Published online Jul 16, 2012. doi: 10.4253/wjge.v4.i7.328
Revised: December 20, 2011
Accepted: March 30, 2012
Published online: July 16, 2012
A case is reported of a 50-year-old woman with a history of small-cell lung cancer admitted with pancreatic head lesions, discovered during investigation for obstructive jaundice. Endoscopic ultrasound assisted fine needle aspiration of the pancreatic mass was consistent with small cell carcinoma, presenting as an isolated metastasis from the previously diagnosed lung cancer. Endoscopic retrograde cholangiopancreatography (ERCP) showed extrinsic compression and a bile duct stricture, requiring sphincterotomy and stent insertion. This case highlights that acute pancreatitis and biliary obstruction can occur as a manifestation of small cell lung cancer metastasizing to the pancreas. EUS is a safe, low risk and rapid diagnostic tool in such cases, and ERCP with stenting offers a safe and effective treatment option.
- Citation: Singh D, Vaidya OU, Sadeddin E, Yousef O. Role of endoscopic ultrasound and endoscopic retrograde cholangiopancreatography in isolated pancreatic metastasis from lung cancer. World J Gastrointest Endosc 2012; 4(7): 328-330
- URL: https://www.wjgnet.com/1948-5190/full/v4/i7/328.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i7.328
The pancreas is a rare site for solitary metastases, but is often involved in diffuse metastatic disease[1]. The literature demonstrates that most of the patients with isolated pancreatic metastases are from renal cell cancer[2]. Our case is one of the extremely rare occurrences[3-5] of small-cell lung cancer, an infrequent form (10%) of lung cancer, metastasizing to the pancreas.
A 50-year-old Caucasian female, with a history of small-cell lung cancer post chemo-radiation, presented with vomiting, severe epigastric pain radiating to the back, and a 10 pound weight loss over a 3 mo period. She was noted to have direct hyperbilirubinemia (1.6 mg/dL, normal 0.1-0.5 mg/dL), elevated alkaline phosphatase (1147 IU, normal 32-91 IU), transaminitis (aspartate aminotransferase 242 IU, normal 15-41 IU; alanine aminotransferase 294 IU, normal 14-54 IU) and elevated lipase (274 IU, normal 22-51 IU).
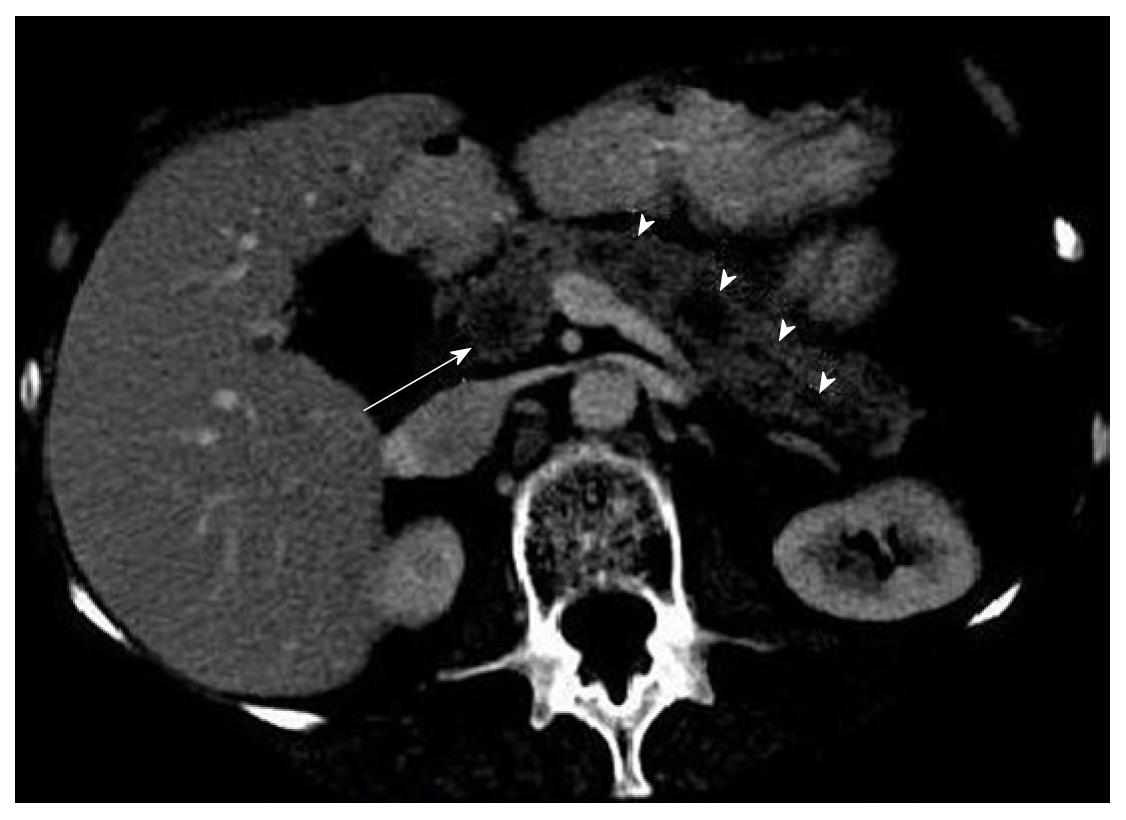
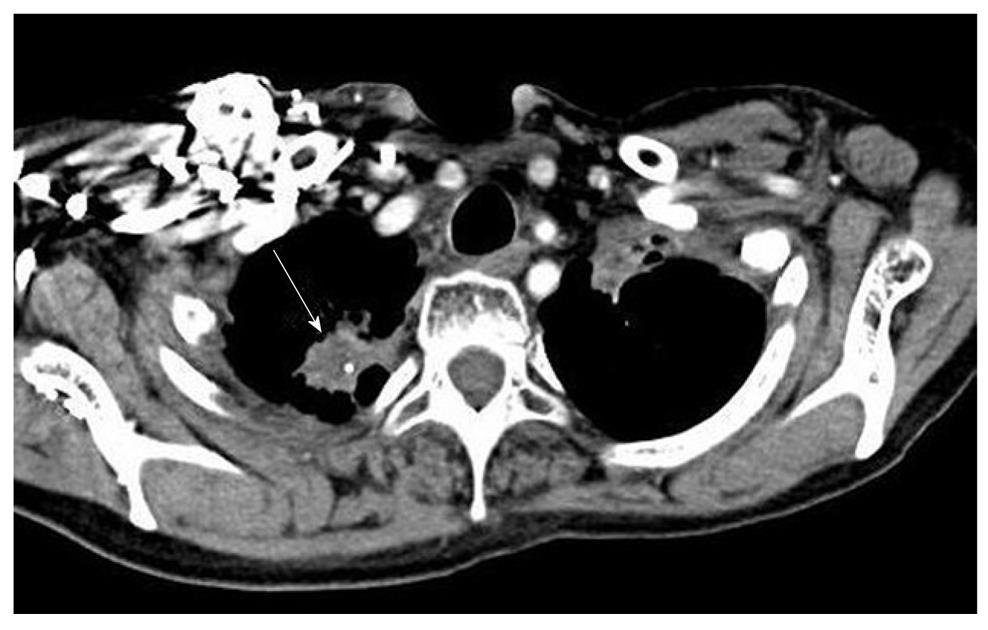
Abdominal computed tomography (CT) scan (Figure 1) demonstrated distended gallbladder, intrahepatic ductal dilatation and multiple hypo-attenuated lesions throughout the pancreas (arrowheads), the largest noted at the pancreatic head measuring 1.7 cm × 1.6 cm (arrow). With suspicion of metastatic disease, an extensive evaluation [whole body CT scan, magnetic resonance imaging brain and a bone scan] was ordered. All these were unremarkable except for the spiculated mass in the right lung apex on chest CT (Figure 2). This was present on prior imaging and represented the previously diagnosed small-cell lung cancer (unchanged size and characteristics).
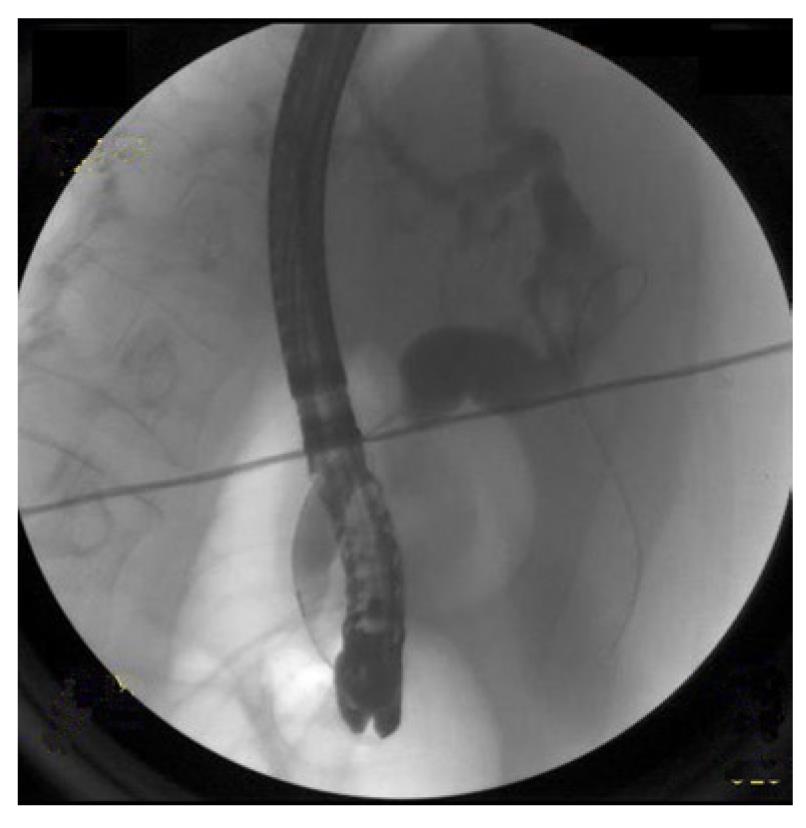
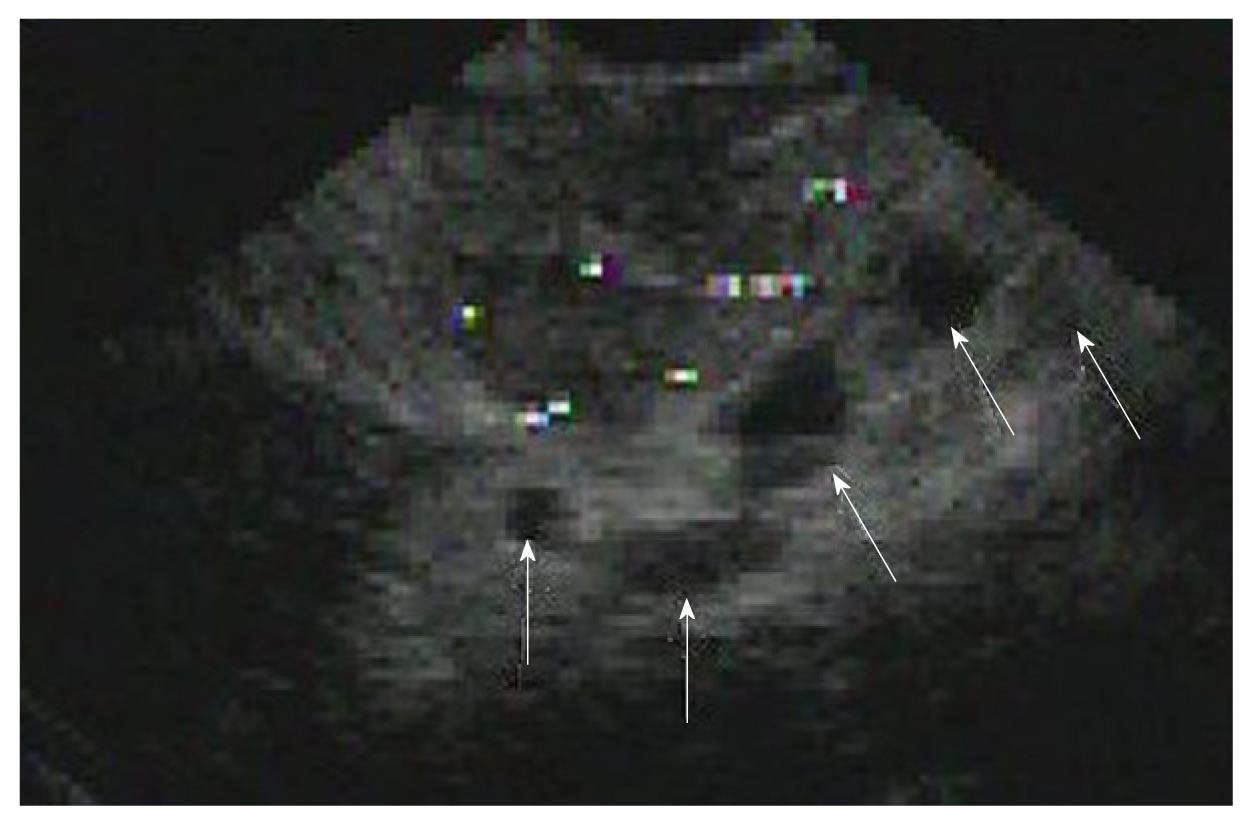
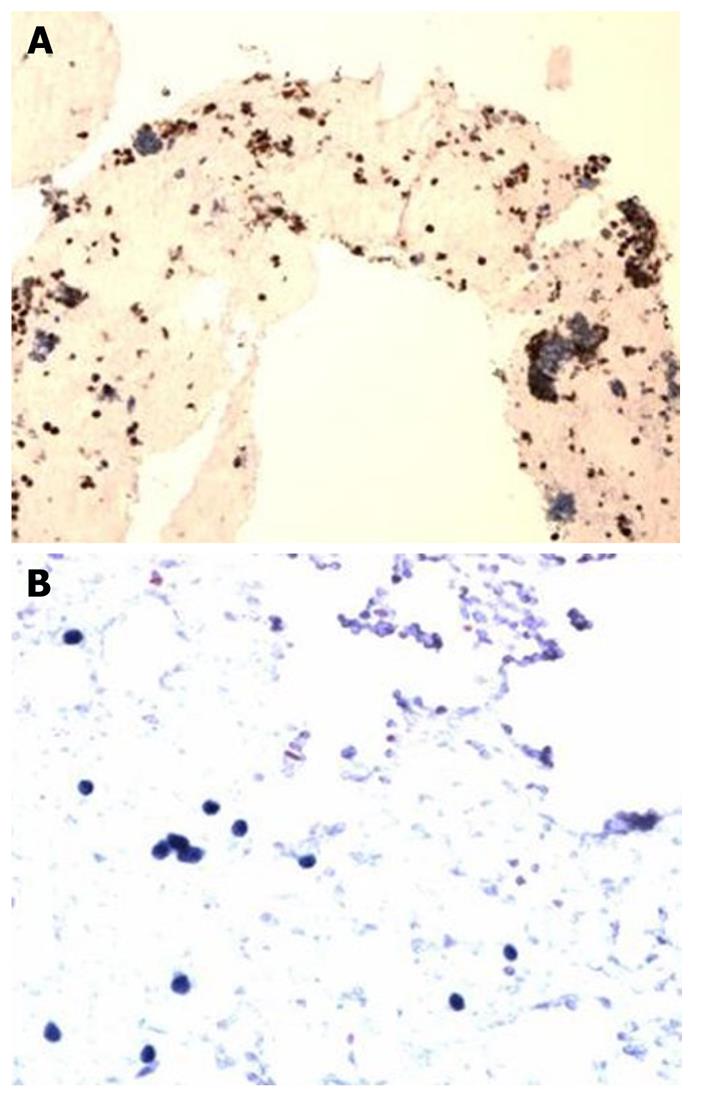
Subsequently, endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate the dilated hepatic ducts (Figure 3) and showed a biliary stricture. A sphincterotomy was performed followed by placement of a biliary stent (10 French by 9 cm) with good drainage. Endoscopic ultrasound (EUS) (Figure 4) with fine needle aspiration of the lesion in the pancreatic head (arrows) was performed and was consistent with small cell carcinoma (Figure 5A and B). The patient eventually decided to receive palliative care and was discharged home with hospice care.
A few years ago, the diagnosis of pancreatic lesions was not feasible without subjecting patients to invasive surgical procedures. Nowadays, EUS-assisted fine-needle aspiration cytology is a safe, low risk and minimally invasive method for diagnosing non-primary pancreatic neoplasms[6], which played a key role in the diagnosis of our case.
It is believed that acute pancreatitis and obstructive jaundice in biliary malignancies results from infiltration of the metastatic tumor into the pancreatic ducts. In such instances, ERCP with stent insertion can be a plausible palliative therapy for biliary drainage[7]. It resulted in dramatic resolution of our patient’s obstructive jaundice. Abdominal pain subsided gradually as the pancreatic inflammation resolved. Generally, the treatment for tumor-induced acute pancreatitis and obstructive jaundice is initially supportive followed by aggressive chemotherapy or surgery. If the patient can tolerate the insertion of an endoscopic stent, then this is performed in addition to chemotherapy and surgery. This approach offers a safe and effective treatment modality for such patients.
Peer reviewer: Fauze Maluf-Filho, MD, Hospital das Clínicas, São Paulo University School of Medicine, 488 Olegario Mariano, São Paulo, SP, Brazil
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
| 1. | Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Machado NO, Chopra P. Pancreatic Metastasis from Renal Carcinoma Managed by Whipple Resection. A Case Report and Literature Review of Metastatic Pattern, Surgical Management and Outcome. JOP. 2009;10:413-418. |
| 3. | Martin A, Castagliuolo I, Mastropaolo G, Del Favero G, Di Mario F, Farinati F, Sturniolo G, Cecchetto A, Naccarato R. Cholestatic jaundice as the presenting symptom of small cell lung cancer. Ital J Gastroenterol. 1990;22:36-39. |
| 4. | Noseda A, Gangji D, Cremer M. Acute pancreatitis as presenting symptom and sole manifestation of small cell lung carcinoma. Dig Dis Sci. 1987;32:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Belhassen-García M, Velasco-Tirado V, Carpio-Pérez A, Soler-Fernández MC, López-Bernús A, Pardo-Lledias J, Fuentes-Pardo L, Iglesias-Gómez A. [Acute pancreatitis and obstructive jaundice secondary to metastases from lung cancer]. Gastroenterol Hepatol. 2009;32:697-701. [PubMed] |
| 6. | DeWitt J, Jowell P, Leblanc J, McHenry L, McGreevy K, Cramer H, Volmar K, Sherman S, Gress F. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc. 2005;61:697-699. [RCA] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Chu D, Adler DG. Malignant biliary tract obstruction: evaluation and therapy. J Natl Compr Canc Netw. 2010;8:1033-1044. [PubMed] |