Published online Apr 16, 2014. doi: 10.4253/wjge.v6.i4.137
Revised: March 4, 2014
Accepted: March 11, 2014
Published online: April 16, 2014
AIM: To evaluate the diagnostic yield (inflammatory activity) and efficiency (size of the biopsy specimen) of SpyGlassTM-guided biopsy vs standard brush cytology in patients with and without primary sclerosing cholangitis (PSC).
METHODS: At the University Medical Center Mainz, Germany, 35 consecutive patients with unclear biliary lesions (16 patients) or long-standing PSC (19 patients) were screened for the study. All patients underwent a physical examination, lab analyses, and abdominal ultrasound. Thirty-one patients with non-PSC strictures or with PSC were scheduled to undergo endoscopic retrograde cholangiography (ERC) and subsequent peroral cholangioscopy (POC). Standard ERC was initially performed, and any lesions or strictures were localized. POC was performed later during the same session. The Boston Scientific SpyGlass SystemTM (Natick, MA, United States) was used for choledochoscopy. The biliary tree was visualized, and suspected lesions or strictures were biopsied, followed by brush cytology of the same area. The study endpoints (for both techniques) were the degree of inflammation, tissue specimen size, and the patient populations (PSC vs non-PSC). Inflammatory changes were divided into three categories: none, low activity, and high activity. The specimen quantity was rated as low, moderate, or sufficient.
RESULTS: SpyGlassTM imaging and brush cytology with material retrieval were performed in 29 of 31 (93.5%) patients (23 of the 29 patients were male). The median patient age was 45 years (min, 20 years; max, 76 years). Nineteen patients had known PSC, and 10 showed non-PSC strictures. No procedure-related complications were encountered. However, for both methods, tissues could only be retrieved from 29 patients. In cases of inflammation of the biliary tract, the diagnostic yield of the SpyGlassTM-directed biopsies was greater than that using brush cytology. More tissue material was obtained for the biopsy method than for the brush cytology method (P = 0.021). The biopsies showed significantly more inflammatory characteristics and greater inflammatory activity compared to the cytological investigation (P = 0.014). The greater quantity of tissue samples proved useful for both PSC and non-PSC patients.
CONCLUSION: SpyGlassTM imaging can be recommended for proper inflammatory diagnosis in PSC patients. However, its value in diagnosing dysplasia was not addressed in this study and requires further investigation.
Core tip: Endoscopic retrograde cholangiography remains the gold standard method for diagnosing biliary tract diseases. However, choledochoscopy with the SpyGlassTM system enables direct visualization of the biliary tract. Furthermore, targeted biopsies can be performed. In our single-center study, the diagnostic yield of SpyGlassTM-directed biopsy for inflammatory changes in primary sclerosing cholangitis (PSC) and non-PSC patients was significantly greater than that of brush cytology. The better diagnostic yield strongly correlated with significantly greater amounts of tissue for histological evaluation.
- Citation: Rey JW, Hansen T, Dümcke S, Tresch A, Kramer K, Galle PR, Goetz M, Schuchmann M, Kiesslich R, Hoffman A. Efficacy of SpyGlassTM-directed biopsy compared to brush cytology in obtaining adequate tissue for diagnosis in patients with biliary strictures. World J Gastrointest Endosc 2014; 6(4): 137-143
- URL: https://www.wjgnet.com/1948-5190/full/v6/i4/137.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i4.137
The precise diagnosis of biliary lesions and strictures is of crucial importance in patients with primary sclerosing cholangitis (PSC) or other biliary strictures because malignant tumors of the bile duct frequently have poor prognoses and high recurrence rates. Furthermore, the precise diagnosis of inflammatory activity influences medical and endoscopic treatments and might affect surveillance intervals.
The accurate assessment of bile duct stenosis (malignant vs inflammatory vs scar) is the ultimate goal of endoscopic retrograde cholangiopancreatography (ERCP) in patients with PSC. However, this differentiation remains challenging because endoscopic retrograde cholangiography (ERC) and other auxiliary fluoroscopy techniques do not permit the reliable diagnostic evaluation of biliary lesions[1,2]. Alternative diagnostic methods, such as endoscopic ultrasonography (EUS) with the use of mini-probes or probe-based endomicroscopy, are still of limited use[3].
Peroral cholangioscopy (POC) provides direct visualization of the biliary tree. This method also permits tissue sampling via targeted biopsies. The additional information provided by POC has been reported to change overall patient management and outcomes[4]. Furthermore, POC appears to be useful for clarifying filling defects during ERCP[5]. Recent data suggest that POC provides sufficient resolution and that in combination with biopsy, it can accurately diagnose biliary tract lesions[6]. POC is not a new process, as it has been used since the 1970s[7]. However, when first introduced, the procedure required two investigators, and the fiber-optic image quality was poor[8].
The first single-operator choledochoscopy system was introduced in 2005 by Boston Scientific and is known as the SpyGlassTM direct visualization system. The system enables a single investigator to perform cholangioscopy and targeted biopsies of bile duct abnormalities[9]. After the SpyGlassTM direct visualization system was introduced, its clinical application was reported in several publications. The main aspects addressed in these studies were the accessibility, direct view, and characterization of abnormal biliary lesions[10,11]. A recent study showed that the sensitivity of SpyGlassTM for gross assessment was significantly superior to that of ERC (81% vs 53%)[12].
However, ERC remains the gold standard for diagnosing biliary lesions in PSC[13]. Although brush cytology is the preferred investigation method for strictures and PSC-associated lesions, the poor sensitivity has been reported to be a major problem. Cytology achieves fairly good specificity, but its sensitivity is poor (approximately 50%)[14-19]. Cholangioscopy-guided biopsy appears to have the potential to overcome the problems associated with inadequate tissue sampling.
Thus, the aim of the present study was to evaluate the diagnostic yield (inflammatory activity) and efficiency (the biopsy specimen size for histological evaluation) of SpyGlassTM-guided biopsy versus standard brush cytology.
From January 2009 to February 2011 at the University Medical Center of Mainz, Germany, 35 consecutive patients with unclear biliary lesions (16 patients) or long-standing PSC (19 patients) were screened for the study. Thirty-one patients were finally included in the study after providing informed consent. All patients underwent a physical examination, lab analyses (Table 1), and abdominal ultrasound prior to ERCP and POC.
| All patients | PSC | Non-PSC | P value | |
| Patients (n) | 29 | 19 | 10 | - |
| Age | (48.9 ± 16.7) | (42.1 ± 13.9) | (61.9 ± 13.9) | 0.00172 |
| ALT | (100.2 ± 129.1) | (69.5 ± 41.1) | (155.4 ± 203.9) | 0.21921 |
| AST | (74.5 ± 69.0) | (63.7 ± 104.7) | (93.9 ± 107.7) | 0.39791 |
| Gamma GT | (411.4 ± 470.7) | (314.7 ± 288.4) | (585.2 ± 674.8) | 0.25288 |
| AP | (310.4 ± 212.6) | (285.0 ± 177.3) | (356.0 ± 269.6) | 0.46748 |
| Bilirubin | (3.0 ± 4.9) | (2.9 ± 5.0) | (3.3 ± 5.1) | 0.83431 |
| CRP | (20.8 ± 32.0) | (15.4 ± 25.9) | (30.3 ± 40.5) | 0.31297 |
| Leukocytes | (8.1 ± 3.1) | (8.2 ± 3.3) | (7.7 ± 2.6) | 0.66937 |
The Boston Scientific SpyGlassTM and the Boston Scientific SpyScopeTM were used for choledoscopy. The choledochoscope was advanced through a standard therapeutic duodenoscope (Pentax ED-3480T, Pentax, Hamburg). The choledochoscope (Boston ScientificTM) was passed through the working channel of the “mother” scope (Pentax ED-3480T duodenoscope). All procedures were performed using Propofol (1% Disoprivan, AstraZeneca, Switzerland) as sedation.
Before POC, a standard retrograde cholangiogram with biliary sphincterotomy was performed to localize the strictures and to facilitate ductal access and therapy. The choledochoscope was introduced into the bile duct through the guidewire via the working channel. For patients in whom the wire could not be advanced beyond a lesion or stenosis, the guidewire was advanced to the stricture under direct visualization of the bile duct whenever possible.
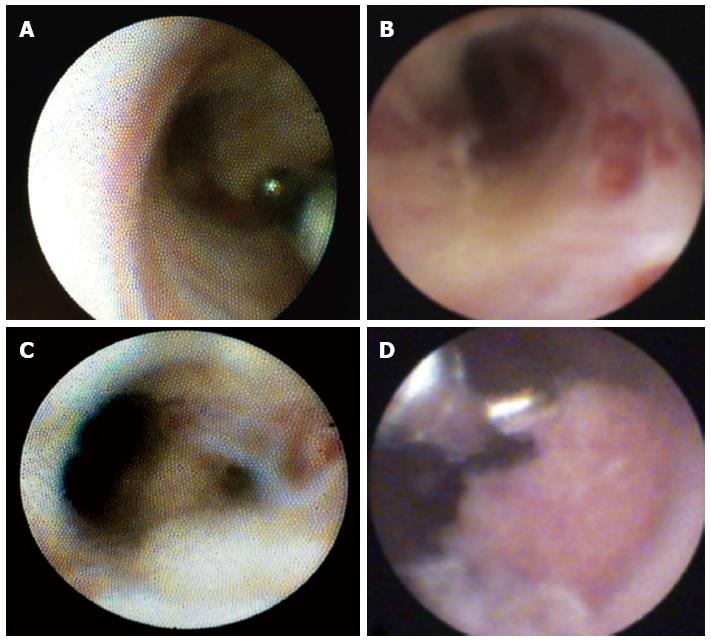
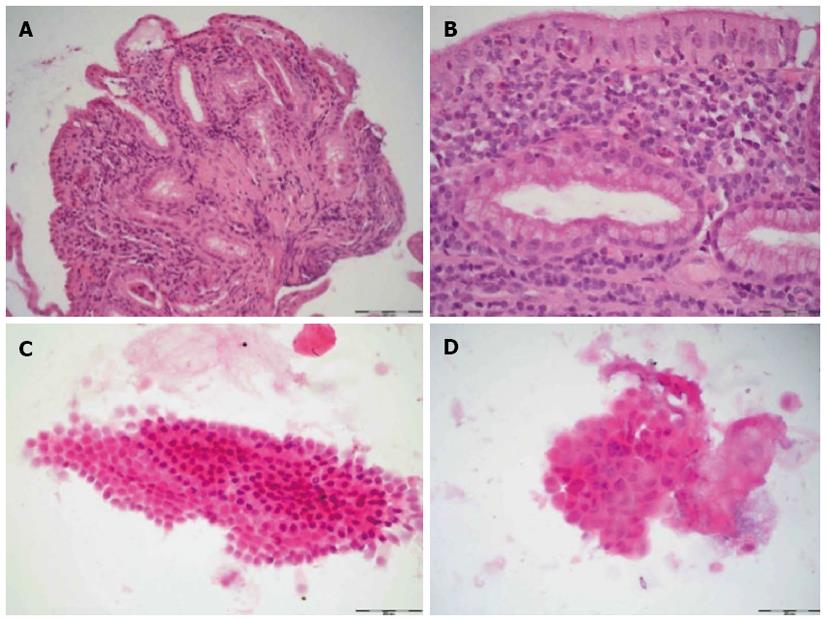
Standard ERC was initially performed, and any lesions or strictures were localized. Subsequently, POC was performed during the same session. The biliary tree was inspected, and suspicious lesions or strictures were biopsied; at least two or three biopsies per lesion or stricture were taken for histological examination (Figure 1). In addition, brush cytology of the same area was performed with a Cook medical Double Lumen Biliary BrushTM (Cytology). A single pathologist who specialized in biliary pathology graded the biopsy specimens and the brush cytology (T.H.) in a standardized manner. The inflammatory changes were divided into 4 categories (none, low, moderate, high) according to the number of leukocytes. For the biopsies, 5 high-power fields (HPFs, 0.309 mm²) were observed, and the leukocytes were semiquantitatively analyzed as follows: no activity, < 10 leukocytes/HPF; low activity < 100 leukocytes/HPF; moderate activity > 100 leukocytes/HPF; and high activity > 150 leukocytes/HPF. In the case of the cytological specimens, semiquantitative evaluation revealed the following activity levels: none, < 5 leukocytes/HPF; low, < 50 leukocytes/HPF; and high, > 50 leukocytes/HPF. The quantity of specimens was rated as low, moderate, or sufficient, according to the cell number in an HPF (0.306 mm²); in the case of cytology, the quantities were as follows: low, < 10 cells/HPF; moderate, < 20 cells/HPF; and sufficient, > 50 cells/HPF). In biopsy specimens, either the number of specimens (low, one tissue fragment; moderate, at least two tissue fragments; sufficient, at least three tissue fragments) or the number of mucosal folds/villi was encountered (low, one villus; moderate, at least two villi; sufficient, at least three villi).
The ethics committee of Rheinland-Pfalz, Germany, approved this study (No. 837.432.07 (5967)).
Practical limitations allowed us to collect material from 31 patients; 2 samples did not meet our quality criteria. The material collection proved to be sufficient to detect the differences between the two groups. Statistical analysis was performed using the R statistical language. Bowker‘s test was used to reject the null hypothesis of symmetry in contingency tables (Tables 2 and 3):
| Brush | Total | ||||
| Small | Moderate | Sufficient | |||
| Biopsy | Small | 3 | 4 | 0 | 7 |
| Moderate | 6 | 4 | 2 | 12 | |
| Sufficient | 9 | 1 | 0 | 10 | |
| Total | 18 | 9 | 2 | 29 | |
| Brush | Total | |||||
| None | Low | Moderate | High | |||
| Biopsy | None | 0 | 1 | 0 | 0 | 1 |
| Low | 0 | 11 | 0 | 0 | 11 | |
| Moderate | 0 | 8 | 0 | 0 | 8 | |
| High | 1 | 6 | 0 | 2 | 9 | |
| Total | 1 | 26 | 0 | 2 | 29 | |
, where B is χ2 distributed with [n (n - 1)]/2 degrees of freedom.
All reported P values are the result of a data exploration process.
All 31 patients underwent brush cytology and biopsy. No procedure-related complications were encountered. However, for both methods, tissues could be retrieved from only 29 patients. In one patient, SpyGlassTM failed to obtain any tissue material; in another patient, no cytological specimens could be obtained using brush cytology.
The patient and laboratory characteristics are summarized in Table 1. Twenty-three of the 29 patients were male, and the median patient age was 45 years (range, 20-76 years). Nineteen patients had known PSC, and 10 showed non-PSC strictures. The patient characteristics did not significantly differ between the two groups. Four patients had a suspicion of malignant strictures during endoscopy that was not confirmed by histologic results.
The biopsy method revealed significantly more tissue material (P = 0.021) than the brush method (Table 2, Figure 2). In 10 patients, the number of biopsy specimens was sufficient; by contrast, only 2 patients demonstrated sufficient numbers of specimens by brush cytology. In 27 patients, no or little inflammatory activity was detected using the brush method, compared to 12 patients using the biopsy method. Using SpyGlassTM-directed biopsy, a greater degree of inflammatory activity (classified as moderate or high) was observed in 17 of 29 (58.62%) patients (P = 0.014) (Table 3). Brush cytology failed to reveal any significant signs of inflammation because of the paucity of material. A common characteristic of the two techniques was that a greater quantity of test material predicted stronger signs of inflammation. The subgroup analysis between the PSC and non-PSC patients did not reveal any significant differences in the assessment of inflammatory activity with regard to the biopsy or brush method, respectively (Tables 4, 5). Neither brush cytology nor biopsy detected any malignant strictures or dysplasia in the patients.
| Brush | Total | |||||
| None | Low | Moderate | High | |||
| Biopsy | None | 0 | 0 | 0 | 0 | 0 |
| Low | 0 | 6 | 0 | 0 | 6 | |
| Moderate | 0 | 4 | 0 | 0 | 4 | |
| High | 1 | 6 | 0 | 2 | 9 | |
| Total | 1 | 16 | 0 | 2 | 19 | |
The brush method demonstrated a positive correlation between the amount of test material and the characteristics of inflammation. This method typically produced little study material and revealed only a few features of inflammation. SpyGlassTM-directed biopsy showed moderate or high levels of inflammation in 17 of 29 cases.
A significantly greater quantity of material was obtained with the biopsy-directed procedure. Brush cytology showed adequate signs of inflammation in two cases (Table 2). We observed no differences in the outcomes of the patients with or without PSC. Furthermore, no significant difference was noted in the patients with elevated laboratory parameters of inflammation with regard to the histopathological signs of inflammation.
SpyGlassTM is a single-operator system that allows direct visualization of the biliary and pancreatic tracts[9,20]. SpyGlassTM provides significantly greater sensitivity to clarify biliary strictures compared to ERCP[12,21,22]. The largest study in the literature (comprising nearly 300 patients) showed that SpyGlassTM could visualize 96% of all strictures and that 88% of the identified strictures or lesions could be successful biopsied[23]. Other studies reported a higher diagnostic value of SpyGlassTM-guided biopsy compared to brush cytology[24-26]. However, to date, the diagnostic yield for PSC has not been clarified. Thus, we investigated the diagnostic value of SpyGlassTM-directed biopsy versus brush cytology in patients with or without PSC. Furthermore, we evaluated whether the biopsy or brush cytology characteristics differed between PSC and non-PSC patients. We clearly demonstrated that SpyGlassTM-guided biopsy obtained greater quantities of tissue specimens and provided a more accurate diagnosis of inflammatory changes. This result is important because the degree of inflammation might alter the medical treatment or refine the surveillance of PSC patients.
Our study focused on the amount of tissue obtained and the presence of inflammatory changes. Although malignant changes were suspected in four of our patients during endoscopy, the specimens could not confirm dysplasia or carcinoma. However, malignancies have been identified using SpyGlassTM, with a reported accuracy of 77% in patients with suspected cholangiocarcinoma[4,25-28]. Our study could not clarify whether SpyGlassTM is beneficial in identifying PSC-associated dysplasia.
In our study, biopsy specimens were obtained using SpyGlassTM in 28 of 29 cases (96.5%). This percentage is greater than that previously reported[11], which might be because we performed at least 2-3 passes of the biopsy forceps (SpybiteTM) at the area of interest.
Brush cytology often failed to reveal signs of inflammation because of the paucity of material. The most important result of our study was that tissue acquired by directed biopsy was associated with greater signs of inflammation that allowed a more precise diagnosis because SpyGlassTM-directed biopsy acquired a greater amount of sample, at quantities adequate for analysis. Pathological examination improved the diagnosis of inflammation by the amount of specimens. This result occurred significantly more often in the biopsied specimens. These data are relevant with regard to patients with unknown biliary strictures and concur with another study in which the initial working diagnosis was modified after a SpyGlassTM investigation in 68.9% of patients with biliary strictures[29]. Specific risk populations (e.g., patients with PSC or prolonged chronic inflammation of the bile duct) are subject to an increased risk of cancer[30,31]. As POC provides direct information about the bile duct, it may serve as an important and informative extension of ERC[22].
Note that there were no complications related to the SpyGlassTM examination. In addition to the expected result of improved detection of inflammation in SpyGlassTM-directed biopsy, we also demonstrated that the method was easy and safe, as previously reported[32].
The present study had some limitations. First, we had to perform brush cytology after biopsy, and the influence of the quantity of the brush cytology specimens remains unknown. Second, this study was performed at a single center with a limited number of patients. Third, a single pathologist performed all the histopathological examinations.
In conclusion, the diagnostic yield of SpyGlassTM-directed biopsy for inflammatory changes in PSC and non-PSC patients was significantly greater than that of brush cytology. The better diagnostic yield strongly correlated with the greater amount of tissue specimens obtained from the SpyGlassTM-directed biopsy. A total of 2-3 biopsies must be obtained from suspicious areas in the biliary tract. Further studies are needed to fully clarify the benefit of the better inflammatory diagnosis in PSC and to investigate the potential of SpyGlassTM in diagnosing PSC-associated dysplasia.
Patients with primary sclerosing cholangitis (PSC) suffer from chronic and relapsing inflammation of the biliary tract. Endoscopic retrograde cholangiopancreatography is recommended procedure to stage the disease and to clarify inflammatory strictures. SpyglassTM as a single operator cholangioscopy system provides direct visualization of the biliary tract with the possibility of direct biopsies.
Cholangioscopy is basically not a new process. It has been introduced since the 1970´s as a so-called mother-baby endoscopy technique, in which a thin choledochoscope (baby-scope) was pushed through the instrumentation channel of the duodenoscope (mother-scope) during the endoscopic retrograde cholangiography (ERC). The procedure required two investigators and the quality of the fiber-optic images was poor. The first single-operator choledochoscopy system was introduced in 2005 by Boston Scientific, and is known as the SpyGlassTM direct visualization system.
Precise diagnosis of biliary lesions and strictures is still difficult but of crucial importance for the patients. However, neither ERC nor other auxiliary fluoroscopy-techniques permit reliable diagnostic evaluation of biliary lesions. The gold standard for the diagnosis of biliary lesions, especially in PSC, is still ERC. A recent study showed that the sensitivity of SpyGlassTM for gross assessment was significantly superior to that of ERC (81% vs 53%) and biliary strictures could be significant better characterized. Furthermore the SpyGlassTM system allows optical guided biopsy sampling with definite histologic diagnosis and high accuracy.
This study indicates that the diagnostic yield of SpyGlassTM-directed biopsies for inflammatory changes in PSC and non-PSC patients is significantly higher than that of brush cytology. The better diagnostic yield is strongly correlated with the larger amount of tissue specimens, which can be obtained with SpyGlassTM directed biopsies.
The SpyGlass System was developed to overcome the limitations of the so called traditional cholangioscopy. Integrated irrigation channels and a 1.2 mm diameter therapeutic channel make for the first time optical guided biopsies and therapeutic stone management possible. Thus, this system enables for a single investigator during ongoing ERC to perform targeted biopsy of bile duct lesions and to perform laser therapy of complicated bile duct stones.
This study is well conducted even if only a few patients were included. In this study the advantages of direct cholangioscopy with the possibility of using a single operator cholangioscopy and with the possibility of direct biopsies are well described. The results showing significant advantages of biopsy versus brush cytology in grading inflammation and non-inflammatory changes in the bile duct.
P- Reviewers: Fabozzi M, Sameer AS S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
| 1. | Harewood GC. Endoscopic tissue diagnosis of cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:627-630. [Cited in This Article: ] |
| 2. | Kawakami H, Kuwatani M, Etoh K, Haba S, Yamato H, Shinada K, Nakanishi Y, Tanaka E, Hirano S, Kondo S. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy. 2009;41:959-964. [Cited in This Article: ] |
| 3. | Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71-78. [Cited in This Article: ] |
| 4. | Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67-73. [Cited in This Article: ] |
| 5. | Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374-382. [Cited in This Article: ] |
| 6. | Osanai M, Itoi T, Igarashi Y, Tanaka K, Kida M, Maguchi H, Yasuda K, Okano N, Imaizumi H, Itokawa F. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013;45:635-642. [Cited in This Article: ] |
| 7. | Urakami Y, Seifert E, Butke H. Peroral direct cholangioscopy (PDCS) using routine straight-view endoscope: first report. Endoscopy. 1977;9:27-30. [Cited in This Article: ] |
| 8. | Meenan J, Schoeman M, Rauws E, Huibregtse K. A video baby cholangioscope. Gastrointest Endosc. 1995;42:584-585. [Cited in This Article: ] |
| 9. | Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303-311. [Cited in This Article: ] |
| 10. | Balderramo D, Sendino O, Miquel R, de Miguel CR, Bordas JM, Martinez-Palli G, Leoz ML, Rimola A, Navasa M, Llach J. Prospective evaluation of single-operator peroral cholangioscopy in liver transplant recipients requiring an evaluation of the biliary tract. Liver Transpl. 2013;19:199-206. [Cited in This Article: ] |
| 11. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [Cited in This Article: ] |
| 12. | Pleskow D, Parsi MA, Chen YK, Neuhaus H, Slivka A, Haluszka O, Petersen BT, Deviere J, Sherman S, Meisner S. Biopsy of indeterminate biliray strictures - does direct visualisation help? - A multicenter experience. Gastrointest Endosc. 2008;67:AB103. [Cited in This Article: ] |
| 13. | Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ, McNair AE, Mulholland M, Norton NJ, Rabeneck L. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803-809. [Cited in This Article: ] |
| 14. | Govil H, Reddy V, Kluskens L, Treaba D, Massarani-Wafai R, Selvaggi S, Gattuso P. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol. 2002;26:273-277. [Cited in This Article: ] |
| 15. | Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671-677. [Cited in This Article: ] |
| 16. | Mohammad Alizadeh AH, Mousavi M, Salehi B, Molaei M, Khodadoostan M, Afzali ES, Dadvar Z, Mirsattari D, Aghdaei HA, Lahmi F. Biliary brush cytology in the assessment of biliary strictures at a tertiary center in Iran. Asian Pac J Cancer Prev. 2011;12:2793-2796. [Cited in This Article: ] |
| 17. | Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064-1072. [Cited in This Article: ] |
| 18. | Selvaggi SM. Biliary brushing cytology. Cytopathology. 2004;15:74-79. [Cited in This Article: ] |
| 19. | Singh V, Bhasin S, Nain CK, Gupta SK, Singh G, Bose SM. Brush cytology in malignant biliary obstruction. Indian J Pathol Microbiol. 2003;46:197-200. [Cited in This Article: ] |
| 20. | Nagayoshi Y, Aso T, Ohtsuka T, Kono H, Ideno N, Igarashi H, Takahata S, Oda Y, Ito T, Tanaka M. Peroral pancreatoscopy using the SpyGlass system for the assessment of intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2013;Epub ahead of print. [Cited in This Article: ] |
| 21. | Abstracts of Digestive Disease Week, May 17-22, 2008 and the ASGE (American Society for Gastrointestinal Endoscopy) Postgraduate Course, May 21-22, 2008. San Diego, California, USA. Gastrointest Endosc. 2008;67:AB57-A349. [Cited in This Article: ] |
| 22. | Tischendorf JJ, Krüger M, Trautwein C, Duckstein N, Schneider A, Manns MP, Meier PN. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665-669. [Cited in This Article: ] |
| 23. | Monga A, Ramchandani M, Reddy DN. Per-oral cholangioscopy. J Interv Gastroenterol. 2011;1:70-77. [Cited in This Article: ] |
| 24. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. [Cited in This Article: ] |
| 25. | Siddiqui AA, Mehendiratta V, Jackson W, Loren DE, Kowalski TE, Eloubeidi MA. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol. 2012;10:466-71; quiz e48. [Cited in This Article: ] |
| 26. | Hartman DJ, Slivka A, Giusto DA, Krasinskas AM. Tissue yield and diagnostic efficacy of fluoroscopic and cholangioscopic techniques to assess indeterminate biliary strictures. Clin Gastroenterol Hepatol. 2012;10:1042-1046. [Cited in This Article: ] |
| 27. | Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635-638. [Cited in This Article: ] |
| 28. | Yeo D, Perini MV, Muralidharan V, Christophi C. Focal intrahepatic strictures: a review of diagnosis and management. HPB (Oxford). 2012;14:425-434. [Cited in This Article: ] |
| 29. | Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353-1358. [Cited in This Article: ] |
| 30. | Ehlken H, Schramm C. Primary sclerosing cholangitis and cholangiocarcinoma: pathogenesis and modes of diagnostics. Dig Dis. 2013;31:118-125. [Cited in This Article: ] |
| 31. | Kalaitzakis E, Webster GJ, Oppong KW, Kallis Y, Vlavianos P, Huggett M, Dawwas MF, Lekharaju V, Hatfield A, Westaby D. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol. 2012;24:656-664. [Cited in This Article: ] |
| 32. | Manta R, Frazzoni M, Conigliaro R, Maccio L, Melotti G, Dabizzi E, Bertani H, Manno M, Castellani D, Villanacci V. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013;27:1569-1572. [Cited in This Article: ] |