Published online Jul 25, 2015. doi: 10.4253/wjge.v7.i9.872
Peer-review started: April 30, 2015
First decision: May 13, 2015
Revised: May 20, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: July 25, 2015
Processing time: 103 Days and 22.3 Hours
Endoscopic diagnosis with histological evidence is necessary to decide the best strategy for treating esophageal squamous cell carcinoma and Barrett’s-associated neoplasia, and the recent development of endoscopic technologies have made possible real-time information of malignant hallmarks. We focused on the development of optical coherence tomography (OCT), the only technology that can depict real-time cross-sectional images with high resolution. With the improvements in image resolution, acquisition rate and demonstrable area of three-dimensional devices with Doppler capability, OCT imaging was shown to enable visualization of structural/functional alterations in the mucosal/submucosal tissue of the esophagus, resulting in more accurate preoperative diagnosis of such malignancies. Moreover, it approved to be useful for targeting malignant areas for biopsy and treatment as well as for predicting the treatment effects. Therefore, further development of this technology is expected to overcome the current clinical issues in management strategies of esophageal malignancies.
Core tip: Optical coherence tomography (OCT) provides real-time cross-sectional images with extremely high resolution. We previously reported that OCT provided significantly more accurate preoperative staging of esophageal squamous carcinoma (ESCC) than endosonography. With remarkable improvements in this technology, such as three-dimensional devices with Doppler capability, for the detection of Barrett’s-associated neoplasia, the diagnostic accuracy gradually became better through enhanced visualization of structural/functional alterations in mucosal/submucosal tissue. Recent reports suggested its usefulness for targeting malignant lesions for endoscopic intervention and for predicting treatment effects. Therefore, further development of OCT should promote improved management strategies for esophageal malignancies, including ESCC.
- Citation: Uno K, Koike T, Shimosegawa T. Recent development of optical coherence tomography for preoperative diagnosis of esophageal malignancies. World J Gastrointest Endosc 2015; 7(9): 872-880
- URL: https://www.wjgnet.com/1948-5190/full/v7/i9/872.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i9.872
Both endoscopic assessment and histological evidence of gastrointestinal malignancies are necessary to decide the best treatment strategy. Notably, image-enhanced endoscopic technologies have been developed to provide real-time information on malignant hallmarks. In this review, we focused on the development of optical coherence tomography (OCT), the only technology that can depict real-time cross-sectional images of biological tissue at a near-microscopic level without contrast agents[1].
OCT images by near infrared light in the wavelength range of 700-1500 nm are similar to the B-mode images of ultrasonography. To construct an image, optical interferometry measures the delay between the emission of an invisible beam and the detection of its reflection to determine the distance from the emitter to the site. Its axial resolution is determined by coherence length of the light source. Most of the OCT devices reported in previous studies were first-generation probe-types [Light Lab Imaging (Boston, United States)] that used a super-luminescent diode light source with a center wavelength of 1300 nm, a bandwidth of 50 nm, and power output of 10 mW[2-7]. They had 10-20 μm of axial resolution, 5-25 times higher than that of high-frequency endosonography, which was another cross-sectional imaging device. Although its image acquisition rate was gradually improved from 1 frame/s to 9.8 frames/s with a lower signal-to-noise ratio, 4.0 frames/s could be used for the easy interpretation of images (Table 1)[8-10]. As a result, detailed OCT images can be constructed in gray-scale.
| OCT device | |||||
| Manuscript | Type | Resolution | Diameter (mm) | Image acquisition rate (frame/s) | |
| Axial (μm) | Transverse (μm) | ||||
| 4 | Probe | 10 | 25 | 4 | |
| 5 | Probe | 10 | 25 | 2.4 | - |
| 6, 7 | Probe | 10 | - | 2.5 | 2 |
| 2 | Probe | 11 | 30 | 1.5 | 4 |
| 3 | |||||
| 33 | Probe | 5 | - | 1.8 | 4 |
| 34 | Probe | Approximately 2 | 5.6 | - | - |
| 39 | Probe | 5 | 14 | - | 60 |
| 41 | Probe-3D | 5 | 15 | - | 60 |
| 10 | Balloon (OFDI) | 7 | 30 | 18 | 4 |
| 46 | Balloon (VLE) | 7 | - | 20 | 10 |
These mechanical characteristics provides several advantages to OCT in comparison with other advanced endoscopic technologies, as follows. First, it provides high-resolution cross-sectional images in real-time. OCT shows tissue structures in the mucosal/sub-mucosal layers at a microscopic scale, such as “pit and gland” morphology, revealing crypts/villi/vessels[4,6,7,11,12], as well as intracellular strictures, such as nuclei and other organelles, based on their different intensity of signal scattering[13]. Second, OCT does not always need tissue contact or coupling, although a biocompatible chemical agent was reported to possibly enhance its signal penetration depth[14]. Actually, we used a probe-type OCT [HOYA (Tokyo, Japan)] to depict detailed structures of the esophageal wall components, regardless of the location, while EUS-based imaging required acoustic coupling with a water preparation or a water-filled balloon, resulting in some difficulty in avoiding artifacts[2]. Third, a prototype OCT has a through-the-endoscope design, which may be easier to handle during endoscopic examination. In the next section, we will describe the technique for acquiring high-quality images using the OCT.
Nowadays, two types of OCT probe-devices, such as a radial-probe/linear-probe, and one balloon-type device are available but only for research[10,11,13]. While linear scanning is able to sample only a small area, radial scanning creates an image similar to that of radial EUS with the potential for assessing larger areas, due to its easier identification of the scanning orientation compared with the linear scanning. Therefore, radial-type probes have been applied in most of the previous studies.
The OCT devices are inserted through the accessory channel of an endoscope and maneuvered under direct endoscopic observation so that the imaging plane is perpendicular to the gastrointestinal wall. Its position when scanning across the tissue surface is monitored using visible light. A series of tomograms are obtained, while its spot diameter is selected for maintaining the appropriate depth of focus, while the distance above the surface is controlled by endoscopic maneuvers. In fact, the distance between the device and the site may affect the penetration depth of its signal. While mucosal structures were well-focused when the probe was held about 1 mm above the surface, the structures in the deeper submucosa (SM) could be revealed when the wall was compressed or collapsed around the probe. Using such a technique, the penetration depth of the OCT signal and consequent image quality in the stomach, duodenum, and colon were reported to be inadequate compared with those in the esophagus, suggesting that the OCT device was most suitable for the esophagus[11].
Previous studies demonstrated close correspondences between the clear, five-layered morphologies in the OCT images and those of a normal esophageal wall in the histological findings[4,15]. It was shown that the first relatively less reflective layer corresponded to stratified squamous epithelium (EP); the second more reflective layer to the lamina propria mucosa (LPM); the third less reflective layer to the muscularis mucosa (MM); fourth more reflective layer to the SM; and fifth less reflective layer to the muscularis propria (MP) with deeper structures of the esophageal wall. Subsequent studies based on such findings promoted the development of OCT devices for the management of Barrett’s-associated neoplasia and esophageal squamous cell carcinoma (ESCC). Originally, the studies aimed to improve the quality of “optical biopsies” of OCT devices for Barrett’s-associated neoplasia and remarkable advances were achieved in the West from the first-generation conventional probe-type OCT to the second-generation OCT (Table 1). In the East, we demonstrated the usefulness of the first-generation OCT in the preoperative staging of superficial ESCCs (SESCCs) (Table 1). Therefore, we review these achievements, and propose future roles for OCT in the management of esophageal disease.
Barrett’s esophagus (BE) is a precursor lesion with a 30-40-fold increased risk of cancer occurrence, i.e., from specialized intestinal metaplasia (SIM) to low grade dysplasia (LGD) and high grade dysplasia (HGD) and, finally, to adenocarcinoma[16]. Based on knowledge of the multi-step transformation, a surveillance program with regular endoscopic examination is recommended, but the prognosis for adenocarcinoma remains poor, with an overall 5-year survival of less than 20%[17]. Previous studies suggested that some dysplasia and intramucosal adenocarcinoma might be overlooked until the advanced stage in the current clinical setting[18]. Most of them were shown to be minute with a patchy distribution in a wide-ranging BE, and subsquamous SIM (SSIM) was found in 71.4% of pre-treatment dysplastic BE when 0.4-6.8 mm of oral extension was observed, although the sampling area and depth by random biopsy were limited[18-22]. Therefore, there still remain controversies about sampling errors and costs/time of endoscopic biopsies in the current surveillance system[18,20,21]. Moreover, several studies have pointed out the low inter- or intra- observer agreement of their histological diagnoses[23-29]. Likewise, cutting-edge endoscopic technologies have difficulties in reaching a consensus on the recognition or interpretation of abnormal patterns, which can limit their clinical usefulness[30]. However, real-time visualization of high-resolution cross-sectional architectural information, even in the SM, analogous to the loupe image, is an important advantage of the OCT imaging. In this section, we list previous achievements by OCT devices employed for endoscopic “optical biopsies” of Barrett’s-associated neoplasm.
Previous studies demonstrated that in vivo or ex vivo use of probe-type OCT devices could provide characteristic images of normal human esophagus, gastric mucosa, BE, dysplastic BE and adenocarcinoma, although subsequent studies showed that the differences in OCT images between non-dysplastic BE and dysplastic BE were subtle. Bouma et al[13] first reported the ability of in vivo OCT to provide detailed images of structures in Barrett’s-associated neoplasia by investigating biopsy-correlated OCT images, and proposed OCT-based grading criteria for characterizing dysplastic BE, as follows: (1) normal squamous epithelium: homogenous layered structures; (2) BE: absence of the layered-structure of normal esophagus in addition to abnormal/disorganized glandular structure of low reflectance within/under the mucosa; (3) dysplastic BE: highly reflective intensity of the background correlated with increased architectural disorder and heterogeneity; and (4) Barrett’s adenocarcinoma: abnormal configuration of neoplastic epithelium containing large pockets and surrounded by cellular stroma.
In 2001, using 288 biopsy-correlated OCT images of 121 patients, Poneros et al[4] demonstrated that in vivo OCT had sensitivity of 97% and specificities of 92% for the diagnosis of BE. In 2005, Isenberg et al[5] conducted a prospective study to evaluate diagnostic accuracy of in vivo OCT for dysplastic/non-dysplastic BE in comparison with the histological diagnosis of jumbo biopsy specimens. They used a 2.4 mm-diameter probe under a two-channel endoscope fitted with a cap attachment, which might stabilize the OCT device on the mucosal surface during the procedure. Using a total of 314 biopsy-correlated OCT images of 33 patients, they reported sensitivity of 68%, specificity of 82%, and positive predictive value of 53%, negative predictive value of 89%, and diagnostic accuracy of 78% for the diagnosis of BE. When the analysis was restricted to the diagnosis of HGD/ adenocarcinoma based on findings, such as: (1) lack of epithelial surface maturation; (2) gland architecture disarray; and (3) cytologic atypia[31,32], its sensitivity and specificity was 54% and 72%, respectively. Although such a negative predictive value may be advantageous for directing the examiners’ attention to malignant areas for the biopsy target, there remained limitations, such as large variability in the endoscopists’ accuracy rates, 56%-98%. Therefore, more refined criteria for differentiating dysplastic BE from non-dysplastic BE were required. In 2006, in a prospective study, Evans et al[6] investigated the relationship between a new scoring system, a “dysplasia index”, based on both the OCT findings of surface maturation and gland architecture, and biopsy-proven histology of HGD/adenocarcinoma in BE subjects. Using a total of 177 biopsy-correlated OCT images, the threshold of > 2 in the scoring system had sensitivity of 83% and specificity of 75% for the diagnosis of HGD/adenocarcinoma. Accordingly, these studies demonstrated that discrimination between non-dysplastic BE and dysplastic BE using OCT devices with standard resolution still remained a challenging issue.
Then, Chen et al[33] developed an ultra-high resolution OCT (UHR-OCT) with 5-μm axial resolution and compared its image quality and diagnostic accuracy with those of a standard OCT with 12-μm axial resolution. Using a total of 233 biopsy-correlated OCT images of 50 patients, the accuracy of UHR-OCT for making a diagnosis of normal squamous epithelium, non-dysplastic BE, HGD and adenocarcinoma was 100%, 98.1%, 83.3% and 100%, respectively. Actually, UHR-OCT depicted smaller/finer structures and sharper layered structures, resulting in improved discrimination and more detailed features of dysplastic BE. In 2010, Cobb et al[34] reported that UHR-OCT detected clearly SSIM as well as abnormal structures of non-dysplastic BE/HGD/adenocarcinoma in 14 post-surgical specimens. Accordingly, these studies suggested that higher-resolution OCT with the developed criteria might be more useful for targeting biopsies to differentiate between BE and normal esophagus, or between dysplastic/cancerous BE and non-dysplastic BE. However, some studies pointed out that the point-sampling nature of a probe-type OCT, similar to those of biopsy, might miss dysplastic lesions in large surface areas of BE[10].
These drawbacks of the probe-type OCT might have been mainly caused by the relatively slow image-acquisition rate, while recent improvements in OCT technology have enabled dramatic increases in imaging speed[35-38]. As a result, three-dimensional balloon-type OCT, referred to optical frequency-domain imaging (OFDI) and three-dimensional probe-type OCT (Light-Lab Imaging, Massachusetts, United States), could be developed with a combination of high-resolution at a near-microscopic level, large field of view, and rapid data acquisition[10].
Three-dimensional probe-type OCT: Volumetric data of a 10-mm circumference and 20-mm length could be acquired in 20 s by the helical scan of a prototype three-dimensional OCT, and each of data set provided comprehensive imaging of the glandular structure over a sampling area of 200 mm2, which was 30-60 times as large as those of approximately 6 mm2 by jumbo biopsy forceps and those of approximately 2.5 mm2 by conventional biopsy forceps[39]. Additionally, the imaging depths of 3D-OCT and biopsy were 1.5-mm and < 1 mm, respectively. Using data of biopsy-correlated OCT images of 3 patients, Adler et al[39] demonstrated the usefulness of a three-dimensional OCT system for the detection of large areas of a normal esophagus, non-dysplastic BE and post-ablative BE. The increase in the data volume of three-dimensional OCT improved the clear detection of SSIM at 300-500 μm depth beneath neosquamous epithelium, and they therefore proposed its use to guide decisions concerning additional treatment sessions or biopsy points with a reduction of sampling error[39]. Subsequent studies demonstrated that the pre-treatment thickness of Barrett’s mucosa and the presence of residual glandular structures immediately after focal radiofrequency ablation (RFA) in the three-dimensional OCT images were correlated with the treatment response determined by surveillance endoscopy with biopsy 6-8 wk after the latest session[40,41]. Accordingly, the three-dimensional OCT findings might be used as a promising real-time predictor of successful ablative therapy for BE.
Use of OFDI/volumetric laser endomicrography: OFDI can provide more than 100-fold faster imaging, compared with the conventional probe-type OCT[42]. The optical components in the inner sheath, positioned at the center of a 1.8 mm-diameter balloon catheter, are rotated helically, and cross-sectional images of the esophageal wall are revealed when the balloon is in contact with the mucosal surface, whose demonstrable area in the circumferential lumen might be affected by the degree of contact. All raw data are simultaneously stored and displayed in real-time. The OFDI/volumetric laser endomicrography (VLE) image with balloon-compression has four advantage, as follows: (1) the acquirement of microstructural data over large areas; (2) increased contrast of anatomical architecture; (3) increased signal penetration depth; and (4) reduced artifacts during imaging process.
Originally, volumetric OFDI images of the mucosa extended to the outer layer of the MP, with clear delineation of each layer, obtained for 4.5-cm-long segments in less than 6 min. In 2008, in a single-center study, complete acquisition of the OFDI data was successfully performed in 8 of 12 patients, and their images were consistent with the histological findings obtained by target/random biopsy specimens[10]. The loss of an appropriate image due to inadequate contact of the balloon was observed in 0.37% ± 0.79% of the total tubular esophageal surface area/patient. More recently, the Nvision Volumetric laser endomicrography Imaging System (Nine Point Medical, Cambridge, MA) was developed as a commercially available device. It is derived from OFDI and provides real-time three-dimensional images of mucosa/SM over a 6-cm length of the esophagus in 90 s. Baron et al[43] demonstrated that in vivo use of VLE clearly depicted SSIM proven by random endoscopic biopsy in 3 post-RFA BE patients, and Leggett et al[44] revealed that ex vivo use of VLE clearly detected subsquamous adenocarcinoma of endoscopic mucosal resection specimens, which could not be seen by conventional endoscopy or confocal laser endomicroscopy (CLE). In a multicenter prospective feasibility study, 4 lesions of HGD/adenocarcinoma were detected by VLE in 74 BE patients[45].
However, there still remain two drawbacks. First, previous studies pointed out that inadequate contact of the balloon, due to the interference of blood/mucus, existing motion artifacts, or excessive compression of the balloon on the mucosal surface, might still reduce the image quality. Especially, in some parts of the esophagus, such as in large hiatal hernias, tissue contact with the balloon surface was not maintained throughout the imaging window. Second, it is impossible to make one-to-one correlations between OFDI/VLE images and the histological evidence, because the balloon-centering system is not suitable for the subsequent biopsy procedure, nor is the technology to localize the region of interest in the three-dimensional data. Unfortunately, unreliable correlations between them may make it difficult to determine whether the possible discrepancies are caused by either a sampling error or misdiagnosis of the images, so we cannot assess abnormal findings detected in only one session of OFDI/VLE. Actually, the true biological significance of SSIM has not been clarified by the current OFDI/VLE system without histological evidence. To overcome this issue, a biopsy guidance platform that provides endoscopically visible laser markings at VLE-determined sites was developed, and its feasibility was demonstrated in a pilot study[46]. During the examination of VLE, the marks were made in 2 s at 410 mW of electric current, with the thermal-damage predominantly limited to the mucosa[47]. The accuracies of endoscopy, VLE intent-to-biopsy, and corrected VLE post-marking images for diagnosing tissue between the marks were 67%, 93%, and 100%, respectively. The transverse and longitudinal targeting error was 1.2 ± 1.3 mm and 0.5 ± 0.9 mm, respectively, while there were no longitudinal targeting errors in 21 of 30 cases. Henceforth, larger trials by VLE-guided biopsy can be expected to evaluate its practical usefulness.
Doppler OCT: Doppler OCT can directly visualize the intensity of the blood-flow data derived from moving erythrocytes, and its velocity resolution was reported to be 10-100-times as high as that of Doppler EUS[48]. Previous studies demonstrated that it could depict dramatic alterations in the functional microvascular network, which might provide additional clues for improved identification of the layer structure, during the sequential development of Barrett’s carcinogenesis[42,49]: (1) Normal esophagus: Distinct layers with small vessels in the LPM and medium vessels in the SM; (2) BE: Absence of the distinct layers with diffuse/small vessels and glandular structure; and (3) Esophageal Adenocarcinoma: Absence of distinct layers with diffuse/small vessels.
Recently, Tsai et al[50] developed OCT-angiography with an ultrahigh-speed (more than 10 times than that of conventional systems) and minimal motion artifacts, enabling imaging of the finer/denser microvascular architecture in BE. With an image acquisition of 400 frames/s, the total area of its image acquisition was improved to > 100 mm2 in 8 s. Because of these technological advances, the OCT-angiography could reveal more detailed structural/functional changes in the subsurface vasculature/glandular structure for early identification of Barrett’s carcinogenesis.
In the East, ESCC is the most predominant type of esophageal carcinoma, and its mortality rate remains still high. With the development of endoscopic technologies, the indication for endoscopic treatment for SESCCs has been expanded, since it is a minimally invasive procedure with few complications and after-effects. According to the esophageal cancer treatment guidelines of the Japanese Society of Esophageal Diseases, the definitive indication for endoscopic resection (ER) is limited to carcinoma in situ and tumors invading the LPM, regardless of tumor size[51]. Although more precise preoperative staging has been required for curative treatment, the accuracy of EUS has not yet been satisfactory, due to its limited visualization[52,53].
Second, we established the criteria of OCT-based staging for SESCCs in a phase I study. We used a probe-type OCT system under endoscopic observation in order to detect every part of a key finding for tumor staging[2]. After we investigated correlation the between OCT-based staging and histological staging of en bloc ESD specimens, the criteria of OCT-based staging for SESCCs were established. The criteria were classified into 3 categories based on the treatment guidelines: clinical EP/LPM, clinical MM, and clinical SM: (1) Clinical EP/LPM: the thick or normal layer I with regular interfacial signal of layer II or involvement of the tumor signal into layer II without involvement of layer III; (2) Clinical MM: involvement of the tumor signal into layer III with regular interfacial signal of layer IV; and (3) Clinical SM: Destruction of layers I to III and irregular interfacial signal of layer IV or loss of layer V architecture by high backscattering.
Thereafter, in a prospective phase II study, we investigated the accuracy based on the criteria in 62 consecutive patients[2]. The overall accuracy was 92.7%, and the accuracy of EP/LPM, MM, and SM cancer was 94.7%, 85.0%, and 90.9%, respectively. Although the staging accuracy was not significantly different among tumor locations (P = 0.79), the 0.46 (range 0.10-1.5) mm thickness of the lesion in the images without deep attenuation was significantly thinner than the 2.5 (1.2-5.0) mm images with deep attenuation. Conversely, this study uncovered the following limitations of this modality: (1) the limited depth of OCT signal penetration; (2) the inability to distinguish between cancer cell invasion and inflammatory cell infiltration; and (3) the inability to distinguish between intraepithelial cancer and normal tissue. Still, this phase-II study suggested that the criteria might be applicable for clinical use with high accuracy of tumor staging for SESCCs.
Finally, we investigated the clinical usefulness of OCT-based staging of SESCCs in a single-center prospective study by comparing the staging accuracy of OCT with that of 20-MHz probe-type EUS (UM-3R; Olympus, Tokyo) without a water-filled balloon for a total of 131 SESCCs in 123 consecutive patients[3]. The histological staging was confirmed by specimens obtained by en bloc ESD or surgical resection. As the primary endpoint, the accuracy for EP/LPM, a definitive indication for ER, by OCT was significantly higher than that by EUS (94.6% vs 80.6%, respectively, P < 0.05). The overall accuracy of OCT and EUS was 90.1% and 77.1%, respectively (P = 0.0046). Although there were no significant differences in the accuracy of OCT among tumor locations, the accuracy of EUS in the distal esophagus was significantly lower than that in the middle esophagus (P = 0.023). Further, due to the inferiority of EUS in image resolution, we found that the accuracy rate in 33.6% of the cases, which had less than 9-layer visualization in the EUS finding, was significantly lower than that in the remaining cases, which showed a clear discrimination of the 9-layer structure (P = 0.015). This study demonstrated that, because of mechanical advantage of OCT compared to EUS, the accuracy of OCT was significantly superior to that of EUS for the preoperative staging of EP/LPM in the clinical management of SESCCs. However, we noted 3 drawbacks of OCT: (1) a limitation in the penetration depth; (2) the limited width of the depiction area (limited to 4 mm); and (3) the inability to distinguish between cancer invasion and inflammatory cell infiltration. Accordingly, since the first-generation OCT-device still had limited usefulness in the management of SESCCs, further development of the OCT devices will be needed.
From the point of view that OCT may have advantages in the real-time visualization of the mucosal/submucosal architecture with/without functional alterations, we review promising research data on OCT-devices for providing “optical biopsies” for early detection of neoplastic changes during Barrett’s carcinogenesis or for accurate staging of SESCCs to improve treatment curability. However, to apply this technology in the clinical setting, the following issues will needed to be addressed, i.e.: (1) easy interpretation with low inter-observer variability; (2) real-time image acquisition for large-areas; and (3) cost effectiveness.
As for the first issue, more refined criteria for easy interpretation with less variability are needed for effective and stable stratification during surveillance. Although accurate interpretation is necessary for both well-trained endoscopists and well-trained pathologists, Qi et al[54] demonstrated 82% sensitivity, and 74% specificity in a computer-aided algorithm for the diagnosis of dysplastic BE based on the current criteria. Hence, future computer-aided algorithms can be realized by easy-to-identify criteria.
For the next two issues, OFDI/VLE may provide great cues toward real-time imaging of structural/functional alterations in the 6 cm-length circumferential esophageal mucosa during cancer development and the after-effects of endotherapies. Although no study has demonstrated a close correspondence between the OFDI/VLE imaging and histological evidence, a monitoring system for occult lesions, such as SSIM and tiny dysplastic Barrett’s mucosa, with a laser marking platform at VLE-determined sites for biopsy-guidance might unmask their true malignant potential during surveillance. Actually, there has been no study of them using conventional endoscopic imaging, CLE or the first-generation OCT, due to the limited sampling width/depth[55]. Instead, recent studies have proposed that OCT devices might be used to guide the biopsy target for enhanced detection of malignant Barrett’s mucosa or to assist in predicting the treatment effect[39,40,49]. Future monitoring by biopsy-correlated OFDI/VLE imaging might yield more effective management strategy with a risk-stratification, which could have the greatest impact on cost-effectiveness and clinical risk-management.
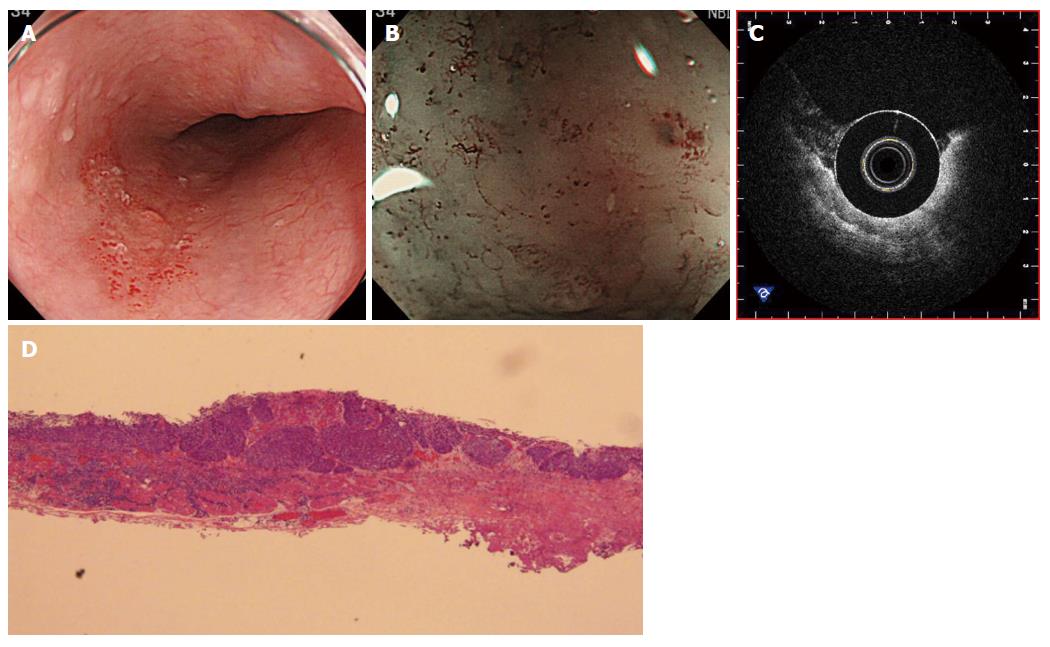
Regarding this point, we also emphasize that the second-generation OCT-devices with marking equipment may have a great impact on the development of new management strategies for SESCCs. In fact, there remain two difficulties in the current strategy for SESCCs. First, accurate staging for large-sized SESCCs by the detection of tiny abnormalities of superficial microvascular structure in the magnifying endoscopic findings with point-sampling characteristics is more difficult than that for small-sized SESCCs[56]. Second, another well-known difficulty is achieving early detection of the subepithelial recurrence of SESCCs after chemo-radiation therapy (Figure 1). However, the newly advanced OCT-devices can help with early detection by revealing tiny and invasive spots in large lesions and small subepithelial lesions[57]. Accordingly, real-time inspection with the OCT devices, after further technologic innovation, may play a central role in the histological diagnosis and choice of management strategies for esophageal malignancies.
In this review, we described previous achievements by which endoscopic OCT enhanced the visualization of structural/functional alterations in mucosal/submucosal tissue of the esophagus, and suggested that it might be useful for guiding/monitoring the area to be targeted for biopsy and treatment as well as to predict the treatment effect. Basically, it is important that the examiner/reviewer have familiarity and expertise in both histopathology and OCT imaging in order to achieve high accuracy in the diagnostic process. However, if reliable criteria of OCT imaging can be developed with computer-aid algorisms, the general use of OCT-related devices may provide “optical biopsies” or “optical staging” of Barrett’s-associated neoplasia and SESCCs. Therefore, further development of OCT technology is required for the future progress of management strategies of the esophageal malignancies.
P- Reviewer: Jiang CM, Kurtoglu E, Nicodeme F, Vynios D, Zhuang ZH S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178-1181. [PubMed] |
| 2. | Hatta W, Uno K, Koike T, Yokosawa S, Iijima K, Imatani A, Shimosegawa T. Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2010;71:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Hatta W, Uno K, Koike T, Iijima K, Asano N, Imatani A, Shimosegawa T. A prospective comparative study of optical coherence tomography and EUS for tumor staging of superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2012;76:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Poneros JM, Brand S, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7-12. [PubMed] |
| 5. | Isenberg G, Sivak MV, Chak A, Wong RC, Willis JE, Wolf B, Rowland DY, Das A, Rollins A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825-831. [PubMed] |
| 6. | Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43. [PubMed] |
| 7. | Evans JA, Bouma BE, Bressner J, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest Endosc. 2007;65:50-56. [PubMed] |
| 8. | Sergeev A, Gelikonov V, Gelikonov G, Feldchtein F, Kuranov R, Gladkova N, Shakhova N, Snopova L, Shakhov A, Kuznetzova I. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt Express. 1997;1:432-440. [PubMed] |
| 9. | Bouma BE, Tearney GJ. Power-efficient nonreciprocal interferometer and linear-scanning fiber-optic catheter for optical coherence tomography. Opt Lett. 1999;24:531-533. [PubMed] |
| 10. | Suter MJ, Vakoc BJ, Yachimski PS, Shishkov M, Lauwers GY, Mino-Kenudson M, Bouma BE, Nishioka NS, Tearney GJ. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc. 2008;68:745-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Sivak MV, Kobayashi K, Izatt JA, Rollins AM, Ung-Runyawee R, Chak A, Wong RC, Isenberg GA, Willis J. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc. 2000;51:474-479. [PubMed] |
| 12. | Brand S, Poneros JM, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Optical coherence tomography in the gastrointestinal tract. Endoscopy. 2000;32:796-803. [PubMed] |
| 13. | Bouma BE, Tearney GJ, Compton CC, Nishioka NS. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest Endosc. 2000;51:467-474. [PubMed] |
| 14. | Wang RK, Elder JB. Propylene glycol as a contrasting agent for optical coherence tomography to image gastrointestinal tissues. Lasers Surg Med. 2002;30:201-208. [PubMed] |
| 15. | Yokosawa S, Koike T, Kitagawa Y, Hatta W, Uno K, Abe Y, Iijima K, Imatani A, Ohara S, Shimosegawa T. Identification of the layered morphology of the esophageal wall by optical coherence tomography. World J Gastroenterol. 2009;15:4402-4409. [PubMed] |
| 16. | Haggitt RC. Barrett’s esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982-993. [PubMed] |
| 17. | Falk GW, Rice TW, Goldblum JR, Richter JE. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170-176. [PubMed] |
| 18. | Cameron AJ, Carpenter HA. Barrett’s esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586-591. [PubMed] |
| 19. | Dulai GS. Surveying the case for surveillance. Gastroenterology. 2002;122:820-823. [PubMed] |
| 20. | Falk GW, Chittajallu R, Goldblum JR, Biscotti CV, Geisinger KR, Petras RE, Birgisson S, Rice TW, Richter JE. Surveillance of patients with Barrett’s esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112:1787-1797. [PubMed] |
| 21. | Hatta W, Uno K, Koike T, Ara N, Asano N, Iijima K, Imatani A, Shimosegawa T. The usefulness of optical coherence tomography in evaluating the extension of Barrett’s mucosa underneath the squamous epithelium. Gastroenterology. 2015;148:Su-1724. |
| 22. | Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566-572. [PubMed] |
| 23. | Khandwalla HE, Graham DY, Kramer JR, Ramsey DJ, Duong N, Green LK, El-Serag HB. Barrett’s esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what’s next? Am J Gastroenterol. 2014;109:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 973] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 25. | Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669-1676. [PubMed] |
| 26. | Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394-398. [PubMed] |
| 27. | Lopes CV, Pereira-Lima JC, Hartmann AA, Tonelotto E, Salgado K. [Dysplasia in Barrett’s esophagus--intra- and interobserver variability in histopathological diagnosis]. Arq Gastroenterol. 2004;41:79-83. [PubMed] |
| 28. | Singh M, Bansal A, Curvers WL, Kara MA, Wani SB, Alvarez Herrero L, Lynch CR, van Kouwen MC, Peters FT, Keighley JD. Observer agreement in the assessment of narrowband imaging system surface patterns in Barrett’s esophagus: a multicenter study. Endoscopy. 2011;43:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Gaddam S, Mathur SC, Singh M, Arora J, Wani SB, Gupta N, Overhiser A, Rastogi A, Singh V, Desai N. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2011;106:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Alvarez Herrero L, Curvers WL, Bansal A, Wani S, Kara M, Schenk E, Schoon EJ, Lynch CR, Rastogi A, Pondugula K. Zooming in on Barrett oesophagus using narrow-band imaging: an international observer agreement study. Eur J Gastroenterol Hepatol. 2009;21:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166-178. [PubMed] |
| 32. | Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368-378. [PubMed] |
| 33. | Chen Y, Aguirre AD, Hsiung PL, Desai S, Herz PR, Pedrosa M, Huang Q, Figueiredo M, Huang SW, Koski A. Ultrahigh resolution optical coherence tomography of Barrett’s esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007;39:599-605. [PubMed] |
| 34. | Cobb MJ, Hwang JH, Upton MP, Chen Y, Oelschlager BK, Wood DE, Kimmey MB, Li X. Imaging of subsquamous Barrett’s epithelium with ultrahigh-resolution optical coherence tomography: a histologic correlation study. Gastrointest Endosc. 2010;71:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067-2069. [PubMed] |
| 36. | Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183-2189. [PubMed] |
| 37. | Yun S, Tearney G, de Boer J, Iftimia N, Bouma B. High-speed optical frequency-domain imaging. Opt Express. 2003;11:2953-2963. [PubMed] |
| 38. | Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, Suter MJ, Chan RC, Evans JA, Jang IK. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429-1433. [PubMed] |
| 39. | Adler DC, Zhou C, Tsai TH, Lee HC, Becker L, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Three-dimensional optical coherence tomography of Barrett’s esophagus and buried glands beneath neosquamous epithelium following radiofrequency ablation. Endoscopy. 2009;41:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Zhou C, Tsai TH, Lee HC, Kirtane T, Figueiredo M, Tao YK, Ahsen OO, Adler DC, Schmitt JM, Huang Q. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc. 2012;76:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Tsai TH, Zhou C, Tao YK, Lee HC, Ahsen OO, Figueiredo M, Kirtane T, Adler DC, Schmitt JM, Huang Q. Structural markers observed with endoscopic 3-dimensional optical coherence tomography correlating with Barrett’s esophagus radiofrequency ablation treatment response (with videos). Gastrointest Endosc. 2012;76:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Vakoc BJ, Shishko M, Yun SH, Oh WY, Suter MJ, Desjardins AE, Evans JA, Nishioka NS, Tearney GJ, Bouma BE. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest Endosc. 2007;65:898-905. [PubMed] |
| 43. | Baron TH, Raju GS. Optical biopsy approaches in Barrett’s esophagus with next-generation optical coherence tomography. Gastrointest Endosc. 2014;80:516-517. [PubMed] |
| 44. | Leggett CL, Gorospe E, Owens VL, Anderson M, Lutzke L, Wang KK. Volumetric laser endomicroscopy detects subsquamous Barrett’s adenocarcinoma. Am J Gastroenterol. 2014;109:298-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Sharma P, Kanakadandi V, Wang KK, Tearney GJ, Giacchino M, Wallace MB. Feasibility of using a novel imaging technique in patients with Barrett’s Esophagus: 3 dimensional volumetric laser endomicroscopy. Gastroenterology. 2013;144:S-892. |
| 46. | Suter MJ, Gora MJ, Lauwers GY, Arnason T, Sauk J, Gallagher KA, Kava L, Tan KM, Soomro AR, Gallagher TP. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest Endosc. 2014;79:886-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Suter MJ, Jillella PA, Vakoc BJ, Halpern EF, Mino-Kenudson M, Lauwers GY, Bouma BE, Nishioka NS, Tearney GJ. Image-guided biopsy in the esophagus through comprehensive optical frequency domain imaging and laser marking: a study in living swine. Gastrointest Endosc. 2010;71:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Hino S, Kakutani H, Ikeda K, Yasue H, Kitamura Y, Sumiyama K, Uchiyama Y, Kuramochi A, Matsuda K, Arakawa H. Hemodynamic analysis of esophageal varices using color Doppler endoscopic ultrasonography to predict recurrence after endoscopic treatment. Endoscopy. 2001;33:869-872. [PubMed] |
| 49. | Standish BA, Yang VX, Munce NR, Wong Kee Song LM, Gardiner G, Lin A, Mao YI, Vitkin A, Marcon NE, Wilson BC. Doppler optical coherence tomography monitoring of microvascular tissue response during photodynamic therapy in an animal model of Barrett’s esophagus. Gastrointest Endosc. 2007;66:326-333. [PubMed] |
| 50. | Tsai TH, Ahsen OO, Lee HC, Liang K, Figueiredo M, Tao YK, Giacomelli MG, Potsaid BM, Jayaraman V, Huang Q. Endoscopic optical coherence angiography enables 3-dimensional visualization of subsurface microvasculature. Gastroenterology. 2014;147:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | The Japanese Society of Esophageal Diseases. Esophageal cancer treatment guidelines [Japanese]. Tokyo: Kanehara 2007; . |
| 52. | Das A, Sivak MV, Chak A, Wong RC, Westphal V, Rollins AM, Willis J, Isenberg G, Izatt JA. High-resolution endoscopic imaging of the GI tract: a comparative study of optical coherence tomography versus high-frequency catheter probe EUS. Gastrointest Endosc. 2001;54:219-224. [PubMed] |
| 53. | Goda K, Tajiri H, Ikegami M, Yoshida Y, Yoshimura N, Kato M, Sumiyama K, Imazu H, Matsuda K, Kaise M. Magnifying endoscopy with narrow band imaging for predicting the invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Qi X, Sivak MV, Isenberg G, Willis JE, Rollins AM. Computer-aided diagnosis of dysplasia in Barrett’s esophagus using endoscopic optical coherence tomography. J Biomed Opt. 2006;11:044010. [PubMed] |
| 55. | Gupta N, Mathur SC, Dumot JA, Singh V, Gaddam S, Wani SB, Bansal A, Rastogi A, Goldblum JR, Sharma P. Adequacy of esophageal squamous mucosa specimens obtained during endoscopy: are standard biopsies sufficient for postablation surveillance in Barrett’s esophagus? Gastrointest Endosc. 2012;75:11-18. [PubMed] |
| 56. | Sato H, Inoue H, Ikeda H, Sato C, Onimaru M, Hayee B, Phlanusi C, Santi EG, Kobayashi Y, Kudo SE. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy. 2015;47:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 57. | Peery AF, Shaheen NJ. Optical coherence tomography in Barrett’s esophagus: the road to clinical utility. Gastrointest Endosc. 2010;71:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |