Published online May 16, 2025. doi: 10.4253/wjge.v17.i5.105158
Revised: March 10, 2025
Accepted: April 22, 2025
Published online: May 16, 2025
Processing time: 119 Days and 0.3 Hours
There is a scarcity of evidence and systematic reviews on endoscopic gastroplasty (EG) compared to other management options for the treatment of obesity.
To assess the published meta-analyses through a systematic review approach and provide further insight into the current status of available evidence through a critical appraisal.
PubMed/MEDLINE, Scopus, Embase and Cochrane Library were searched from inception to November 2022. The meta-analyses that compared the efficacy and safety of EG to other interventions were considered for this overview. The out
A total of six meta-analyses out of 364 records were considered for this review with a major contribution from the United States. Overall methodological quality of included studies were moderate to good. EG treatments were significantly better in terms of TBWL, excessive weight loss, and average weight loss. Ho
Though EG was significantly effective for treatment of obesity, there is limited comparative evidence on this topic. High-quality well-controlled evidence is required to strengthen the current evidence base on EG treatment for obesity.
Core Tip: Endoscopic gastroplasty treatments were significantly better in reducing total body weight, excessive weight, and average weight among obese population. No significant difference between endoscopic transoral outlet reduction and full-thickness suturing plus argon plasma mucosal coagulation. Lack of comparative, long-term follow-up and randomized studies, reporting and selection bias, high level of heterogeneity were the major limitations in the currently available meta-analyses.
- Citation: Abdulla M, Mohammed N, AlQamish J, Arau RT. Efficacy and safety of endoscopic gastroplasty for treatment of obesity: An overview of comparative meta-analyses. World J Gastrointest Endosc 2025; 17(5): 105158
- URL: https://www.wjgnet.com/1948-5190/full/v17/i5/105158.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i5.105158
Obesity and excess weight are a major threat across the world that has grown to a level of epidemic proportion with a contribution of at least 2.8 million deaths annually. As per the global statistics, 13% of adults with obesity, and 39% are overweight. Among children and adolescents, one in five are overweight[1]. Numerous approaches such as lifestyle modifications, dietary interventions, physical activity, behavioral therapy, pharmacological interventions, and surgical procedures have been adopted for the treatment of obesity[2].
Bariatric surgery plays an inevitable role in this area, however the unavoidable morbidity and mortality, limited access to surgery, high surgical risk, cost, and patient preference mean that bariatric surgery is limited to a set amount of patients[3]. Endoscopic gastroplasty (EG) methods are excellent substitutes for conventional surgery for obesity, with the advantage of being suited to high-risk patients, less invasive, and more effective. Three EG techniques are widely used which include endoscopic sleeve gastroplasty (ESG), primary obesity surgery endoluminal procedure, and transoral endoscopic vertical gastroplasty [endoscopic transoral outlet reduction (TORe)][4].
Many primary research reviews have assessed the effectiveness of EG in obesity management, though the findings are inconclusive[5,6]. Meta-analysis employs statistical measures to pool the outcomes from different studies into a single estimate. It is considered to be the highest level of evidence and the findings are easily transferred to clinical practice[7,8]. There are inconclusive findings among the published meta-analyses concerning the effectiveness of EG in the treatment of obesity compared to other management options[9-11]. Hence, this study aimed to perform an overview of meta-analyses to give a clear picture of this topic and provide further insights into efficacy and safety of EG in patients with obesity. This overview also assessed the quality of included studies/risk of bias, heterogeneity, and limitations in the available meta-analyses.
Participants, intervention, comparator, outcomes, and study design framework to perform this overview and reported the findings as per the PRISMA guidelines to report this systematic review[12]. Ethical approval was not required as this was a review of published literature evidence. The protocol was not registered with any repository; however, this review was conducted as per a pre-defined methodology.
Study design: Only the meta-analyses with a comprehensive search strategy in at least one literature database and that were considered the primary research were included in this overview. Only studies comparing the effect of EG with other treatments written in English were considered. Systematic reviews without a proper research question, search strategy, defined process of study selection, or pooled estimations were excluded from this overview. Those meta-analyses which did not having a comparative analysis or only had a pooled estimation of single intervention were not considered. Also, research articles, narrative reviews, overviews, study protocols, and duplicates were excluded.
Participants: The studies considered adult patients (≥ 18 years old) with obesity who had undergone surgical treatment were included in this overview. Patients who had weight regain after the initial surgical management were also considered. The diagnosis of obesity and inclusion of participants was in accordance with any international guidelines or as per the author’s discretion.
Intervention: The intervention considered was any of the methods of EG for the management of obesity, excess weight, or weight regain. Studies that did not include any surgical methods other than EG were not considered for inclusion.
Comparator: Any comparator including surgical and non-surgical methods of weight loss were considered in this study. This included bariatric surgery techniques other than EG and non-surgical methods like sham, or diet and lifestyle changes. Meta-analyses that had not performed a comparative analysis were excluded from this review.
Outcomes and outcome measures: The primary outcomes were efficacy measures such as total body weight loss (TBWL), excess weight loss (EWL), absolute weight loss (AWL), or percentage (%) TBWL, EWL, or AWL measured at any time point. The secondary endpoint was safety measures like adverse events or adverse drug reactions (ADR). To be included in this review, the meta-analyses had to report their outcome in terms of mean difference with its 95%CI or mean estimate with SD from its pooled analysis after a follow-up period.
Electronic databases including PubMed/MEDLINE, Scopus, Cochrane Library, and Embase were searched using a comprehensive search strategy from inception to November 2022 to identify all the studies. A snowball search on Google, Google Scholar, and Research Gate was also performed to identify any additional studies. The bibliographic lists of all identified articles were screened to check for the addition of any potentially relevant articles. A detailed search strategy in various databases is provided as Supplementary Table 1 and Supplementary Figures 1-3.
All the studies identified from the databases were retrieved to a Microsoft Excel sheet and their titles and abstracts were screened initially as per the predefined criteria. Consequently, the full texts of the included studies were screened for eligibility using the same criteria. First author’s last name and year of publication were used to identify the studies. All the relevant data such as study characteristics, eligibility criteria, literature search, quality assessment, heterogeneity, and study limitations were extracted to the data extraction grid. Two independent reviewers were involved in the study selection and data extraction and any disagreements between the reviewers were resolved through consensus or discussion with another reviewer.
All the evidence extracted through the systematic process was presented in tabular form and summarised narratively. The evidence from the studies was classified in accordance with the clinical outcomes. A quantitative estimation was not performed as the data used in this review was already from a meta-analysis and another meta-analysis on the same data would exaggerate the actual effect of intervention. Hence, the synthesis was restricted it to the narrative synthesis.
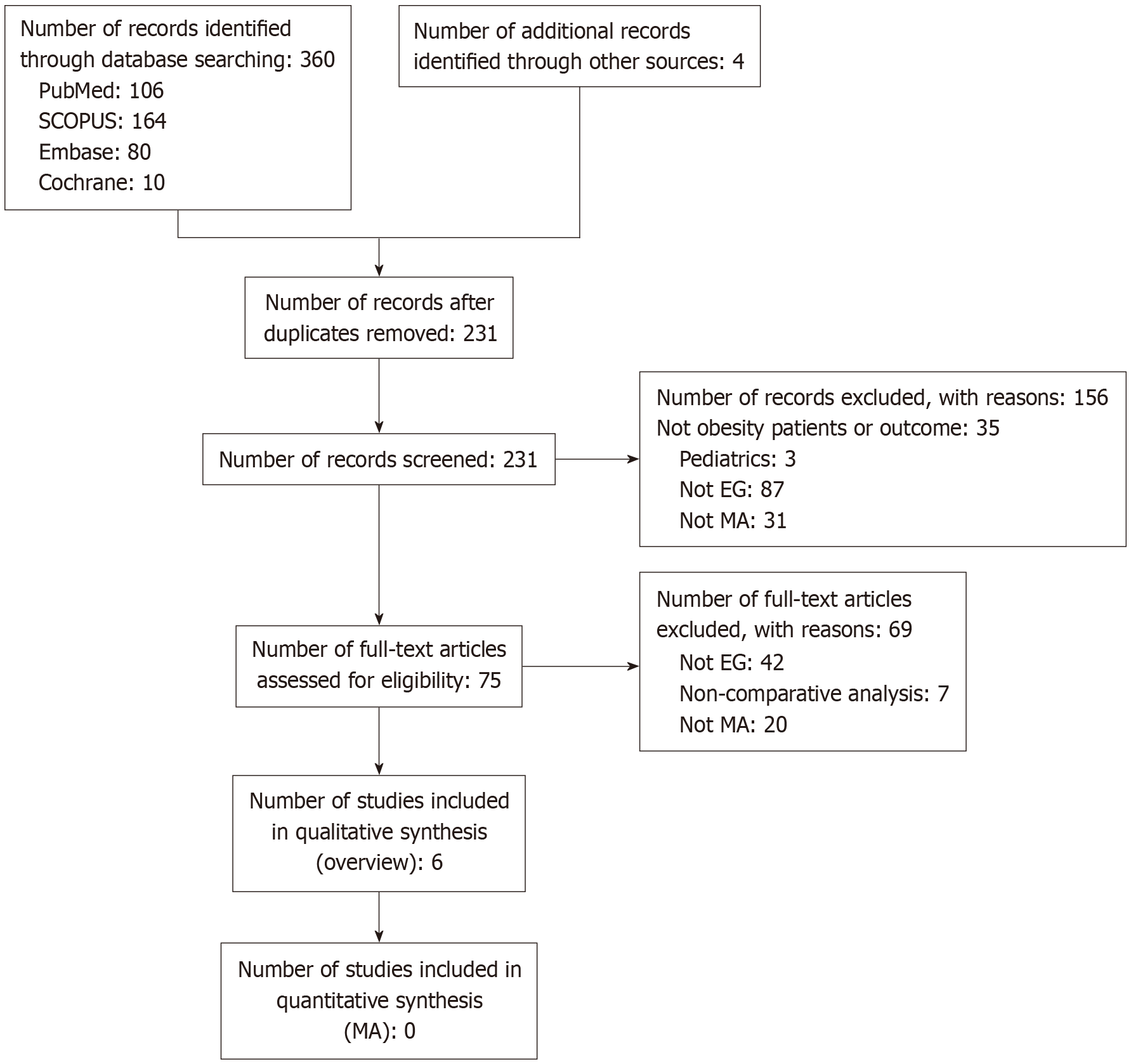
Out of 364 identified studies, a total of 231 studies were screened for their title and abstract. A total of 75 studies were found eligible for full-text screening, and 156 records were excluded. Finally, a total of six meta-analyses[4,9,11,13,14] were included for this overview. A detailed description of the study selection process is provided in Figure 1.
The included meta-analyses were published between the years 2017 to 2020 in the United States (n = 3, 50%), Brazil (n = 2, 33.3%) and Australia (n = 1, 16.7%). The included patients were people with obesity or those who had gained weight after undergoing Roux-en-Y Gastric Bypass. The interventions included were ESG, Endoscopic TORe, full-thickness suturing (FTS) plus argon plasma mucosal coagulation (ft-TORe), argon plasma mucosal coagulation alone (APMC-TORe); EG with full-thickness suture or plication devices. These were compared with various techniques like intragastric balloon (IGB), laparoscopic sleeve gastroplasty (LSG), sham, diet, and lifestyle changes. All the studies reported any of the pre-defined outcomes. The included studies in the considered meta-analyses were randomized controlled trials (RCTs), observational studies, case-control studies, and cohort studies. The follow-up and outcome measurement in each study varied from 3 to 24 months. The characteristics of included studies are presented in Table 1.
| No. | Ref. | Country | Eligibility/inclusion criteria | Search characteristics | Number of studies and total number of patients | |
| Databases searched | Search period and limitations | |||||
| 14 | Singh et al[9], 2020 | United States | Inclusion criteria: P: Patients underwent EBMT for obesity; I: ESG; C: IGB; O: %TWL, %EWL and adverse events; SD: All RCTs and observational studies with a minimal follow-up of 12 months; studies with more than one treatment arm, patients who underwent ESG or IGB alone with or without lifestyle modification | MEDLINE (PubMed), Scopus, Cochrane Register of Controlled Trials, and Web of Science databases | Inception to August 2019; restricted to observational and RCTs | 28 studies; 1 study with direct comparison |
| Exclusion criteria: Patients with prior or sequential EBMTs or bariatric surgery; Case reports and study with < 25 patients; studies with endoscopic gastroplasty techniques using devices other than the OverStitch endoscopic suturing system; studies with IGBs not approved by the United States Food and Drug Administration; Letters, editorials, expert opinions, and reviews without original data and studies with overlapping patient cohorts | ||||||
| 42 | Jaruvongvanich et al[11], 2020 | United States | Inclusion criteria: P: Adults aged 18 years or older with obesity; I: ft-TORe or APMC-TORe to manage weight regain after RYGB; C: Any; O: TBWL; SD: case-control studies, cohort studies, RCTs | Ovid MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus | Inception to February 10, 2020, limited to the English language and excluding animal studies | 16 studies; 1625 participants |
| 43 | Mohan et al[13], 2020 | United States | Inclusion criteria: P: Patients with moderate to severe obesity; I: ESG; C: LSG on published 2013; O: Pooled and difference in %TWL, %EWL and BMI with ESG at 1, 6, and 12 months; and adverse events; SD: All studies were included irrespective of the study sample-size, inpatient/outpatient setting, and geography as long as they provided data needed for the analysis | PubMed, EMBASE, Google-Scholar, Scopus, and Web of Science databases | Inception to August 2019; restricted to studies in human subjects and published in English language in peer-reviewed journals | 15 studies; 3994 patients |
| Exclusion criteria: Studies on LSG published until December 31, 2012; studies on LSG published as abstracts; studies on robot-assisted LSG; studies that did not report outcomes on weight loss, in terms of total weight loss and/or excess weight loss and/or BMI; studies that did not report first 12 months’ outcome data; studies done exclusively in elderly and/or geriatric population (age > 60 years); studies done in a pediatric population (age < 18 years); and studies not published in English language | ||||||
| 50 | Jalal et al[10], 2020 | Australia | Inclusion criteria: P: Adult patient study populations greater or equal to 20 with obesity; I: ESG; C: LSG; O: Weight loss potential of ESG, ESG complications, rates of conversion to surgery and cost; SD: English studies as Case-control, cohort and RCTs | PubMed/MEDLINE, Cochrane Library, EMBASE/OVID, and the World Wide Web | NR | 2 studies; 348 participants |
| Exclusion criteria: Case reports or case series; Articles that did not have extractable ESG data; Same centre studies or studies with cross over patient populations; Patient cohort less than 20 | ||||||
| 56 | Madruga-Neto et al[4], 2018 | Brazil | Inclusion criteria: P: Patients with obesity (BMI > 30 kg/m2); I: EG with full-thickness suture or plication devices; C: sham or diet and lifestyle changes; O: AWL, %EWL, responder rate (%TWL ≥ 5%), and potential complications in 6 and 12 months; SD: Only RCTs without restrictions on language or publication year | MEDLINE [PubMed], Embase, Cochrane Central, and LILACS/BIREME | Inception to November 2017 | 3 studies; 459 patients |
| Exclusion criteria: studies with follow-up periods < 1 month, those involving revision endoscopic procedures after bariatric surgery, and those involving patients who were overweight (BMI: 25-30 kg/m2) | ||||||
| 57 | Brunaldi et al[14], 2018 | Brazil | Inclusion criteria: P: Patients with RYGB who presented with weight regain; I: Endoscopic therapy for weight regain following RYGB; C: Any comparator; O: AWL, EWL, and TBWL; SD: RCT, observational cohort studies, and case series and Conference abstracts if they met the eligibility criteria | MEDLINE, Embase, Scopus, Web of Science, Cochrane, OVID, CINAHL/EBSCo, LILACS/Bireme, and gray literature | Inception to October 31, 2016 | 15 studies; 882 patients |
| Exclusion criteria: Reviews, editorials, case-control studies, studies using non-human subjects, articles without English translation, did not describe the endoscopic method clearly; follow-up weight or BMI less than 1 month; with endoscopic treatment for other indications besides weight regain, such as dumping syndrome or fistula closure; did not report baseline BMI; included patients with weight regain who had already undergone other endoscopic or surgical treatment of weight regain | ||||||
The databases searched were MEDLINE (PubMed), Scopus, Cochrane Register of Controlled Trials, Web of Science databases, Google Scholar, World Wide Web, LILACS/ BIREME, CINAHL/EBSCo, and Gray literature. The latest search was on February 10, 2020. The applied search restrictions were English language, RCTs, and human studies. The details are presented in Table 1.
The various tools such as Cochrane Collaboration risk assessment tool, New Castle Ottawa scale, National Institutes of Health quality assessment scale, GRADE standards, and Joanna Briggs Institute Checklist were used for the quality assessment of included studies. The quality of included studies ranged from very low to high quality. Only four studies[4,9,10,11], reported the heterogeneity in the analysis ranging it from low to high, while two studies did not report the heterogeneity. The quality assessment and heterogeneity characteristics of the included studies are provided in Table 2.
| Ref. | Country | Quality assessment/risk of bias | Author reported factors affecting the quality | Heterogeneity in the analysis | Author reported limitations in the review |
| Singh et al[9], 2020 | United States | NOS; Score of 8 | NR | Considerable | Limited quality of the included studies, lack of controlled ESG studies, lack of long-term follow-up data, lack of clarify the concomitant use of weight loss medication, considerable heterogeneity, lack of standardized definition for SAE |
| Jaruvongvanich et al[11], 2020 | United States | RCTs: Cochrane Collaboration risk assessment tool; high quality (1), low to medium quality (2); Observational studies: National Institutes of Health quality assessment scale for pre-post Studies; high quality (7) and low to medium quality (6) | Reporting bias, inappropriate patient selection, blinding of personals and outcome assessors, loss to follow-up | High (0%-87%) | Small sample size, limited follow-up duration, small number of studies in each analysis, low to moderate methodological quality of the included studies, the small number of comparative trials between the 2 techniques, substantial heterogeneities, influence of concomitant pharmacotherapy, limiting generalizability of findings, reporting bias |
| Mohan et al[13], 2020 | United States | NOS; 3 were high quality and the rest were medium quality | Included studies were not entirely representative of the general population and community practice, selection bias, inadequate follow-up | NR | Effect of additional confounding factors, included studies were not entirely representative of the general population and community practice, retrospective nature of studies, selection bias, lack of subgroup data based on the presence of comorbidities, lack of data on success/failure of procedure |
| Jalal et al[10], 2020 | Australia | NR: Limited quality | High loss to follow-up rates | Low (19%) | Lack of studies, retrospective nature of cohort studies, lack of control groups, heterogeneity, lack of long-term study data, high loss to follow-up rates |
| Madruga-Neto et al[4], 2018 | Brazil | JADAD quality score: 3; GRADE standards: Very low to moderate | Inappropriate randomization, inappropriate blinding | Moderate (I2: 50% to 68%) | Biases, heterogeneity, inadequate data |
| Brunaldi et al[14], 2018 | Brazil | Joanna Briggs Institute Checklist; very low | Selection bias, unclear reporting of information | NR | Lack of RCT, bias, heterogeneity, low methodological quality, lack of comparative studies |
Many factors were reported to affect the methodological quality of the included studies. This includes reporting bias, inappropriate patient selection, blinding of outcome assessors, lack of or inadequate follow-up, the inclusion of studies that were not entirely representative of the general population and community practice, and unclear reporting of information. The factors affecting the quality of included meta-analyses are provided in Table 2.
The following limitations were reported in the included studies: Limited quality of the included studies, lack of controlled ESG studies, lack of long-term follow-up data, lack of clarification of the confounding factors such as concomitant use of weight loss medication, considerable heterogeneity, lack of standardized definition for serious adverse events, small sample size, small number of studies in each analysis, limiting generalizability of findings, reporting bias, retrospective nature of studies, selection bias, lack of subgroup data based on the presence of comor
TBWL: (1) At 3 months: A total of 3 studies reported the effect of EG in TBWL outcomes. Among them, two studies reported a better effect in the intervention group, whereas one study reported no significant difference. The meta-analysis by Brunaldi et al[14], from eight studies with 320 participants recorded significantly (P < 0.001) greater weight loss with FTS with argon plasma coagulation (APC) group compared to FTS alone. The study by Jaruvongvanich et al[11] demonstrated significantly greater weight loss with TORe than TORe-gastroplasty from two studies. However, non-significant difference in weight loss was observed between ft-TORe and APMC-TORe[11]. The detailed information is provided in Table 3; (2) At six months: A total of 3 studies with 4 analyses reported the outcomes of EG at six months, of which 2 studies reported on ESG. The study by Singh et al[9] observed significantly (P = 0.002) superior weight loss with ESG than IGB, whereas Jalal et al[10], reported significantly (P < 0.00001) superior weight loss with LSG than ESG. The remaining two studies recorded a non-significant difference between ft-TORe vs APMC-TORe,11 and TORe vs TORe-gastroplasty[11]. The detailed information is provided in Table 3; (3) At 12 months: A total of three studies reported the outcomes of EG at 12 months, out of which two studies reported on ESG. The study by Singh et al[9] observed sig
| Ref. | Country | Number of studies & participants | Intervention and comparator | Effect measure | Effect size (95%CI) | Interpretation with respect to intervention |
| 3 months | ||||||
| Brunaldi et al[14], 2018 | Brazil | 8 studies; 320 participants | FTS-APC vs FTS-alone | % mean | FTS-APC: 25.0 ± 1.99; FTS-alone: 15.3 ± 9.88 | The FTS-APC group and demonstrated significantly a greater weight loss compared to FTS alone (P < 0.001) |
| Jaruvongvanich et al[11], 2020 | United States | 2 studies | ft-TORe vs APMC-TORe | % mean difference | -0.1 (-4.6 to 4.4) | There was no significant difference between two treatments |
| Jaruvongvanich et al[11], 2020 | United States | 2 studies | TORe vs TORe-gastroplasty | % mean difference | 2.6 (0.5 to 4.7) | The TORe group and demonstrated significantly a greater weight loss compared to TORe-gastroplasty |
| 6 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 3.07 (1.46–4.67) | ESG achieved significantly (P = 0.002) superior weight loss compared to IGB |
| Jalal et al[10], 2020 | Australia | 2 studies; 348 participants | ESG vs LSG | % mean difference | 8.52 (6.35–0.69) | LSG appeared to have a significantly (P < 0.00001) superior weight loss compared to ESG |
| Jaruvongvanich et al[11], 2020 | United States | 3 studies | ft-TORe vs APMC-TORe | % mean difference | 0.3 (-5.5 to 6.0) | There was no significant difference between two treatments |
| Jaruvongvanich et al[11], 2020 | United States | 2 studies | TORe vs TORe-gastroplasty | % mean difference | 0.8 (-2.3 to 3.9) | There was no significant difference between two treatments |
| 12 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 7.33 (5.22-9.44) | ESG achieved significantly (P = 0.0001) superior weight loss compared to IGB |
| Mohan et al[13], 2020 | United States | 9 studies | ESG vs LSG | % mean | ESG: 17.08 (15.05-19.10); LSG: 30.5 (27.4-33.5) | LSG appeared to have a significantly (P = 0.001) superior weight loss compared to ESG |
| Madruga-Neto et al[4], 2018 | Brazil | 2 studies; 376 participants | EG vs Sham | % mean difference | 4.8 (1.1-8.51) | The difference between the groups was significantly (P = 0.01) higher in the intervention group than in the control group |
| 18-24 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 11.51 (5.33-17.69) | ESG achieved significantly (P = 0.0003) superior weight loss compared to IGB |
Excessive weight loss: (1) At six months: Only two studies reported the EWL following EG treatment at six months of which, Singh et al[9], recorded significantly (P = 0.0001) better weight loss with ESG compared to IGB, whereas the study by Madruga-Neto et al[4] reported a non-significant (P = 0.07) difference between EG and conventional treatment. The detailed information is provided in Table 4; (2) At 12 months: A total of four studies recorded EWL at 12 months of which the study by Singh et al[9], recorded significantly (P = 0.0001) superior weight loss with ESG compared to IGB. Similarly, Brunaldi et al[14], demonstrated significantly (P < 0.001) greater weight loss in FTS-APC group compared to FTS alone, whereas another two studies, Mohan et al[13], and Madruga-Neto et al[4], observed a non-significant difference in EWL between LSG vs ESG and EG vs conventional therapy procedures. The detailed information is provided in Table 4; and (3) At 18-24 months: Two studies reported EWL at 18-24 months of which Singh et al[9], reported significantly (P = 0.0001) superior weight loss with ESG compared to IGB, and Brunaldi et al[14], recorded significantly (P < 0.0001) superior weight loss with FTS-APC compared to FTS-alone. The detailed information is provided in Table 4.
| Ref. | Country | Number of studies & participants | Intervention and comparator | Effect measure | Effect size (95%CI) | Interpretation with respect to intervention |
| 6 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 20.80 (12.50-29.10) | ESG achieved significantly (P = 0.0001) superior weight loss compared to IGB |
| Madruga-Neto et al[4], 2018 | Brazil | 2 studies; 127 participants | EG vs CT | % mean difference | 17.87 (-1.8 to 37.54) | There was no significant (P = 0.07) difference between the groups, although the EG group presented higher effect than the control group |
| 12 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 30.99 (22.81-39.16) | ESG achieved significantly (P = 0.0001) superior weight loss compared to IGB |
| Mohan et al[13], 2020 | United States | 9 studies | ESG vs LSG | % pooled mean | ESG: 62.3 (51.3-74.6); LSG: 69.3 (60.1-78.4) | There was no significant (P = 0.4) difference between LSG and ESG |
| Madruga-Neto et al[4], 2018 | Brazil | 2 studies; 127 participants | EG vs CT | % mean difference | 16.01 (-1.48 to 33.5) | There was no significant (P = 0.07) difference between the groups, although the EG group presented higher effect than the control group |
| Brunaldi et al[14], 2018 | Brazil | 14 studies; 619 participants | FTS-APC vs FTS-alone | % mean difference | FTS-APC: 27.0 ± 2.91; FTS-alone: 17.8 ± 15.3 | The FTS-APC group and demonstrated significantly a greater weight loss compared to FTS alone (P < 0.001) |
| 18-24 months | ||||||
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | % mean difference | 43.78 (35.98-51.58) | ESG achieved significantly (P = 0.0001) superior weight loss compared to IGB |
| Brunaldi et al[14], 2018 | Brazil | 10 studies; 455 participants | FTS-APC vs FTS-alone | % mean difference | FTS-APC: 24.2 ± 0.84; FTS-alone: 11.7 ± 21.6 | The FTS-APC group and demonstrated significantly a greater weight loss compared to FTS alone (P < 0.001) |
AWL: (1) At six months: Only the meta-analysis by Madruga-Neto et al[4], reported the AWL at six months which indicated that there was significant (P < 0.0001) weight reduction in the EG group compared to the control group. The detailed information is provided in Table 5; and (2) At 12 months: Only two studies recorded the AWL at 12 months. The meta-analysis by Madruga-Neto et al[4], recorded significant (P = 0.03) weight reduction in the EG group compared to the control group. The analysis by Mohan et al[12], recorded a non-significant (P = 0.65) difference in AWL between LSG and ESG groups. The detailed information is provided in Table 5. No meta-analyses analyzed the outcomes with more than 12-month follow-up. The detailed information is provided in Table 6.
| Ref. | Country | Number of studies & participants | Intervention and comparator | Effect measure | Effect size (95%CI) | Interpretation with respect to intervention |
| 6 months | ||||||
| Madruga-Neto et al[4], 2018 | Brazil | 2 studies; 127 participants | EG vs CT | % mean difference | 7.05 (3.83 to 10.28) | There was significant (P < 0.0001) weight reduction in the EG group than the control group |
| 12 months | ||||||
| Mohan et al[13], 2020 | United States | 11 studies | ESG vs LSG | % pooled mean | ESG: 29.3 (27.6-32.3) LSG: 29.3 (27.1-31.4) | There was no significant (P = 0.65) difference between LSG and ESG |
| Madruga-Neto et al[4], 2018 | Brazil | 2 studies; 127 participants | EG vs CT | % mean difference | 4.99 (0.52 to 9.46) | There was a significant (P = 0.03) weight reduction in EG group than the control group |
| Ref. | Country | Number of studies & participants | Intervention and comparator | Adverse events reported |
| Singh et al[9], 2020 | United States | 1 study; 58 participants | ESG vs IGB | 3 (2 upper gastrointestinal bleeding, 1 peri gastric fluid collection) |
| Mohan et al[13], 2020 | United States | 15 studies | ESG vs LSG | All ADRs: ESG: 2.9 % (1.8-4.4); LSG: 11.8 % (8.4-16.4); P = 0.001; Bleeding: ESG: 1.1 % (0.7-1.8); LSG: 2.6 % (1.9-3.7); P = 0.005; GERD: ESG: 0.4 % (0.1-1.1); LSG: 5.8 % (3.5-9.3, 73); P = 0.001 |
| Madruga-Neto et al[4], 2018 | Brazil | 3 studies; 459 patients | ESG vs CT | The total rate of adverse events in the EG group was 52.9%-77.8%, of which 5.0%-5.2% of the events were severe |
| Jaruvongvanich et al[11], 2020 | United States | 16 studies; 1625 participants | ft-TORe vs APMC-TORe | APMC-TORe: GI bleeding (1); Overall AE rates: ft-TORe: 9.3% (8-17.8); APMC-TORe: 6.4% (1.9-10.9); stricture rates: ft-TORe: 3.3% (1.4-5.3); APMC-TORe: 4.8% (2.3-7.2) |
Adverse effects: Only 4 analyses reported the safety outcomes in their meta-analyses. However, there was no compa
Excess weight can impact all domains of human health including physical, mental, spiritual, and social[15]. Obesity can contribute to many comorbidities like hyperglycemia, hypertension, hypercholesterolemia, and metabolic dysfunction-associated steatotic liver disease[16]. It should be managed appropriately through suitable non-surgical and surgical interventional strategies to control the morbidities and mortalities associated with it. Many surgical strategies were employed for obesity management and ESG is an excellent and effective strategy that is minimally invasive[17]. How
This overview revealed that there were six meta-analyses assessing the effectiveness of EG among obese patients and 50% of the included studies were from the United States. These studies reported their outcomes in three to 24 months. All the studies searched the major databases, without many limitations other than English language which is acceptable. However, the lack of primary research raised questions about the validity of conclusions from the meta-analyses.
All the included studies derived their findings of comparative analysis from very few studies. This is due to the lack of comparative analysis available on the efficacy and safety of EG in the treatment of obesity. The quality of included studies varied among the meta-analyses and there was a considerable level of heterogeneity in the analyses. Many factors contributed to the risk of bias in its all aspects and future studies should attempt to reduce the risk of bias. It’s a fact that it may not be possible to blind the participants and study personals in all circumstances, but blinding of outcome assessors will be an effective method to control the bias[18].
The non-randomized nature as well as the retrospective or cohort nature of the studies also contributed to the bias in the included meta-analyses. In some clinical settings, it may be difficult to randomize the surgical intervention and, in some cases, researchers usually prefer to go with the non-randomized design. In those cases, researchers can use methodological quality tools such as ROBINS-I and II tool[19], and Downs and Black[20], checklists for non-interventions. The use of stringent critical appraisal tools like Cochrane Risk of Bias Assessment will significantly reduce the quality of the studies. Unclear reporting as well as undefined uniform criteria for outcomes assessments also contributed to the risk of bias, therefore further meta-analyses should be planned appropriately. In cases of heterogeneous clinical outcomes in the included studies, a subgroup analysis or meta-regression analysis should be planned and performed to understand the influence of potential factors or to understand the effect of intervention on a specific population or a set of characteristics[21].
Though the outcomes in the included meta-analyses had a follow-up of three to 24 months, there was not much data available with a longer follow-up. There was a conflicting finding with respect to the effect of ESG on TBWL compared to the LSG[9,10]. Also, a non-significant difference was observed between ft-TORe vs APMC-TORe and TORe vs TORe-gastroplasty[11]. Altogether, this overview demonstrates the superior effect of EG over other methods which is backed by recent clinical evidence. The new literature evidence confirms the proven efficacy and safety of EG procedure for treating obesity and controlling associated comorbidities[22].
The effect of EG in EWL and AWL is very effective and ESG is shown to be a promising safer option for obesity. Similarly, FTS-APC also appeared to be a better option than FTS-alone for EWL and AWL in patients with obesity or excess weight, and the present literature supports this fact[16,23]. Furthermore, there was a great technical success rate and post-procedure gastric sleeve and suture intactness following ESG[24,25]. The safety concerns were tolerable among patients undergoing EG management for obesity. ADRs such as upper gastrointestinal bleeding, peri gastric fluid collection, bleeding, and GERD were observed among the patients[4,9,11,13]; however, more data is required on this aspect.
The high level of hetrogeneity and the potential risk of bias might have hampered the robustness of the existing findings. Multiple mitigation strategies such as adequate reporting, bias controlled long-term prospective comparative studies, adapting the appropriate randomization technique, controlling the confounding factors, adequate follow-up, adequately powered with a homogenized larger sample sized multi-centric studies, standardized definition to the outcomes, and its reporting, subgroup and sensitivity analyses along with adequate reporting of the findings as per the validated guidelines[8,26-30] will help the future studies to bring a robust and stronger conclusion.
There are some limitations to this study. Firstly, there are very limited data available on the efficacy and safety of EG for obesity, hence more research needs to be conducted to get a clear picture. Secondly, the generalizability of the infor
The limited available evidence indicates that EG was effective and safe in the treatment of obesity compared to other interventional strategies. The quality of evidence was moderate to high. Further good quality well-controlled comparative studies with long-term follow-up is needed to strengthen this evidence. Additionally, the subgroup and sensitivity analyses will increase the generalizability of the findings.
| 1. | Smith KB, Smith MS. Obesity Statistics. Prim Care. 2016;43:121-135, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 2. | Reid TJ, Korner J. Medical and Surgical Treatment of Obesity. Med Clin North Am. 2022;106:837-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Gulliford MC, Charlton J, Prevost T, Booth H, Fildes A, Ashworth M, Littlejohns P, Reddy M, Khan O, Rudisill C. Costs and Outcomes of Increasing Access to Bariatric Surgery: Cohort Study and Cost-Effectiveness Analysis Using Electronic Health Records. Value Health. 2017;20:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Madruga-Neto AC, Bernardo WM, de Moura DTH, Brunaldi VO, Martins RK, Josino IR, de Moura ETH, de Souza TF, Santo MA, de Moura EGH. The Effectiveness of Endoscopic Gastroplasty for Obesity Treatment According to FDA Thresholds: Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Obes Surg. 2018;28:2932-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Sullivan S, Swain JM, Woodman G, Antonetti M, De La Cruz-Muñoz N, Jonnalagadda SS, Ujiki M, Ikramuddin S, Ponce J, Ryou M, Reynoso J, Chhabra R, Sorenson GB, Clarkston WK, Edmundowicz SA, Eagon JC, Mullady DK, Leslie D, Lavin TE, Thompson CC. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. Obesity (Silver Spring). 2017;25:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST Multicenter Randomized Controlled Trial: 12-Month Weight Loss and Satiety Outcomes After pose (SM) vs. Medical Therapy. Obes Surg. 2017;27:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Khan S, Gionfriddo MR, Cortes-Penfield N, Thunga G, Rashid M. The trade-off dilemma in pharmacotherapy of COVID-19: systematic review, meta-analysis, and implications. Expert Opin Pharmacother. 2020;21:1821-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Rashid M, Khan S, Datta D, Thunga G, Chandran VP, Balakrishnan A, Shanbhag V, Acharya RV, Nair S. Efficacy and safety of corticosteroids in acute respiratory distress syndrome: An overview of meta-analyses. Int J Clin Pract. 2021;75:e14645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Singh S, de Moura DTH, Khan A, Bilal M, Chowdhry M, Ryan MB, Bazarbashi AN, Thompson CC. Intragastric Balloon Versus Endoscopic Sleeve Gastroplasty for the Treatment of Obesity: a Systematic Review and Meta-analysis. Obes Surg. 2020;30:3010-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Jalal MA, Cheng Q, Edye MB. Systematic Review and Meta-Analysis of Endoscopic Sleeve Gastroplasty with Comparison to Laparoscopic Sleeve Gastrectomy. Obes Surg. 2020;30:2754-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Jaruvongvanich V, Vantanasiri K, Laoveeravat P, Matar RH, Vargas EJ, Maselli DB, Alkhatry M, Fayad L, Kumbhari V, Fittipaldi-Fernandez RJ, Hollenbach M, Watson RR, Gustavo de Quadros L, Galvao Neto M, Aepli P, Staudenmann D, Brunaldi VO, Storm AC, Martin JA, Gomez V, Abu Dayyeh BK. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:1164-1175.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40136] [Article Influence: 10034.0] [Reference Citation Analysis (2)] |
| 13. | Mohan BP, Asokkumar R, Khan SR, Kotagiri R, Sridharan GK, Chandan S, Ravikumar NP, Ponnada S, Jayaraj M, Adler DG. Outcomes of endoscopic sleeve gastroplasty; how does it compare to laparoscopic sleeve gastrectomy? A systematic review and meta-analysis. Endosc Int Open. 2020;8:E558-E565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Brunaldi VO, Jirapinyo P, de Moura DTH, Okazaki O, Bernardo WM, Galvão Neto M, Campos JM, Santo MA, de Moura EGH. Endoscopic Treatment of Weight Regain Following Roux-en-Y Gastric Bypass: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Djalalinia S, Qorbani M, Peykari N, Kelishadi R. Health impacts of Obesity. Pak J Med Sci. 2015;31:239-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Jain D, Bhandari BS, Arora A, Singhal S. Endoscopic Sleeve Gastroplasty - A New Tool to Manage Obesity. Clin Endosc. 2017;50:552-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Li P, Ma B, Gong S, Zhang X, Li W. Efficacy and safety of endoscopic sleeve gastroplasty for obesity patients: a meta-analysis. Surg Endosc. 2020;34:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. Br J Surg. 2009;96:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 20. | Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5177] [Cited by in RCA: 5929] [Article Influence: 219.6] [Reference Citation Analysis (0)] |
| 21. | Bellavia A, Murphy SA. Heterogeneity of Treatment Effects in Clinical Trials: Overview of Multivariable Approaches and Practical Recommendations. Circulation. 2024;150:978-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Goyal H, Kopel J, Perisetti A, Mann R, Ali A, Tharian B, Saligram S, Inamdar S. Endobariatric procedures for obesity: clinical indications and available options. Ther Adv Gastrointest Endosc. 2021;14:2631774520984627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Lopez-Nava G, Galvao M, Bautista-Castaño I, Fernandez-Corbelle JP, Trell M. Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success. Endosc Int Open. 2016;4:E222-E227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, Gostout CJ. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin Gastroenterol Hepatol. 2017;15:37-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 26. | Dayyeh BKA, Stier C, Alqahtani A, Sharaiha R, Bandhari M, Perretta S, Jirapinyo SP, Prager G, Cohen RV. IFSO Bariatric Endoscopy Committee Evidence-Based Review and Position Statement on Endoscopic Sleeve Gastroplasty for Obesity Management. Obes Surg. 2024;34:4318-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Nduma BN, Mofor KA, Tatang J, Amougou L, Nkeonye S, Chineme P, Ekhator C, Ambe S. Endoscopic Sleeve Gastroplasty (ESG) Versus Laparoscopic Sleeve Gastroplasty (LSG): A Comparative Review. Cureus. 2023;15:e41466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Abuawwad M, Tibude A, Bansi D, Idris I, Madhok B. A commentary review on endoscopic sleeve gastroplasty: Indications, outcomes and future implications. Diabetes Obes Metab. 2024;26:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Nadolsky K, Addison B, Agarwal M, Almandoz JP, Bird MD, DeGeeter Chaplin M, Garvey WT, Kyle TK. American Association of Clinical Endocrinology Consensus Statement: Addressing Stigma and Bias in the Diagnosis and Management of Patients with Obesity/Adiposity-Based Chronic Disease and Assessing Bias and Stigmatization as Determinants of Disease Severity. Endocr Pract. 2023;29:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Nair AS, Borkar N. Sensitivity and subgroup analysis in a meta-analysis - What we should know? Indian J Anaesth. 2024;68:922-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |