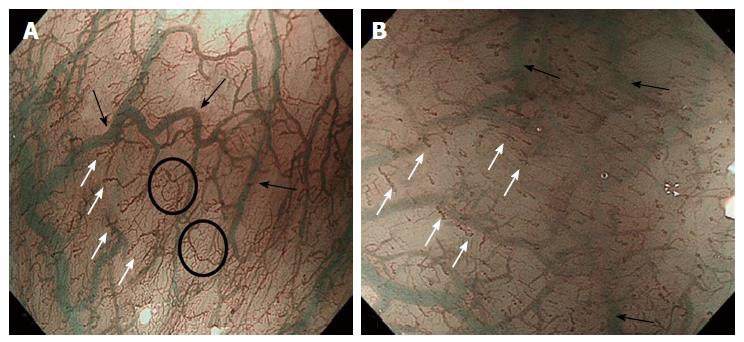
The GEJ is usually endoscopically defined as that area where the palisade vessels encounter gastric longitudinal mucosal folds[17-19]. These structures can be directly seen by entering in the submucosal space: From this internal point of view, on the mucosal side, the branching vessels appears neighboring with palisade vessels, running in the same plane, just above the muscolaris mucosae.
In the submucosal layer, immediately below the GEJ, small veins are laying, running regularly and parallel to each other, perpendicularly to the muscular layer, found in most of the patients (Figure 5). These “spindle veins” can be considered a reliable landmark of the GEJ already been passed through.
The first descriptions of the esophageal vasculature and its connection with the portal system span from Vesalius in 1543 to Bartholin in 1673 and Dionis in 1703. In 1951 Butler recorded a more detailed description, categorizing the intramural esophageal vessels into intrinsic veins, venae commitantes of the vagus and extrinsic veins[20].
Subsequent descriptions have concentrated largely on abnormalities due to esophageal varices, but these were limited to post-mortem, ex vivo analysis, frequently employing the corrosion-cast technique, or scanning electron microscopy (visualizing vessels down to 200 μm)[21]. These studies demonstrated the existence of a SECN, a draining submucosal venous plexus, and the anastomoses between these two.
Advanced therapeutic endoscopy allows, for the first time, the direct in vivo observation of the deeper layers of the esophageal wall. Many of these structures are of interest and key importance to endoscopists undertaking advanced therapeutic procedures.
Previous studies of the esophageal vasculature have yielded conflicting observations. Palisade vessels were first described using microangiography, then in 1984 endoscopically identified as “sudare-like veins” [19]. In 1987 Vianna et al[22] performed a study on the normal esophageal venous circulation and defined in particular the palisade zone located at the gastroesophageal junction. The veins in this zone were distributed uniformly, running longitudinally and parallel to each other. The submucosal veins of the gastric zone were described as piercing the muscularis mucosae at the GEJ, running in the lamina propria, with the exception of a small number which seemed to remain in the submucosal space[22]. In contrast, Aharinejad et al[23] demonstrated that submucosal veins maintain their general longitudinal course when passing through the GEJ. Using M-NBI, Kumagai and colleagues observations of the GEJ and its vessels corresponded to the ex vivo description of Kagaries and Butler: They described in the lamina propria a longitudinal plexus of small vessels and in the submucosa at the GEJ, the palisade vessels, with a caliber of 150-170 μm. They demonstrated that the density of palisade vessels is highest near the squamo-columnar junction and that starting form their proximal ends they gradually increase in thickness and become confluent[24]. Using M-NBI, our endoscopic findings, approaching the submucosal space, correspond most closely to those of Aharinejad, with the palisade vessels at the GEJ lying in the lamina propria. In other words, the palisade vessels are continuations of the branching vessels, but we postulate that the vessels appear elongated as a result of the high pressure forces present at the GEJ. This is supported by the presence of similar vessels at the UES level (Figure 6).
The GEJ has previously been divided into four distinct zones (the first two immediately below and the second two above the “Z” line). In zone 1, the most caudally zone directly connected with the gastric side, a complex of small twisted veins, with circumscript, ampullar bulges[21] - has been found. These veins correspond to the so-called “spindle veins”, found in more than 70% of the total cases of our personal series and clearly visible during submucosal endoscopy.
The architecture of IPCLs has been evaluated ex vivo in the normal esophagus, with microfilm[19]; comparing these stereoscopic microscopic images with magnifying endoscopic images, at a magnification of approximately 80 times, small vessels coming up from the mucosal vessels could be seen originating and running obliquely upward toward the epithelium and then toward to the intrapapillary capillaries. At a magnification of more than 100 × each intrapapillary capillary can be observed as a single distinct loop[25].
As endoscopists have become more familiar with M-NBI, it became apparent that characteristic morphological changes were associated with the development of malignancy[26]. These observations finally led to the development of the IPCL classification[1,27].
Esophageal vasculature is now in vivo evaluable with advanced endoscopic technology and techniques. Our knowledge of the characteristic changes in health and disease, as well definition of anatomical landmarks, will serve to the practice of endoscopic diagnostics and treatment in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lirici MM, Teoh AY S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Inoue H. Magnification endoscopy in the esophagus and stomach. Digest Endosc. 2001;13:S40-S41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 2. | Inoue H, Honda T, Nagai K, Kawano T, Yoshino K, Takeshita K, Endo M. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1997;9:16-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Inoue H, Honda T, Yoshida T, Nishikage T, Nagahama T, Yano K, Nagai K, Kawano T, Yoshino K, Tani M. Ultra-high magnification endoscopy of the normal esophageal mucosa. Dig Endosc. 1996;8:134-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 4. | Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Dig Endosc. 2002;14:131-137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Gheorghe C. Narrow-band imaging endoscopy for diagnosis of malignant and premalignant gastrointestinal lesions. J Gastrointestin Liver Dis. 2006;15:77-82. [PubMed] |
| 6. | Sambongi M, Igarashi M, Obi T. Analysis of spectral reflectance of mucous membrane for endoscopic diagnosis. Med Phys. 2000;27:1396-1398. |
| 7. | Kiesslich R, Jung M. Magnification endoscopy: does it improve mucosal surface analysis for the diagnosis of gastrointestinal neoplasias? Endoscopy. 2002;34:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Carns J, Keahey P, Quang T, Anandasabapathy S, Richards-Kortum R. Optical molecular imaging in the gastrointestinal tract. Gastrointest Endosc Clin N Am. 2013;23:707-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Wong Kee Song LM, Wilson BC. Endoscopic detection of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:833-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Huang Q. Definition of the esophagogastric junction: a critical mini review. Arch Pathol Lab Med. 2011;135:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1234] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 14. | Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | von Renteln D, Inoue H, Minami H, Werner YB, Pace A, Kersten JF, Much CC, Schachschal G, Mann O, Keller J. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Shiwaku H, Inoue H, Beppu R, Nakashima R, Minami H, Shiroshita T, Yamauchi Y, Hoshino S, Yamashita Y. Successful treatment of diffuse esophageal spasm by peroral endoscopic myotomy. Gastrointest Endosc. 2013;77:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Hoshihara Y, Kogure T. What are longitudinal vessels? Endoscopic observation and clinical significance of longitudinal vessels in the lower esophagus. Esophagus. 2006;3:145-150. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Noda T. Angioarchitectural study of esophageal varices. With special reference to variceal rupture. Virchows Arch A Pathol Anat Histopathol. 1984;404:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Butler H. The veins of the oesophagus. Thorax. 1951;6:276-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ferraz-de-Carvalho CA, Liberti EA, Fujimura I, Nogueira JO. Scanning electron microscope study of the veins at the human esophago-gastric junction. Rev Hosp Clin Fac Med Sao Paulo. 1994;49:49-52. [PubMed] |
| 22. | Vianna A, Hayes PC, Moscoso G, Driver M, Portmann B, Westaby D, Williams R. Normal venous circulation of the gastroesophageal junction. A route to understanding varices. Gastroenterology. 1987;93:876-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 134] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Aharinejad S, Böck P, Lametschwandtner A. Scanning electron microscopy of esophageal microvasculature in human infants and rabbits. Anat Embryol (Berl). 1992;186:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kumagai Y, Yagi M, Aida J, Ishida H, Suzuki S, Hashimoto T, Amanuma Y, Kusano M, Mukai S, Yamazaki S. Detailed features of palisade vessels as a marker of the esophageal mucosa revealed by magnifying endoscopy with narrow band imaging. Dis Esophagus. 2012;25:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular arc–hitecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Arima H, Arima M, Tada T. Microvascular Patterns Of Esophageal Micro Squamous Cell Carcinoma On Magnifying Endoscopy. Digest Endosc. 2008;20:6-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Maselli R, Inoue H, Ikeda H, Onimaru M, Yoshida A, Santi EG, Sato H, Kaga M, Hayee B, Kudo S. In vivo Observation of the Esophagus by Intraluminal and Transmural Endoscopy: Anatomical Lessons from Advanced Endoscopy. Treatment Strategies - Gastroenterology. 2013;2:48-49 Available from: http://viewer.zmags.com/publication/69da19a6#/69da19a6/48. |