Published online Apr 16, 2014. doi: 10.4253/wjge.v6.i4.128
Revised: January 17, 2014
Accepted: March 3, 2014
Published online: April 16, 2014
Processing time: 129 Days and 11.2 Hours
AIM: To assess feasibility of unsedated esophagoscopy using a small-caliber disposable transnasal esophagoscopy and to compare its accuracy with standard endoscopy.
METHODS: We prospectively included subjects who were referred for upper endoscopy. All subjects underwent transnasal endoscopy with E.G. Scan™. The disposable probe has a 3.6 mm gauge and at its distal end there is a 6 mm optical capsule, with a viewing angle of 125°. Patients underwent conventional endoscopy after the completion of E.G. Scan™. We describe the findings detected by the E.G. Scan™ and calculate the diagnostic accuracy, sensitivity, specificity, positive predictive value, negative predictive value and Kappa index for esophageal diagnosis.
RESULTS: A total of 96 patients (54 women), mean age of 50.12 years (14 to 79), were evaluated. In all cases we were able to perform esophagoscopy with E.G. Scan™. The average realization time was 5 min. A total of 58 alterations were detected in the esophagus, 49 gastric abnormalities and 13 duodenal abnormalities. We found that for esophageal varices, E.G. Scan™ has sensitivity, specificity and diagnostic accuracy of 95%, 97% and 97%, respectively. Kappa coefficients were 0.32 for hiatal hernia, 0.409 for erosive gastroesophageal reflux disease, 0.617 for Barrett’s esophagus, and 0.909 for esophageal varices.
CONCLUSION: Esophagoscopy with E.G. Scan™ is a well-tolerated, fast and safe procedure. It has an appropriate diagnostic accuracy for esophageal varices when compared with conventional endoscopy.
Core tip: Although esophagogastroduodenoscopy (EGD) is considered the gold standard technique for evaluation of mucosal esophageal diseases, the cost and invasiveness of this diagnostic tool limits its utilization in some patients. Thus, in recent years several endoscopy techniques have been developed as alternatives and less invasive diagnostic tools for evaluating gastroesophageal reflux disease and esophageal varices. Here, in this study we have shown that unsedated esophagoscopy using a novel disposable transnasal esophagoscope (E.G. Scan™) is a safe, well-tolerated, effective and accurate screening tool for esophageal diseases, specifically for esophageal varices.
- Citation: Aedo MR, Zavala-González M&, Meixueiro-Daza A, Remes-Troche JM. Accuracy of transnasal endoscopy with a disposable esophagoscope compared to conventional endoscopy. World J Gastrointest Endosc 2014; 6(4): 128-136
- URL: https://www.wjgnet.com/1948-5190/full/v6/i4/128.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i4.128
Esophagogastroduodenoscopy (EGD) is the most effective method to investigate disorders affecting the upper digestive tract. In particular, EGD is the gold standard technique for the evaluation, diagnosis, screening and surveillance of esophageal diseases. Among patients with gastroesophageal reflux disease (GERD) symptoms, up to one-third of patients have endoscopic evidence of erosive esophagitis and up to one-fifth have complicated reflux disease, such as esophageal strictures and Barrett’s esophagus (BE)[1,2]. In subjects with portal hypertension, EGD is used for both screening and surveillance purposes because the presence and the size of esophageal varices correlates with severity of liver disease and determines the prognosis[3,4].
Although EGD is widely used and available, the procedure is costly, may be unpleasant, and still has a small but potential risk of complications[5,6]. Frequently, patients are routinely sedated with intravenous diazepam or midazolam, often complemented with a narcotic such as meperidine, fentanyl or propofol[6]. There is a small but definite risk of cardiopulmonary complications, which may be related to a combination of oversedation and preexisting cardiopulmonary disease[6]. In addition, sedated patients require close monitoring during and after procedures, cannot drive or return to work on the day of the procedure, and may have post-procedure amnesia with poor recall of instructions.
Over the last years, several noninvasive or minimally invasive methods have been proposed as alternatives to conventional EGD for the diagnosis of esophageal diseases, such as esophageal capsule endoscopy (ECE) and ultra-thin small caliber esophagoscopes[7-12]. Several studies have shown that ECE is safe and has an acceptable accuracy for the evaluation of esophageal varices and can be used as an alternative to EGD for the screening of portal hypertension, especially in patients unable or unwilling to undergo EGD[7,9].
Unsedated small-caliber transnasal esophagoscopy offers the possibility of efficient and accurate endoscopic assessment of the esophagus, with less cost and fewer risks compared with sedated upper endoscopy, and can be used as a method to screen for esophageal disease in a primary care population[11-15]. Recently, Chung et al[16], in a case series study, reported the use of a novel disposable transnasal esophagoscope, the E.G. Scan™ (IntroMedic Co. Ltd., Seoul, South Korea). This transnasal esophagoscope does not require a large endoscopy system or special equipment for disinfection; it is portable, disposable and well tolerated.
The aim of the study was to assess the feasibility of unsedated routine upper esophagoscopy using the E.G. Scan™ and to compare its optical quality and diagnostic accuracy to that of a standard EGD in the general medical outpatient setting as a screening method for esophageal disease.
We performed a prospective study conducted from November 2011 to February 2012 at the Instituto de Investigaciones Medico Biologicas de la Universidad Veracruzana, Veracruz, México. Consecutive patients referred for the evaluation of esophageal diseases were enrolled in the study. Inclusion criteria were: age 20 years or older; reflux symptoms (heartburn, epigastric soreness and/or regurgitation); non-cardiogenic chest pain; and known or suspected esophageal varices. Exclusion criteria included: history or symptoms of severe rhinitis and sinusitis; acute respiratory inflammation at the time of examination; and known abnormal anatomy of the nasal cavity or nasopharynx. All patients provided written informed consent before enrollment and the study received approval from the institution’s ethics committee.
All conventional EGD and E.G. Scan™ procedures were performed by two experienced endoscopists (J.M.R.T. and A.M.D) after written informed consent was obtained. Randomization was performed by using a computer-generated randomization (http://www.randomization.com), which allocated patients on a one-to-one basis to the investigator who will perform the EG Scan procedure. Thus if one investigator performed the E.G. Scan™, the other performed the conventional EGD, and investigators were blinded each other. Also, endoscopists were blinded to the indication for endoscopy. The E.G. Scan™ procedure was performed first and 45 min later a conventional sedated endoscopy was performed.
E.G. Scan™: After an overnight fast, patients were referred to the endoscopy unit. For the procedure, patients were seated with their neck at a 30° angle and 2 puffs of a nasal spray containing oxymetazoline hydrochloride, a selective alpha-1 agonist and partial alpha-2 agonist topical nasal decongestant, was sprayed in each nostril (Afrin, Merck Consumer Care, Inc. Mexico). After 5 min, lidocaine hydrochloride 10mg/dose (Xylocaine 10% Pump Spray AstraZeneca, London, United Kingdom) was sprayed into the nasal cavity and oropharynx for topical anesthesia. The endoscope, moistened with Lidocaine HCL jelly 2% (lidocaine hydrochloride 2% 20 mg/mL; Lubricaine, Mexico), was inserted under visual control through the nostril to the pharynx. Upper esophageal sphincter intubation was facilitated by asking the patient to ingest water through a straw with endoscope advancement. No sedatives or antispasmodics were used during the procedure.
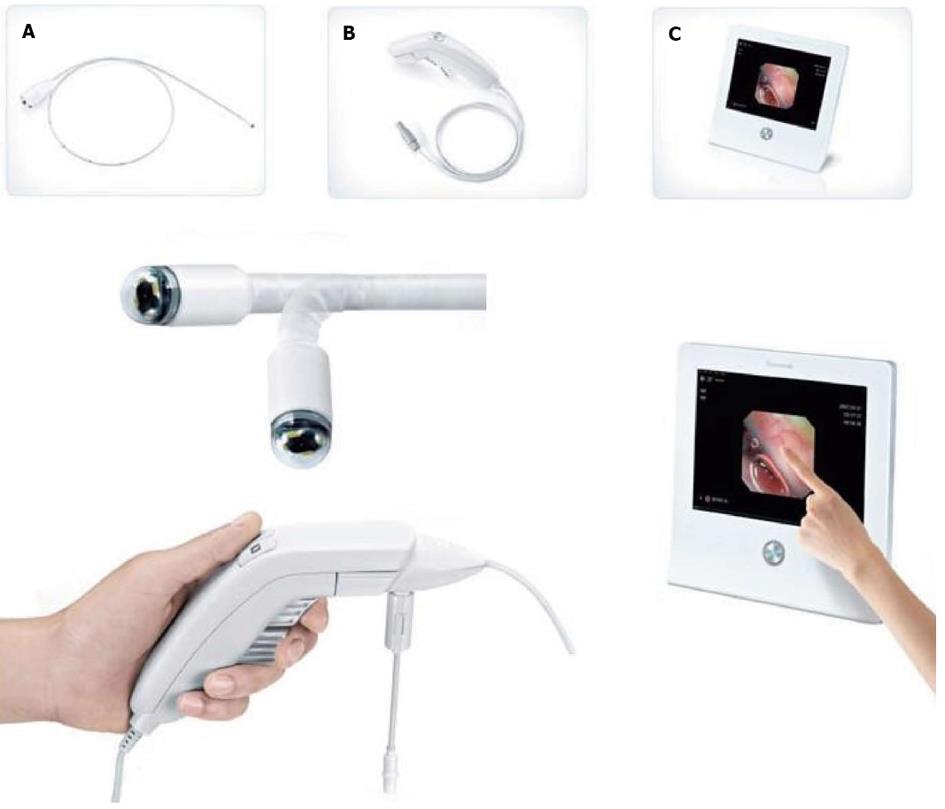
The E.G. Scan™ system (first generation) consists of four main subsystems: a probe (containing the camera capsule, bending module and data connector), controller, display system and computer software (EG View) to display the images (Figure 1). The connection tube, which does not have suction or an air channel, is 3.6 mm in diameter and the camera capsule at the tip head is 6 mm in diameter. The tip deflection capability is 60° up and 60° down. The camera capsule comprises four white light emitting diodes (LEDs) and a complementary metal-oxide semiconductor (CMOS), with a field of view of 125° and a resolution of 400 × 400 pixels. The probe is disposable. The controller has both freeze-capture buttons and an up-down lever at the handle. The display system consists of a liquid crystal display (LCD) monitor, keyboard and display software (EG View) to allow playback and storage of images taken during the procedure; this system is light enough to carry.
During the procedure, the posterior pharynx, esophagus, esophagogastric junction (EGJ) and proximal stomach were routinely examined. EGJ examination was considered appropriate if at least 75% of the Z-line was visualized[16]. If possible, the mid-stomach, pylorus and duodenum were examined. Any pathological lesions were photographed and recorded on the display system. The investigators documented the duration time of the study, presence or absence of suspected BE, presence or absence of erosive esophagitis, Los Angeles grade of erosive esophagitis (if present), presence or absence of hiatal hernia (documented and measured at the nares in centimeters beginning at the crural pinch distally to the most proximal extent of the gastric folds), esophageal varices were graded according to the size of varices (small or large), the presence or absence of red spots on esophageal varices was also noted, and any other abnormal findings discovered during the study. These findings were recorded on a data sheet. After the procedure, patients completed a written questionnaire to assess their satisfaction with the E.G. Scan™ and level of discomfort for nasal pain and nausea using a 4 point type Likert scale (0 = none, 1 = mild, 2 = moderate and 3 = severe)[17,18].
EGD: Sedated endoscopy was performed with the Olympus XGIF-160 with patients under local anesthetic with lidocaine spray (Xylocaine; AstraZeneca, United Kingdom) and conscious sedation with midazolam, according to our standard practice. Blood pressure, pulse, cardiac rhythm and oxygen saturation were monitored and recorded every 2 min. In all cases, the endoscope was inserted under visual control through the mouth to the pharynx. The upper esophageal sphincter was crossed under direct vision and the esophagus, stomach and first and second portions of the duodenum were examined as usual. Endoscopic findings were reported using the definitions previously mentioned. Histological confirmation of esophageal biopsies from endoscopically suspected esophageal metaplasia was considered as the “gold standard” for the diagnosis of BE.
EGD was considered to be the “gold standard” for the diagnosis of esophageal diseases. According to the Standards for Reporting of Diagnostic Accuracy (STARD) initiative on assessment of diagnostic tests[19], analysis was performed on an intention-to-diagnose (ITD) basis, with all patients enrolled in the trial included in the analysis. The diagnostic performance was expressed in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
At the end of the enrollment period and in a blinded fashion, both endoscopists reviewed the printed images from all E.G. Scan™ studies and interobserver agreement analysis was performed. Concordance among the different E.G. Scan™ observers and between the E.G. Scan™ and EGD final diagnoses was performed using kappa statistics. Our sample size was decided arbitrarily, according to the available material to perform the studies (E.G. Scan™) during the frame time when the study was performed. All other statistics were descriptive and the results are reported in terms of the mean (with 95% confidence interval in brackets) or median and ranges, depending on the distribution of data values. P values less than 0.05 were considered statistically significant.
During the study period, a total of 96 patients (54 women) were included. Mean age was 50.12 years (range 18 to 79). Baseline characteristics and symptoms are described in Table 1. In all cases, we were able to perform esophagoscopy with E.G. Scan™ and the mean duration of the procedure was 5 min (range 3-7.5).
| Age (yr, mean, range) | 50.12 (18-79) |
| Gender (male/female) | 42/54 |
| Predominant symptoms | |
| Reflux symptoms | 41 (43) |
| Suspect of esophageal varices | 23 (24) |
| Epigastric pain | 14 (15) |
| Upper GI bleeding | 11 (11) |
| Dysphagia | 4 (4) |
| Weight loss | 3 (3) |
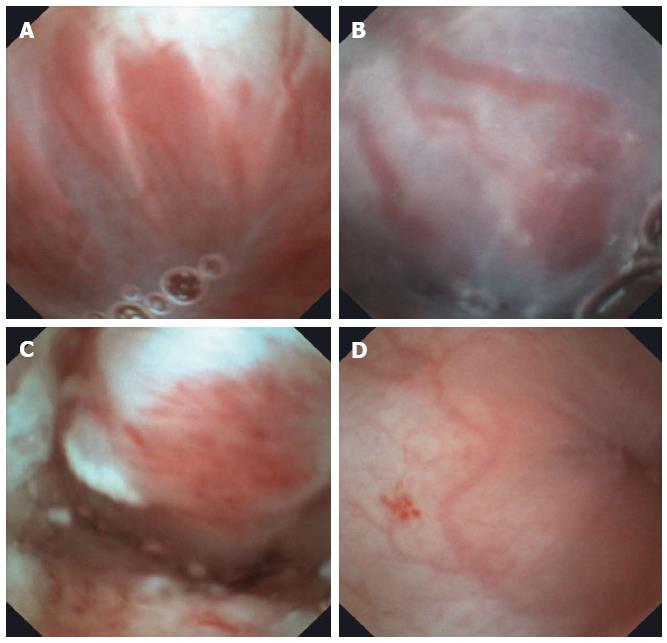
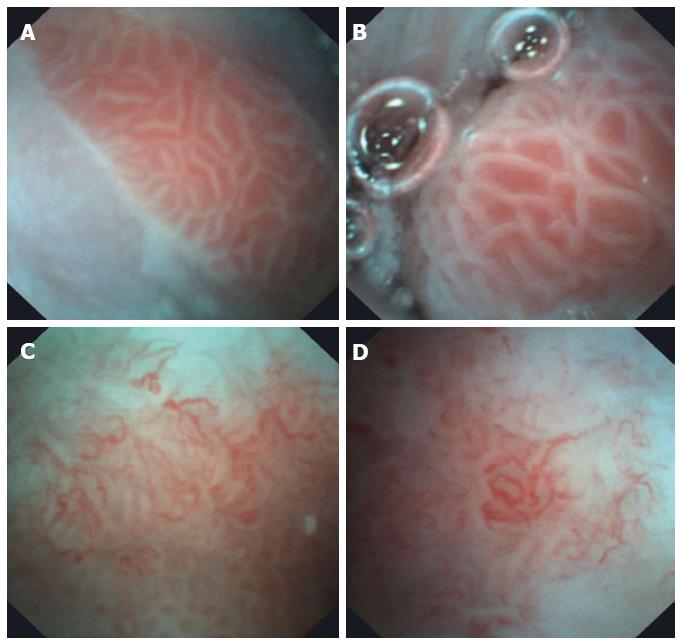
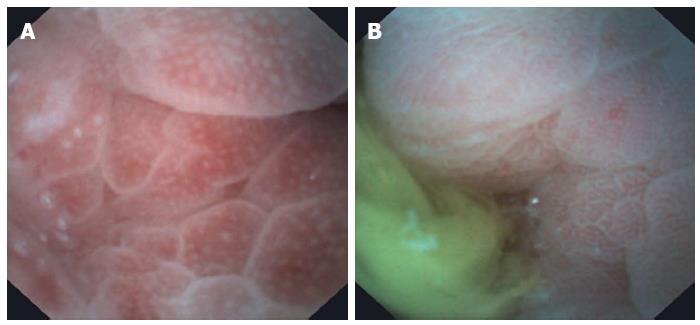
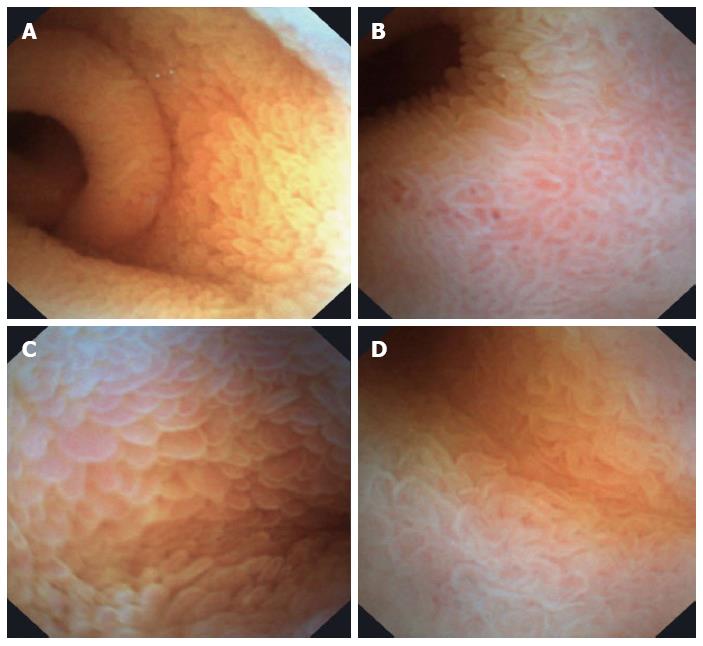
Using the E.G. Scan™ in all cases, the EGJ was evaluated; in 43% the pylorus was visualized and we reach the duodenum in 36% cases. Appropriate evaluation of the EGJ junction was achieved in 95% (n = 91). A total of 58 alterations were detected in the esophagus (Table 2), 49 gastric abnormalities (18 portal hypertension gastritis, 18 mild erythematous gastritis, 10 bile gastropathy, 3 gastric polyps) and 13 duodenal abnormalities (9 duodenitis, findings suggestive of celiac disease in 2, 1 duodenal ulcer and 1 angiodysplasia) (Figures 2-5).
| Finding | E.G. Scan™ | Conventional EGD |
| Esophagus | ||
| Esophageal varices (overall) | 20 | 21 |
| Small | 5 | 8 |
| Large | 11 | 13 |
| Erosive GERD | 13 | 29 |
| Grade A-B | 10 | 20 |
| Grade C | 2 | 8 |
| Grade D | 1 | 1 |
| Hiatal hernia | 13 | 33 |
| Barrett’s esophagus | 8 | 12 |
| Esophageal carcinoma | 2 | 2 |
| Esophageal angiodysplasia | 1 | 1 |
| Gastric heterotopic mucosa | 1 | 1 |
Using conventional endoscopy, a total of 99 esophageal diagnoses were made (Table 2). In addition, 71 gastric abnormalities were detected (23 portal hypertension gastritis, 21 erythematous and/or erosive gastritis, 15 bile gastropathy, 8 fundic polyps and 4 fundic varices), and 25 duodenal abnormalities (19 duodenitis, 3 findings suggestive for celiac disease and 3 duodenal ulcers).
The diagnostic performance of E.G. Scan™ compared to EGD for erosive GERD, Barrett’s esophagus, esophageal varices and hiatal hernia is shown in Table 3. Regarding the agreement between E.G. Scan™ and EGD, the kappa values for esophageal diagnoses is shown in Table 4.
| Prevalence % (95%CI) | Sensitivity % (95%CI) | Specificity % (95%CI) | PPV % (95%CI) | NPV % (95%CI) | Accuracy % (95%CI) | |
| Erosive GERD | 30.1 (21.5-40.6) | 44.8 (27-64) | 91 (80.9-96.3) | 68.4 (43.5-86.4) | 79.2 (68.2-87.3) | 77.1 (67.2-84.8) |
| Barrett’s esophagus | 12.5 (6.9-21.2) | 66.7 (35.4-88.7) | 95 (87.6-98.5) | 66.7 (35.4-88.7) | 95.2 (87.6-98.5) | 91.7 (83.8-96.1) |
| Esophageal varices | 21.8 (14.4-31.7) | 95.2 (74.1-99.8) | 97.3 (89.8-99.5) | 90.9 (69.3-98.4) | 98.6 (91.7-99.9) | 96.8 (90.5-99.1) |
| Hiatal Hernia | 34.4 (25.1-44.8) | 39.4 (23.4-57.7) | 88.9 (77.8-95) | 65 (41-83.7) | 73.7 (62.1-82.8) | 71.9 (61.6-80.3) |
| Kappa value | Standard error of Kappa | 95%CI | |
| Esophageal varices | 0.910 | 0.051 | 0.810-1.010 |
| Large esophageal varices | 0.822 | 0.086 | 0.653-0.911 |
| Small esophageal varices | 0.591 | 0.151 | 0.294-0.880 |
| Barrett’s esophagus | 0.619 | 0.123 | 0.378-0.860 |
| Hiatal Hernia | 0.398 | 0.103 | 0.196-0.600 |
| Erosive esophagitis | 0.398 | 0.100 | 0.196-0.600 |
The mean kappa values for interobserver agreement for each esophageal condition were: for hiatal hernia 0.762 (0.506-1.018); for erosive esophagitis 0.832 (0.606-1.058); for BE 0.554 (0.207-0.901); for esophageal varices 0.903 (0.796-1.011); for large esophageal varices 0.911 (0.739-1.083); and for small esophageal varices 0.832 (0.606-1.058).
Nasal introduction caused no or only mild pain in 77 of 96 patients (80%) and moderate pain in 19 patients (20%). The majority of patients did not experience nausea (88%).
Although EGD is considered the gold standard technique for evaluation of mucosal esophageal diseases, the cost and invasiveness of this diagnostic tool limits its utilization in many patients[6]. Thus, several endoscopy techniques have recently been developed as alternatives and less invasive diagnostic tools for evaluating GERD and esophageal varices[12-16,20].
Here, in this study we have shown that unsedated esophagoscopy using a novel disposable transnasal esophagoscope (E.G. Scan™) is a safe, well-tolerated, effective and accurate screening tool for esophageal diseases, specifically for esophageal varices. In recent years, the use of transnasal endoscopy (TNE) has had a boom and several studies have evaluated the usefulness of this technique. For example, Peery et al[20] in one of the largest studies (n = 426) found that TNE is a safe and good method to screen for esophageal disease in a primary care population. In this study, mean examination time with TNE was 3.7 ± 1.8 min and there were no serious adverse events. Our results are similar, but the E.G. Scan™ has some advantages compared to other transnasal endoscopy systems. Although the tip of the probe is 6 mm in diameter, the connection tube (which does not have suction or an air channel) is 3.6 mm in diameter; thus, the probe is smaller than other TNE (range from 4.1 to 5.9 mm) and could minimize the gag reflex and vomiting. Another advantage is that no disinfection is required because the probe is designed for single use and is disposable.
Chung et al[16], in the first study published with E.G. Scan™, evaluated 46 patients with suspected or known esophageal disease and found that in almost all cases, the Z line was appropriately evaluated and abnormalities were identified in 27 patients. In this small sample size pilot study, the authors concluded that although E.G. Scan™ has some technical limitations compared with conventional EGD, its convenience, good tolerance, rapid access, cost-effectiveness and good safety profile indicate that it may be an acceptable alternative to conventional esophagoscopy for surveillance.
Compared to the pilot study by Chung et al[16], our study included a larger sample size, we performed the first randomized and blinded evaluation, but most remarkably, we compared the results with the gold standard technique, the EGD. Because conventional endoscopy with sedation may be associated with complications, especially in critically ill patients such as subjects with cirrhosis, the use of an alternative and safe method for evaluating the esophagus, especially in the setting of a screening strategy (i.e., esophageal varices), is needed[5,6]. We found that for esophageal varices (independently of the size), E.G. Scan™ is an excellent option, with sensitivity, specificity and diagnostic accuracy of 95%, 97% and 97%, respectively. These results are similar to that reported by Choe et al[21], in a study where 100 cirrhotics were evaluated both by transnasal and standard endoscopy, showing that diagnostic accuracies of transnasal non sedated EGD for detecting esophageal varices, gastric varices and red color signs were 98%, 98% and 96%, respectively. Also, as in the Choe et al[21] study, we found that concordance rates on grading esophageal varices were excellent at 95% (κ = 0.91). These results are better than those reported by using endoscopy capsule for detection of esophageal varices[7-9].
With regards to erosive esophagitis diagnosed at upper endoscopy, E.G. Scan™ showed a sensitivity of 45% and specificity of 91%. These results are very similar to those reported by Sharma et al[8] in a study comparing ECE versus conventional endoscopy. In a recent study, Shariff et al[11] found that using a transnasal endoscope, a correct diagnosis of BE was obtained in 48 of 49 cases compared with the criterion standard, giving sensitivity and specificity of 98% and 100%, respectively. Although in our study the sensitivity was lower (67%), the specificity was 95% for the diagnosis of BE. In another study, Jobe et al[12] found that, in a cohort of 274 eligible adults scheduled for endoscopic screening for gastroesophageal reflux symptoms or surveillance of BE in a tertiary care center, the prevalence of BE was 26% using conventional endoscopy and 30% using unsedated small-caliber endoscopy (P = 0.503). In this study, the level of agreement between the two approaches was “moderate” (κ = 0.591). In our study, we found that agreement between E.G. Scan™ and EGD was 0.619. However, E.G. Scan™ misses about half of the cases of erosive esophagitis and one third of patients with Barrett’s esophagus. It appears therefore that this version of E.G. Scan™ is not sufficiently sensitive for evaluation of acid reflux evaluation.
It is important to remark that, even although E.G. Scan™ has been developed for esophageal evaluation, in almost 40% of the cases we could reach the pylorus and the duodenum. We could do that because we asked patients to lie down in the left lateral position and then under direct visualization we advanced the probe. As shown in the figures, good quality images from the duodenum of patients with celiac disease and inflammatory duodenitis were obtained.
Regarding tolerability, we found that, as was reported by Chung et al[16], most of the patients experienced mild or no symptoms during the procedure and even if they reported mild symptoms, we could perform the evaluation in all cases. An unusual 100% success rate of nasal intubation with this device was found in our study, contrasting with other reports on transnasal endoscopy that present an average of 8% failure rate for nasal intubation due to anatomic nasal limitation or patient intolerance[22,23]. Although the probe shaft is 3 mm, its tip is 6 mm, a little larger than an ultra-slim endoscope, and we believe that the routine use of oxymetazoline hydrochloride, a selective alpha-1 agonist and partial alpha-2 agonist topical, influences such a high success rate for nasal intubation. Previous studies have shown that the use of oxymetazoline for pediatric nasendoscopy is effective, safe and allows an ease of performance and cooperation of the patients[24]. Although we prepared the patient with an assurance of a successful nasal intubation, we did not use simethicone routinely, a compound that has been used in several studies to improve visibility[25].
Regarding costs, in our country the cost for the E.G. Scan™ device is 8000 USD and each probe costs 140 USD. However, costs can vary among countries and further cost-effectiveness studies are required. Although our study has the strength of a large, blinded evaluation and comparative study with conventional EGD, there are some limitations and technical issues that should be remarked on. The current version of the E.G. Scan™ does not have a channel for air insufflation or water ejection for wash or suction water and bubble air to improve the quality of images. Another major limitation is that it also does not have a biopsy channel to corroborate some conditions, such as BE or malignant lesions. Recently, the manufacturer has provided a new version of the E.G. Scan™ that has an insufflation channel and the bending angle of the tip probe is closed to 180°; thus retroversion at the stomach fundus can now be performed. Nowadays, slim endoscopes have much better quality than in the past with high-resolution images and digital chromoendoscopy and a complete EGD. E.G. Scan™ seems to be an alternative to ultra-slim endoscopes for transnasal examination. In the future, an ideal comparative trial will be performed between E.G. Scan™ and nasogastroscopes.
According to our results, we conclude that E.G. Scan™ might represent an easy, safe and well tolerated procedure to investigate patients with suspected esophageal varices in the medical outpatient setting.
Esophagogastroduodenoscopy (EGD) is the most effective method to investigate disorders affecting the upper digestive tract. In particular, EGD is considered as the gold standard technique for the evaluation, diagnosis, screening and surveillance of esophageal diseases. Although EGD is widely used and available, the procedure is costly, may be unpleasant, and still has a small but potential risk of complications.
Over the last years, a research hotspot has been the development of alternative methods to conventional EGD for the noninvasive or minimally invasive diagnosis of esophageal diseases, such as ultra-thin small caliber esophagoscopes.
Recently, a novel disposable transnasal esophagoscope, the E.G. Scan™ (IntroMedic Co. Ltd., Seoul, South Korea) has been developed. This transnasal esophagoscope does not require either a large endoscopy system or special equipment for disinfection; it is portable, disposable and well tolerated. In our study, we found that that E.G. Scan™ might represent an easy, safe and well tolerated first-line procedure to investigate patients with suspected esophageal varices in the medical outpatient setting.
Unsedated small-caliber transnasal esophagoscopy offers the possibility of efficient and accurate endoscopic assessment of the esophagus, with less cost and fewer risks compared with sedated upper endoscopy, and can be used as a method to screen for esophageal disease in a primary care population.
Esophagogastroduodenoscopy: Esophagogastroduodenoscopy or panendoscopy is a diagnostic endoscopic procedure that visualizes the upper part of the gastrointestinal tract up to the duodenum. It is considered a minimally invasive procedure since it does not require an incision into one of the major body cavities and does not require any significant recovery after the procedure (unless sedation or anesthesia has been used). Esophagoscopy: Esophagoscopy is a procedure in which a flexible endoscope is inserted through the mouth, or more rarely through the nares, and into the esophagus. The endoscope uses a charge-coupled device to display magnified images on a video screen. The procedure allows visualization of the esophageal mucosa from the upper esophageal sphincter all the way to the esophageal gastric junction or esophagogastric junction.
This is an interesting and well-designed prospective study that evaluated a new device called E.G Scan™ for endoscopic esophageal examination through the transnasal route compared to conventional EGD, with better results seen with the modified version of the scope. E.G. Scan™ seems to be an alternative to ultra-slim endoscopes for transnasal examination and the ideal comparative trial would have been between E.G. Scan™ and nasogastroscopes. Nowadays slim endoscopes have much better quality than in the past, with high-resolution images and digital chromoendoscopy, and they permit a complete EGD.
P- Reviewers: Alsolaiman M, Arantes V, Bak YT, Eysselein VE S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. |
| 2. | Frazzoni M, De Micheli E, Savarino V. Different patterns of oesophageal acid exposure distinguish complicated reflux disease from either erosive reflux oesophagitis or non-erosive reflux disease. Aliment Pharmacol Ther. 2003;18:1091-1098. |
| 3. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. |
| 4. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. |
| 5. | Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut. 1991;32:12-15. |
| 6. | Froehlich F, Gonvers JJ, Fried M. Conscious sedation, clinically relevant complications and monitoring of endoscopy: results of a nationwide survey in Switzerland. Endoscopy. 1994;26:231-234. |
| 7. | Lapalus MG, Ben Soussan E, Gaudric M, Saurin JC, D’Halluin PN, Favre O, Filoche B, Cholet F, de Leusse A, Antonietti M. Esophageal capsule endoscopy vs. EGD for the evaluation of portal hypertension: a French prospective multicenter comparative study. Am J Gastroenterol. 2009;104:1112-1118. |
| 8. | Sharma P, Wani S, Rastogi A, Bansal A, Higbee A, Mathur S, Esquivel R, Camargo L, Sampliner RE. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: a blinded, prospective study. Am J Gastroenterol. 2008;103:525-532. |
| 9. | Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol. 2009;15:1254-1258. |
| 10. | Galmiche JP, Sacher-Huvelin S, Coron E, Cholet F, Soussan EB, Sébille V, Filoche B, d’Abrigeon G, Antonietti M, Robaszkiewicz M. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538-545. |
| 11. | Shariff MK, Bird-Lieberman EL, O’Donovan M, Abdullahi Z, Liu X, Blazeby J, Fitzgerald R. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc. 2012;75:954-961. |
| 12. | Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD, Diggs BS, O’Rourke RW, Rader AE, Schipper P. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693-2703. |
| 13. | Wilkins T, Gillies RA. Office-based unsedated ultrathin esophagoscopy in a primary care setting. Ann Fam Med. 2005;3:126-130. |
| 14. | Madhotra R, Mokhashi M, Willner I, Hawes RH, Reuben A. Prospective evaluation of a 3.1-mm battery-powered esophagoscope in screening for esophageal varices in cirrhotic patients. Am J Gastroenterol. 2003;98:807-812. |
| 15. | Thota PN, Zuccaro G, Vargo JJ, Conwell DL, Dumot JA, Xu M. A randomized prospective trial comparing unsedated esophagoscopy via transnasal and transoral routes using a 4-mm video endoscope with conventional endoscopy with sedation. Endoscopy. 2005;37:559-565. |
| 16. | Chung JW, Park S, Chung MJ, Park JY, Park SW, Chung JB, Song SY. A novel disposable, transnasal esophagoscope: a pilot trial of feasibility, safety, and tolerance. Endoscopy. 2012;44:206-209. |
| 17. | Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117-126. |
| 18. | Zaman A, Hapke R, Sahagun G, Katon RM. Unsedated peroral endoscopy with a video ultrathin endoscope: patient acceptance, tolerance, and diagnostic accuracy. Am J Gastroenterol. 1998;93:1260-1263. |
| 19. | Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40-44. |
| 20. | Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S, Sanz AF, Davison J, Spacek M, Connors D. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video). Gastrointest Endosc. 2012;75:945-953.e2. |
| 21. | Choe WH, Kim JH, Ko SY, Kwon SY, Kim BK, Rhee KH, Seo TH, Lee TY, Hong SN, Lee SY. Comparison of transnasal small-caliber vs. peroral conventional esophagogastroduodenoscopy for evaluating varices in unsedated cirrhotic patients. Endoscopy. 2011;43:649-656. |
| 22. | Preiss C, Charton JP, Schumacher B, Neuhaus H. A randomized trial of unsedated transnasal small-caliber esophagogastroduodenoscopy (EGD) versus peroral small-caliber EGD versus conventional EGD. Endoscopy. 2003;35:641-646. |
| 23. | Yagi J, Adachi K, Arima N, Tanaka S, Ose T, Azumi T, Sasaki H, Sato M, Kinoshita Y. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy. 2005;37:1226-1231. |
| 24. | Jonas NE, Visser MF, Oomen A, Albertyn R, van Dijk M, Prescott CA. Is topical local anaesthesia necessary when performing paediatric flexible nasendoscopy? A double-blind randomized controlled trial. Int J Pediatr Otorhinolaryngol. 2007;71:1687-1692. |
| 25. | Arantes V, Albuquerque W, Salles JM, Freitas Dias CA, Alberti LR, Kahaleh M, Ferrari TC, Coelho LG. Effectiveness of unsedated transnasal endoscopy with white-light, flexible spectral imaging color enhancement, and lugol staining for esophageal cancer screening in high-risk patients. J Clin Gastroenterol. 2013;47:314-321. |