Published online May 16, 2025. doi: 10.4253/wjge.v17.i5.101322
Revised: February 26, 2025
Accepted: April 11, 2025
Published online: May 16, 2025
Processing time: 243 Days and 9.3 Hours
Surveillance colonoscopies are predominantly normal, identifying patients for potential polypectomy is advantageous.
To assess colon capsule endoscopy (CCE) and/or faecal immunochemical test (FIT) as filters in surveillance.
Patients aged ≥ 18 due for polyp surveillance were invited for CCE and FIT. Identifying polyps or colorectal cancer resulted in a positive CCE. Significant lesions (≥ 3 polyps or ≥ 6 mm polyps), incomplete studies and positive FITs (≥ 225 ng/mL) were referred for endoscopy. CCE and endoscopy results, FIT accuracy and patient preference were assessed.
From a total of 126 CCEs [mean age 64 (31-80), 67 (53.2%) males), 70.6% (89/126) were excreted, 86.5% (109/126) had adequate image quality. CCE positivity was 70.6% (89/126), 42.9% (54/126) having significant polyps with 63.5% (80/126) referred for endoscopy (19 sigmoidoscopies, 61 colonoscopies). CCE reduced endoscopy need by 36.5% (46/126) and 51.6% (65/126) were spared a colonos
CCE appears effective in low-risk polyp surveillance. FIT does not appear to be of benefit in surveillance.
Core Tip: This is a prospective study uniquely combining colon capsule endoscopy and the faecal immunochemical test as potential filter tests for colonic polyp surveillance patients. Given the burden of surveillance on limited endoscopy resources, we believe surveillance practice can be more balanced, in particular with respect to low risk patients who are less likely to need an invasive test and polypectomy. Our study shows that more than 50% percent of patients can avoid a full colonoscopy and that colon capsule endoscopy in particular can serve as an alternative to colonoscopy in surveillance. In contrast, faecal immunochemical test on its own underperformed in this cohort.
- Citation: Semenov S, Ismail MS, Sihag S, Manoharan T, Reilly P, Boran G, Ryan B, Breslin N, O’Connor A, O’Donnell S, McNamara D. Colon capsule endoscopy is an effective filter test for colonic polyp surveillance. World J Gastrointest Endosc 2025; 17(5): 101322
- URL: https://www.wjgnet.com/1948-5190/full/v17/i5/101322.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i5.101322
Colorectal cancer (CRC) has the third highest worldwide incidence among malignancies, but second for mortality, accounting for 9.4% of all cancer deaths. It is the 2nd and 3rd most common in women and men, respectively, according to the American Cancer Society and World Health Organisation[1]. Published literature suggests an association between the risk of developing CRC and obesity, alcohol excess, personal/family history of CRC, personal history of colorectal polyps, inflammatory bowel disease, smoking, and consuming red meat[2,3]. Around 2%-5% of CRCs arise in well-defined inherited syndromes; hereditary nonpolyposis CRC, familial adenomatous polyposis, MUTYH-associated polyposis, and hamartomatous polyposis conditions[4].
A clear majority of CRCs arise from adenomas, a histologically specific type of colonic polyp. These form from sporadic mutations in the adenomatous polyposis coli pathway or DNA mismatch repair. The adenoma-carcinoma sequence refers to the stepwise progression of an adenoma as it grows and changes from low-grade to high-grade dysplasia, to carcinoma-in-situ, to invasive carcinoma[5]. It is also believed that specific subsets of serrated polyps account for > 30% of CRCs and arise through the serrated neoplasia pathway[6]. Current knowledge of adenomas and their link with CRC forms the basis of CRC surveillance guidelines. CRC screening programmes are associated with a substantial decreased mortality risk[7]. While surveillance colonoscopy and polypectomy reduces CRC incidence and mortality, the per-individual polyp/CRC yield is low with a large proportion of surveillance procedures being normal, particularly in the low-risk groups[8]. It is believed that the greatest benefit of CRC prevention is derived from the initial polypectomy, rather than from subsequent surveillance colonoscopies[9]. Furthermore, given the invasive nature of colonoscopy and its associated risks, and the increasing demand on endoscopy services, a more efficient surveillance model is required.
At present, colonoscopy is the gold standard technique in polyp surveillance. After a patient undergoes an index colonoscopy and polypectomy, a risk stratification process separates low and high-risk groups by assessing size, number of adenomas and grade of dysplasia. British and European guidelines suggest 3-year surveillance for high-risk groups[9,10]. Estimates suggest that surveillance accounts for 17%-30% of capacity in endoscopy departments[3,11]. Colonoscopy quality is influenced by bowel preparation, endoscopist experience, withdrawal time and many others. Colonoscopy has risks, with some studies reporting serious complications of 2.8/1000 procedures in a 2008 systematic review[12]. Naturally, this risk increases with age and co-morbidities. Colonoscopy requires a day ward admission, sedation and a period of observation by nursing staff. Putting risks and service demand aside, colonoscopy can lead to a burden on the patient as they undergo bowel preparation, take time off work and likely experience intra-procedural discomfort[3].
Colonoscopy referrals have increased substantially due to screening and surveillance programmes. This is responsible for longer colonoscopy waiting times with potential impact on symptomatic and high-risk asymptomatic individuals. In contrast to colonoscopy, which is perceived as an invasive and uncomfortable procedure, colon capsule endoscopy (CCE) is viewed by some patients as a minimally invasive and patient friendly method to investigate the bowel[13]. With respect to adenoma detection rates, a recent metanalysis reported pooled sensitivities and specificities for polyps ≥ 6 mm of 87% [95% confidence interval (CI): 83%-90%] and 87% (95%CI: 76%-93%) in 8 studies, respectively. For polyps ≥ 10 mm, pooled estimates for sensitivities and specificities were 87% (95%CI: 83%-90%) and 95% (95%CI: 92%-97%) in 9 studies, respectively[14]. A recent Danish paper, evaluating CCE as a screening tool reported higher polyp detection rates compared to colonoscopy, particularly for right sided polyps[15]. Another paper published by a Danish group suggests that CCE can be valuable in surveillance by reducing colonoscopy referrals by 43%[16].
Stool biomarkers, like faecal immunochemical test (FIT), have also been proposed as a tool in prioritising surveillance colonoscopies. FIT’s ability to detect patients with recurrent polyps is less clear when comparing to its well established role in detecting colorectal cancer. FIT is a quantitative test and cut-offs can be set, effective and safe cut-offs have already been established for screening. The optimal FIT cut-off in a surveillance population, who have already undergone polypectomy, has not been defined. In one study, annual FIT in post polypectomy patients achieved relatively high sensitivity for CRC over three years. If this strategy was to replace three-yearly surveillance, the number of colonoscopies could potentially be reduced by 70%[17]. Our aim in this study was to assess CCE and/or FIT as a potential filter in surveillance.
This was a prospective single-center study taking place in a tertiary capsule endoscopy referral center (Tallaght University Hospital, Dublin, Ireland) over a 16-month period. Ethical approval was received from the St James’s Hospital/Tallaght University Hospital Research Ethics Committee. Patients who were due/overdue surveillance, were selected from a colonoscopy waiting list issued by our local endoscopy department. Patients aged ≥ 18 awaiting surveillance due to a personal history of colonic polyps and/or a family history of CRC were included. Of note, these patients were originally stratified using the older 2010 British Society of Gastroenterology (BSG) polyp surveillance guidelines into low (5 year interval), intermediate (3 year interval) and high (1 year interval) risk[18]. Patients with a personal history of CRC, polyposis syndromes, attending for inflammatory bowel disease surveillance and who were not yet due for surveillance based on updated European Society of Gastrointestinal Endoscopy (ESGE) criteria were not included in the waiting list search. Patients were then invited by post for a CCE and FIT instead of colonoscopy. All patients who expressed interest were contacted by telephone by a research team member, scheduled for a CCE and were sent a FIT test kit by post along with instructions on correct stool sample collection. All patients who did not respond to the invite, remained on the colonoscopy waiting list.
FIT samples of recruited patients were collected and delivered within 24 hours directly to the hospital clinical chemistry laboratory and processed as standard and in accordance with manufacturers’ guidelines. The FIT positivity threshold was modelled after the Irish bowel screening cut-off value, therefore a result of ≥ 225 ng/mL (≥ 45 μg Hb/g) was considered positive. All CCE patients were screened for capsule retention risk factors (bowel surgery, chronic nonsteroidal anti-inflammatory drug use, abdominal radiotherapy, previous capsule retention, history of bowel obstruction, etc.) and if present, underwent a capsule patency test prior to CCE. Any patients who failed the patency test did not proceed with the study and were returned to the surveillance colonoscopy waiting list. All CCEs were performed using PillCam COLON 2 (Medtronic, Minneapolis, United States) with a 2 Litre split-dose bowel preparation MoviPrep (Norgine, Mid Glamorgan, United Kingdom) and booster regimen (MoviPrep based with 500 mL for booster 1 mL and 250 mL for booster 2) as per our capsule centre protocol.
CCE procedures were read by experienced capsule endoscopists and in keeping with our practice, were reviewed and approved at our institution’s weekly capsule review board. A CCE procedure with continuous image capture from the first caecal image to the dentate line was considered complete. Colonic image quality, based on the reader’s overall impression of the bowel preparation was recorded as either “adequate” or “inadequate” in the report. The cleansing level was evaluated based on a previously validated scale and classified as poor (large amount of faecal residue), fair (enough residue to preclude a completely reliable examination), good (small amount of residue, not enough to interfere with examination) and excellent (no more than small amounts of adherent faeces) for each colonic segment. Examinations scored as “poor” or “fair” in any segment were considered “inadequate”, whereas those scored as “good” or “excellent” in all segments were considered “adequate”[19,20].
A CCE was considered positive if polyps or CRC were identified. CCE significant polyps were defined as ≥ 3 in number and/or ≥ 6mm in size, based on current CCE ESGE guidelines[21]. Patients with a significant lesion on CCE and/or an incomplete study, due to either poor image quality or delayed capsule excretion, were referred for endoscopy. In cases of significant lesions in the left colon only and adequate right colon visualisation, patients were referred for a left sided colonoscopy/sigmoidoscopy instead of a colonoscopy. Any patient with a positive FIT irrespective of CCE result was referred for a colonoscopy. Patients with a negative FIT and a CCE not reaching colonoscopy referral criteria were referred for a further surveillance CCE as per ESGE guidelines.
Following CCE, patients were followed up in our hospital’s gastroenterology clinic and appropriate management was instigated where appropriate. In addition, CCE and colonoscopy patient feedback and satisfaction were assessed by interview, a minimum of 3 months after their final procedure. The survey included overall procedural satisfaction using a Likert scale, assessed the impact of each procedure on daily activities (mild, moderate or severe), work activity (number of days missed from work), procedure preference and preference depending on CCE outcome.
Basic patient demographics, indication (family CRC history or personal history of polyps), FIT results, all CCE and subsequent colonoscopy/sigmoidoscopy findings were recorded. FIT diagnostic accuracy was correlated with CCE, by plotting a receiver operating characteristic curve (ROC) with the use of StatsDirect 3.0 software. An intention to treat analysis of CCE performance was completed including completion, image quality, complication, positivity (including significant and non-significant findings) and colonoscopy referral rates. CCE results and subsequent colonoscopy/sigmoidoscopy results were compared, with colonoscopy defined as the gold standard. Further assessment of positive CCE predictive factors was undertaken using a χ2 test. Patient surveys were analyzed: Satisfaction (Likert scale good or excellent) and moderate to severe impact on daily activity were compared for CCE and colonoscopy using a Fisher’s exact test. Days missed from work and preference were also compared between procedures using a student t-test.
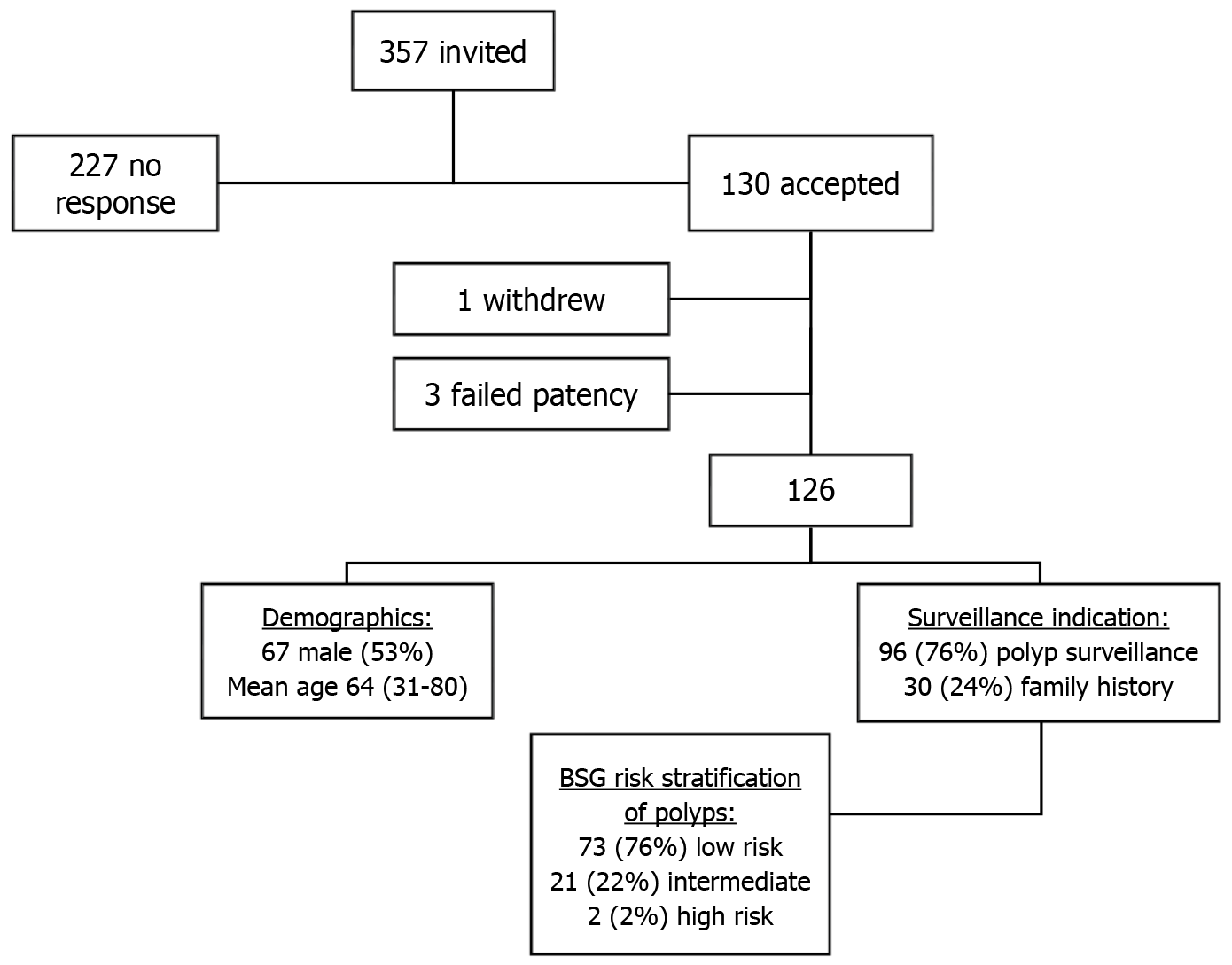
Following a comprehensive review of our centre’s colonoscopy waiting list, 357 patients were invited during the study period, of which, 130 accepted a CCE and FIT instead of a colonoscopy. Overall, 126 patients had a CCE (Figure 1), as 3 failed the capsule patency test and one patient withdrew from the study. Out of 126 participants, 114 (90.5%) returned a FIT test prior to CCE. The mean age was 64 (range: 31-80) and 53.2% were males (67/126). A larger portion of participants had a personal history of polyps as their CCE indication 76% (96/126) vs family history of CRC 24% (30/126). Of the patients with a history of polyps, 2010 BSG polyp surveillance risk stratification guidelines classified 73 (76%) as low, 21 (22%) as intermediate and 2 (2%) as high risk.
CCE excretion rates were 70.6% (89/126) with adequate image quality in 86.5% (109/126). Combining adequate image quality and CCE excretion resulted in an overall completion rate of 65.9% (83/126). There were no complications recorded. The overall CCE positivity rate was 70.6% (89/126) where any significant finding was recorded including polyps, inflammation, or cancer. CCE polyp detection rate was also 70.6% (89/126). Of the positive CCEs, 54 (60.6%) studies yielded significant polyps/high risk (43% of all CCEs), defined in ESGE guidelines as ≥ 3 in number and/or ≥ 6 mm in size, and 35 (39.4%) studies with low-risk polyps. Large polyps (≥ 6 mm) were found in 77.8% (42/54) of the significant CCE studies. In addition, 2 CCEs revealed colitis, these studies also identified colonic polyps, resulting in identical CCE positivity and CCE polyp detection rates. There were no cancers recorded.
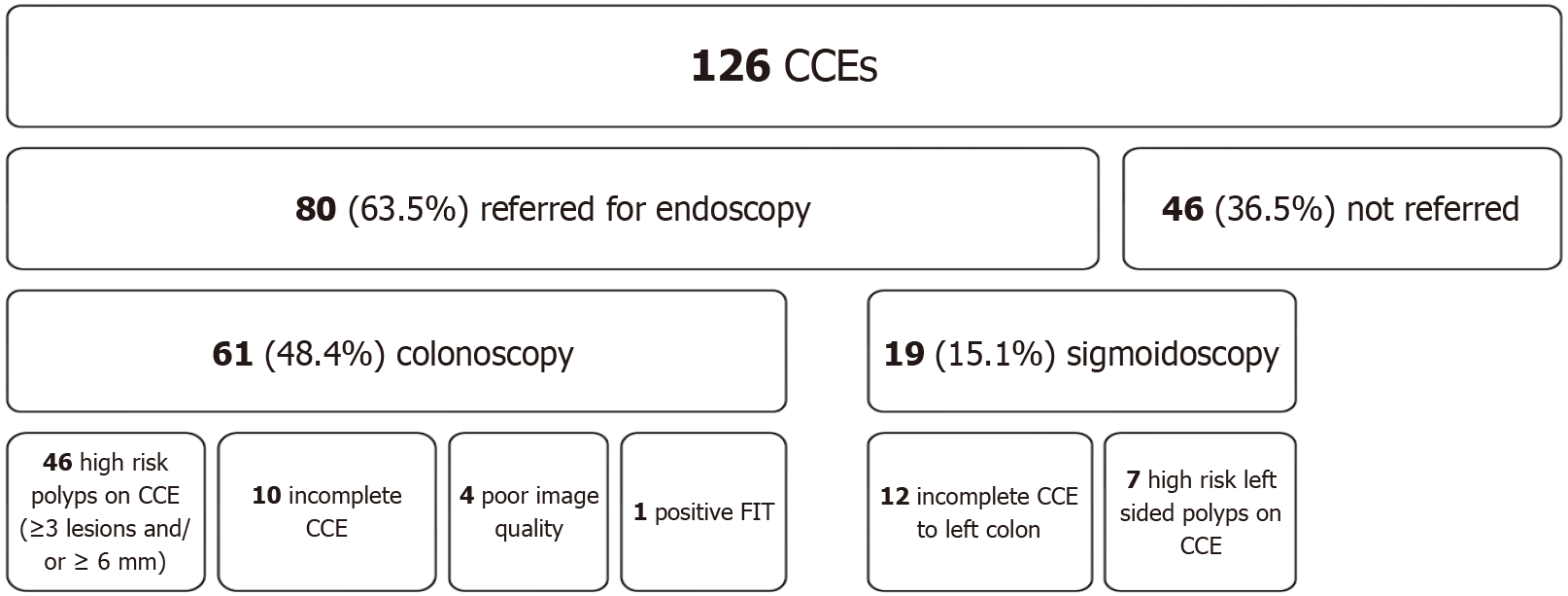
Extracolonic findings (small bowel and gastric) were reported in 23.8% (30/126) studies, of which 3.2% (4/126) were significant, including small bowel polyps and enteritis. Based on CCE findings, 63.5% (80/126) were referred for a follow up endoscopic procedure for polypectomy or as a completion study (Figure 2); 48.4% (61/126) for a full colonoscopy and 15.1% (19/126) for a sigmoidoscopy/left-sided colonoscopy. In all, 36.5% (46/126) of participants were spared an invasive procedure. Most colonoscopy referrals were due to high-risk polyps (46) with the remainder due to incomplete CCE (10 non-excreted and 4 poor image quality) or a positive FIT test (1). Sigmoidoscopy referrals included 12 incomplete CCEs and 7 left sided high-risk polyps.
In total, 93.8% (75/80) of endoscopic procedures were completed within the study period. Polyp detection rate was 81.3% (61/75), of which 27.9% (17/61) were deemed high risk lesions based on the updated 2020 ESGE polyp surveillance guidelines. Histology was available for 59 of the 61 procedures which resulted in polypectomy, 45 of these (76.3%) had at least one adenoma resected. There were no cancers identified on follow up endoscopy. Overall positive predictive value of CCE was 88.2% (45/51).
When comparing CCE and colonoscopy findings by indication (Table 1), higher risk (“intermediate” and “high” as per 2010 BSG guidelines) polyp patients were more likely to have a positive CCE compared to “low risk” (including family history), 91.3% (21/23) vs 66% (68/103), P = 0.0156, odds ratio (OR) = 5.4 (95%CI: 1.1979-24.3824). In terms of significant CCE lesions (Table 2), there remained a statistically significant difference between the two groups where higher risk surveillance patients were more likely to be referred for polypectomy post CCE, OR = 2.5 (95%CI: 0.9701-6.1874), P = 0.0448. There was good correlation between CCE and colonoscopy findings for significant polyps in both groups of patients, 3/16 (19%) vs 14/59 (24%), OR = 0.7418 (95%CI: 0.1845-2.9820), P = 0.3366.
| Variable | Positive CCE | Negative CCE | P value (χ2) | OR | 95%CI | OR P value |
| BSG risk grouping | ||||||
| High/intermediate | 21 | 2 | - | - | - | - |
| Low | 68 | 35 | 0.0156 | 5.4044 | 1.1979-24.3824 | 0.0282 |
| Indication | ||||||
| History of polyps | 72 | 24 | - | - | - | - |
| Family history | 17 | 13 | 0.0450 | 2.2941 | 0.9734-5.4066 | 0.0576 |
| Age (year) | ||||||
| ≤ 65 | 35 | 23 | - | - | - | - |
| > 65 | 54 | 14 | 0.0159 | 2.5347 | 1.1517-5.5787 | 0.0208 |
| Gender | ||||||
| Male | 51 | 16 | - | - | - | - |
| Female | 38 | 21 | 0.1067 | 1.7615 | 0.8121-3.8206 | 0.1518 |
| Variable | Significant CCE | Non-significant CCE | P value (χ2) | OR | 95%CI | OR P value |
| BSG risk grouping | ||||||
| High/intermediate | 14 | 9 | 0.0448 | 2.4500 | 0.9701-6.1874 | 0.0580 |
| Low | 40 | 63 | - | - | - | - |
| Indication | ||||||
| History of polyps | 43 | 53 | 0.2831 | 1.4014 | 0.6022-3.2609 | 0.4335 |
| Family history | 11 | 19 | - | - | - | - |
| Age (year) | ||||||
| ≤ 65 | 21 | 37 | 0.1127 | 1.6612 | 0.8117-3.3999 | 0.1648 |
| > 65 | 33 | 35 | - | - | - | - |
| Gender | ||||||
| Male | 31 | 36 | 0.2597 | 1.3478 | 0.6625-2.7420 | 0.4101 |
| Female | 23 | 36 | - | - | - | - |
Further analysis revealed that older patients (> 65 years) were more likely to have a positive CCE, OR = 2.5 (95%CI: 1.1517-5.5787), P = 0.0159, but this did not influence the likelihood of a significant CCE lesion, OR = 1.7 (95%CI: 0.8117-3.3999), P = 0.1127. Having a personal history of colonic polyps also seemed to increase the chance of a positive CCE, OR = 2.3 (95%CI: 0.9734-5.4066), P = 0.045, but this change was not significant when considering the requirement for polypectomy, OR = 1.4 (95%CI: 0.6022-3.2609), P = 0.2831. Gender did not influence CCE positivity rates (Tables 1 and 2).
Out of 126 patients, 114 (90.5%) returned a FIT at the time of their CCE procedure. Based on a FIT cut-off of 225 ng/mL (45 μg Hb/g), only 5 (4.4%) FIT tests were positive. FIT range was 0 ng/mL - 1394 ng/mL (0 μg Hb/g - 279 μg Hb/g) and mean 54 ng/mL (11 μg Hb/g). All patients with a positive FIT had a polyp on subsequent colonoscopy but only one (20%) was an advanced polyp on colonoscopy based on current ESGE polyp risk stratification. Most patients with a negative FIT sample who underwent endoscopy based on other study referral criteria had a polyp identified, 79.7% (47/59) resulting in a low sensitivity of 9.6% and a negative predictive value of only 20.3%. Of these false negative cases, over a quarter (12/47) had high risk polyps on colonoscopy.
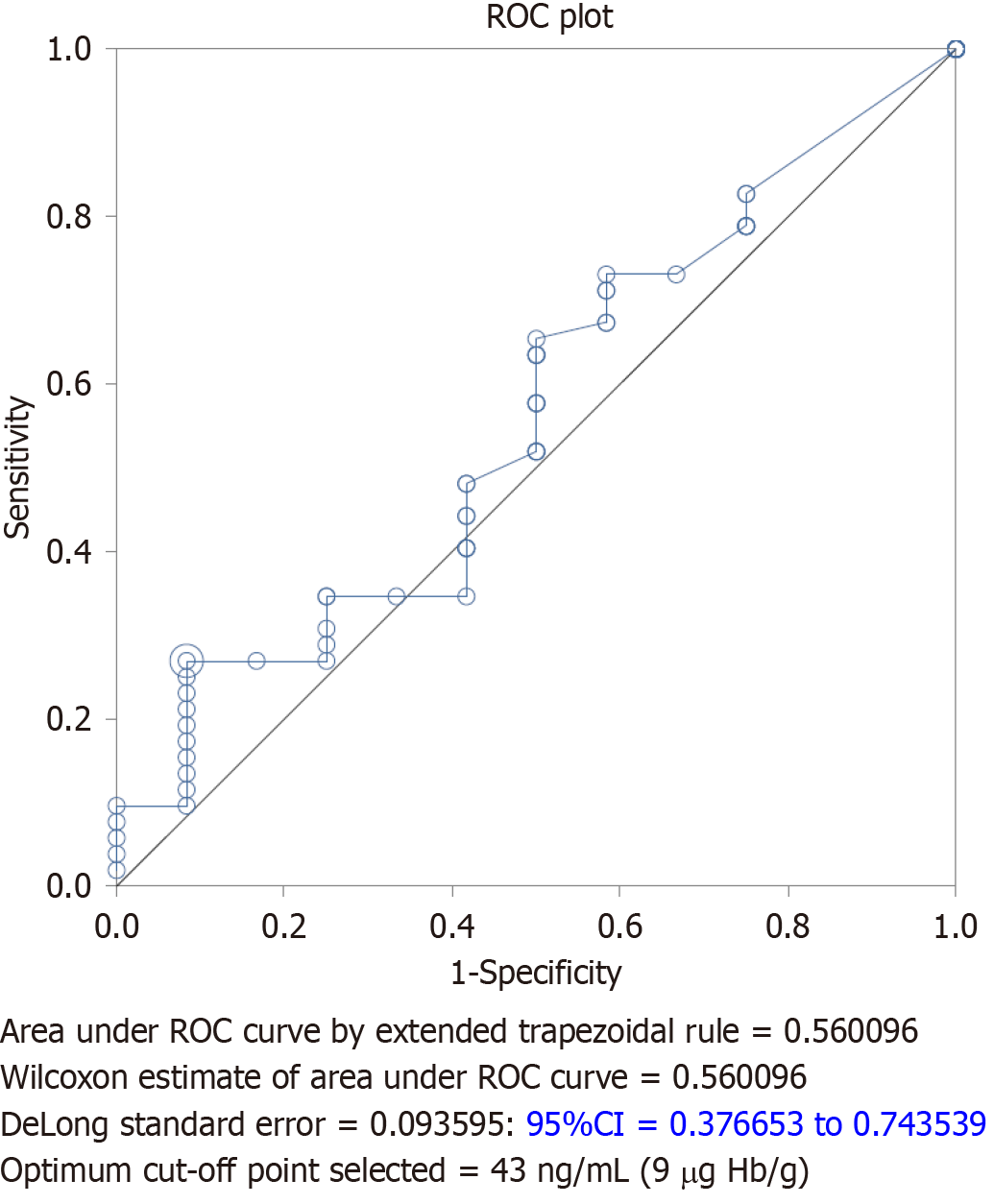
When calculating the ROC for FIT results and follow up colonoscopy polyp yield (Figure 3), the optimal cut-off FIT value of 43 ng/mL (9 μg Hb/g) was determined. If we were to adapt a new cut-off based on the ROC analysis, the sensitivity would increase by 17.3% and colonoscopy referral rates would increase to 19.3% (22/114), more than quadrupling referral rates. Despite a lower cut-off, based on our study data, 16/22 (73%) of colonoscopies with advanced polyps would still have had a negative FIT.
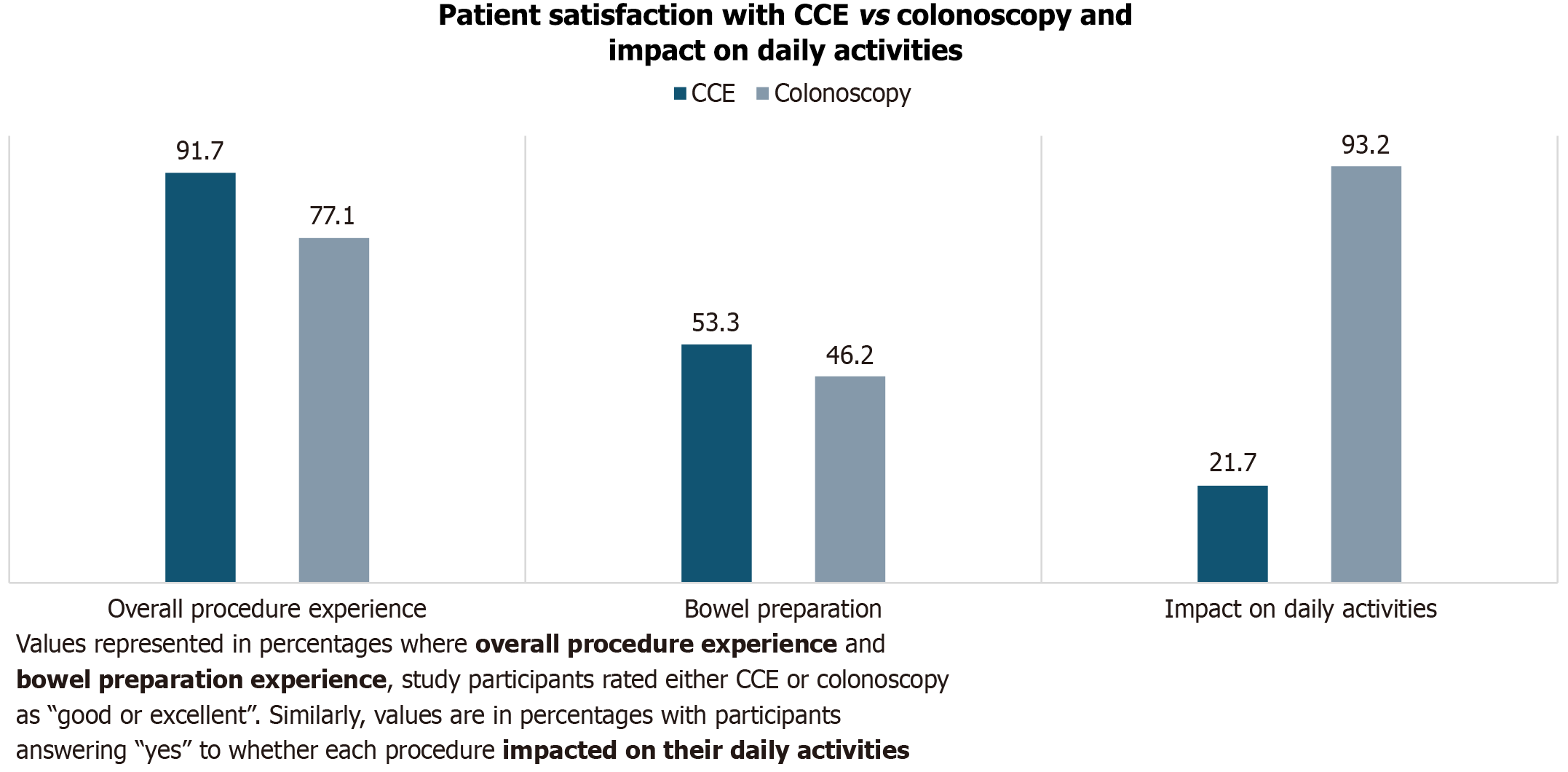
As part of follow up, 95.2% (120/126) patients were available to complete a post-procedure survey with a member of the research team. Having experienced both procedures at one point in their lives, 63.3% (76/120) of the patients expressed an overall preference for CCE vs colonoscopy. Only 4 patients changed their mind when reminded of the possibility of requiring a colonoscopy after CCE. By applying a Likert scale with respect to bowel preparation for CCE vs colonoscopy, the interview yielded 53.3% (64/120) satisfaction (good or excellent experience) vs 46.2% (55/119), respectively, excluding one patient in the colonoscopy group who was not able to provide an answer due to poor recall. The difference in these groups was not statistically significant, P = 0.3017. A similar Likert scale enquiry was made about the overall procedure experience where a larger proportion of patients found the CCE “good or excellent” 91.7% (110/120) vs colonoscopy 77.1% (91/118), P = 0.0022, similarly by excluding 2 patients with poor recall in the colonoscopy group.
When asked about the impact of both procedures on daily activities (cooking, cleaning, etc.), 21.7% (26/120) answered “yes” to CCE having an impact on their daily activities (11 mild, 10 moderate, 5 severe) vs 93.2% (109/117) when asked the same question about the impact of colonoscopy (78 mild, 23 moderate, 8 severe), excluding 3 patients who were not able to accurately recall the events of the day of their colonoscopy. When considering moderate - severe impact on daily activities, CCE appeared more favorable P = 0.0083 (Figure 4). Out of 38 patients employed at the time of their CCE procedure, 30 (79%) required time off; range 0.5 days - 3 days, mode = 1 day, average = 0.9 days. In contrast, 98% (51/52) required time off work at the time of their colonoscopy, ranging from 0.5 days to 3 days, mode = 1 day, average = 1.2 days. The difference in time off work required between both procedures reached statistical significance, P = 0.0201.
Colonoscopy is a finite resource and careful consideration should be given when booking or re-booking this procedure given the burden of surveillance procedures on waiting lists. Some studies suggest the yield of advanced neoplasia at surveillance is low in “low-risk” patients[22] and that CRC incidence is comparable between low/intermediate risk polyp surveillance patients and that of the general population[23]. Given that most polyp surveillance patients fall into this category, this poses the question of whether it’s a good use of resources to carry out full colonoscopies on these patients or whether a less invasive approach is favorable. Similarly in our cohort, low-risk polyp surveillance patients were significantly less likely to require a colonoscopy for polypectomy post CCE (OR = 2.5). In our study, other factors like gender, age and personal or family history of polyps/CRC did not appear to have a significant impact on polyp detection on CCE and the need for polypectomy.
We found that despite a disappointing overall completion rate of 65.9%, more than half of patients (51.6%) were still spared a full colonoscopy. Out of the patients who did require a follow up endoscopy, almost a quarter (19/80) only needed a sigmoidoscopy. These endoscopy referral rates will likely further improve with advances in CCE bowel preparation, including the addition of either using castor oil or prucalopride to the booster regimen, both of which have improved completion rates[24,25]. By using 2012 ESGE CCE guidelines, over a fifth (22.2%) of CCE patients referred for polypectomy were due to having more than two diminutive polyps. Considering that diminutive colorectal polyps < 6 mm are common and almost universally benign[26], there may be room for adjusting the aging ESGE guidelines, in turn, reducing unnecessary/premature polypectomy referrals. Reassuringly, unlike CT colonography[27], additional investigations for significant extra-colonic findings on CCE were low (3.2%).
When choosing a procedure for polyp surveillance, patient satisfaction should also be factored in, especially in an asymptomatic cohort who may choose to avoid a test potentially associated with anxiety, embarrassment, discomfort, or pain. Higher patient preference rates for CCE have been seen in some studies, some as high as 77.5%[28], but pooled preferences from a recent meta-analysis have failed to show a statistical difference[13]. The CCE procedure itself is acceptable to our study participants following a patient preference survey. In comparison to colonoscopy, CCE was preferrable to almost two thirds of patients (63.3%). No significant difference in bowel preparation preference was recorded despite larger volumes being used in CCE procedures. CCE also appears more favorable with respect to missed time from work or even a patient’s ability to carry out their daily tasks on or around the day of the procedure.
In contrast to CCE, FIT underperformed as a polyp surveillance tool, failing to accurately predict absence of colonic polyps with a disappointing negative predictive value of 20.3%. Optimising FIT cut-off ranges following ROC analysis did not significantly improve polyp detection and would result in increased colonoscopy referrals. The application of FIT in surveillance has failed to gain traction in other larger studies, some reporting sensitivities for advanced adenomas as low as 28%[29]. One English group studied the application of annual FITs instead of a 3-yearly colonoscopy for inter
It is worth mentioning that our study has a few limitations. Firstly, this is a single centre study which contributes to a lack of heterogeneity in our patient cohort who are referred primarily from one catchment area. Secondly, the patient preference surveys were not anonymized and were conducted one-on-one with a member of the research team, leading to potential unconscious interviewer bias. Recall bias was also a factor as some patients interviewed had their colonoscopy over 5 years ago. Thirdly, overall CCE uptake was low at 36.4% (130/357) following a single written invitation, however, this method was undertaken to avoid an even larger bias through contacting and persuading patients directly. Despite the resulting small sample size our prospective study compares favorably with similar published data[16]. Finally, we accept that using older and different polyp surveillance criteria than those currently recommended could overestimate the benefit of CCE as a filter test, however, these patients were selected from a historical waiting list. Despite changes in polyp and patient risk stratification, it is likely that CCE, based on its performance in this study, will remain a useful tool in polyp surveillance in patients at lower risk of having an advanced lesion. Therefore, when considering the use of CCE as a filter test vs colonoscopy alone, our study highlights that the former could improve endoscopy burden, safety, patient experience and preference as well as diagnostic performance in a low-risk group while being able to select out a higher risk group for subsequent colonoscopy and polypectomy.
Our prospective evaluation of CCE in a polyp surveillance cohort adds further evidence that it is an appropriate, effective and acceptable test particularly in a cohort at low risk of significant lesions. Disappointingly, either alone or in combination with CCE, FIT does not appear to be of benefit in surveillance.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 2. | Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 524] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 3. | Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol. 2016;22:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 4. | Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 5. | Calderwood AH, Lasser KE, Roy HK. Colon adenoma features and their impact on risk of future advanced adenomas and colorectal cancer. World J Gastrointest Oncol. 2016;8:826-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 6. | Obuch JC, Pigott CM, Ahnen DJ. Sessile serrated polyps: detection, eradication, and prevention of the evil twin. Curr Treat Options Gastroenterol. 2015;13:156-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, Schottinger J, Doria-Rose VP, Levin TR, Weiss NS, Fletcher RH. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 8. | Morelli MS, Glowinski EA, Juluri R, Johnson CS, Imperiale TF. Yield of the second surveillance colonoscopy based on the results of the index and first surveillance colonoscopies. Endoscopy. 2013;45:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, Kaye PV, Monahan KJ, Novelli MR, Plumb A, Saunders BP, Thomas-Gibson S, Tolan DJM, Whyte S, Bonnington S, Scope A, Wong R, Hibbert B, Marsh J, Moores B, Cross A, Sharp L. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 10. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 11. | Gimeno-García AZ, Quintero E. Colonoscopy appropriateness: Really needed or a waste of time? World J Gastrointest Endosc. 2015;7:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 515] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 13. | Deding U, Cortegoso Valdivia P, Koulaouzidis A, Baatrup G, Toth E, Spada C, Fernández-Urién I, Pennazio M, Bjørsum-Meyer T. Patient-Reported Outcomes and Preferences for Colon Capsule Endoscopy and Colonoscopy: A Systematic Review with Meta-Analysis. Diagnostics (Basel). 2021;11:1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Möllers T, Schwab M, Gildein L, Hoffmeister M, Albert J, Brenner H, Jäger S. Second-generation colon capsule endoscopy for detection of colorectal polyps: Systematic review and meta-analysis of clinical trials. Endosc Int Open. 2021;9:E562-E571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kobaek-Larsen M, Kroijer R, Dyrvig AK, Buijs MM, Steele RJC, Qvist N, Baatrup G. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Colorectal Dis. 2018;20:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Kroijer R, Kobaek-Larsen M, Qvist N, Knudsen T, Baatrup G. Colon capsule endoscopy for colonic surveillance. Colorectal Dis. 2019;21:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Cross AJ, Wooldrage K, Robbins EC, Kralj-Hans I, MacRae E, Piggott C, Stenson I, Prendergast A, Patel B, Pack K, Howe R, Swart N, Snowball J, Duffy SW, Morris S, von Wagner C, Halloran SP, Atkin WS. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: a diagnostic accuracy and cost-effectiveness study. Gut. 2019;68:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 19. | Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | González-Suárez B, Pagés M, Araujo IK, Romero C, Rodríguez de Miguel C, Ayuso JR, Pozo À, Vila-Casadesús M, Serradesanferm A, Ginès À, Fernández-Esparrach G, Pellisé M, López-Cerón M, Flores D, Córdova H, Sendino O, Grau J, Llach J, Serra-Burriel M, Cárdenas A, Balaguer F, Castells A. Colon capsule endoscopy versus CT colonography in FIT-positive colorectal cancer screening subjects: a prospective randomised trial-the VICOCA study. BMC Med. 2020;18:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, Benamouzig R, de Franchis R, Delvaux M, Devière J, Eliakim R, Fraser C, Hagenmuller F, Herrerias JM, Keuchel M, Macrae F, Munoz-Navas M, Ponchon T, Quintero E, Riccioni ME, Rondonotti E, Marmo R, Sung JJ, Tajiri H, Toth E, Triantafyllou K, Van Gossum A, Costamagna G; European Society of Gastrointestinal Endoscopy. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Hornung TA, Bevan R, Mumtaz S, Hornung BR, Rutter MD. Surveillance colonoscopy in low-risk postpolypectomy patients: Is it necessary? Frontline Gastroenterol. 2015;6:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Cross AJ, Robbins EC, Pack K, Stenson I, Patel B, Rutter MD, Veitch AM, Saunders BP, Duffy SW, Wooldrage K. Colorectal cancer risk following polypectomy in a multicentre, retrospective, cohort study: an evaluation of the 2020 UK post-polypectomy surveillance guidelines. Gut. 2021;70:2307-2320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Semenov S, Ismail MS, O'Hara F, Sihag S, Ryan B, O'Connor A, O'Donnell S, McNamara D. Addition of castor oil as a booster in colon capsule regimens significantly improves completion rates and polyp detection. World J Gastrointest Pharmacol Ther. 2021;12:103-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Deding U, Kaalby L, Baatrup G, Kobaek-Larsen M, Thygesen MK, Epstein O, Bjørsum-Meyer T. The Effect of Prucalopride on the Completion Rate and Polyp Detection Rate of Colon Capsule Endoscopies. Clin Epidemiol. 2022;14:437-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Kandel P, Wallace MB. Should We Resect and Discard Low Risk Diminutive Colon Polyps. Clin Endosc. 2019;52:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Deding U, Kaalby L, Bøggild H, Plantener E, Wollesen MK, Kobaek-Larsen M, Hansen SJ, Baatrup G. Colon Capsule Endoscopy vs. CT Colonography Following Incomplete Colonoscopy: A Systematic Review with Meta-Analysis. Cancers (Basel). 2020;12:3367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ismail MS, Murphy G, Semenov S, McNamara D. Comparing Colon Capsule Endoscopy to colonoscopy; a symptomatic patient's perspective. BMC Gastroenterol. 2022;22:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Terhaar sive Droste JS, van Turenhout ST, Oort FA, van der Hulst RW, Steeman VA, Coblijn U, van der Eem L, Duijkers R, Bouman AA, Meijer GA, Depla AC, Scholten P, Loffeld RJ, Coupé VM, Mulder CJ. Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multi-centre cohort study. BMC Gastroenterol. 2012;12:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |