Published online Feb 16, 2023. doi: 10.4253/wjge.v15.i2.64
Peer-review started: November 29, 2022
First decision: December 19, 2022
Revised: December 28, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 16, 2023
Processing time: 75 Days and 22.1 Hours
Stenting as a bridge to curative surgery (SBTS) for obstructing colon cancer (OCC) has been associated with possibly worse oncological outcomes.
To evaluate the recurrence patterns, survival outcomes, and colorectal cancer (CRC)-specific death in patients undergoing SBTS for OCC.
Data from 62 patients undergoing SBTS at a single tertiary centre over ten years between 2007 and 2016 were retrospectively examined. Primary outcomes were recurrence patterns, overall survival (OS), cancer-specific survival (CSS), and CRC-specific death. OS and CSS were estimated using the Kaplan-Meier curves. Competing risk analysis with cumulative incidence function (CIF) was used to estimate CRC-specific mortality with other cause-specific death as a competing event. Fine-Gray regressions were performed to determine prognostic factors of CRC-specific death. Univariate and multivariate subdistribution hazard ratios and their corresponding Wald test P values were calculated.
28 patients (45.2%) developed metastases after a median period of 16 mo. Among the 18 patients with single-site metastases: Four had lung-only metastases (14.3%), four had liver-only metastases (14.3%), and 10 had peritoneum-only metastases (35.7%), while 10 patients had two or more sites of metastatic disease (35.7%). The peritoneum was the most prevalent (60.7%) site of metastatic involvement (17/28). The median follow-up duration was 46 mo. 26 (41.9%) of the 62 patients died, of which 16 (61.5%) were CRC-specific deaths and 10 (38.5%) were deaths owing to other causes. The 1-, 3-, and 5-year OS probabilities were 88%, 74%, and 59%; 1-, 3-, and 5-year CSS probabilities were 97%, 83%, and 67%. The highest CIF for CRC-specific death at 60 mo was liver-only recurrence (0.69). Liver-only recurrence, peritoneum-only recurrence, and two or more recurrence sites were predictive of CRC-specific death.
The peritoneum was the most common metastatic site among patients undergoing SBTS. Liver-only recurrence, peritoneum-only recurrence, and two or more recurrence sites were predictors of CRC-specific death.
Core Tip: This is the first retrospective study with a 10-year period using the competing risk analysis of cumulative incidence function to evaluate survival and estimate colorectal cancer (CRC)-specific death based on the Fine-Gray model in patients undergoing stenting as a bridge to curative surgery (SBTS) for obstructing colon cancer (OCC). The duration of this study allows a thorough examination of the long-term oncological outcomes of SBTS, survival rates, recurrence patterns, and prognostic factors contributing to CRC-specific death. Our results showed that liver-only recurrence, peritoneum-only recurrence, and more than two recurrence sites are significantly associated with poor survival and prognostic factors for CRC-specific death in patients undergoing SBTS for OCC.
- Citation: Chok AY, Zhao Y, Lim HJ, Ng YYR, Tan EJKW. Stenting as a bridge to surgery in obstructing colon cancer: Long-term recurrence pattern and competing risk of mortality. World J Gastrointest Endosc 2023; 15(2): 64-76
- URL: https://www.wjgnet.com/1948-5190/full/v15/i2/64.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i2.64
Colorectal cancer (CRC) ranks as the second most prevalent malignant neoplasm and the third leading cause of cancer-related death worldwide[1]. Malignant bowel obstruction at presentation can occur in approximately 8% to 25% of CRC patients[2-4]. Emergency surgery is the conventional treatment for acute malignant colonic obstruction but is often associated with substantial morbidity (40%-60%), mortality (15%-34%) rates, worse oncological outcomes, and higher rates of stoma formation[5-7]. Since the 1990s, self-expanding metal stents (SEMS) have been accepted and increasingly utilized for palliation of malignant colorectal obstruction, as well as stenting as a bridge to curative surgery (SBTS), as a feasible alternative to emergency surgery[8-14].
Despite the fact that SEMS had been reported to have relatively high technical success rates between 70.1% and 91.9%, and clinical success rates of 69.0% to 71.7%, SBTS with curative intent remains debatable primarily due to possibly worse oncological outcomes[15,16]. Stent-related tumour perforations and subclinical micro-perforations may result in tumour dissemination and seeding, hence likely increasing the risk of recurrence. The effects of tumour perforation, silent stent-related micro-perforation, and the potential risks of tumour seeding on recurrence and survival have been reported[17]. Moreover, among patients with CRC recurrence, comorbidities such as cardiovascular and pulmonary diseases typically compete with CRC as the cause of death. To date, however, no studies have investigated the long-term oncological effects of SBTS on CRC-specific death under the competing risk of other cause-specific death.
This study aimed to evaluate the recurrence patterns, survival outcomes, and CRC-specific death in patients undergoing SBTS for obstructing colon cancer (OCC). The traditional Kaplan-Meier survival function would filter non-CRC related mortality rather than recognizing that patients dying from other causes are no longer at risk of CRC-specific death and consequently skew the results without considering competing risks[18]. Similarly, covariate effects in the cause-specific Cox regression model refer exclusively to CRC-specific death without considering how covariates could influence competing risk events[19]. Therefore, competing risk analysis with cumulative incidence function (CIF) was used in this study to estimate the probability of CRC-specific death over time, treating other cause-specific death as a competing risk. The covariate effects of clinical characteristics and recurrence patterns on the CIF for CRC-specific death were analysed with the Fine-Gray model[20].
Our institutional review board approved this study (IRB No. 2017/2481). 114 consecutive patients underwent SBTS for OCC over ten years from 2007 to 2016 at Singapore General Hospital. All patients underwent computed tomography (CT) scans of the abdomen and pelvis at presentation, and OCC was confirmed clinically and radiologically. Full-staging CT scans were performed at the time of diagnosis or within 30 days of presentation. Data from 62 patients with non-metastatic OCC who underwent SBTS were analysed after excluding patients with stage IV disease at diagnosis and those with endoscopic stenting deployment for anastomotic recurrence.
Clinical, histopathological, biochemical, and oncological data were collected from our electronic health record system (Sunrise Clinical Manager version 5.8, Eclipsys Corp., Atlanta, GA, United States). Patient demographics, clinical and surgical characteristics, and recurrence patterns were analysed. Follow-up data included time to recurrence and date and cause of death. After CRC resection with curative intent, all patients were considered for adjuvant chemotherapy consisting of capecitabine and oxaliplatin. The protocol for clinical management and postoperative surveillance has been established in an earlier study[13].
Overall survival (OS) and cancer-specific survival (CSS) were estimated using the Kaplan-Meier curves. OS is defined as the elapsed time from the date of diagnosis to the date of death or last follow-up, while CSS is defined as the elapsed time from the date of diagnosis to the date of death from CRC. Clinical variables correlated with CRC-specific death were categorized and included in the competing risk analysis. Cumulative incidence function (CIF) was applied to account for the competing event, with other cause-specific mortality treated as a competing risk for CRC-specific mortality. CIF of death by each level of prognostic covariates was estimated and tabulated. CIF curves of CRC-specific death and other cause-specific death were estimated and visualized. The Fine-Gray competing risk model, which is based on the subdistribution hazard ratio (SHR), was used to examine the probabilities of CRC-specific death and other cause-specific[20]. Univariate and multivariate SHR and their corresponding Wald test P values were calculated. The Fine-Gray regression is a multivariate time-to-event model considering that a person can only experience one of the two competing events. This model also considers censoring among patients who experienced no events throughout the follow-up duration.
All statistical analyses were performed in R statistical software (version 4.2.1). Results were presented as median (range) for continuous variables and count (percentage) for categorical variables. Statistical significance was set at P value < 0.05.
There were 62 patients with OCC undergoing SBTS with curative intent. None of them had distant metastases at presentation. 57 patients had successful stenting procedures. On the same day, one stent technical failure and one stent perforation required emergency surgery. Three patients had post-stenting minimal bowel decompression and were operated on within 48 h.
Patient demographics and clinicopathological information are summarized in Table 1. The median age was 70 (range: 37-90) years. 87.1% of the patients were ASA classification I-II. 75.8% of tumours were T3 staging, whereas 22.6% were T4 staging. 95.2% of tumours were moderately differentiated adenocarcinoma. Only three tumours (4.8%) had a mucinous component. 19.4% of patients had at least one extra-nodal tumour deposit. The median time to elective CRC resection was 10 (range: 5-23) d. Laparoscopic approach was performed in 46.8% of the cases, while three cases were converted to open surgery. During the elective surgery, one patient was discovered to have a sealed perforation at the stented tumour site. The postoperative complication rate was 21%, and 30-day and 90-day mortality rates were 1.6% and 3.2%, respectively. One patient sustained an anastomotic leak and died 12 d after surgery, while the second succumbed to pneumonia 46 days after surgery. Postoperative adjuvant chemotherapy was given to 50% of patients.
| Variables | n = 62 |
| Age (yr, median [range]) | 70.0 [37.0, 90.0] |
| Sex | |
| Female | 25 (40.3) |
| Male | 37 (59.7) |
| ASA classification | |
| I | 11 (17.7) |
| II | 43 (69.4) |
| III | 8 (12.9) |
| IV | 0 (0.0) |
| Diabetes mellitus | |
| No | 50 (80.6) |
| Yes | 12 (19.4) |
| Albumin (g/dL) | |
| Median [range] | 3.65 [1.90, 4.60] |
| ≥ 3.0 | 52 (83.9) |
| < 3.0 | 10 (16.1) |
| CEA (µg/L) | |
| Median [range] | 5.75 [0.95, 84.4] |
| < 5.3 | 28 (45.2) |
| ≥ 5.3 | 34 (54.8) |
| Tumour location | |
| Rectosigmoid | 8 (12.9) |
| Sigmoid | 26 (41.9) |
| Descending | 17 (27.4) |
| Splenic flexure | 11 (17.7) |
| Tumour staging | |
| T2 | 1 (1.6) |
| T3 | 47 (75.8) |
| T4 | 14 (22.6) |
| Nodal involvement | |
| N0 | 27 (43.5) |
| N1 | 23 (37.1) |
| N2 | 12 (19.4) |
| Tumour differentiation | |
| Well differentiated | 2 (3.2) |
| Moderately differentiated | 59 (95.2) |
| Poorly differentiated | 1 (1.6) |
| Histology | |
| Adenocarcinoma | 59 (95.2) |
| Mucinous adenocarcinoma | 3 (4.8) |
| Tumour deposit(s) | |
| No | 50 (80.6) |
| Yes | 12 (19.4) |
| Microscopic margin involvement (R1 resection) | |
| No | 58 (93.5) |
| Yes | 4 (6.5) |
| Perineural infiltration | |
| No | 40 (64.5) |
| Yes | 22 (35.5) |
| Lymphovascular invasion | |
| No | 43 (69.4) |
| Yes | 19 (30.6) |
| Pericolic microabscess | |
| No | 54 (87.1) |
| Yes | 8 (12.9) |
| Stent failure | |
| No | 57 (91.9) |
| Yes | 5 (8.1) |
| Surgical approach | |
| Open | 33 (53.2) |
| Laparoscopic | 29 (46.8) |
| Stoma formation | |
| No | 58 (93.5) |
| Yes | 4 (6.5) |
| Adjuvant chemotherapy | |
| No | 31 (50.0) |
| Yes | 31 (50.0) |
| Perioperative major complication(s) | |
| No | 58 (93.5) |
| Yes | 4 (6.5) |
| Postoperative 30 d mortality | |
| No | 61 (98.4) |
| Yes | 1 (1.6) |
| Postoperative 90 d mortality | |
| No | 60 (96.8) |
| Yes | 2 (3.2) |
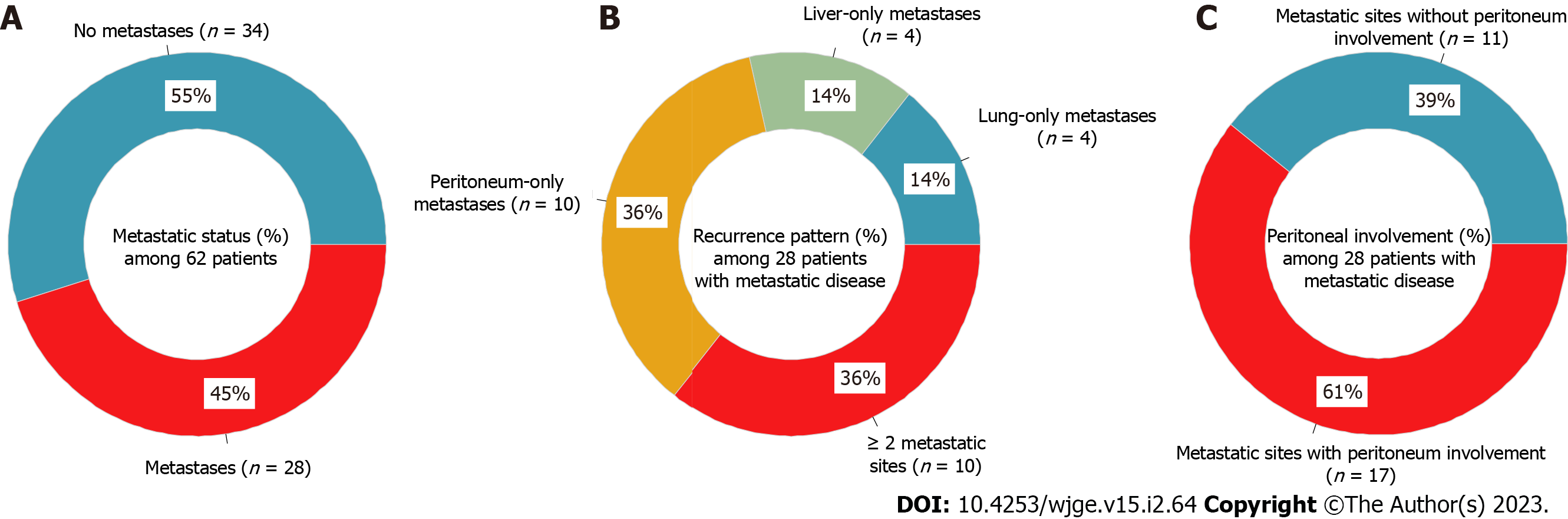
Percentages of metastases status, recurrence patterns, and peritoneal involvement are shown in Figure 1. During the study period, 28 patients (45.2%) developed metastases (Figure 1A). The median time to detection of metastases was 16 (range: 3-69) mo. Among the 18 patients with single-site metastases: Four had lung-only metastases (14.3%), four had liver-only metastases (14.3%), and 10 had peritoneum-only metastases (35.7%); while another 10 patients had two or more sites of metastatic disease (35.7%; Figure 1B). The peritoneum was the most prevalent site of metastatic involvement, with 17 out of 28 patients (60.7%) having peritoneal involvement (Figure 1C).
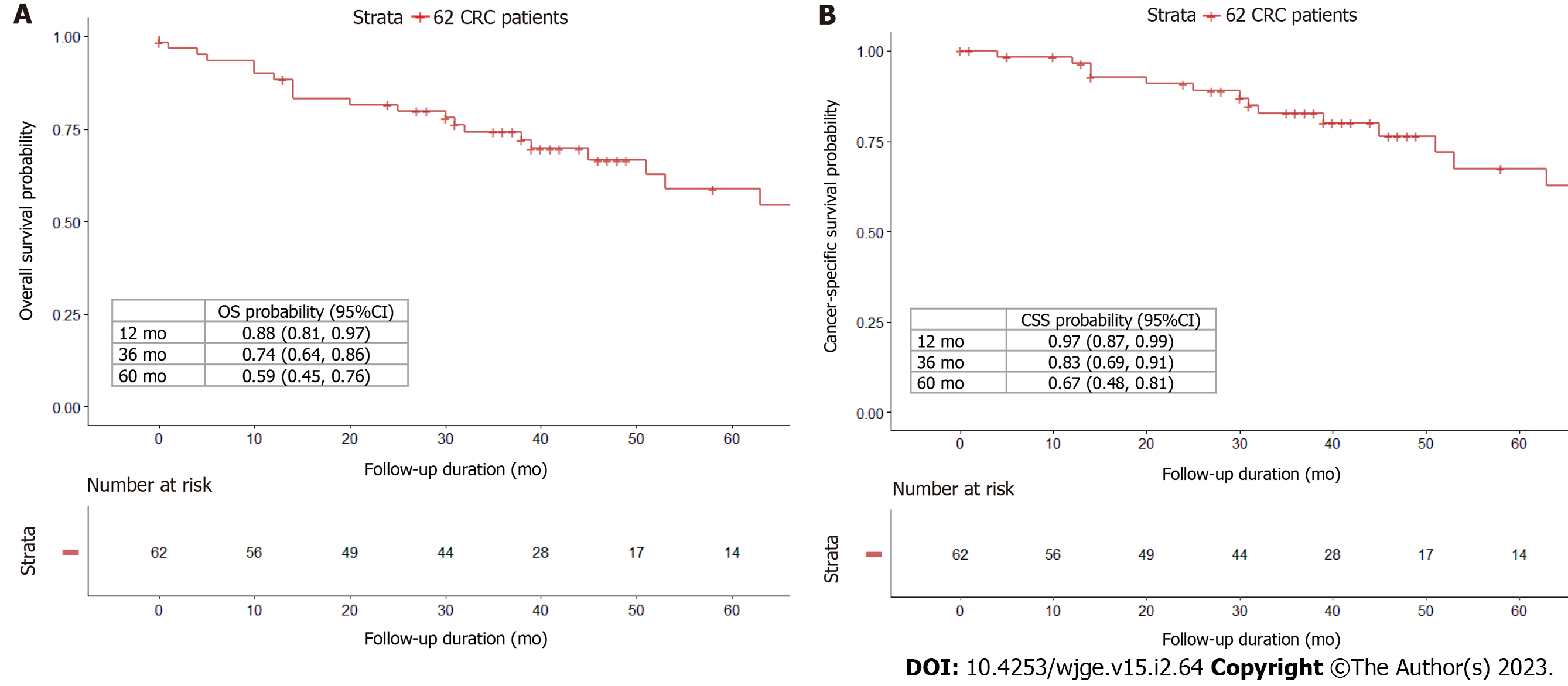
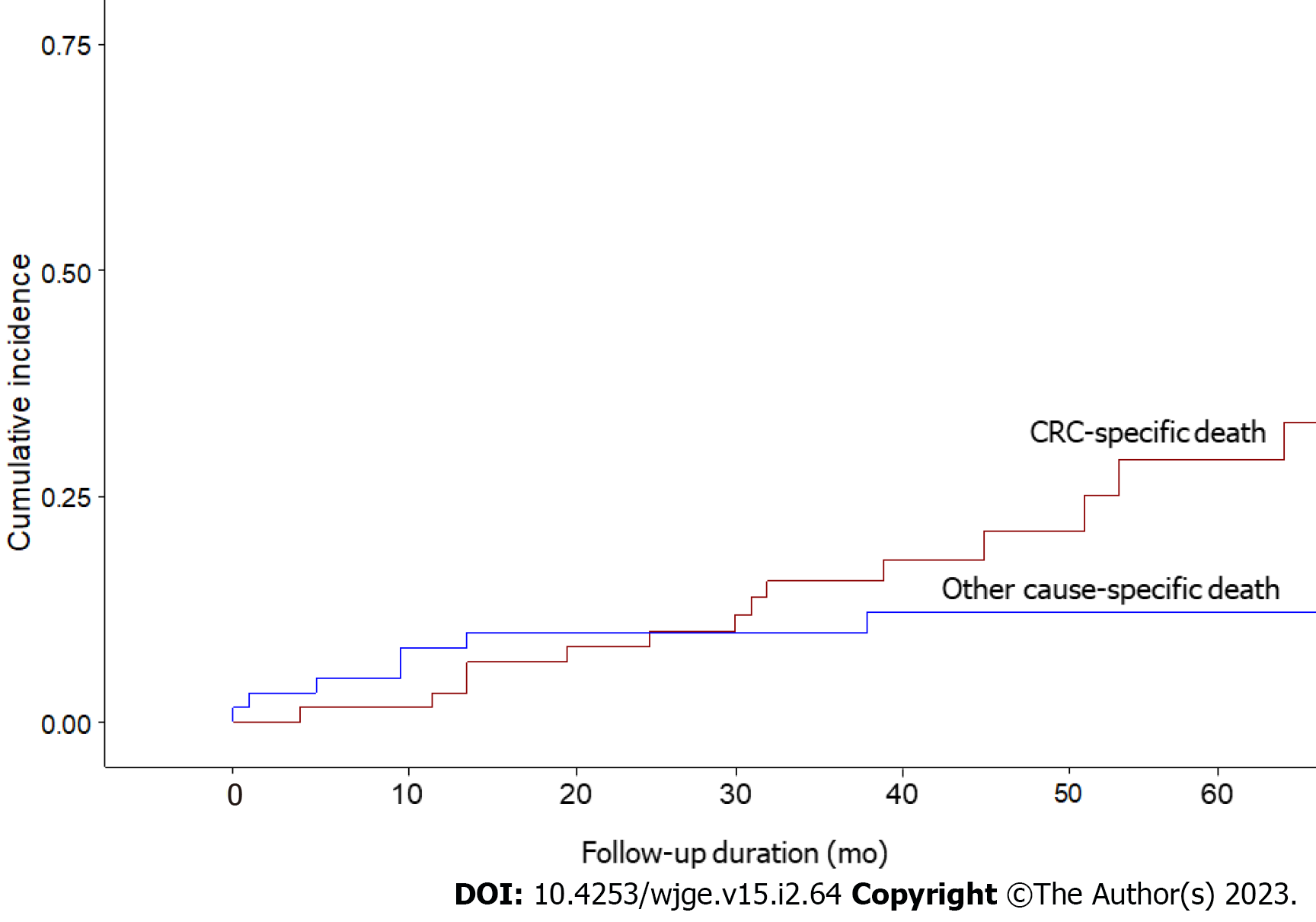
The median follow-up duration was 46 (range: 0-154) mo. 26 (41.9%) of the 62 patients died, with 16 (61.5%) deaths attributable to CRC and 10 (38.5%) deaths owing to other causes. The 1-, 3-, and 5-year OS probabilities were 88%, 74%, and 59% (Figure 2A), while the 1-, 3-, and 5-year CSS probabilities were 97%, 83%, and 67% (Figure 2B). CIF curves for CRC-specific death under the competing risk of other cause-specific death are shown in Figure 3. The CIF curve for CRC-specific death climbed steadily and continuously, whereas the CIF curve for other-cause specific death climbed rapidly from 0 to 13 mo and subsequently steadied. This result suggests that most deaths unrelated to CRC occurred earlier after SBTS, between 0 and 13 mo. At 12-, 36-, and 60-month after endoscopic stenting followed by curative surgery, the CIF for CRC-specific death was 0.03, 0.16, and 0.29, whereas the CIF for other cause-specific death was 0.08, 0.10, and 0.12. CIF estimates for CRC-specific death by potential risk factors at 12, 36, and 60 mo are shown in Table 2. The highest CIF value at 60 mo was seen at liver-only recurrence (0.69), followed by peritoneum-only recurrence (0.65), lymphovascular invasion (0.64), ≥ 2 sites of recurrences (0.63), and T4 staging (0.62). The Fine-Gray regression of modelling SHR that corresponded to the CIF for CRC-specific death is displayed in Table 3. Poor differentiation and lymphovascular invasion (LVI) were strongly associated with CRC-specific death on univariate analysis, with SHR of 2.67 (95%CI: 1.50-4.76, P < 0.001) and 3.99 (95%CI: 1.55-10.3, P = 0.004) respectively. Liver-only recurrence, peritoneum-only recurrence, and ≥ 2 sites of recurrences were adverse prognostic factors on both univariate and multivariate analyses. Lung-only recurrence was not statistically significantly associated with CRC-specific death in our study (P = 0.570).
| Variable | CRC-specific death (mo) | ||
| 12 | 36 | 60 | |
| Age | |||
| < 70 yr | 0.00 | 0.22 | 0.37 |
| ≥ 70 yr | 0.06 | 0.10 | 0.21 |
| Sex | |||
| Female | 0.04 | 0.27 | 0.45 |
| Male | 0.03 | 0.09 | 0.20 |
| Stent failure | |||
| No | 0.04 | 0.15 | 0.31 |
| Yes | 0.00 | 0.25 | 0.25 |
| Surgical approach | |||
| Open | 0.06 | 0.16 | 0.22 |
| Laparoscopic | 0.00 | 0.15 | 0.40 |
| T4 staging | |||
| No | 0.04 | 0.14 | 0.18 |
| Yes | 0.00 | 0.23 | 0.62 |
| N2 | |||
| No | 0.02 | 0.13 | 0.26 |
| Yes | 0.09 | 0.27 | 0.36 |
| Tumour deposit(s) | |||
| No | 0.04 | 0.13 | 0.26 |
| Yes | 0.00 | 0.28 | 0.40 |
| Microscopic margin involvement (R1 resection) | |||
| No | 0.04 | 0.15 | 0.29 |
| Yes | 0.00 | 0.25 | 0.25 |
| Histology | |||
| Adenocarcinoma | 0.02 | 0.15 | 0.29 |
| Mucinous adenocarcinoma | 0.33 | 0.33 | 0.33 |
| Poorly differentiated | |||
| No | 0.03 | 0.16 | 0.30 |
| Yes | 0.00 | 0.00 | 0.00 |
| Perineural infiltration | |||
| No | 0.05 | 0.08 | 0.17 |
| Yes | 0.00 | 0.30 | 0.52 |
| Lymphovascular invasion | |||
| No | 0.02 | 0.10 | 0.10 |
| Yes | 0.06 | 0.30 | 0.64 |
| Pericolic microabscess | |||
| No | 0.04 | 0.14 | 0.29 |
| Yes | 0.00 | 0.29 | 0.29 |
| Albumin (g/dL) | |||
| ≥ 3.0 | 0.02 | 0.15 | 0.26 |
| < 3.0 | 0.10 | 0.21 | 0.56 |
| CEA (µg/L) | |||
| < 5.3 | 0.00 | 0.13 | 0.22 |
| ≥ 5.3 | 0.06 | 0.18 | 0.35 |
| ASA classification | |||
| I/II | 0.02 | 0.12 | 0.28 |
| III | 0.13 | 0.38 | 0.38 |
| Diabetes mellitus | |||
| No | 0.04 | 0.13 | 0.28 |
| Yes | 0.00 | 0.25 | 0.35 |
| Perioperative major complication(s) | |||
| No | 0.04 | 0.15 | 0.29 |
| Yes | 0.00 | - | - |
| Adjuvant chemotherapy | |||
| No | 0.03 | 0.07 | 0.21 |
| Yes | 0.03 | 0.24 | 0.37 |
| Lung-only recurrence | |||
| No | 0.04 | 0.16 | 0.28 |
| Yes | 0.00 | 0.13 | 0.34 |
| Liver-only recurrence | |||
| No | 0.04 | 0.12 | 0.24 |
| Yes | 0.00 | 0.38 | 0.69 |
| Peritoneum-only recurrence | |||
| No | 0.00 | 0.07 | 0.12 |
| Yes | 0.12 | 0.38 | 0.65 |
| ≥ 2 sites of recurrences | |||
| No | 0.04 | 0.10 | 0.23 |
| Yes | 0.00 | 0.44 | 0.63 |
| Variable | CRC-specific death | |||
| Univariate | Multivariate | |||
| SHR (95%CI) | P value | SHR (95%CI) | P value | |
| Age ≥ 70 yr | 0.84 (0.33, 2.15) | 0.710 | ||
| Sex (Male) | 0.49 (0.19, 1.28) | 0.150 | ||
| Laparoscopic surgery | 1.28 (0.49, 3.33) | 0.610 | ||
| Stent failure | 0.58 (0.06, 5.51) | 0.630 | ||
| T4 staging | 1.23 (0.97, 1.57) | 0.088 | ||
| N2 | 2.44 (0.88, 6.75) | 0.086 | ||
| Tumour deposit(s) | 2.02 (0.74, 5.56) | 0.170 | ||
| Microscopic margin involvement (R1 resection) | 1.68 (0.58, 4.84) | 0.340 | ||
| Mucinous components | 3.35 (0.72, 15.5) | 0.120 | ||
| Poorly differentiated | 2.67 (1.50, 4.76) | < 0.001 | 1.11 (0.32, 3.83) | 0.870 |
| Perineural infiltration | 2.34 (0.89, 6.17) | 0.086 | ||
| Lymphovascular invasion | 3.99 (1.55, 10.3) | 0.004 | 1.98 (0.61, 6.49) | 0.260 |
| Pericolic microabscess | 1.12 (0.25, 5.04) | 0.880 | ||
| Albumin < 3.0 g/dL | 1.36 (0.38, 4.90) | 0.640 | ||
| CEA ≥ 5.3 µg/L | 2.45 (0.80, 7.53) | 0.120 | ||
| ASA classification III | 1.10 (0.68, 1.80) | 0.700 | ||
| Diabetes mellitus | 2.02 (0.75, 5.49) | 0.170 | ||
| Perioperative major complication(s) | 1.26 (0.16, 9.78) | 0.820 | ||
| Adjuvant chemotherapy | 1.37 (0.54, 3.46) | 0.500 | ||
| Lung-only recurrence | 0.69 (0.19, 2.51) | 0.570 | ||
| Liver-only recurrence | 4.25 (0.98, 18.4) | 0.049 | 41.0 (5.01, 336) | < 0.001 |
| Peritoneum-only recurrence | 4.53 (1.79, 11.5) | 0.001 | 23.2 (2.92, 185) | 0.003 |
| ≥ 2 sites of recurrences | 1.96 (1.19, 3.23) | 0.008 | 5.28 (1.80, 15.4) | 0.002 |
The use of SBTS in OCC offers advantages, including minimally invasive resection, reduced perioperative complications, and lower stoma formation rates. However, wider-scale adoption of this approach remains limited owing to worse oncological outcomes. To the best of our knowledge, this is the first study reporting the long-term recurrence pattern and competing risk analysis to evaluate CRC-specific death among this group of patients.
Successful bowel decompression after SEMS deployment permits not only the optimisation of comorbidities, hydration, and nutrition but also complete staging and assessment for synchronous cancers[21]. 46.8% of the patients underwent laparoscopic CRC resection, which has been associated with reduced postoperative discomfort, lower incidence of infectious complications, and attenuated immune response to surgery. The stoma formation rate of 6.5% in our study was close to the rate of 4.3% reported in another multi-centre retrospective study[22]. Moreover, our overall morbidity and mortality rates compare favourably against other similar cohorts[17,23]. Although the short-term outcomes of SEMS, including successful primary anastomosis and decreased morbidity and mortality rates, have been well established in several randomised controlled trials, controversy remains regarding their long-term oncological effects and impact on tumour recurrence[24-28].
A randomised study published in 2011 comparing 15 patients in the SBTS group vs 13 patients in the upfront emergency surgery group, reported a higher recurrence rate in the SBTS group (53.3% vs 15.4%, P = 0.055) after a mean follow-up of 37.6 mo, although the overall survival rates were similar between the two groups[24]. In our study, 45.2% of the patients (28/62) developed metastases after a median period of 16 mo. A clear predominance of 60.7% (17/28) in peritoneal metastatic involvement was observed among the 28 patients. Furthermore, 36% of these patients (10/28) had two or more sites of metastases, upon detection of recurrence during the follow-up period. The adverse oncological repercussions among patients with OCC treated with SBTS are clear. While stent-related tumour perforation can result in intraperitoneal seeding of tumour cells, the radial expansion of the obstructing tumour caused by SEMS might promote tumour cell migration, elevating the risk of systemic metastasis[29,30]. Subclinical micro-perforations among these patients may contribute to tumour dissemination and seeding, thereby increasing the risk of peritoneal recurrence.
Recurrence, together with the presence and degree of lymph node metastasis, and LVI, are well-known prognostic factors influencing CRC survival. In our study, 41.9% of the patients died after SBTS, with 61.5% of deaths attributable to CRC. Our cohort’s 5-year OS rate of 59% is comparable to similar patients undergoing SBTS reported by a previous study (5-year OS: 60%)[31]. The CRC-specific mortality was measured against the competing risk of other cause-specific mortality. The factors with the highest CIF (at 60 mo) of CRC-specific mortality were liver-only recurrence, followed by peritoneum-only recurrence, LVI, ≥ 2 sites of recurrences, and T4 staging. Liver-only recurrence, peritoneum-only recurrence, and ≥ 2 sites of recurrences were highly associated with CRC-specific mortality on both univariate and multivariate Fine-Gray regressions. Lung metastases were not associated with poor survival and CRC-specific death in our study.
Our findings are consistent with other studies, which have shown that CRC patients with liver metastases had considerably worse survival[32]. In addition, patients with peritoneal metastases had very limited survival, with only a median of 12 mo with systemic chemotherapy[33]. LVI has also been identified as an independent risk factor associated with decreased 5-year survival rates in CRC patients[34]. The prognosis for patients with LVI-positive tumours is poorer than those with LVI-negative tumours[35]. Furthermore, the prognostic heterogeneity in metastatic CRC is mainly attributable to primary tumour characteristics, the number of metastatic sites, and the pattern of metastasis, particularly peritoneal involvement, which portends a worse prognosis[36-38]. Survival probabilities are drastically reduced with multiple metastatic sites and the presence of peritoneal metastases. Our results highlight a substantial proportion of peritoneal metastatic disease developing among patients treated with SBTS, with the presence of peritoneum-only recurrence strongly associated with CRC-specific mortality.
The main limitations of this study are its retrospective nature and the relatively small cohort size. Nevertheless, the long-term recurrence and survival outcomes reported should offer a note of caution in the routine use of SBTS among patients with OCC. Future randomised comparative studies may be able to further evaluate the oncological impact of this treatment strategy.
The peritoneum was the most common metastatic site among patients undergoing SBTS for OCC. Liver-only recurrence, peritoneum-only recurrence, and two or more recurrence sites were predictors of CRC-specific death.
Stenting as a bridge to curative surgery (SBTS) for obstructing colon cancer (OCC) has been associated with concerns regarding long-term oncological outcomes.
While SBTS may be associated with worse oncological outcomes, there are other competing risks that can affect colorectal cancer (CRC)-specific mortality among patients with OCC.
To evaluate the long-term oncological effects, recurrence patterns, survival outcomes, and CRC-specific mortality in patients who underwent SBTS for OCC.
This study retrospectively examined long-term data from 62 patients who underwent SBTS at our institution over ten years from 2007 to 2016. CRC-specific mortality was evaluated by the competing risk analysis with cumulative incidence function. Fine-Gray analyses were performed to identify prognostic factors of CRC-specific mortality.
28 of 62 patients developed metastases after a median of 16 mo, with the peritoneum being the most prevalent (60.7%) metastatic site. In 46 mo of median follow-up, 26 (41.9%) patients died, of which 16 (61.5%) were CRC-specific deaths. Liver-only recurrence, peritoneum-only recurrence, and two or more recurrence sites were determined to be prognostic factors of CRC-specific mortality.
The peritoneum was the most prevalent metastatic site among patients who underwent SBTS for OCC in this study. CRC-specific mortality most likely occurred in patients with liver-only recurrence, peritoneum-only recurrence, or two or more recurrence sites.
The long-term recurrence pattern and factors contributing to CRC-specific mortality were reported.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng X, China; Qin J, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castañeda-Orjuela CA, Catalá-López F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 1168] [Article Influence: 166.9] [Reference Citation Analysis (0)] |
| 2. | Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. 1985;72:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 270] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Aslam MI, Kelkar A, Sharpe D, Jameson JS. Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg. 2010;8:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kim EJ, Kim YJ. Stents for colorectal obstruction: Past, present, and future. World J Gastroenterol. 2016;22:842-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 5. | Breitenstein S, Rickenbacher A, Berdajs D, Puhan M, Clavien PA, Demartines N. Systematic evaluation of surgical strategies for acute malignant left-sided colonic obstruction. Br J Surg. 2007;94:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. 2003;46:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD; Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Repici A, Pagano N, Hervoso CM, Danese S, Nicita R, Preatoni P, Malesci A. Metal stents for malignant colorectal obstruction. Minim Invasive Ther Allied Technol. 2006;15:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Law WL, Choi HK, Chu KW. Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg. 2003;90:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | De Ceglie A, Filiberti R, Baron TH, Ceppi M, Conio M. A meta-analysis of endoscopic stenting as bridge to surgery versus emergency surgery for left-sided colorectal cancer obstruction. Crit Rev Oncol Hematol. 2013;88:387-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Siddiqui A, Cosgrove N, Yan LH, Brandt D, Janowski R, Kalra A, Zhan T, Baron TH, Repici A, Taylor LJ, Adler DG. Long-term outcomes of palliative colonic stenting versus emergency surgery for acute proximal malignant colonic obstruction: a multicenter trial. Endosc Int Open. 2017;5:E232-E238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chok AY, Lim HJ, Lye WK, Samarakoon LB, Guo J, Tang CL, Mathew R. Stenting as a bridge to surgery for obstructed stage IV colorectal cancers - long-term outcomes of a 10-year study. Asian J Endosc Surg. 2020;13:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, Kishi T, Uchida E. Comparison of long-term outcomes of colonic stent as "bridge to surgery" and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 16. | Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Kavanagh DO, Nolan B, Judge C, Hyland JM, Mulcahy HE, O'Connell PR, Winter DC, Doherty GA. A comparative study of short- and medium-term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum. 2013;56:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 712] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 19. | Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391-4400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 21. | Brehant O, Fuks D, Bartoli E, Yzet T, Verhaeghe P, Regimbeau JM. Elective (planned) colectomy in patients with colorectal obstruction after placement of a self-expanding metallic stent as a bridge to surgery: the results of a prospective study. Colorectal Dis. 2009;11:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Bae SU, Yang CS, Kim S, Lim DR, Jeong WK, Dong Kim D, Kim JH, Shin EJ, Lee YJ, Lee JY, Kim NK, Baek SK. Long-term oncologic outcomes of laparoscopic versus open resection following stent insertion for obstructing colon cancer: a multi-center retrospective study. Surg Endosc. 2019;33:3937-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ; UK Emergency Laparotomy Network. Variations in mortality after emergency laparotomy: the first report of the UK Emergency Laparotomy Network. Br J Anaesth. 2012;109:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg. 2011;35:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg. 2009;144:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 28. | Huang X, Lv B, Zhang S, Meng L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg. 2014;18:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM. Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc. 2015;29:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg. 2007;94:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Knight AL, Trompetas V, Saunders MP, Anderson HJ. Does stenting of left-sided colorectal cancer as a "bridge to surgery" adversely affect oncological outcomes? Int J Colorectal Dis. 2012;27:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 599] [Article Influence: 85.6] [Reference Citation Analysis (1)] |
| 33. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1513] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 34. | Courtney ED, West NJ, Kaur C, Ho J, Kalber B, Hagger R, Finlayson C, Leicester RJ. Extramural vascular invasion is an adverse prognostic indicator of survival in patients with colorectal cancer. Colorectal Dis. 2009;11:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Horn A, Dahl O, Morild I. The role of venous and neural invasion on survival in rectal adenocarcinoma. Dis Colon Rectum. 1990;33:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, Saltz L, Punt CJA, Koopman M, Tournigand C, Tebbutt NC, Diaz-Rubio E, Souglakos J, Falcone A, Chibaudel B, Heinemann V, Moen J, De Gramont A, Sargent DJ, Grothey A; Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 37. | Arakawa K, Kawai K, Ishihara S, Hata K, Nozawa H, Oba K, Sugihara K, Watanabe T. Prognostic Significance of Peritoneal Metastasis in Stage IV Colorectal Cancer Patients With R0 Resection: A Multicenter, Retrospective Study. Dis Colon Rectum. 2017;60:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Fujita S, Shimoda T, Yoshimura K, Yamamoto S, Akasu T, Moriya Y. Prospective evaluation of prognostic factors in patients with colorectal cancer undergoing curative resection. J Surg Oncol. 2003;84:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |