INTRODUCTION
Aberrant pancreas is defined as pancreatic tissue that lacks anatomic and vascular continuity with the main body of the pancreas. Aberrant pancreas is frequently seen in the stomach. Most patients with gastric aberrant pancreas are asymptomatic. They rarely present with clinical symptoms, such as abdominal pain and bleeding[1,2]. A few cases of aberrant pancreas complicated with acute inflammation like that of pancreatitis have been reported, and they are treated surgically when diagnosed with submucosal lesion (SML) of the stomach with symptoms, for which surgical pathology reveals pancreatitis with aberrant pancreas[3-5]. We experienced a patient with acute inflammation occurring in the gastric aberrant pancreas, who was followed up by endoscopic ultrasonography (EUS) before and after the diagnosis. No report in the literature has described a similar case, although EUS images of aberrant pancreas have been presented[6]. Consequently, our case is extremely valuable from the perspective of diagnostic imaging for acute inflammation of gastrointestinal aberrant pancreas.
CASE REPORT
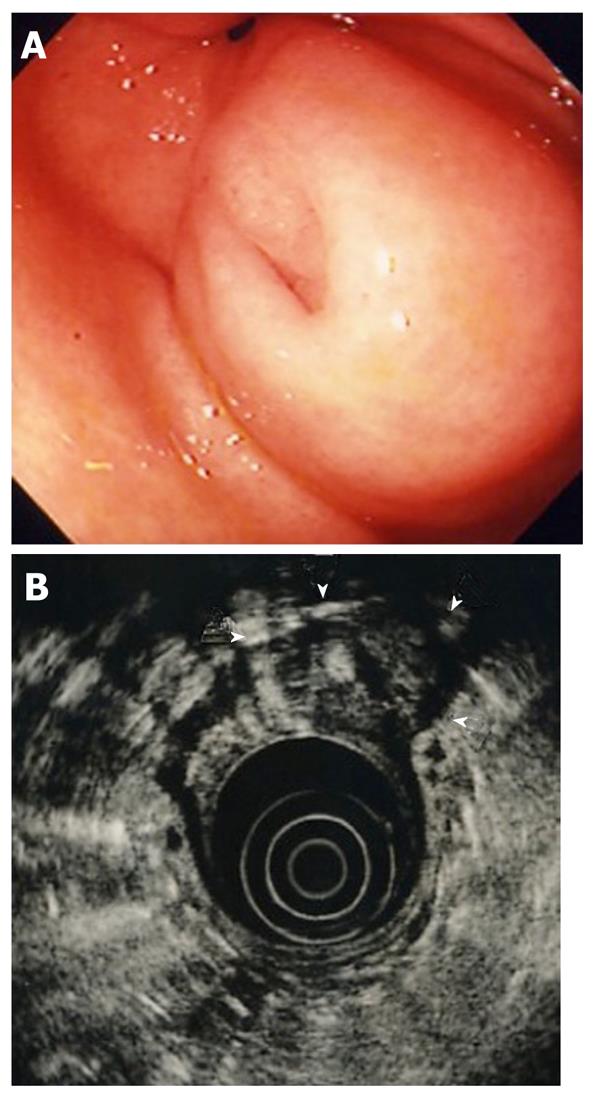
A 20-year-old woman with no remarkable medical history presented with epigastralgia that had continued for 3 d. Esophagogastroduodenoscopy (EGD) revealed an SML in the antrum of the stomach, but the cause of the pain was not identified. To clarify the SML pathogenesis, EUS was subsequently performed, revealing a mass located in the third layer (submucosa) with a diameter of 20 mm. The internal echo pattern was slightly more hypoechoic than the normal echo level of the third layer. It contained an anechoic lumen in the mass, which was regarded as the duct. We made a diagnosis of the aberrant pancreas of the stomach based on EGD (Figure 1A) and EUS (Figure 1B) findings. After initial examination, she had been followed up once a year using EUS to observe the changes of the SML. Four years later, she was admitted to our hospital because of severe epigastralgia.
Figure 1 Esophagogastroduodenoscopic and endoscopic ultrasonographic images of submucosal lesion at the first examination.
A: Esophagogastroduodenoscopy. Submucosal lesion was visible in the antrum of the stomach; B: Endoscopic ultrasonography. Lesion (arrow heads) located in the third layer (submucosa) with a diameter of 20 mm, slightly hypoechoic internal echo, and anechoic lumen, regarded as the duct.
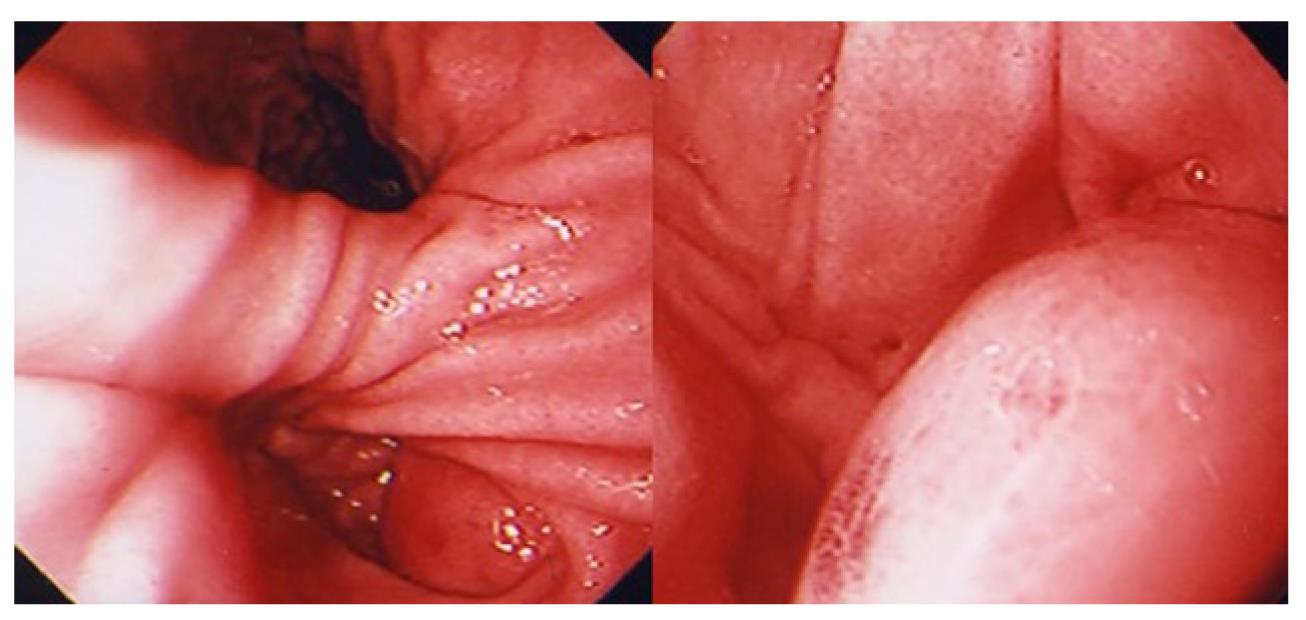
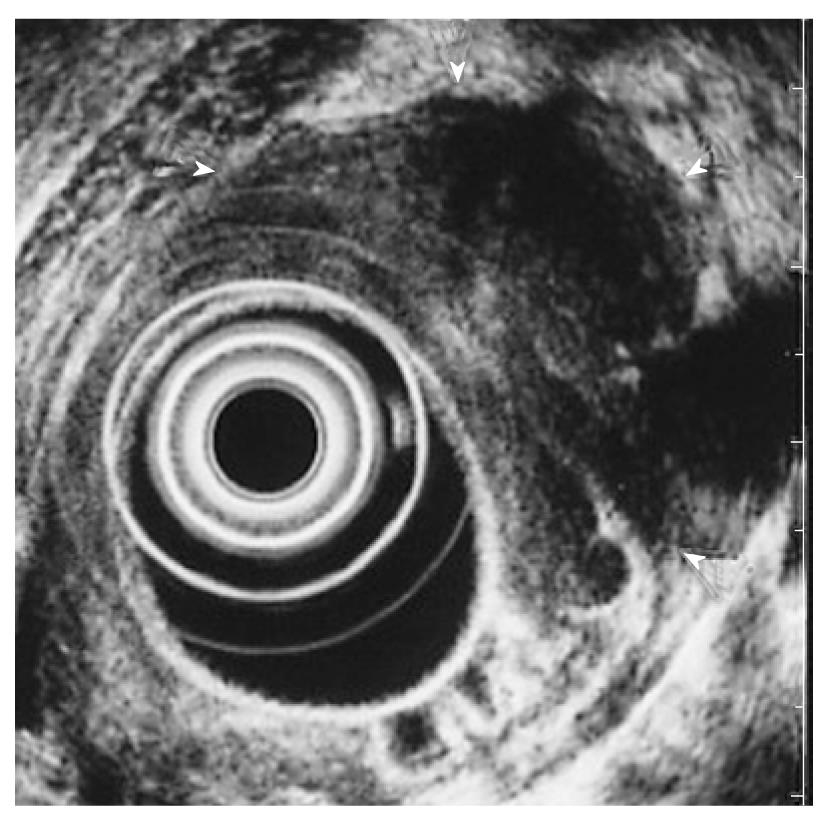
Laboratory tests revealed that white blood cells (WBC) were 6600/μL (normal range: 3000-9800/μL); amylase (AMY), 170 IU/L (normal range, 70-240 IU/L); and C reactive protein, 2.0 mg/dL (under 0.3 mg/dL). Percutaneous ultrasound revealed an irregular large hypoechoic mass in the gastric wall with an anechoic area. The pancreas was normal. We thought that the severe epigastralgia might imply some sort of inflammation in the SML. Therefore, we performed EGD and it revealed a more swollen SML compared with the previous EGD (Figure 2). On subsequent EUS, the lesion was more swollen, with a diameter of 35 mm, and the internal echo-pattern was more hypoechoic than in the previous EUS. The border between the fourth layer (muscularis propria) and the SML was unclear (Figure 3). Based on the results of these examinations, we diagnosed the patient as having acute inflammation like pancreatitis in the aberrant pancreas.
Figure 2 Esophagogastroduodenoscopy after inflammation.
Esophagogastroduodenoscopy (EGD) revealed a more swollen submucosal lesion compared with the previous EGD.
Figure 3 Endoscopic ultrasonographic image after inflammation.
On endoscopic ultrasonography (EUS), the lesion was more swollen, with a diameter of 35 mm. The internal echo-pattern was more hypoechoic than in the previous EUS (arrow heads).
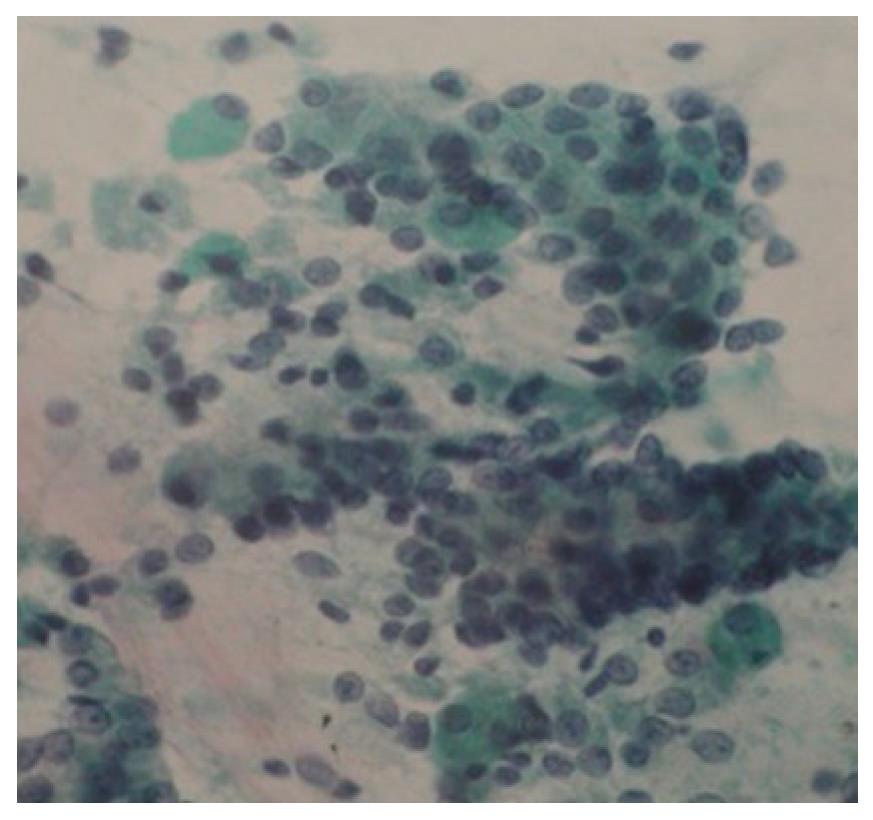
Treatment was given following the diagnosis of acute pancreatitis. A protease inhibitor, gabexate mesylate (300 mg/d), was administrated for three days. Two months later, EUS was performed again to observe the status, which revealed that the size of SML decreased from 35 mm to 25 mm in diameter, and the internal echo pattern had improved. To establish a definite diagnosis of SML, we performed EUS-guided fine needle aspiration biopsy (EUS-FNA). The pathological examination showed ductal epithelial cells and acinar cells (Figure 4), and the SML was definitely diagnosed as an aberrant pancreas. This case was followed up without surgical operation.
Figure 4 Pathological findings of submucosal lesion.
Pathologic examination showed ductal epithelial cells and acinar cells.
DISCUSSION
Aberrant pancreas is a congenital anomaly found in the gastrointestinal tract and adjacent structures in 0.55%-14% of autopsy series; approximately 70% of all such tissues are found in the stomach, duodenum, and jejunum[3,7,8]. Patients with aberrant pancreas are usually asymptomatic. Therefore, the lesion is often found incidentally during clinical investigation for other gastroduodenal diseases[9]. However, aberrant pancreas sometimes produces symptoms associated with pancreatitis, cyst formation, ulceration, bleeding, obstructive jaundice, and gastric outlet obstruction[10].
Acute pancreatitis occurring in the gastrointestinal aberrant pancreas is rare. In the relevant literature, a major symptom of this pathosis was described as abdominal pain. Almost all cases show slight elevation of serum amylase. Aberrant pancreas can be classified into three histological types. Type I designates a typical pancreatic tissue with acini, duct, and islet cells resembling those of a normal pancreas. Type II aberrant pancreatic tissue comprises a pancreatic tissue with numerous acini and a few ducts lacking islet cells. Finally, type III in which only excretory ducts were observed[11]. Therefore, aberrant pancreas (especially types I and II) might demonstrate the full range of pancreatic pathologies including pancreatitis (acute and chronic) as well as benign and malignant neoplastic transformations[12,13]. To date, some reports have described acute or chronic pancreatitis occurring in the aberrant pancreas[3-5,13,14]. It remains controversial whether acute or chronic inflammatory changes in aberrant pancreas are induced by similar pathogenesis to that of pancreatitis in anatomical pancreas. In a reported case of chronic pancreatitis derived from Heinrich type II (no drainage duct microscopically) aberrant pancreas, aberrant pancreatic tissue might be more susceptible to pancreatitis because of the lack of drainage ducts in Heinrich type II[13]. Such a pathosis as in this case will also apply to the etiology of acute pancreatitis in aberrant pancreas. However, it was reported that in patients with chronic alcoholic pancreatitis associated with aberrant pancreas, microscopic examination of the aberrant pancreas showed no changes that were suggestive of chronic pancreatitis, despite severe chronic pancreatitis in the main pancreas[15]. Consequently, these reports suggest that the acute or chronic pancreatitis derived from aberrant pancreas might occur because of some sort of ductal obstruction, but not from direct injury of acini caused by heavy alcohol consumption. In the case described herein, the pathological findings obtained by EUS-FNA showed ductal epithelial cells and acinar cells, indicating Heinrich type I. The patient reported no alcohol abuse. Therefore, we assume that the pathogenesis of acute pancreatitis of the aberrant pancreas might be obstruction of ductal system in the ectopic tissue from any cause.
It is notable in our case that the inflammation of aberrant pancreas was followed up by EUS. No report in the literature describes such a case and provides images of acute pancreatitis derived from aberrant pancreas using EUS. The image of acute pancreatitis in aberrant pancreas is similar to acute inflammation of anatomic pancreas: swelling with heterogeneous parenchymal echo pattern. These images do not resemble those of a tumor with necrosis such as gastrointestinal stromal tumor, or abscess/phlegmonous gastritis with SML[16]. We believe that images such as those related to our case were not observed on EUS except for acute inflammation of aberrant pancreas. Therefore, we should be aware that a gastric aberrant pancreas can cause acute pancreatitis and must be suspected in patients with atypical abdominal pain and SMT in the stomach. EUS is strongly recommended for the definite diagnosis in such a case.
Peer reviewers: Thawatchai Akaraviputh, MD, Associated Professor, Minimally Invasive Surgery Center, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand; Jong Ho Moon, MD, PhD, Digestive Disease Center, Soon Chun Hyang University Bucheon Hospital, No. 1174 Jung-Dong, Wonmi-Ku, Bucheon 420-767, South Korea
S- Editor Yang XC L- Editor Ma JY E- Editor Yang XC