Published online Mar 16, 2014. doi: 10.4253/wjge.v6.i3.82
Revised: January 26, 2014
Accepted: February 16, 2014
Published online: March 16, 2014
Processing time: 112 Days and 12.5 Hours
AIM: To determine whether a newly developed respiratory rate monitor can practically and accurately monitor ventilation under propofol sedation in combination with standard monitoring.
METHODS: Patients [American Society of Anesthesiologists (ASA) Classification I-III] scheduled for elective colonoscopy under propofol sedation were monitored with a new device that measures the respiratory rate based on humidity in expired air. Patients with clinically significant cardiac disorders or pulmonary disease and patients requiring emergency procedures were excluded from study participation. All of the patients also received standard monitoring with pulse oximetry. This was a single-center study conducted in a community hospital in Switzerland. After obtaining written informed consent from all subjects, 76 patients (51 females and 25 males) were monitored during colonoscopy under propofol sedation. The primary endpoint was the occurrence of any respiratory event (apnea or hypopnea). Apnea was defined as the cessation of breathing for a minimum of 10 s. Significant apnea was defined as the cessation of breathing for more than 30 s. Hypopnea was defined as a reduction in the respiratory rate below 6/min for a minimum of 10 s. Any cases of significant apnea triggered interventions by the endoscopy team. The interventions included withholding propofol, verbal stimulation of the patients, and increased oxygen supplementation or the chin lift maneuver. A secondary endpoint was the correlation of apnea or hypopnea with hypoxemia (measured as a decrease in SaO2 of at least 5% from baseline or less than 90%).
RESULTS: At least one respiratory event was detected in thirty-seven patients (48.7%). In total, there were 73 respiratory events, ranging from one to six events in a single patient. Significant apnea (> 30 s) occurred in five patients (6%). Only one episode of apnea led to a relative SaO2 reduction (from 98% to 93%) after a 50 s lag time. No event requiring assisted ventilation was recorded. Our analysis revealed that the total propofol dose was an independent risk factor for respiratory events (P = 0.01). Artifacts developed with the same frequency with the new device as with conventional pulse oximetry. Compared with pulse oximetry alone, this new monitoring device detected more respiratory events and may provide earlier warning of impending respiratory abnormalities.
CONCLUSION: Apnea commonly occurs during endoscopy under sedation and may precede hypoxemia. We recommend this respiration rate monitor as an alternative to capnography to aid in detecting ventilatory problems.
Core tip: Apnea monitoring is a useful adjunct in assessing the ventilatory status of patients undergoing sedation. Capnography is too expensive to be used during normal endoscopic procedures. A newly developed respiratory rate-monitoring device based on the humidity of expired air enables the real-time assessment of ventilation. Compared with pulse oximetry alone, this new monitoring device detected more respiratory events and may provide earlier warning of impending respiratory abnormalities.
- Citation: Anand GW, Heuss LT. Feasibility of breath monitoring in patients undergoing elective colonoscopy under propofol sedation: A single-center pilot study. World J Gastrointest Endosc 2014; 6(3): 82-87
- URL: https://www.wjgnet.com/1948-5190/full/v6/i3/82.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i3.82
Colonoscopy is established as a routine intervention to diagnose and treat colonic diseases. It is recommended as the most efficient screening procedure to detect colon cancer[1,2]. The acceptance and tolerance of the procedure has remarkably increased with the use of a sedative agent[3,4]. The short-acting sedative propofol is an ideal drug with a superior pharmacokinetic profile: an excellent amnestic effect, a rapid onset of action, and a short half-life of 4 min[5-7]. In many European countries (e.g., Switzerland), propofol is administered under the guidance of a gastroenterologist without the assistance of an anesthesiologist in most routine endoscopic procedures[8-11].
Propofol is administered as a bolus at repeated intervals to achieve a level of moderate sedation in which the patient is still responsive to verbal or tactile stimulation[12]. Nevertheless, carefully sedated patients can potentially progress into deeper levels of sedation[10,13]. Drug-induced respiratory depression and airway obstruction are the leading causes of morbidity during sedation and are the most feared complications of propofol because there is no antidote[6,12]. Thus, standard patient monitoring includes pulse oximetry as a surrogate measure of ventilation[12,14]. Normal SaO2 does not ensure adequate ventilation. Apnea and hypoventilation precede hypoxemia with a significant lag time of up to 2 min. In previous studies, capnography was shown to increase the detection of adverse respiratory events[15-17]. However, capnography is expensive, and its use relies on the observation of the breath curve.
In this study, we sought to determine whether breath monitoring with a newly developed respiratory rate-monitoring device based on the humidity of expired air could be a practical and accurate method of monitoring ventilation under sedation in addition to standard monitoring with pulse oximetry.
This was a single-center pilot study to assess the practicability of breath monitoring with a newly developed respiratory rate-monitoring device (respiR8™) during propofol sedation in patients undergoing colonoscopy. The study protocol was reviewed and approved by a local ethics committee in Zurich, Switzerland.
All consecutive patients presenting for an inpatient or outpatient diagnostic colonoscopy in a community hospital endoscopy center (Zollikerberg Hospital, Zurich, Switzerland) were considered for enrollment if they met all of the following inclusion criteria: (1) age ≥ 18 years; (2) ASA class I-III; and (3) ability to provide informed consent.
Patients were excluded from enrollment if they met any of the following exclusion criteria: (1) ASA class IV or V; (2) inability to give informed consent; (3) emergency procedure, (4) allergy to propofol; and (5) pregnant. After providing written informed consent, 76 patients (51 females and 25 males) were included in this study.
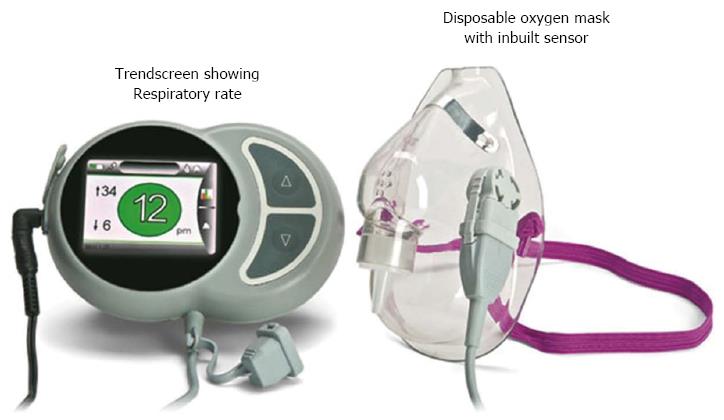
RespiR8™ (Anaxsys Technology Ltd., United Kingdom) is a European community-certified device specifically developed to allow continuous monitoring of the respiratory rate of patients requiring oxygen delivered by a face mask. Its use in the postoperative recovery setting has been tested successfully[18]. The device comprises a disposable oxygen mask fitted with Anaxsys’ patented sensor, which measures moisture in the exhaled breath. The respiR8™ monitor displays the current respiratory rate and has a trend screen that shows the respiratory rate over time (Figure 1).
The respiR8™ humidity sensor consists of a ceramic substrate printed with two gold interdigitated electrodes that are coated with an ion exchange polymer. This polymer is a highly proton-conductive fluoropolymer. A small current is applied across the electrodes, and as the patient exhales, moisture condenses on the surface of the sensor. Due to the presence of the moisture, ions from the coating migrate between the electrodes and produce an electric signal (the greater the amount of moisture, the greater the signal). As the patient inhales, the surface of the sensor is dried by a flow of ambient air passing over the surface, and the signal returns to baseline. The analog signal is then converted to a digital signal. An algorithm in the monitor detects the peaks in the signal and converts the signal into a respiratory rate value.
Each patient’s age, gender, ASA status, and indication for colonoscopy were recorded. The ASA status and the indication for colonoscopy were determined by the endoscopist. The duration of colonoscopy was defined as the time from the introduction to the withdrawal of the endoscope. The total dose of propofol used during the procedure was considered a procedural variable.
Sedation: A gastroenterologist-directed sedation technique was applied to achieve and maintain an adequate level of sedation, as described elsewhere[14]. After an initial intravenous dose of 20 mg of propofol (Disoprivan 1%, AstraZeneca, London, GB), repeated doses of 10-20 mg of propofol were administered intravenously (with no limit on the total dose). All monitoring values were confirmed before administering doses of propofol. No other sedation medication was used during the procedure.
Monitoring: According to the standard operation procedures of our unit, all of the patients underwent continuous monitoring of their heart rate and SaO2 with a pulse oximeter and BP measurement at 5-min intervals[14]. Additionally, all of the patients were continuously monitored with respiR8™ to record respiratory events. A disposable respiR8™ mask covering the patient’s nose and mouth was attached to an oxygen supply, which provided continuous oxygen at a rate of 2 L/min.
The SaO2 display and respiR8™ display were placed near the endoscopy monitor to facilitate continuous observation by the gastroenterologist and the endoscopy nurse. The data from the respiR8™ device and the pulse oximeter were saved directly in a personal computer using a USB connection. All of the recorded data were transferred into an individual patient file numbered according to the usual case numbering system in our hospital. The clocks on the endoscopy monitor, the SaO2 device, and the respiR8™ device were all synchronized.
Approximately four minutes following completion of the colonoscopy, the patients were disconnected from the monitoring device when they could give meaningful verbal responses and their vital signs were stable.
All of the patients were monitored for several minutes before the first dose of propofol was administered, which signaled the beginning of sedation. Any sign of apnea or hypoxemia prompted a clinical observation of chest movement and patient stimulation to exclude artifacts in the respiR8™ or pulse oximeter and signs of mask displacement. Significant apnea permitted the use of a chin lift or jaw thrust maneuver, increasing the oxygen supplementation, and withholding further propofol doses where appropriate.
The primary study outcome was the occurrence of any respiratory event (apnea or hypopnea). Apnea was defined as the cessation of breathing for at least 10 s. Significant apnea was defined as the cessation of breathing for more than 30 s[19]. Hypopnea was defined as a reduction in the respiratory rate below 6/min for a minimum of 10 s. The secondary end point was the correlation of apnea or hypopnea with hypoxemia (defined as a decrease in SaO2 of at least 5% from baseline or less than 90%)[15]. The time of sedation was defined from the start of the first administration of propofol until the disconnection of electronic monitoring devices after the procedure was complete.
Continuous variables and count data are presented as means with standard deviations or as medians with ranges. The data were compared between patients with and without respiratory events using the Mann-Whitney test with exact P-values. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test where appropriate. A two-tailed P-value of less than 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics 20 (SPSS Inc., Chicago, IL).
From July 2011 to November 2011, 76 patients presenting for an elective diagnostic colonoscopy were enrolled in the study. The mean procedure duration was 17.2 ± 6.4 min. The demographic data are shown in Table 1.
| Event (n = 37) | No event (n = 39) | P value | |
| Age (yr) | 60.8 (± 18.6) | 67.8 (± 18.9) | NS |
| Male:female (25:51) | 12:25 | 13:26 | NS |
| ASA I (21) | 12 | 9 | NS |
| ASA II (22) | 12 | 10 | NS |
| ASA III (33) | 13 | 20 | NS |
In this study, 37 (48.7%) patients developed at least one respiratory event (Table 1). There were 73 respiratory events, ranging from one to a maximum of six events in a single patient. Significant apnea lasting for more than 30 s occurred in five patients (6%).
In one case, apnea led to a relative SaO2 reduction within the physiologic range (from 98% to 93%) after a lag time of 50 s. There were no serious hypoxemia events in our study.
The mean propofol dose used in patients who had no respiratory events was 189.5 ± 81.6 mg compared with 228.7 ± 70.8 mg in patients who had respiratory events (P = 0.01). The mean SaO2 values did not differ significantly in patients with or without respiratory events. Our analysis revealed that the total propofol dose was an independent risk factor for respiratory events (P = 0.01) (Table 2).
| Event (n = 37) | No event(n = 39) | P value | |
| Total propofol dose (mg) | 228.6 (± 78.8) | 189.5 (± 81.6) | 0.01 |
| SaO2 mean (%) | 99.86 (± 0.4) | 99.73 (± 0.7) | NS |
Artifacts affecting the results of the respiration counter occurred in 36.5% of patients without respiratory events and in 41.6% of patients with respiratory events. These artifacts were related to superficial breathing, incorrect positioning of the oxygen mask, coughing, snoring, or a chin drop. Superficial breathing could be verified by palpating the chest movement of the patient.
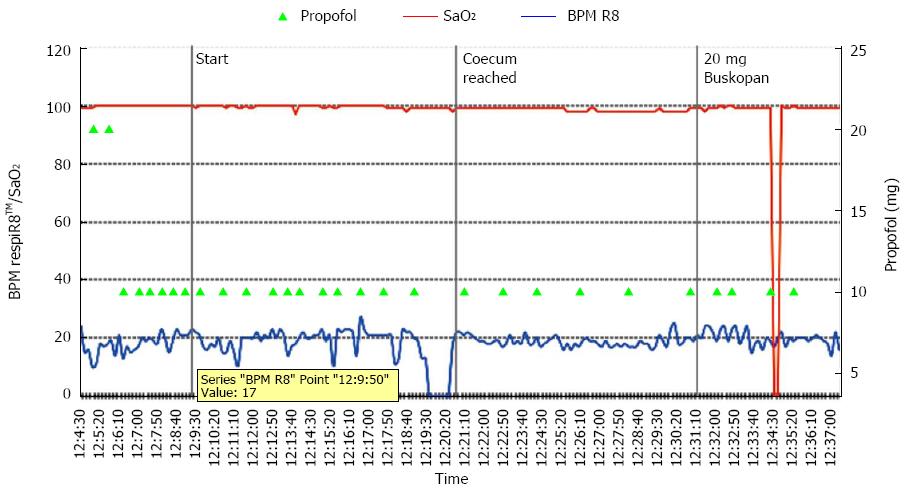
Graphic monitoring of SaO2 and the respiratory rate is shown in Figure 2. Artifacts from the pulse oximetry device occurred in 36.9% of patients without respiratory events and in 41.2% of patients with respiratory events. SaO2 artifacts were related to the dysfunction of the SaO2 monitor or the displacement of the SaO2 sensor on the patient’s finger during repositioning. No event requiring assisted ventilation was recorded.
Our study is the first prospective evaluation of the continuous monitoring of breathing during propofol sedation in patients undergoing colonoscopy with a new respiratory rate-monitoring device. In 48.7% of our patients, the device detected at least one episode of a respiratory event defined as apnea or hypopnea. Notably, none of the events were detected by monitoring with pulse oximetry alone. This result correlates with previous studies demonstrating that abnormal respiratory events detected with capnography occurred in more than 50% of patients[6,15]. Although pulse oximetry is considered the de facto standard of care for the detection of respiratory depression during endoscopic procedures, our study confirms that it could be potentially misleading to rely only on pulse oximetry as a surrogate marker of alveolar hypoventilation[20-22]. As shown by Vargo et al[19], significant alveolar hypoventilation can occur during endoscopic procedures even in the presence of a normal level of SaO2, as measured by pulse oximetry. Despite the identified episodes of hypoventilation in our study, the measured mean SaO2 did not differ significantly. In one case, an apnea registered by the respiR8™ device led to a relative SaO2 reduction of 5% within the physiologic range (from 98% to 93%) after a lag time of 50 s. Therefore, we assume that short episodes of apnea or hypoventilation occur at a higher rate than expected based on pulse oximetry monitoring.
Guidelines for propofol sedation by non-anesthesiologists recommend ventilation monitoring[12]. This monitoring can be accomplished by direct observation or the palpation of chest wall excursions[23]. However, these approaches may be impractical in darkened endoscopy rooms and require additional attention from the available personnel for visual or tactile assessment. Routine ventilation monitoring using an oxygen mask could facilitate patient observation and enable the assistant to perform tasks that are more demanding. Many gastroenterologists use capnography to monitor the respiratory rate and for the early detection of apnea. The respiR8™ device may be a suitable alternative for these physicians. Compared to capnography, the studied device is less expensive and easier to use because the focus is on the respiratory rate and not on the measured value of CO2. The costs associated with the use of the respiR8™ device include the onetime cost of the device (approximately USD 940) and the cost of a disposable mask (approximately USD 10) that allows concurrent oxygen administration.
In this study, 73 respiratory events were detected, but no serious respiratory events requiring bag mask ventilation occurred. The patient sample size was likely too small to observe such an event, which occurs in only 0.1% of routine sedations[10]. Therefore, we speculate that respiratory events detected with the respiR8™ device may precede the development of reduced SaO2 and could serve as an early warning system for impending respiratory compromise. Further studies are required to confirm the potential benefit of additional respiratory rate monitoring.
Consistent with previous findings, there was no difference among patients with or without respiratory events with respect to ASA classification, age, or indication of colonoscopy[14]. The total propofol dose used was an independent predictor of respiratory events in our study, which confirmed the observation by Beitz et al[15].
Unfortunately, the respiratory rate displayed by the respiR8™ device in clinical circumstances can be impaired by artifacts. These artifacts are typically related to an involuntary slipping of the oxygen mask, which occurs with the same frequency as displacements of the fingertip sensor used for pulse oximetry. Similar artifacts were shown to occur after nasal cannula dislocation and with device dysfunction in the capnographic monitoring of ventilator activity in a study from Beitz[15].
Our study has certain limitations. We included only patients with ASA I-III, and these findings may not hold true in ASA IV-V patients, who have a higher risk of developing respiratory and other adverse events. Whether special subgroups of patients (e.g., COPD, asthma, ASA III-V, obese patients) may benefit from additional monitoring should be addressed in further studies.
We conclude that apnea occurs commonly during endoscopy under sedation and most likely precedes the development of hypoxemia. The newly developed respiratory rate-monitoring device based on humidity in expired air detects more respiratory events and may provide an earlier warning of impending respiratory abnormalities that are not detected with pulse oximetry alone. We recommend using this method as an alternative to capnography in endoscopy to facilitate the earlier detection of ventilatory problems.
Our endoscopy staff, including Peter Meier-Gräub, MD, Karolina Zdrnja, RN, Tatjana Rechsteiner, RN, and Christine Nüesch, RN, participated in patient care and data collection; Burkhard Seifert, PhD, was responsible for the statistical analysis. Anaxsys provided the equipment for respiratory monitoring. The company had no role in the study design, data collection, data analysis, or manuscript preparation. No specific funding was received for this study.
Colonoscopies are easier and more comfortable if they are performed under sedation. Nevertheless, the use of sedative agents can lead to cardiopulmonary complications, most notably incidents of apnea.
Pulse oximetry and capnography, the widely established monitoring techniques to prevent oversedation or apnea, are inaccurate and/or expensive. There is a significant need for the development of newer monitoring devices.
In this pilot-study, the authors demonstrate the feasibility of breath monitoring with a newly developed respiratory rate-monitoring device that measures the moisture in exhaled breath.
The device is easy to use in any endoscopy suite.
A good pilot study requires a larger sample size to validate this new respiratory rate monitor under propofol sedation. The authors detected 73 respiratory events in a cohort of 76 patients. This is most likely due to the use of bolus doses of propofol. It would be more prudent to administer propofol via a target controlled infusion pump that would not produce peaks and valleys in the therapeutic plasma propofol level.
P- Reviewers: Shah OJ, Tischendorf JJW, Wong KKY, Zavoral M S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. |
| 2. | Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90:353-365. |
| 3. | Froehlich F, Gonvers JJ. Patient tolerance: an important factor of dissatisfaction for colonoscopy. Am J Gastroenterol. 1995;90:2068-2069. |
| 4. | Terruzzi V, Meucci G, Radaelli F, Terreni N, Minoli G. Routine versus “on demand” sedation and analgesia for colonoscopy: a prospective randomized controlled trial. Gastrointest Endosc. 2001;54:169-174. |
| 5. | Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049-1056. |
| 6. | Vargo JJ, Zuccaro G, Dumot JA, Shay SS, Conwell DL, Morrow JB. Gastroenterologist-administered propofol for therapeutic upper endoscopy with graphic assessment of respiratory activity: a case series. Gastrointest Endosc. 2000;52:250-255. |
| 7. | Singh H, Poluha W, Cheung M, Choptain N, Baron KI, Taback SP. Propofol for sedation during colonoscopy. Cochrane Database Syst Rev. 2008;CD006268. |
| 8. | Heuss LT, Froehlich F, Beglinger C. Changing patterns of sedation and monitoring practice during endoscopy: results of a nationwide survey in Switzerland. Endoscopy. 2005;37:161-166. |
| 9. | Heuss LT, Froehlich F, Beglinger C. Nonanesthesiologist-administered propofol sedation: from the exception to standard practice. Sedation and monitoring trends over 20 years. Endoscopy. 2012;44:504-511. |
| 10. | Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229-137; quiz 1229-137;. |
| 11. | Rex DK, Heuss LT, Walker JA, Qi R. Trained registered nurses/endoscopy teams can administer propofol safely for endoscopy. Gastroenterology. 2005;129:1384-1391. |
| 12. | Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol. 2010;27:1016-1030. |
| 13. | Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol. 2008;43:368-374. |
| 14. | Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Risk stratification and safe administration of propofol by registered nurses supervised by the gastroenterologist: a prospective observational study of more than 2000 cases. Gastrointest Endosc. 2003;57:664-671. |
| 15. | Beitz A, Riphaus A, Meining A, Kronshage T, Geist C, Wagenpfeil S, Weber A, Jung A, Bajbouj M, Pox C. Capnographic monitoring reduces the incidence of arterial oxygen desaturation and hypoxemia during propofol sedation for colonoscopy: a randomized, controlled study (ColoCap Study). Am J Gastroenterol. 2012;107:1205-1212. |
| 16. | Radaelli F, Terruzzi V, Minoli G. Extended/advanced monitoring techniques in gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 2004;14:335-352. |
| 17. | Froehlich F, Milliet N. Propofol sedation during endoscopic procedures in private practice: the case for capnography to make 1-nurse endoscopy acceptable. Gastrointest Endosc. 2008;67:1008. |
| 18. | Smith I, Macka J, Farid N, Krucheck D. Respiratory rate measurement: a comparison of methods. Brit J Healthcare Assist. 2011;5:18-23. |
| 19. | Vargo JJ, Zuccaro G, Dumot JA, Conwell DL, Morrow JB, Shay SS. Automated graphic assessment of respiratory activity is superior to pulse oximetry and visual assessment for the detection of early respiratory depression during therapeutic upper endoscopy. Gastrointest Endosc. 2002;55:826-831. |
| 20. | Freeman ML, Hennessy JT, Cass OW, Pheley AM. Carbon dioxide retention and oxygen desaturation during gastrointestinal endoscopy. Gastroenterology. 1993;105:331-339. |
| 21. | Müller S, Prolla JC, Maguilnik I, Breyer HP. Predictive factors of oxygen desaturation of patients submitted to endoscopic retrograde cholangiopancreatography under conscious sedation. Arq Gastroenterol. 2004;41:162-166. |
| 22. | Arakawa H, Kaise M, Sumiyama K, Saito S, Suzuki T, Tajiri H. Does pulse oximetry accurately monitor a patient’s ventilation during sedated endoscopy under oxygen supplementation? Singapore Med J. 2013;54:212-215. |
| 23. | Walker JA, McIntyre RD, Schleinitz PF, Jacobson KN, Haulk AA, Adesman P, Tolleson S, Parent R, Donnelly R, Rex DK. Nurse-administered propofol sedation without anesthesia specialists in 9152 endoscopic cases in an ambulatory surgery center. Am J Gastroenterol. 2003;98:1744-1750. |