Published online Aug 16, 2025. doi: 10.4253/wjge.v17.i8.109176
Revised: May 10, 2025
Accepted: June 27, 2025
Published online: August 16, 2025
Processing time: 105 Days and 23 Hours
Sessile serrated lesions (SSLs) are premalignant polyps implicated in up to 30% of colorectal cancers. Australia reports high SSL detection rates (SSL-DRs), yet with marked variability (3.1%-24%). This substantial variation raises concerns about missed lesions and post-colonoscopy colorectal cancer. This study investigates determinants associated with SSL-DR variation in regional Australia.
To study how patient, clinical, and colonoscopy factors are associated with SSL detection in a regional Australian practice. We aimed to contribute high-detection data to the literature by analyzing the association of SSL detection with various determinants.
This retrospective, cross-sectional analysis examined 1450 colonoscopies per
The overall SSL-DR was 30.7%. Multivariate analysis identified several independent predictors: Clinical indication, bowel preparation quality, inflammatory bowel disease status, and serrated polyposis syndrome. The faecal occult blood test positive (FOBT) (+) cohort showed the highest predicted SSL detection probability (39.8%), while clinical symptoms showed the lowest (22.3%). After adjustment, SSL detection odds were 2.3 times greater among FOBT (+) patients than those with clinical symptoms (adjusted odds ratio = 2.30, 95% confidence interval: 1.20-4.40, P = 0.004).
SSL-DR as a quality indicator requires contextualization regarding clinical indications, bowel preparation quality, and comorbidities. There was a significantly higher prevalence of SSLs in FOBT (+) patients. Despite comprehensive adjustment, this study cannot fully explain the wide SSL-DR variation in Australia, highlighting the need for standardized detection protocols and further research to ensure optimal cancer prevention outcomes.
Core Tip: This study reports an exceptionally high sessile serrated lesion detection rate of 30.7% in regional Australia, with faecal occult blood test positive-positive patients showing the highest rate (42%). Clinical indication, bowel preparation quality, inflammatory bowel disease status, and serrated polyposis syndrome were identified as independent predictors. The marked variability in sessile serrated lesion detection rate across Australia (3.1%-24%) highlights the need for standardized detection protocols and context-specific quality indicators to optimize colorectal cancer prevention.
- Citation: Williams H, Dierick NR, Lee C, Sundaralingam P, Kostalas SN. Determinants of high sessile serrated lesion detection: Role of faecal occult blood test and colonoscopy quality indicators. World J Gastrointest Endosc 2025; 17(8): 109176
- URL: https://www.wjgnet.com/1948-5190/full/v17/i8/109176.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i8.109176
Colorectal cancer (CRC) represents the third most frequently diagnosed non-cutaneous malignancy globally and the second leading cause of cancer mortality[1-4]. While the traditional “adenoma-carcinoma sequence” explains the majority of cases, emerging evidence indicates that up to 30% of sporadic CRC cases arise via the “serrated neoplasia pathway” from serrated lesions[1,5-7].
Recent World Health Organization classification updates replaced the term sessile serrated adenoma/polyp with sessile serrated lesion (SSL), simplifying diagnostic criteria to require only one unequivocal aberrant crypt[8,9]. This clarification has improved inter-observer agreement among pathologists and increased SSL diagnosis rates[10-13].
SSLs have established malignant potential, with dysplastic SSLs (dSSLs) representing an intermediate step toward CRC[14]. Though dSSLs comprise only 2%-5% of all SSLs[1,15-17], this likely reflects rapid progression to carcinoma rather than rare dysplastic transformation[18]. Once dysplasia develops, progression to invasive carcinoma accelerates signifi
Due to challenges in endoscopic differentiation, current guidelines recommend removing all serrated lesions except diminutive distal hyperplastic polyps[20,21]. CRCs originating from serrated lesions are disproportionately represented among post-colonoscopy CRCs (PCCRCs), attributed to rapid malignant transformation, detection difficulties, and in
International studies show marked variation in SSL detection rates (SSL-DRs)[5,23,24]. Meta-analyses reveal significant geographic disparities: Western countries demonstrate rates of 4.3% [95% confidence interval (CI):1.2%-6.0%] vs 0.4% (95%CI: 0.2%-0.9%) in Eastern countries[23]. Notably, Australia reports significantly higher SSL-DRs at 10.5% (95%CI: 2.8-18.2%) compared to the United States at 5.1% (P = 0.03) - providing important context for the present study[24,25].
Crockett and Nagtegaal[5] reported international SSL-DRs generally between 2% to 8%, with high-detection centers exceeding 20%. They suggested that rates achieved by high-detectors may better reflect true SSL prevalence, implying that significant variations in SSL-DR indicate many SSLs go undetected[5,26].
Recent studies confirm the clinical significance of SSL-DRs, with higher detection rates protecting against PCCRC[27]. Anderson et al[27] demonstrated a 71% PCCRC reduction in the highest SSL-DR quintile (> 6%; hazard ratio = 0.29; 95%CI: 0.16-0.50). European research shows similar protective effects independent of adenoma detection rates (ADRs)[26,28,29].
Australian data remains limited without a comprehensive national colonoscopy registry outside the National Bowel Cancer Screening Program[30]. Recent Australian studies report SSL-DRs ranging from 3.1% to 24% - an almost eight-fold variation (Table 1). Higher SSL-DRs correlate with quality indicators including adequate bowel preparation, high caecal intubation rates, longer withdrawal times, and higher ADRs[2,10,11,21,24], though all cited studies reported acceptable colonoscopy quality metrics[1,2,10,11,17,20,21,25].
| Ref. | Study focus | Number of colonoscopies | Colonoscopist | SSL-DR | ||
| Specialty | Number | Rate | Value | |||
| Zhang et al[1], 2023 | SSLDRs prevalence | 21903 | NR | NR | SSL-DR | 24.0% |
| Haga et al[2], 2022 | Rural QI performance | 1674 | GP | 3 | CSSDR | 14.2% |
| PSDR | 16.0% | |||||
| Watson et al[10], 2023 | Rural QI performance | 3497 | GS | 24 | CSSDR | 5.4% |
| GS Reg | 12 | |||||
| Wong et al[11], 2020 | Withdrawal times | 1579 | GS | NR | SSL-DR | 5.4% |
| Zorron Cheng Tao Pu et al[21], 2019 | Specialty and time of day | 2657 | GE | 9 | SSL-DR | 7.1% |
| Surgeons | 6 | 4.9% | ||||
| Wong et al[36], 2017 | Age in years, < 50 vs ≥ 50 | 2013 | NR | NR | SSL-DR | 3.1% |
| Bettington et al[17], 2017 | SSL prevalence | 707 | GE | 1 | SSL-DR | 19.8% |
| Kumbhari et al[25], 2013 | Caucasian vs Asian | 1000 | GE | 1 | SSL-DR | 5.3% |
A retrospective, single-centre, cross-sectional study evaluated SSL-DRs and associated factors among patients undergoing colonoscopy between 1 January 2023 and 31 December 2023.
The study was conducted at Port Macquarie Gastroenterology (PMG), which serves the local community and greater Mid North Coast region of New South Wales, Australia. All procedures were performed at Port Macquarie Private Hospital (PMPH), the only accredited private facility for colonoscopy in the area. Two consultant gastroenterologists with current Gastroenterological Society of Australia recertification in Adult Colonoscopy performed all colonoscopies during 2023.
The study included all patients who underwent colonoscopy with PMG at PMPH during the study period. The exclusion criteria were: (1) Patients who underwent sigmoidoscopy only; and (2) For patients with multiple colonoscopies in 2023, only the first procedure was included in the analysis to avoid bias from repeat procedures.
Patients received split-dose polyethylene glycol solution (MoviPrep®, Norgine) for bowel preparation, with sodium pico
Data was manually extracted from PMG practice software (Genie version 9.2.2., Magic Carpet Software Solutions). The schedule for each day in 2023 was reviewed, and each patient listed as having undergone a colonoscopy had relevant data collected from their profile, endoscopy report and pathology report.
Patient demographics were obtained from practice software, while procedural information (indication, endoscopist, Boston Bowel Preparation Score, polypectomy number, and intubation depth) came from endoscopy reports. Histopathological reports provided polypectomy details including polyp type and location. Inflammatory bowel disease (IBD) and serrated polyposis syndrome (SPS) were presumed absent unless documented otherwise. Table 2 outlines the methodo
| Characteristic | Terms | Explanation |
| Variables | Indication | For the purpose of this study, each colonoscopy was assigned only a single indication using a hierarchical coding system, with the following priority order: (1) Clinical symptoms; (2) FOBT (+); (3) Surveillance (previous patient); (4) Surveillance (new patient); and (5) Screening |
| Bowel preparation status | Boston Bowel Preparation Scores were collated into three broad groups: Inadequate (BBPS ≤ 5), adequate (BBPS 6 or 7), good (BBPS 8 or 9) | |
| Serrated polyposis syndrome status | This variable amalgates the distinction between those with SPS diagnosed in a previous colonscopy and those with SPS diagnosed from the present colonoscopy. It represents whether SPS classified as present or absent | |
| Polypectomy quality indicators | Polypectomy rate | The proportion of colonoscopies during which at least one polypectomy was performed |
| ADR | The proportion of colonoscopies during which at least one conventional adenomatous polyp, (tubular adenomas, tubulovillous adenomas, or villous adenomas) was detected. Excludes malignancies | |
| ASLDR | The proportion of colonoscopies during which at least one serrated lesion was detected | |
| SPDR | The proportion of colonoscopies during which at least one SSL/dSSL or TSA is detected. Excludes HPs | |
| PSDR SDR | The propotion of colonoscopies during which at least one of any serrated lesion (HP, SSL, dSSL, or TSA) is detected proximal to the border between the descending and sigmoid colon | |
| SSL-DR | The proportion of colonoscopies during which at least one SSL, dSSL is detected |
Data was collated and analyzed using Microsoft Excel (Microsoft Corporation, 2024) and RStudio (Posit Software, 2024). Descriptive statistics summarized patient demographics, clinical features, and quality indicators. Continuous variables were presented as means with standard deviations, and medians with ranges. Categorical variables were summarized as counts and percentages. We performed multivariable logistic regression to examine conditional associations between SSL detection and other variables. Specifically, we modelled SSL detection with respect to patient age at colonoscopy, patient sex, indication for colonoscopy, endoscopist, bowel preparation quality, IBD status, and sessile serrated polyposis status. Model assumptions were verified using quantile residual plots. Estimated marginal means were calculated to provide predicted probabilities of SSL detection for each factor and odds ratios from pairwise contrasts with Tukey’s method for all categorical data. While the conventional alpha of 0.05 was used, as an exploratory study, greater emphasis was placed on trends and effect sizes. Similarly, a formal power analysis was not performed.
Ethical approval was obtained from the University of New South Wales Human Research Ethics Advisory Panel D, No. IRECS0829. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Between 1 January 2023 and 31 December 2023, the two gastroenterologists at PMG performed a total of 1462 colonoscopies at the PMPH. Both Gastroenterologist had over 10 years’ experience with lifetime procedural volume of over 5000 colonoscopies each. Of these, 12 colonoscopies occurred in patients who had a previous colonoscopy with PMG during 2023, and data from these colonoscopies was removed. The remaining 1450 colonoscopies represent the only/first colonoscopy of each patient in 2023 and formed the study sample presented subsequently.
In the study sample, females accounted for 59.3% of patients, and the median patient age at colonoscopy was 69.2 years, ranging from 16.3 years to 93.5 years. The most common indication was surveillance of a previous patient (54.6%), followed by clinical symptoms (28.9%). A diagnosis of IBD was present in 3.7% of patients, and there was a new or existing diagnosis of SPS in 7.9% of patients. Of note, there were 10 new diagnoses of SPS, or 0.7% of the colonoscopy sample. Approximately 65.0% of patients achieved good bowel preparation with a BBPS of 8% or 9%, 30.3% achieved adequate bowel preparation with a BBPS of 6% or 7%, and the remaining 4.8% had inadequate bowel preparation with a BBPS of 5% or less (including those not scored). The caecal intubation rate was high, with the terminal ilium intubated in 99.0% of colonoscopies. Endoscopist 1 completed 86.5% of colonoscopies, and the remainder were completed by endo
| Characteristic | SSL detections | Overall (n = 1450) | Respective SSL-DRs, % | |
| None (n = 1005) | At least one (n = 445) | |||
| Age at colonoscopy | ||||
| mean ± SD | 66.6 ± 13.2 | 66.7 ± 13.3 | 66.6 ± 13.2 | N/A |
| Median (minimum-maximum) | 68.8 (17.0-93.5) | 69.8 (16.3-92.2) | 69.2 (16.3-93.5) | N/A |
| Patient sex | ||||
| Male | 408 (40.6) | 182 (40.9) | 590 (40.7) | 30.8 |
| Female | 597 (59.4) | 263 (59.1) | 860 (59.3) | 30.6 |
| Indication | ||||
| Clinical symptoms | 317 (31.5) | 102 (22.9) | 419 (28.9) | 24.3 |
| FOBT (+) | 58 (5.8) | 42 (9.4) | 100 (6.9) | 42.0 |
| Surveillance (previous patient) | 541 (53.8) | 251 (56.4) | 792 (54.6) | 31.7 |
| Surveillance (new patient) | 45 (4.5) | 29 (6.5) | 74 (5.1) | 39.2 |
| Screening | 44 (4.4) | 21 (4.7) | 65 (4.5) | 32.3 |
| Bowel preparation status | ||||
| Inadequate (BBPS ≤ 5) | 58 (5.8) | 11 (2.5) | 69 (4.8) | 15.9 |
| Adequate (BBPS 6 or 7) | 311 (30.9) | 128 (28.8) | 439 (30.3) | 29.2 |
| Good (BBPS 8 or 9) | 636 (63.3) | 306 (68.8) | 942 (65.0) | 32.5 |
| Serrated polyposis syndrome diagnosis | ||||
| Absent | 959 (95.4) | 377 (84.7) | 1336 (92.1) | 28.2 |
| Prior | 46 (4.6) | 58 (13.0) | 104 (7.2) | 55.8 |
| New | 0 (0) | 10 (2.2) | 10 (0.7) | 100.0 |
| Inflammatory bowel disease | ||||
| Absent | 959 (95.4) | 437 (98.2) | 1396 (96.3) | 31.3 |
| Present | 46 (4.6) | 8 (1.8) | 54 (3.7) | 14.8 |
| Endoscopist | ||||
| 1 | 857 (85.3) | 397 (89.2) | 1254 (86.5) | 31.6 |
| 2 | 148 (14.7) | 48 (10.8) | 196 (13.5) | 24.5 |
| Intubation depth | ||||
| Terminal ilium | 992 (98.7) | 443 (99.6) | 1435 (99.0) | 30.9 |
| Caecum | 3 (0.3) | 2 (0.4) | 5 (0.3) | 40.0 |
| Not to caecum | 7 (0.7) | 0 (0) | 7 (0.5) | 0.0 |
| No comment | 3 (0.3) | 0 (0) | 3 (0.2) | 0.0 |
| Colonoscopy occurrences in 2023 | ||||
| Sole colonoscopy | 996 (99.1) | 442 (99.3) | 1438 (99.2) | 30.7 |
| First colonoscopy | 9 (0.9) | 3 (0.7) | 12 (0.8) | 25.0 |
Histological assessment of all polypectomy samples revealed tubular adenomas as the most common finding (48.5%), followed by non-dSSLs (20.4%) and hyperplastic polyps (14.4%). Malignancy was identified in 11 of 3766 samples (0.3%), each from a distinct patient, representing 0.76% of the 1450 patients studied. The dSSLs were diagnosed in 14 samples (0.4%), each from unique patients (0.97% of total patients), and constituted 1.8% of all SSLs. Among the 14 patients with dSSLs, 3 had pre-existing SPS diagnoses, 2 received new SPS diagnoses, and 9 had no SPS diagnosis. The mean age for dSSL detection (72.5 years) was notably higher than for non-dSSL detection (65.05 years). Of all samples, 12.3% were within normal limits and 1.3% were inadequate for assessment. Notably, no villous adenomas were identified. Among the 237 patients (16.34%) without any polypectomy, only 3 had documented incomplete colonoscopies (Table 4).
| Finding | Location | Overall (n = 3749) | |||||||||
| Caecum (n = 385) | Ascending colon (n = 740) | Hepatic flexure (n = 45) | Transverse colon (n = 1371) | Splenic flexure (n = 5) | Descending colon (n = 298) | Sigmoid colon (n = 722) | Rectosigmoid junction (n = 6) | Rectum (n = 175) | Unknown (n = 2) | ||
| Normal | 54 (14.0) | 117 (15.8) | 2 (4.4) | 206 (15.0) | 0 (0) | 25 (8.4) | 52 (7.2) | 0 (0) | 6 (3.4) | 0 (0) | 462 (12.3) |
| TA | 211 (54.8) | 427 (57.7) | 32 (71.1) | 660 (48.1) | 1 (20.0) | 124 (41.6) | 318 (44.0) | 1 (16.7) | 44 (25.1) | 0 (0) | 1818 (48.5) |
| TVA | 16 (4.2) | 9 (1.2) | 1 (2.2) | 11 (0.8) | 1 (20.0) | 3 (1.0) | 34 (4.7) | 1 (16.7) | 11 (6.3) | 0 (0) | 87 (2.3) |
| HP | 14 (3.6) | 31 (4.2) | 3 (6.7) | 163 (11.9) | 1 (20.0) | 66 (22.1) | 168 (23.3) | 2 (33.3) | 89 (50.9) | 1 (50.0) | 538 (14.4) |
| SSL | 85 (22.1) | 141 (19.1) | 6 (13.3) | 303 (22.1) | 2 (40.0) | 68 (22.8) | 135 (18.7) | 0 (0) | 23 (13.1) | 0 (0) | 763 (20.4) |
| dSSL | 1 (0.3) | 1 (0.1) | 0 (0) | 8 (0.6) | 0 (0) | 2 (0.7) | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | 14 (0.4) |
| TSA | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 2 (0.7) | 3 (0.4) | 1 (16.7) | 0 (0) | 0 (0) | 7 (0.2) |
| Malignancy | 0 (0) | 5 (0.7) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 2 (0.3) | 1 (16.7) | 1 (0.6) | 0 (0) | 10 (0.3) |
| Inadequate sample | 4 (1.0) | 9 (1.2) | 1 (2.2) | 18 (1.3) | 0 (0) | 8 (2.7) | 8 (1.1) | 0 (0) | 1 (0.6) | 0 (0) | 49 (1.3) |
| Lost | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50.0) | 1 (0.0) |
Across all colonoscopy indications, the overall polypectomy rate was 83.66%, with an adenoma detection rate (ADR) of 57.03%, proximal serrated detection rate of 37.1%, serrated polyp detection rate of 30.97%, and SSL-DR of 30.69%. SSL-DR after excluding both IBD and SPS patients (n = 1282): SSL-DR: 28.78%. The faecal occult blood test positive (FOBT)-positive cohort demonstrated the highest ADR at 81% and highest SSL-DR at 42%, while the clinical symptoms cohort showed the lowest rates (ADR: 51.31%, SSL-DR: 24.34%). Notably, all detection metrics consistently followed this pattern, with the FOBT-positive group having the highest rates and the clinical symptoms group the lowest, while patients under
| Indication | Proportions (%) | |||||
| Polypectomy rate | ADR | ASLDR | PSDR | SPDR | SSL-DR | |
| Overall (n = 1450) | 83.66 | 57.03 | 48 | 37.1 | 30.97 | 30.69 |
| Clinical symptoms (n = 419) | 75.1 | 51.31 | 39.38 | 29.83 | 24.34 | 24.34 |
| FOBT (+) (n = 100) | 90 | 81 | 58 | 43 | 43 | 42 |
| Surveillance (previous patient) (n = 792) | 86.49 | 56.19 | 49.62 | 38.64 | 31.94 | 31.69 |
| Surveillance (new patient) (n = 74) | 87.84 | 63.51 | 55.41 | 45.95 | 39.19 | 39.19 |
| Screening (n = 65) | 89.23 | 60 | 60 | 46.15 | 33.85 | 32.31 |
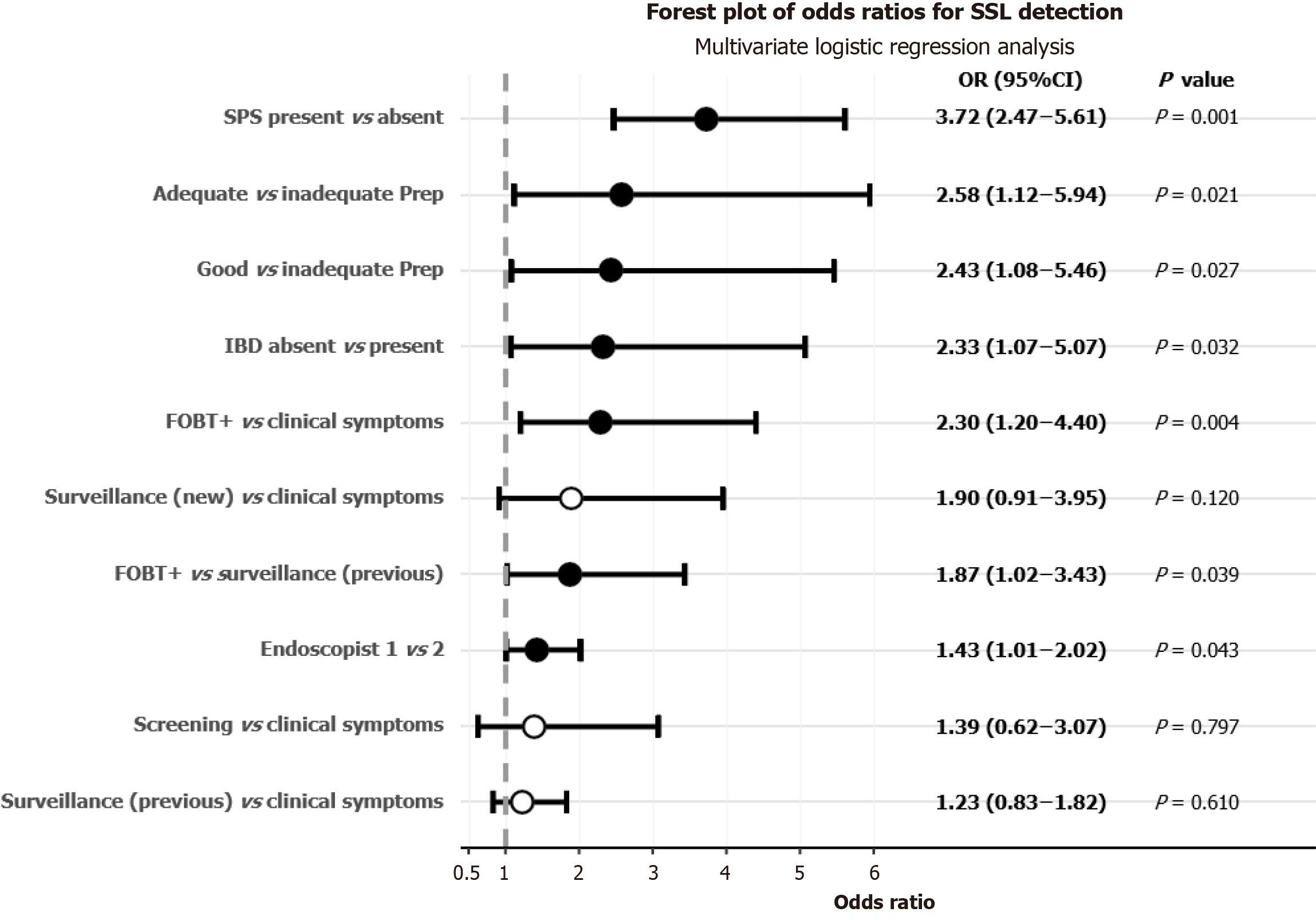
The adjusted odds ratios for predictor variables in the final multivariate model are presented in Figure 1; these pa
Bowel preparation quality exhibited a significant association with SSL-DRs. Compared to subjects with inadequate bowel preparation, those with adequate preparation demonstrated substantially higher odds of SSL detection (OR = 2.58, 95%CI: 1.12-5.94, P = 0.021), with a similar effect observed for good preparation quality (OR 2.43, 95%CI: 1.08-5.46, P = 0.027). The presence of SPS markedly increased the likelihood of SSL detection, with patients having either new or established SPS diagnoses exhibiting 3.72-fold higher odds compared to non-SPS subjects (95%CI: 2.47-5.61, P < 0.001). Notably, patients without IBD demonstrated significantly higher odds of SSL detection compared to those with IBD (OR = 2.33, 95%CI: 1.07-5.07, P = 0.032; Table 6).
| Comparisons | Statistics | ||
| OR | 95%CI | P value | |
| Indication | |||
| FOBT (+)/clinical symptoms | 2.299 | 1.203-4.396 | 0.004a |
| Surveillance (previous patient)/clinical symptoms | 1.229 | 0.829-1.824 | 0.610 |
| FOBT (+)/surveillance (previous patient) | 1.870 | 1.02-3.43 | 0.039a |
| Surveillance (new patient)/clinical symptoms | 1.898 | 0.912-3.953 | 0.120 |
| FOBT (+)/surveillance (new patient) | 1.211 | 0.508-2.888 | 0.975 |
| Surveillance (new patient)/surveillance (previous patient) | 1.544 | 0.767-3.108 | 0.437 |
| Screening/clinical symptoms | 1.386 | 0.625-3.073 | 0.797 |
| FOBT (+)/screening | 1.659 | 0.656-4.194 | 0.570 |
| Screening/surveillance (previous patient) | 1.128 | 0.52-2.444 | 0.993 |
| Surveillance (new patient)/screening | 1.370 | 0.51-3.679 | 0.909 |
| Bowel preparation status | |||
| Adequate/inadequate | 2.578 | 1.119-5.939 | 0.021a |
| Good/inadequate | 2.430 | 1.082-5.458 | 0.027a |
| Adequate /good | 1.061 | 0.748-1.504 | 0.917 |
| Serrated polyposis syndrome status (grouped diagnoses) | |||
| SPS diagnosis (new or prior)/SPS absent | 3.723 | 2.473-5.606 | < 0.001a |
| Inflammatory bowel disease status | |||
| IBD absent/IBD present | 2.332 | 1.073-5.065 | 0.032a |
| Endoscopist | |||
| Endoscopist 1/endoscopist 2 | 1.43 | 1.01-2.02 | 0.043a |
| Patient sex | |||
| Male/female | 1.021 | 0.805-1.296 | 0.861 |
This multivariate analysis demonstrated statistically significant associations between SSL-DRs and several key varia
This study aimed to contribute to the sparse Australian literature on SSL-DRs and factors associated with their variability. Unlike other countries with comprehensive colonoscopy registries, Australia lacks standardized SL reporting metrics and centralized data collection beyond the National Bowel Cancer Screening Program, which represents only approximately 5% of the more than 900000 colonoscopies performed annually[31].
The SSL-DR of 30.7% reported in this study substantially exceeds both international and Australian literature bench
Several factors may contribute to this marked variation. While age-related differences in SSL detection have been suggested, Wong et al[11] found no statistically significant difference between age groups (2.3% in patients < 50 years; 3.4% in patients ≥ 50 years) - rates approximately ten times lower than our findings. Ethnicity may also influence SSL prevalence, with evidence suggesting higher rates among Caucasians, though such data was unavailable for comparison in our study[33]. The simplified World Health Organization diagnostic criteria for SSLs likely contributed to increased detection, though this effect appears too modest to explain the several-fold inter-study variation[13,17].
The indication for colonoscopy significantly influenced SSL-DR. While our overall pattern of detection across indica
Patients with SPS were overrepresented in our study (7.8%) compared to international estimates of approximately 1 in 111 in screening populations[33-35]. However, even after excluding SPS patients, our SSL-DR of 28.2% remains higher than typically reported. The lower SSL-DR observed among IBD patients aligns with limited existing evidence, though more research is needed to understand this relationship.
Endoscopist variation was observed and was statistically significant (31.6% vs 24.5%; OR = 1.43, 95%CI: 1.01-2.02, P = 0.043), with the caveat that endoscopist 2 had a considerably smaller sample size. Notably, both endoscopists achieved SSL-DRs substantially exceeding typical Australian reports. The use of the EndoCuff® device by endoscopist 1 may have contributed to enhanced detection rates[34,35], though endoscopist 2 also achieved comparatively high rates without this device, suggesting that other factors beyond equipment may influence detection capability.
Regarding dSSLs, our finding that 1.8% of SSLs were dysplastic compares favorably with Zhang et al[1] reported 1.3%. Similarly, our per-patient dSSL-DR (0.97%) exceeds their 0.26%, despite similar SPS prevalence. The higher mean age for dSSL detection compared to non-dSSLs aligns with current understanding of the serrated neoplasia pathway[1]. While our study demonstrates exceptionally high SSL-DRs, it remains unclear whether this reflects a unique regional population with higher SSL prevalence or superior detection methods leading to greater ascertainment of SSLs and consequent SPS diagnoses. Further research with larger, diverse Australian cohorts is needed to resolve this question.
This study addresses the significant knowledge gap regarding factors influencing SSL-DR variability in Australia, pro
A key strength of this study was its comprehensive data acquisition methodology, which enabled robust statistical modelling of multiple factors potentially associated with SSL-DRs. This multivariable analysis allowed for better isolation of independent predictors while controlling for potential confounders.
Several methodological limitations warrant critical examination. Our single-center, retrospective study design in
While all histopathological assessments were conducted by Douglas Hanley Moir Pathology with specialist gastro
In conclusion, this study documents one of Australia's highest reported SSL-DRs at 30.7% across 1450 colonoscopies from a regional population, further highlighting the marked variability in Australian SSL-DRs. Notably, the FOBT-positive cohort demonstrated an unprecedented 40% SSL-DR, substantially exceeding previously reported rates. While our findings identify several factors associated with SSL detection variability, the substantial inter-study differences observed in the Australian literature remain largely unexplained, suggesting the need for standardized detection protocols and further investigation.
A/Professor Stuart Kostalas would like to acknowledge Professor Barabra Leggett for her support, ideas and suggestions. He would also like to acknowledge Professors Brian Nicholson and Professor Eva Morris for their ongoing research support.
| 1. | Zhang LL, Nicholas T, Mark B. S196 Prevalence of Dysplastic Sessile Serrated Lesions in a Large Colonoscopy Cohort in Australia. Am J Gastroenterol. 2023;118:S147. [DOI] [Full Text] |
| 2. | Haga H, Tran E, Rieger N. Colonoscopy quality of GP endoscopists in three rural hospitals in Queensland, Australia. Aust J Gen Pract. 2022;51:979-985. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2949] [Article Influence: 737.3] [Reference Citation Analysis (7)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64494] [Article Influence: 16123.5] [Reference Citation Analysis (176)] |
| 5. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Brown I, Bettington M. Sporadic Polyps of the Colorectum. Gastroenterol Clin North Am. 2024;53:155-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 240] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2425] [Article Influence: 485.0] [Reference Citation Analysis (3)] |
| 9. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 10. | Watson MM; South Australian Rural Surgical Research Group, Watson DC, Maddern GJ, Wichmann MW. Quality of rural colonoscopy outperforms key performance indicators in a multi-centre prospective clinical study. ANZ J Surg. 2023;93:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Wong WJ, Arafat Y, Wang S, Hawes S, Hung K. Colonoscopy withdrawal time and polyp/adenoma detection rate: a single-site retrospective study in regional Queensland. ANZ J Surg. 2020;90:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Vennelaganti S, Cuatrecasas M, Vennalaganti P, Kennedy KF, Srinivasan S, Patil DT, Plesec T, Lanas A, Hörndler C, Andraws N, Cherian R, Mathur S, Hassan C, Repici A, Klotz D, Musulen E, Risio M, Castells A, Gupta N, Sharma P. Interobserver Agreement Among Pathologists in the Differentiation of Sessile Serrated From Hyperplastic Polyps. Gastroenterology. 2021;160:452-454.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Murakami T, Kurosawa T, Fukushima H, Shibuya T, Yao T, Nagahara A. Sessile serrated lesions: Clinicopathological characteristics, endoscopic diagnosis, and management. Dig Endosc. 2022;34:1096-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Lamba M, Brown I, Bettington M, Ryan K, Hanigan K, Lasenby K, Dixon A, Grimpen F, Gan C, Tutticci N, Appleyard M, Leggett B. Clinicopathological Correlates of Dysplastic Sessile Serrated Lesion: A Prospective Cohort Study With a High Detection Rate. Gastro Hep Adv. 2022;1:313-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 17. | Bettington M, Walker N, Rahman T, Vandeleur A, Whitehall V, Leggett B, Croese J. High prevalence of sessile serrated adenomas in contemporary outpatient colonoscopy practice. Intern Med J. 2017;47:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Utsumi T, Yamada Y, Diaz-Meco MT, Moscat J, Nakanishi Y. Sessile serrated lesions with dysplasia: is it possible to nip them in the bud? J Gastroenterol. 2023;58:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Leggett B, Whitehall V. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 20. | IJspeert JE, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Zorron Cheng Tao Pu L, Lu K, Ovenden A, Rana K, Singh G, Krishnamurthi S, Edwards S, Wilson B, Nakamura M, Yamamura T, Ruszkiewicz A, Hirooka Y, Burt AD, Singh R. Effect of time of day and specialty on polyp detection rates in Australia. J Gastroenterol Hepatol. 2019;34:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:760-7; quiz e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Shiu SI, Kashida H, Komeda Y. The prevalence of sessile serrated lesion in the colorectum and its relationship to synchronous colorectal advanced neoplasia: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1495-1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Meester RGS, van Herk MMAGC, Lansdorp-Vogelaar I, Ladabaum U. Prevalence and Clinical Features of Sessile Serrated Polyps: A Systematic Review. Gastroenterology. 2020;159:105-118.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Kumbhari V, Behary J, Hui JM. Prevalence of adenomas and sessile serrated adenomas in Chinese compared with Caucasians. J Gastroenterol Hepatol. 2013;28:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Ross WA, Thirumurthi S, Lynch PM, Rashid A, Pande M, Shafi MA, Lee JH, Raju GS. Detection rates of premalignant polyps during screening colonoscopy: time to revise quality standards? Gastrointest Endosc. 2015;81:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Anderson JC, Rex DK, Mackenzie TA, Hisey W, Robinson CM, Butterly LF. Higher Serrated Polyp Detection Rates Are Associated With Lower Risk of Postcolonoscopy Colorectal Cancer: Data From the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2023;118:1927-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | van Toledo DEFWM, IJspeert JEG, Bossuyt PMM, Bleijenberg AGC, van Leerdam ME, van der Vlugt M, Lansdorp-Vogelaar I, Spaander MCW, Dekker E. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022;7:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 29. | Zessner-Spitzenberg J, Waldmann E, Jiricka L, Rockenbauer LM, Hinterberger A, Cook J, Asaturi A, Szymanska A, Majcher B, Trauner M, Ferlitsch M. Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients. Endoscopy. 2023;55:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Australian Institute of Health and Welfare. Cancer data in Australia. Dec 9, 2024. [cited 1 May 2025]. Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/about. |
| 31. | Australian Commission on Safety and Quality in Health Care. Colonoscopy Clinical Care Standard. Jan, 2020 [Internet]. [cited 1 March 2025]. Available from: https://www.safetyandquality.gov.au/sites/default/files/2020-04/colonoscopy_clinical_care_standard_updated_2020.pdf. |
| 32. | Cheng CL, Kuo YL, Liu NJ, Lin CH, Tang JH, Tsui YN, Lee BP, Su MY, Chiu CT. Impact of Bowel Preparation with Low-Volume (2-Liter) and Intermediate-Volume (3-Liter) Polyethylene Glycol on Colonoscopy Quality: A Prospective Observational Study. Digestion. 2015;92:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Parian AM, Lazarev MG. Serrated Colorectal Lesions in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2018;14:19-25. [PubMed] |
| 34. | Aziz M, Fatima R, Lee-Smith W, Khuder S, Nawras A. Comparing endoscopic interventions to improve serrated adenoma detection rates during colonoscopy: a systematic review and network meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2020;32:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Wang J, Ye C, Fei S. Endocuff-assisted versus standard colonoscopy for improving adenoma detection rate: meta-analysis of randomized controlled trials. Tech Coloproctol. 2023;27:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Wong S, Lidums I, Rosty C, Ruszkiewicz A, Parry S, Win AK, Tomita Y, Vatandoust S, Townsend A, Patel D, Hardingham JE, Roder D, Smith E, Drew P, Marker J, Uylaki W, Hewett P, Worthley DL, Symonds E, Young GP, Price TJ, Young JP. Findings in young adults at colonoscopy from a hospital service database audit. BMC Gastroenterol. 2017;17:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |