Published online Apr 16, 2022. doi: 10.4253/wjge.v14.i4.191
Peer-review started: May 7, 2021
First decision: July 27, 2021
Revised: August 9, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 16, 2022
Processing time: 336 Days and 6.3 Hours
There has been a growing interest in developing endoscopic ultrasound (EUS)-guided interventions for pancreatic cancer, some of which have become standard of care. There are two main factors that drive these advancements to facilitate treatment of patients with pancreatic cancer, ranging from direct locoregional therapy to palliation of symptoms related to inoperable pancreatic cancer. Firstly, an upper EUS has the capability to access the entire pancreas–lesions in the pancreatic head and uncinate process can be accessed from the duodenum, and lesions in the pancreatic body and tail can be accessed from the stomach. Secondly, there has been a robust development of devices that allow through-the-needle interventions, such as placement of fiducial markers, brachytherapy, intratumoral injection, gastroenterostomy creation, and ablation. While these techniques are rapidly emerging, data from a multicenter randomized controlled trial for some procedures are awaited prior to their adoption in clinical settings.
Core Tip: Interventional endoscopic ultrasound in pancreatic cancer has been developed via a through-the-needle fashion, using 2 techniques: Injection and/or placement. Examples of through-the-needle injection techniques include intratumoral therapy, injection of alcohol and bupivacaine for celiac plexus neurolysis, and hydrogel for bleb formation to create space in the pancreaticoduodenal groove for dose-escalation stereotactic body radiation therapy. Examples of through-the-needle placement techniques include placement of fiducial markers, placement of ablative probes for non-thermal and thermal therapies, placement of radioactive seeds for brachytherapy, and placement of a lumen-apposing metal stent to create a gastrojejunostomy in patients with gastric outlet obstruction. The vast majority of these techniques have shown comparable or superior outcomes when compared to conventional interventions and therapies.
- Citation: Kerdsirichairat T, Shin EJ. Endoscopic ultrasound guided interventions in the management of pancreatic cancer. World J Gastrointest Endosc 2022; 14(4): 191-204
- URL: https://www.wjgnet.com/1948-5190/full/v14/i4/191.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i4.191
Pancreatic ductal adenocarcinoma has increased in incidence by 0.3% annually since 2006 and is expected to become the second cause of cancer-related death in the year 2030. It has the lowest 5-year relative survival of 11% compared to other solid organ malignancies, with an estimated death toll of 49830 which closely reflects its incidence of 62210 in 2021[1]. Approximately more than half of the patients presented at the metastatic stage, the highest proportion compared to other solid malignancies, while 13% and 29% presented at localized and regional stages, respectively. For those who present without overt evidence of metastasis, surgical resection is the ultimate goal to hopefully provide curative treatment. With the advancement of endoscopic ultrasound (EUS) in both diagnostic and therapeutic aspects of pancreatic cancer management, it has provided treatment options not only by tissue acquisition to get the definitive diagnosis of pancreatic cancer but also by more accurate local disease control in regional or locally advanced stages while awaiting definitive curative surgical resection and through palliative treatments in those with metastasis or advanced disease[2,3]. This review does not include EUS-guided intervention for malignant biliary obstruction.
An initial randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions showed comparable diagnostic efficacy, technical performance, and safety profile without a significant difference in yield or quality of the histologic core between the two needle types[4]. Subsequent randomized trials with larger sample sizes were able to demonstrate that fewer passes were required to establish a diagnosis of pancreatic malignancy with improved histopathological quality using a fine needle biopsy (FNB) needle[5-7]. The use of the 25 gauge FNB needle was technically feasible, safe, efficient and was comparable to the standard 22 gauge fine needle aspiration (FNA) needle in patients with solid pancreatic masses in the absence of an on-site cytopathologist. The cytological sample quality in the liquid-based preparation and the histological diagnostic yield for specific tumor discrimination of EUS-guided sampling using a 25 gauge FNB needle were significantly higher than those using a 22 gauge FNA needle[8]. In terms of designs of FNB needle, an opposing bevel design provided significantly superior tissue yield and diagnostic performance when compared to a reverse bevel needle[9]. For second generation FNB needles, the diagnostic yield when used primarily without rapid on-site evaluation, was higher when a fork-tip needle, in comparison to a Franseen needle or FNA needle, was used[10,11]. However, a subsequent larger trial revealed that samples with the highest degree of cellularity in a single biopsy, resulting in a diagnostic accuracy of 90% or higher, were collected by FNB needles using the Franseen or fork-tip needle[12]. Another study showed that a 22-gauge Franseen needle provided more tissue for histologic evaluation and better diagnostic accuracy than a 20-gauge lateral bevel needle. These studies led to the technical guideline from the European Society of Gastrointestinal Endoscopy in 2017 suggesting performance of 3-4 needle passes with an FNA needle or 2-3 passes with an FNB needle when on-site cytologic evaluation is unavailable[13]. There may be some theoretical concern that the high yield of FNB needles might come with the cost of possibly higher risk of tract seeding, especially in patients with a resectable solid pancreatic mass, unless the tract itself is planned to be resected[14]. In terms of technique, the stylet slow pullback technique might enable better acquisition of tissue and increased cellularity for the diagnosis of pancreatic tumors suspected to be malignant, compared to the conventional negative suction after stylet removal technique or the non-suction after stylet removal technique, in the absence of an on-site cytopathologist.
In the era of personalized medicine, next-generation sequencing (NGS) can serve as a complementary diagnostic test and unveil potentially predictive genomic biomarkers for treatment response[15,16]. An initial experience revealed that NGS can be performed on EUS-FNA-derived samples to provide information on KRAS mutation status and 160 other cancer genes such as TP53, SMAD4, KMT2D, NOTCH2, MSH2, RB1, SMARCA4, PPP2R1A, PIK3R1, SCL7A8, ATM and FANCD2, to supplement cytological evaluation[17-21]. Similar to the efficacy of FNB over FNA for cellularity, FNB should be considered when tumor genotyping is requested, as it was associated with a higher yield of sufficient sampling for genomic testing, especially in tumors of 3 cm or smaller, and tumors located in the head/neck of the pancreas[22]. Moreover, recent data indicated that studying the expression of a selected gene set could inform the selection of the most appropriate treatment for patients, moving towards an individualized medicine approach. To accomplish this, adequate EUS tissue acquisition will allow providers to build organoids platform that can allow determination of the transcription level of informative genes[23]. Early studies were able to demonstrate the successful isolation of organoids using samples obtained from a 22-gauge FNB needle at the time of the initial diagnosis, which may be helpful in patients with pancreatic cancer that are not surgically resectable[24,25].
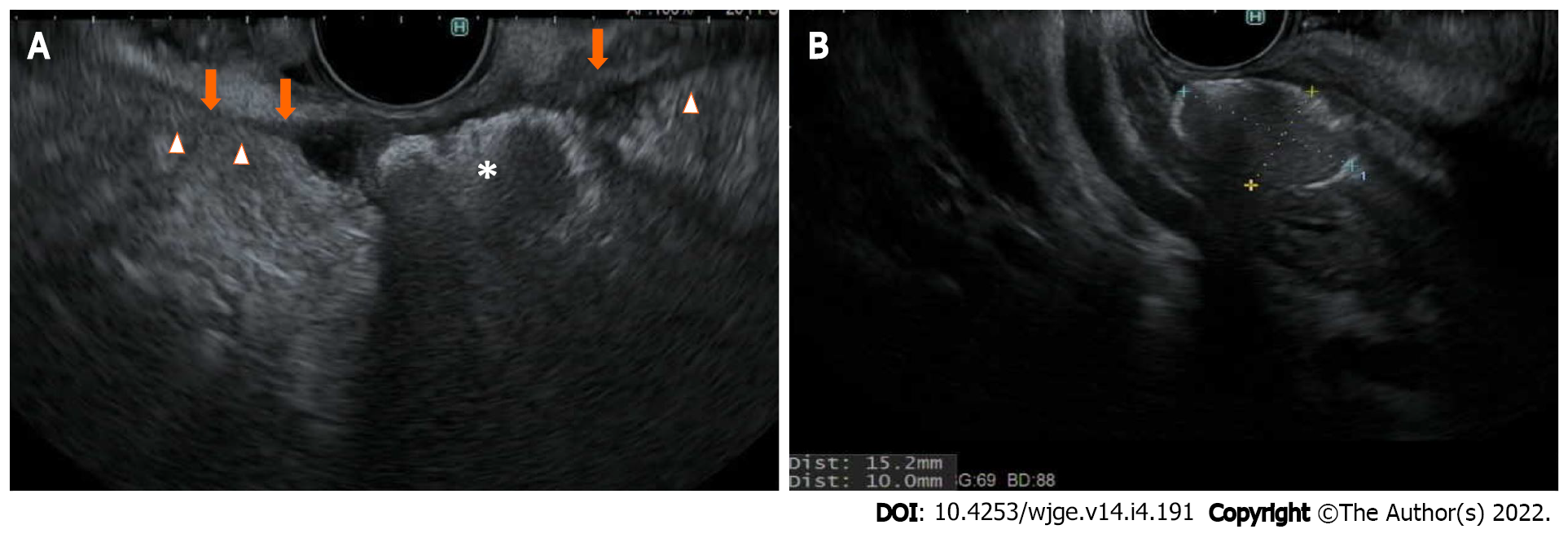
For patients with borderline resectable or locally advanced pancreatic cancer, neoadjuvant chemoradiation plays a vital role. While chemotherapy can potentially control systemic disease, local disease control by radiation therapy has shown additional benefit to hopefully reduce local recurrence after surgical resection[26,27]. Stereotactic body radiation therapy (SBRT) and image guided radiation therapy (IGRT) have increasingly been used in clinical practice since they can provide a higher dose of radiation with a shorter duration of treatment and acceptable rates of toxicity[28]. To be able to focally deliver radiation to the pancreas, which is an organ that moves following respiratory cycles, fiducial marker placement is recommended[29]. The markers are traditionally metallic, made of gold or platinum, or more recently, in hydrogel form, to serve as reference points for planning as well as follow-up daily image guidance over a short course of SBRT/IGRT. EUS-guided fiducial placement has evolved to become the technique of choice to place these fiducial markers, compared to conventional techniques where the markers are either placed surgically or percutaneously under cross-sectional imaging guidance such as computed tomography (CT) or transabdominal ultrasound[30]. The ideal characteristics of fiducial markers should have good visibility, minimal artifacts, and minimal migration over the course of SBRT/IGRT. Fiducials with larger diameters usually provide better visibility, at the cost of greater artifact. Furthermore, fiducial delivery systems that require a 19-gauge needle can pose challenges for EUS-guided fiducial placement when lesions are located at the pancreatic uncinate process. Therefore, the fine balance and preferred types of fiducials should be discussed in a multi-disciplinary tumor board setting, especially between the endosonographers and the radiation oncologists. Generally, balanced visibility and artifacts can be achieved with a 0.35- to 0.43-mm diameter, 5- to 10- mm length, coiled or cylindrical gold fiducials[31]. A comparison study of these types of gold fiducials and the newer generations of fiducials, such as platinum or hydrogel, is still in process. A theoretical benefit of hydrogel compared to other metallic fiducials is that it can be injected via EUS in a liquid bleb formation to create additional space in the pancreaticoduodenal groove to separate the pancreatic head/neck cancer from the adjacent duodenal C loop (Figure 1) to allow for dose escalation during SBRT/IGRT while avoiding mucosal toxicity to the duodenum[32,33].
Given the close proximity of the probe of the therapeutic echoendoscope and several technologies that can be delivered through FNA needles, multiple modalities for local therapies of pancreatic cancer have been developed. These include placement of radiosensitive devices for brachytherapy, injections of antitumoral agents, access for passing through-the-needle probe for ablative devices, and photodynamic therapy.
Intraoperative interstitial brachytherapy when used at laparotomy can improve local disease control in locally advanced pancreatic cancer. An initial animal study from China implementing EUS as a route for the implantation of radioactive seeds was proven safe and feasible. Shortly after, the group conducted a feasibility study in 15 patients who suffered from unresectable pancreatic cancer, showing 30% of patients had clinical benefit, with complications including pancreatitis and pancreatic fluid collection in 20% of patients. This was followed by a prospective cohort of 22 patients with unresectable pancreatic cancer who were treated with radioactive iodine 125 seeds, which resulted in 14% partial remission at 4 wk, 45% with stable disease, and 91% later succumbed to the disease at 2-year follow-up. Another group in China conducted a pilot study in 8 patients with T4 pancreatic cancer, using both intratumoral radioactive seeds and 5-fluorouracil, resulting in a 12% partial response at 3 mo, with overall 50% clinical benefits including a reduction in pain, without complications or hematologic toxicity[34]. Another prospective study showed that EUS-guided implantation of iodine-125 around the celiac ganglia can reduce pain visual analog scale score and analgesic drug consumption in patients with unresectable pancreatic cancer. A special EUS treatment planning system software may play a role in EUS-guided brachytherapy in patients with unresectable cancer, as it demonstrated a rate of partial remission of up to 80% in patients whose minimal peripheral dose was larger than 90 Gy, with a median survival time of 9 mo[35]. In addition to survival benefits, iodine-125 seed implantation placed percutaneously or via EUS after relief of obstructive jaundice via ERCP can improve biliary stent patency, time to development of gastric outlet obstruction, and improve quality of life by pain relief[36]. More recently, EUS guided placement of phosphorus-32 microparticles alone or with gemcitabine with or without nab-paclitaxel in unresectable locally advanced pancreatic cancer has been reported as alternative brachytherapy options[37,38]. The latter is an ongoing trial.
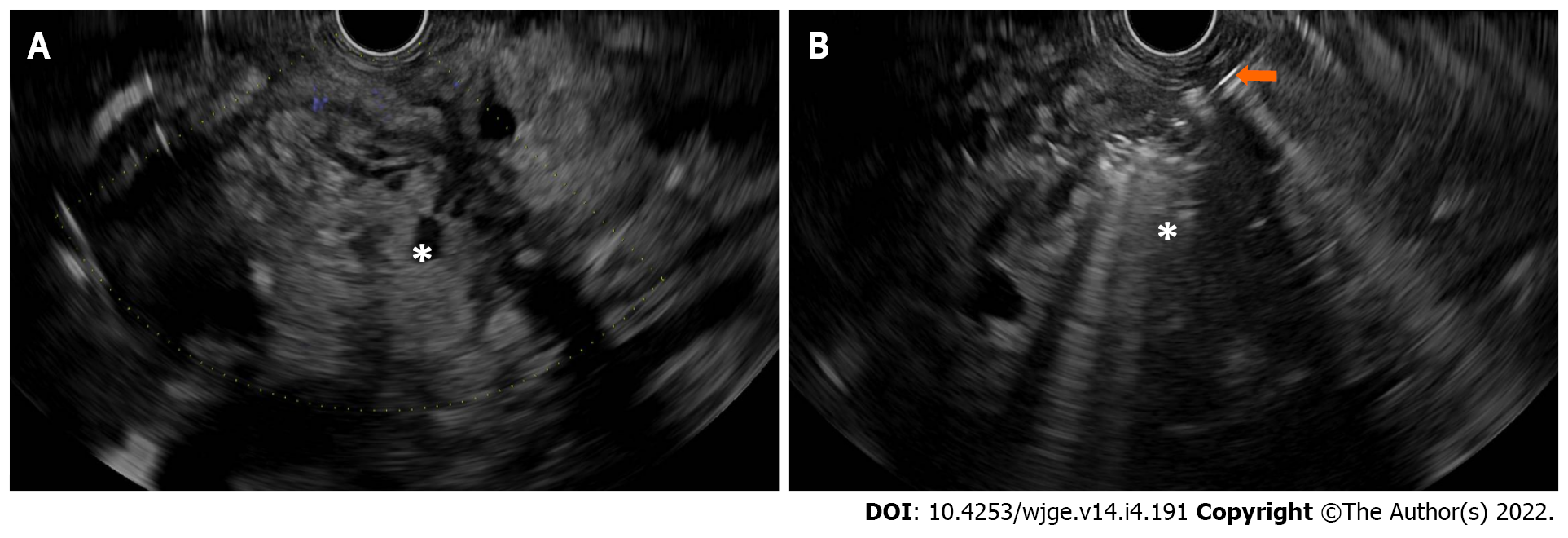
The hypothesis of intratumoral therapy was based on that of other malignancies where both local disease control effect and systemic response effect (i.e., metastasis) can be achieved through the immune response against the tumors, including breast cancer, renal cell carcinoma, and melanoma[39-43]. In addition, immunological responses induced by zoledronate-pulsed dendritic cell-based vaccines have been associated with therapeutic effects in clinical trials[44,45]. The first pilot study in patients with unresectable pancreatic cancer treated with EUS-guided injection of allogeneic mixed lymphocyte culture proved its feasibility and safety profile[46]. Subsequent pilot studies included an injection of immature dendritic cells in pancreatic cancer refractory to gemcitabine[47], a combination of systemic gemcitabine and intratumoral OK-432-pulsed dendritic cell therapy, followed by an intravenous infusion of lymphokine-activated killer cells stimulated with an anti-CD3 monoclonal antibody[48], and dendritic cell-based vaccination and concomitant chemotherapy in patients with advanced or recurrent pancreatic cancer[49]. The first phase 1 comparative trial of intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients had one in nine patients with transient fever. Two out of nine patients treated with immunotherapy, one of whom had stage IV with distant lymph node metastasis, survived five years without further adjuvant therapy[50]. In a phase I/II trial of comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocytes, and gemcitabine in unresectable locally advanced pancreatic cancer, a synergistic therapeutic response was shown with overall survival and progression-free survival of 12 and 5.5 mo, respectively[51]. To date, there has not been a study of EUS-guided intratumoral injection of other types of immunotherapy such as ipilimumab or nivolumab (Figure 2).
Pancreatic cancer is unfortunately insensitive to many chemotherapeutic drugs. It is thought that inefficient delivery of chemotherapy into the tumor plays an important role in chemoresistance in pancreatic cancer. A combination therapy that can increase intratumoral vascular density and intramural concentration of gemcitabine was shown to lead to a transient stabilization of disease[52]. The initial experience using OncoGel (Regel/paclitaxel) for local tumor management via EUS guided 22-gauge needle in a pig model provided high and sustained localized concentrations of paclitaxel. A feasibility study using EUS-guided injection of gemcitabine in 38 patients with locally advanced and metastatic pancreatic cancer confirmed the safety and efficacy of the technique. More recently, a feasibility study of EUS guided injection of a novel polymer-based microparticles for a drug delivery system in a pig model appeared promising[53]. A phase I study evaluating the role of EUS guided injection of epidermal growth factor receptor antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer in 16 patients proved its feasibility and safety profile when administered with abdominal radiation and concurrent gemcitabine. The incidence of grade 1-2 adverse events was 96% and the incidence of grade 3-4 adverse events was 9%[54].
An initial feasibility study in 21 patients with locally advanced or metastatic pancreatic cancer treated with EUS guided injection of ONYX-015 (dl1520), an E1B-55kD gene-deleted replication-selective adenovirus that preferentially replicates in and kills malignant cells, was promising and generally well-tolerated either alone or in combination with gemcitabine[55]. In a multi-center feasibility study of 50 patients, intratumor delivery of TNFerade biologic (AdGVEFR.TNF.11D), a replication-deficient adenoviral vector that expresses tumor necrosis factor-alpha under the control of the Egr-1 promotor, by EUS-guided injection or percutaneously, combined with chemoradiation in the treatment of locally advanced pancreatic cancer, appeared promising, especially at the maximal tolerated doses. Adverse events such as cholangitis and pancreatitis were observed in 6%. The rate of patients who were able to proceed with surgery and achieve negative margin resection was 12%. In a randomized trial of 304 patients, treatment with TNFerade plus standard of care was safe but not effective for prolonging survival in patients with locally advanced pancreatic cancer[56].
For patients with unresectable pancreatic cancer, an open-label, dose-escalation trial using BC-819, which is a DNA plasmid developed to target the expression of diphtheria-toxin gene under the control of H19 regulatory sequences, in combination with systemic chemotherapy, may provide an additional therapeutic benefit, with minimal adverse events such as asymptomatic elevation of lipase[57]. EUS-guided injection of HF10, a spontaneously mutated oncolytic virus derived from herpes simplex virus 1 that has the potential to show a strong antitumor effect against malignancies without damaging normal tissue, in combination with erlotinib and gemcitabine, was a safe treatment for unresectable locally advanced pancreatic cancer[58]. The EUS-guided injection of STNM01, the double-stranded RNA oligonucleotide that specifically represses carbohydrate sulfotransferase-15, was safe and feasible without any adverse events. The authors also proposed that injections of STNM01 during the start of treatment could lower carbohydrate sulfotransferase-15 level, while its overexpression was associated with worse prognosis[59,60].
An open-label phase 1/2a study in the first-line setting of patients with inoperable locally advanced pancreatic cancer using an EUS guided injection of siG12D-LODER to release a siRNA drug against KRAS (G12D), along with systemic chemotherapy, was promising in terms of potential efficacy that 70% had a reduction in tumor marker CA 19-9, and 80% of patients had either stable disease or partial response with a median overall survival of 15 mo. However, one third of patients experienced serious adverse events.
Radiofrequency ablation is a local ablative method that can destroy the tumor by thermal coagulation and protein denaturation[61]. A phase II pilot study using radiofrequency ablation via a laparotomy in patients with locally advanced pancreatic cancer showed its feasibility and safety profiles with a 24% complication rate, with 9% requiring a reoperation. After a feasibility study in a porcine model, a feasibility study of using EUS-guided radiofrequency ablation of unresectable pancreatic cancer showed promising safety data, with one-third of the patients only developing mild abdominal pain without pancreatitis. The safety profile of the technique was later confirmed by subsequent feasibility studies showing no evidence of early or late major adverse events[62,63]. However, it required an 18-gauge electrode, which could be challenging for the treatment of lesions located in the pancreatic head or uncinate process. A new monopolar radiofrequency probe may be technically more versatile because it can be used through a 22-gauge needle[64]. In patients with locally advanced pancreatic cancer treated with EUS-guided radiofrequency ablation, those with wild-type SMAD4 may have improved survival benefits after treatment[65]. For other solid pancreatic lesions such as pancreatic neuroendocrine tumors and pancreatic insulinoma, EUS-guided radiofrequency ablation has shown clinical benefits such as fewer episodes of hypoglycemia[66,67], regression of neuroendocrine syndromes, improved pancreatic cystic sizes, and complete radiological ablation[64] A prospective study of 29 patients using EUS-guided radiofrequency ablation for pancreatic neuroendocrine tumors (PNET) and pancreatic cystic neoplasms revealed an overall tumor resolution of 86% in PNET and a significant response rate of 71% of patients with cystic neoplasms, with an overall complication rate of 10%.
Another application of radiofrequency ablation is to use it along with a simultaneous cryogenic cooling of carbon dioxide. An animal feasibility study was promising, given that only 14% of pigs developed histochemical pancreatitis after the procedure. The group has expanded this technique to 16 explanted pancreatic tumors from 16 patients, showing that the flexible bipolar ablation device, combining radiofrequency and cryotechnology, can create an ablation zone, defined by histological signs of coagulative necrosis, and that the extent of the ablation zone was related to the duration of application. However, data on this technique in in-vivo studies are still forthcoming.
An initial animal study using a neodymium-doped:yttrium aluminum garnet (Nd:YAG) was based on the finding that the ablation resulted in a high rate of tissue necrosis and can be considered as a palliative option in patients with hepatocellular carcinoma, liver metastases in colorectal cancer, and malignant thyroid nodules[68-72]. There was no major post-procedural complication and all 8 pigs survived at 24 h after EUS-guided laser ablation of normal pancreatic tissue. The same group conducted another animal study to evaluate tissue temperature distribution, which plays a crucial role in the outcome laser-induced thermal therapy, proving that the tissue downward from the tip is mostly heated at 60 Celsius degree. The authors further conducted a human feasibility study in nine patients with unresectable pancreatic cancer who were unresponsive to previous chemoradiotherapy. Laser ablation was performed by using a 300-micrometer flexible fiber preloaded onto a 22-gauge fine needle. A 1064-nanometer wavelength Nd:YAG was used at different settings (2-4 Watts and 800-1200 Joules), resulting in an ablation area ranging from 0.4 cm3 with the setting of 2 Watts and 800 Joules, to 6.4 cm3 with the setting of 4 Watts and 1000 Joules, without adverse events. A comparative study using laser ablation compared to other EUS-guided techniques for patients with unresectable pancreatic cancer is awaiting.
EUS-guided photodynamic therapy has two steps: An injection of a photosensitizing agent, followed by the insertion of a 19-gauge needle into the targeted area to pass a small quartz optical fiber to illuminate and ablate tissue with the laser light. Initial pilot studies in porcine models using EUS-guided photodynamic therapy appeared promising. In a rabbit model, the efficacy of verteporfin delivery in tumors can be estimated by perfusion CT, to serve as a non-invasive method of mapping photosensitizer dose to enhance the outcomes of ablation with photodynamic therapy[73]. A human feasibility study in four patients with locally advanced pancreaticobiliary malignancies using a second-generation photosensitizer, a chlorin e6 derivative, and a flexible laser probe was promising, with a median volume of necrosis of up to 4 cm3, no progression of disease over a median follow-up of five months, and no post-procedural complications. A prospective dose-escalation phase 1 study in 12 patients with treatment-naive locally advanced pancreatic cancer using intravenous porfimer sodium and illumination with a 630-nanometer light, followed by a CT scan to document change in pancreatic necrosis, and nab-paclitaxel and gemcitabine, showed an increased volume and percentage of tumor necrosis in 50% of patients after EUS-guided photodynamic therapy, without procedurally related adverse events. Another human feasibility study, which excluded patients with significant metastatic disease burden, disease involving > 50% duodenal or major artery circumference, and recent treatment with curative intent, investigated EUS-guided photodynamic therapy using a different photosensitizer, verteporfin, resulting in tissue necrosis in 62.5% of patients, with a mean diameter of 15.7 mm, and no post-procedural related complications.
The vast majority of studies using EUS-guided ethanol ablation for solid pancreatic tumors are focused on non-functioning pancreatic neuroendocrine tumors and insulinoma[74-76]. Data of EUS-guided ethanol ablation in pancreatic ductal adenocarcinoma, especially in combination with EUS-guided celiac plexus neurolysis, are still needed.
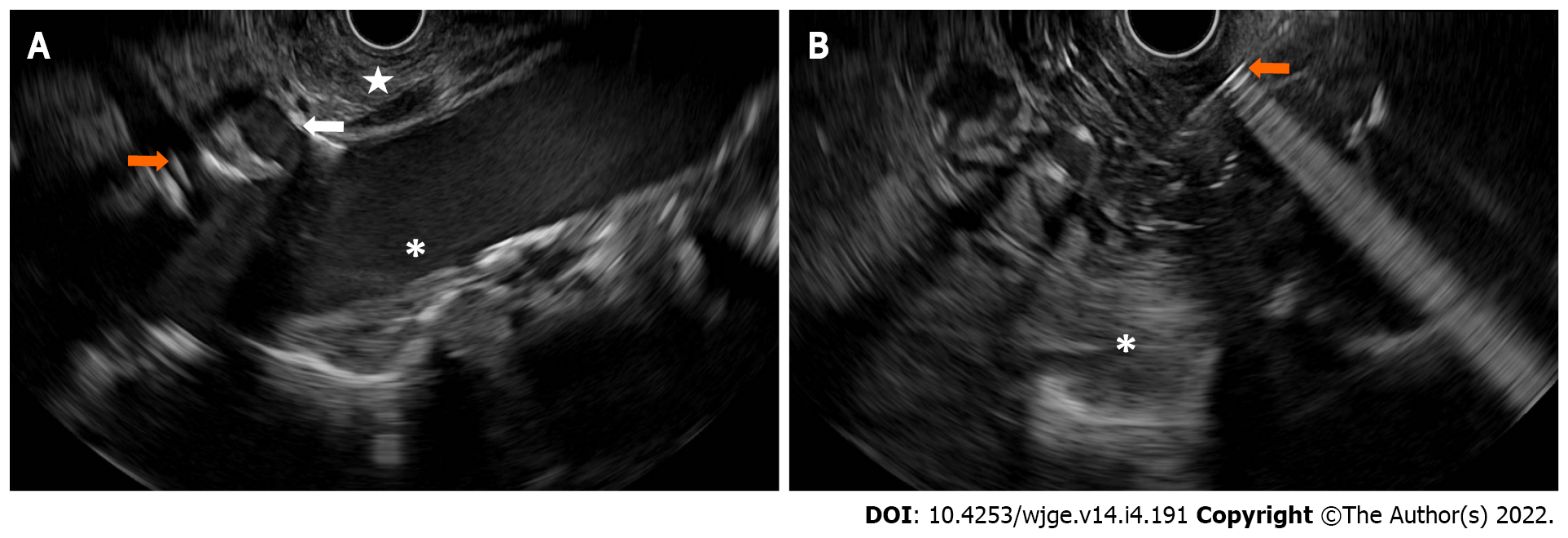
EUS-guided celiac plexus intervention has gained popularity in the management of pain from pancreatic cancer due to its safety profile when compared to narcotics[77]. An initial meta-analysis and systematic review showed that the pooled proportion of patients with pancreatic cancer treated with EUS-guided celiac plexus neurolysis had pain relief up to 53%-80% of the time[78-80]. The first randomized controlled trial in 96 patients assigned to either EUS-guided celiac plexus neurolysis or conventional pain management, showed that early EUS intervention reduced pain and may have moderated morphine consumption in patients with painful, inoperable pancreatic cancer, especially at 3 mo after treatment[81]. While the number of injections might not improve the degree of pain relief[82], the targeted celiac ganglia neurolysis was superior to celiac plexus neurolysis. EUS-guided radiofrequency ablation, using a 1 French monopolar probe passed through a 19-gauge targeting the area of celiac plexus or visualized ganglia, showed superiority in pain relief and improved quality of life when compared to traditional EUS-guided celiac plexus neurolysis. However, a recent study raised the concern that combined celiac ganglion and plexus neurolysis may reduce median survival time without improving pain, quality of life, or adverse events when compared to traditional celiac plexus neurolysis. Furthermore, newer generations of opioids such as oxycodone and fentanyl may be comparable to EUS-guided celiac plexus neurolysis in terms of pain relief, quality of life, and opioid consumption (Figure 3).
Approximately 50% of patients with pancreatic cancer develop nausea and vomiting from malignant gastric outlet obstruction[83]. In patients with an inoperable stage, this was traditionally managed by endoscopic enteral stent placement or surgical gastrojejunostomy creation, depending on life expectancy. EUS-guided gastroenterostomy creation using a lumen apposing metal stent has emerged and gained in popularity due to a higher rate of initial clinical success and/or a lower rate of stent failure requiring repeat intervention when compared to enteral stent placement[84-86]. Compared to surgical approaches for gastrojejunostomy, EUS-guided gastroenterostomy was associated with fewer adverse events[87,88], shorter time to resume oral intake and chemotherapy, shorter lengths of stay, and reduced hospital costs. The technique of EUS-guided gastroenterostomy has been developed over time. The direct technique, defined by using an electrocautery-enhanced lumen-apposing metal stent, rather than a balloon-assisted approach, resulted in shorter procedure time and comparable clinical success (> 90%). In addition, the clinical success of direct-EUS-guided gastroenterostomy is durable with a low rate of re-intervention based on a long-term cohort[89]. Randomized trials comparing these endoscopic and surgical interventions for palliation of malignant gastric outlet obstruction caused by pancreatic cancer are awaiting. It should be noted that the learning curve of the technique can be challenging as it requires up to 40 procedures to achieve competency, otherwise fatal adverse events can occur at a very high rate (> 10%).
Immune checkpoint inhibition targeted against cytotoxic T-lymphocyte-associated antigen 4 and programmed cell death protein 1 has shown survival benefit to treat multiple types of advanced cancer, including pancreatic cancer. Hepatotoxicity from checkpoint Inhibitors is a less common type of immune related adverse events, and it is often mild[90,91]. Concurrent treatment with nivolumab and ipilimumab, which is commonly used in pancreatic cancer, increases the risk of hepatotoxicity up to 37% and the risk of high-grade toxicity by up to 15%[92,93]. In complicated or severe forms, or unclear etiologies, liver biopsy can be used to confirm the etiology of injury[93,94], and/or to clarify the diagnosis in those with elevated liver enzymes refractory to steroid or immunosuppressant treatment[95].
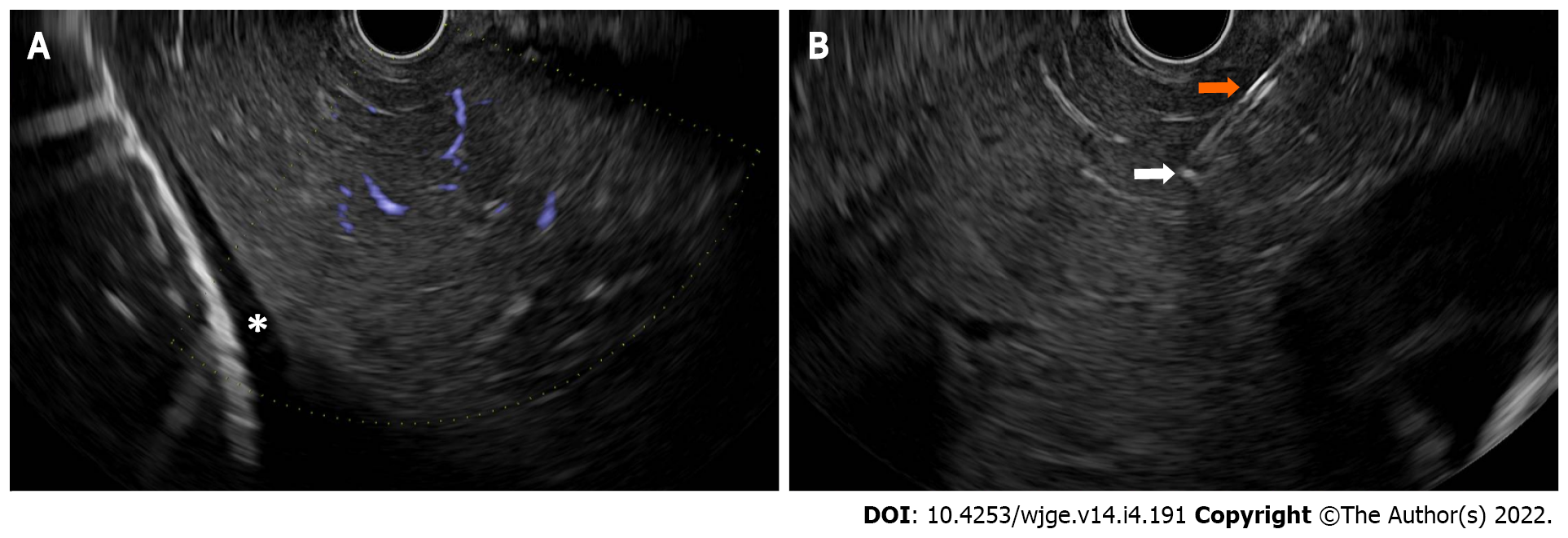
EUS-guided liver biopsies have increased in popularity due to their decreased invasiveness compared to surgical routes and comparable tissue acquisition compared to transjugular or percutaneous route[96]. Bilobar liver biopsies, with one needle pass with three to-and-fro needle movements to each lobe of the liver, enhanced the assessment of disease severity due to an increased number of complete portal tracts, and longer aggregate specimen length, without severe adverse events[97]. A 19-guage Franseen-tip or reverse bevel core needle outperformed FNA needles or other types of core needles, resulting in longer aggregate length, more complete portal tracts, and more adequate specimens despite fewer passes. A heparinized wet suction technique can improve tissue adequacy compared with dry needle techniques. A randomized trial using these specific techniques for EUS-guided liver biopsies, compared to other conventional approaches, is needed (Figure 4)[98].
EUS-guided interventions provide a broad spectrum of treatment modalities for patients with borderline resectable, locally advanced, and inoperable pancreatic cancer. These include direct treatment for locoregional stages such as ablative therapies, brachytherapy, placement of fiducial markers for SBRT/IGRT, as well as palliative treatments such as EUS-guided gastroenterostomy creation for malignant gastric outlet obstruction and EUS-guided celiac plexus neurolysis to manage pain. While many of these procedures are considered investigational with limited data, particularly those from randomized controlled trials, the vast majority of these techniques have been widely used in clinical practice. For patient safety, it is important to note that most of these procedures should be performed at a facility with a multi-disciplinary tumor board and experienced interventional endosonographers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lachter J, Israel S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11440] [Article Influence: 3813.3] [Reference Citation Analysis (4)] |
| 2. | Larghi A, Rimbaș M, Rizzatti G, Carbone C, Gasbarrini A, Costamagna G, Alfieri S, Tortora G. Endoscopic ultrasound-guided therapies for pancreatic solid tumors: An overview. Semin Oncol. 2021;48:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Cazacu IM, Singh BS, Saftoiu A, Bhutani MS. Endoscopic Ultrasound-Guided Treatment of Pancreatic Cancer. Curr Gastroenterol Rep. 2020;22:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Cho YD, Park SH. Core biopsy needle vs standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Alatawi A, Beuvon F, Grabar S, Leblanc S, Chaussade S, Terris B, Barret M, Prat F. Comparison of 22G reverse-beveled vs standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Cheng B, Zhang Y, Chen Q, Sun B, Deng Z, Shan H, Dou L, Wang J, Li Y, Yang X, Jiang T, Xu G, Wang G. Analysis of Fine-Needle Biopsy vs Fine-Needle Aspiration in Diagnosis of Pancreatic and Abdominal Masses: A Prospective, Multicenter, Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2018;16:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Yang MJ, Yim H, Hwang JC, Lee D, Kim YB, Lim SG, Kim SS, Kang JK, Yoo BM, Kim JH. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration vs 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Nayar MK, Paranandi B, Dawwas MF, Leeds JS, Darne A, Haugk B, Majumdar D, Ahmed MM, Oppong KW. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017;85:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Abdelfatah MM, Grimm IS, Gangarosa LM, Baron TH. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018;87:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Oppong KW, Bekkali NLH, Leeds JS, Johnson SJ, Nayar MK, Darné A, Egan M, Bassett P, Haugk B. Fork-tip needle biopsy vs fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized crossover study. Endoscopy. 2020;52:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Young Bang J, Krall K, Jhala N, Singh C, Tejani M, Arnoletti JP, Navaneethan U, Hawes R, Varadarajulu S. Comparing Needles and Methods of Endoscopic Ultrasound-Guided Fine-Needle Biopsy to Optimize Specimen Quality and Diagnostic Accuracy for Patients With Pancreatic Masses in a Randomized Trial. Clin Gastroenterol Hepatol. 2021;19:825-835.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | Nakatsubo R, Yamamoto K, Itoi T, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Tonozuka R, Mukai S, Nagai K, Yamaguchi H, Nagakawa Y. Histopathological evaluation of needle tract seeding caused by EUS-fine-needle biopsy based on resected specimens from patients with solid pancreatic masses: An analysis of 73 consecutive cases. Endosc Ultrasound. 2021;10:207-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Imaoka H, Sasaki M, Hashimoto Y, Watanabe K, Ikeda M. New Era of Endoscopic Ultrasound-Guided Tissue Acquisition: Next-Generation Sequencing by Endoscopic Ultrasound-Guided Sampling for Pancreatic Cancer. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Takano S, Fukasawa M, Shindo H, Takahashi E, Hirose S, Fukasawa Y, Kawakami S, Hayakawa H, Kuratomi N, Kadokura M, Maekawa S, Sato T, Enomoto N. Clinical significance of genetic alterations in endoscopically obtained pancreatic cancer specimens. Cancer Med. 2021;10:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | de Biase D, de Luca C, Gragnano G, Visani M, Bellevicine C, Malapelle U, Tallini G, Troncone G. Fully automated PCR detection of KRAS mutations on pancreatic endoscopic ultrasound fine-needle aspirates. J Clin Pathol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Gleeson FC, Kerr SE, Kipp BR, Voss JS, Minot DM, Tu ZJ, Henry MR, Graham RP, Vasmatzis G, Cheville JC, Lazaridis KN, Levy MJ. Targeted next generation sequencing of endoscopic ultrasound acquired cytology from ampullary and pancreatic adenocarcinoma has the potential to aid patient stratification for optimal therapy selection. Oncotarget. 2016;7:54526-54536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Kameta E, Sugimori K, Kaneko T, Ishii T, Miwa H, Sato T, Ishii Y, Sue S, Sasaki T, Yamashita Y, Shibata W, Matsumoto N, Maeda S. Diagnosis of pancreatic lesions collected by endoscopic ultrasound-guided fine-needle aspiration using next-generation sequencing. Oncol Lett. 2016;12:3875-3881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | de Biase D, Visani M, Acquaviva G, Fornelli A, Masetti M, Fabbri C, Pession A, Tallini G. The Role of Next-Generation Sequencing in the Cytologic Diagnosis of Pancreatic Lesions. Arch Pathol Lab Med. 2018;142:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Larson BK, Tuli R, Jamil LH, Lo SK, Deng N, Hendifar AE. Utility of Endoscopic Ultrasound-Guided Biopsy for Next-Generation Sequencing of Pancreatic Exocrine Malignancies. Pancreas. 2018;47:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Elhanafi S, Mahmud N, Vergara N, Kochman ML, Das KK, Ginsberg GG, Rajala M, Chandrasekhara V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J Gastroenterol Hepatol. 2019;34:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Iovanna J, Dusetti N. Speeding towards individualized treatment for pancreatic cancer by taking an alternative road. Cancer Lett. 2017;410:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Bian B, Juiz NA, Gayet O, Bigonnet M, Brandone N, Roques J, Cros J, Wang N, Dusetti N, Iovanna J. Pancreatic Cancer Organoids for Determining Sensitivity to Bromodomain and Extra-Terminal Inhibitors (BETi). Front Oncol. 2019;9:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Lacomb JF, Plenker D, Tiriac H, Bucobo JC, D'souza LS, Khokhar AS, Patel H, Channer B, Joseph D, Wu M, Tuveson DA, Li E, Buscaglia JM. Single-Pass vs 2-Pass Endoscopic Ultrasound-Guided Fine-Needle Biopsy Sample Collection for Creation of Pancreatic Adenocarcinoma Organoids. Clin Gastroenterol Hepatol. 2021;19:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4890] [Article Influence: 407.5] [Reference Citation Analysis (0)] |
| 27. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1946] [Article Influence: 278.0] [Reference Citation Analysis (0)] |
| 28. | Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, Iacobuzio-Donahue CA, Griffith ME, Pawlik TM, Pai JS, O'Reilly E, Fisher GA, Wild AT, Rosati LM, Zheng L, Wolfgang CL, Laheru DA, Columbo LA, Sugar EA, Koong AC. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 29. | Palta M, Godfrey D, Goodman KA, Hoffe S, Dawson LA, Dessert D, Hall WA, Herman JM, Khorana AA, Merchant N, Parekh A, Patton C, Pepek JM, Salama JK, Tuli R, Koong AC. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2019;9:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 30. | Kothary N, Heit JJ, Louie JD, Kuo WT, Loo BW Jr, Koong A, Chang DT, Hovsepian D, Sze DY, Hofmann LV. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Slagowski JM, Colbert LE, Cazacu IM, Singh BS, Martin R, Koay EJ, Taniguchi CM, Koong AC, Bhutani MS, Herman JM, Beddar S. Evaluation of the Visibility and Artifacts of 11 Common Fiducial Markers for Image Guided Stereotactic Body Radiation Therapy in the Abdomen. Pract Radiat Oncol. 2020;10:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Kerdsirichairat T, Narang AK, Thompson E, Kim SH, Rao A, Ding K, Shin EJ. Feasibility of Using Hydrogel Spacers for Borderline-Resectable and Locally Advanced Pancreatic Tumors. Gastroenterology. 2019;157:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Rao AD, Shin EJ, Meyer J, Thompson EL, Fu W, Hu C, Fishman EK, Weiss M, Wolfgang C, Burkhart R, He J, Kerdsirichairat T, Herman JM, Ding K, Narang A. Evaluation of a Novel Absorbable Radiopaque Hydrogel in Patients Undergoing Image Guided Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma. Pract Radiat Oncol. 2020;10:e508-e513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Sun S, Ge N, Wang S, Liu X, Wang G, Guo J. Pilot trial of endoscopic ultrasound-guided interstitial chemoradiation of UICC-T4 pancreatic cancer. Endosc Ultrasound. 2012;1:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Sun X, Lu Z, Wu Y, Min M, Bi Y, Shen W, Xu Y, Li Z, Jin Z, Liu Y. An endoscopic ultrasonography-guided interstitial brachytherapy based special treatment-planning system for unresectable pancreatic cancer. Oncotarget. 2017;8:79099-79110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Li W, Wang X, Wang Z, Zhang T, Cai F, Tang P, Meng J, Du H, Wang H, Li M, Li S. The role of seed implantation in patients with unresectable pancreatic carcinoma after relief of obstructive jaundice using ERCP. Brachytherapy. 2020;19:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Bhutani MS, Cazacu IM, Luzuriaga Chavez AA, Singh BS, Wong FCL, Erwin WD, Tamm EP, Mathew GG, Le DB, Koay EJ, Taniguchi CM, Minsky BD, Pant S, Tzeng CD, Koong AC, Varadhachary GR, Katz MHG, Wolff RA, Fogelman DR, Herman JM. Novel EUS-guided brachytherapy treatment of pancreatic cancer with phosphorus-32 microparticles: first United States experience. VideoGIE. 2019;4:223-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Bhutani MS, Klapman JB, Tuli R, El-Haddad G, Hoffe S, Wong FCL, Chasen B, Fogelman DR, Lo SK, Nissen NN, Hendifar AE, Varadhachary G, Katz MHG, Erwin WD, Koay EJ, Tamm EP, Singh BS, Mehta R, Wolff RA, Soman A, Cazacu IM, Herman JM. An open-label, single-arm pilot study of EUS-guided brachytherapy with phosphorus-32 microparticles in combination with gemcitabine +/- nab-paclitaxel in unresectable locally advanced pancreatic cancer (OncoPaC-1): Technical details and study protocol. Endosc Ultrasound. 2020;9:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Gasser O, Sharples KJ, Barrow C, Williams GM, Bauer E, Wood CE, Mester B, Dzhelali M, Caygill G, Jones J, Hayman CM, Hinder VA, Macapagal J, McCusker M, Weinkove R, Painter GF, Brimble MA, Findlay MP, Dunbar PR, Hermans IF. A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients. Cancer Immunol Immunother. 2018;67:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Marabelle A, Tselikas L, de Baere T, Houot R. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol. 2017;28:xii33-xii43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 41. | Soldevilla MM, Villanueva H, Martinez-Velez N, Meraviglia-Crivelli D, Alonso MM, Cebollero J, Menon AP, Puigdelloses M, Pastor F. Intratumoral injection of activated B lymphoblast in combination with PD-1 blockade induces systemic antitumor immunity with reduction of local and distal tumors. Oncoimmunology. 2018;7:e1450711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Laurell A, Lönnemark M, Brekkan E, Magnusson A, Tolf A, Wallgren AC, Andersson B, Adamson L, Kiessling R, Karlsson-Parra A. Intratumorally injected pro-inflammatory allogeneic dendritic cells as immune enhancers: a first-in-human study in unfavourable risk patients with metastatic renal cell carcinoma. J Immunother Cancer. 2017;5:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Choi AH, O'Leary MP, Chaurasiya S, Lu J, Kim SI, Fong Y, Chen NG. Novel chimeric parapoxvirus CF189 as an oncolytic immunotherapy in triple-negative breast cancer. Surgery. 2018;163:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Kamigaki T, Takahara M, Maekawa R, Goto S. Zoledronate-pulsed dendritic cell-based anticancer vaccines. Oncoimmunology. 2013;2:e25636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Osada T, Nagaoka K, Takahara M, Yang XY, Liu CX, Guo H, Roy Choudhury K, Hobeika A, Hartman Z, Morse MA, Lyerly HK. Precision cancer immunotherapy: optimizing dendritic cell-based strategies to induce tumor antigen-specific T-cell responses against individual patient tumors. J Immunother. 2015;38:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 47. | Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, Ohto H, Ohira H. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Yamamoto K, Kaneko T, Nieda M, Yokokawa K, Goto H. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Bauer C, Dauer M, Saraj S, Schnurr M, Bauernfeind F, Sterzik A, Junkmann J, Jakl V, Kiefl R, Oduncu F, Emmerich B, Mayr D, Mussack T, Bruns C, Rüttinger D, Conrad C, Jauch KW, Endres S, Eigler A. Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot study. Cancer Immunol Immunother. 2011;60:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Endo H, Saito T, Kenjo A, Hoshino M, Terashima M, Sato T, Anazawa T, Kimura T, Tsuchiya T, Irisawa A, Ohira H, Hikichi T, Takagi T, Gotoh M. Phase I trial of preoperative intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients. J Hepatobiliary Pancreat Sci. 2012;19:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Kamigaki T, Goto S, Takahara M, Goto H. Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: a phase I/II trial. Oncotarget. 2018;9:2838-2847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2543] [Article Influence: 158.9] [Reference Citation Analysis (0)] |
| 53. | Caceres J, Munoz-Sagastibelza M, Hossian AKMN, Paredes J, Barrera K, Mattheolabakis G, Martello LA, Vignesh S. Evaluation of the feasibility of intrapancreatic delivery of drug-loaded microparticles via EUS-guided fine needle injection using a swine model. Endosc Int Open. 2019;7:E1008-E1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Arnoletti JP, Frolov A, Eloubeidi M, Keene K, Posey J, Wood T, Greeno E, Jhala N, Varadarajulu S, Russo S, Christein J, Oster R, Buchsbaum DJ, Vickers SM. A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2011;67:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. [PubMed] |
| 56. | Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H, Savage DT, Canto MI, Klapman J, Reid T, Shah RJ, Hoffe SE, Rosemurgy A, Wolfgang CL, Laheru DA. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 57. | Hanna N, Ohana P, Konikoff FM, Leichtmann G, Hubert A, Appelbaum L, Kopelman Y, Czerniak A, Hochberg A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 58. | Hirooka Y, Kasuya H, Ishikawa T, Kawashima H, Ohno E, Villalobos IB, Naoe Y, Ichinose T, Koyama N, Tanaka M, Kodera Y, Goto H. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer. 2018;18:596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 59. | Matsuda Y, Fujii Y, Matsukawa M, Ishiwata T, Nishimura M, Arai T. Overexpression of carbohydrate sulfotransferase 15 in pancreatic cancer stroma is associated with worse prognosis. Oncol Lett. 2019;18:4100-4105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Ito Z, Takakura K, Suka M, Kanai T, Saito R, Fujioka S, Kajihara M, Yanagisawa H, Misawa T, Akiba T, Koido S, Ohkusa T. Prognostic impact of carbohydrate sulfotransferase 15 in patients with pancreatic ductal adenocarcinoma. Oncol Lett. 2017;13:4799-4805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Crinò SF, D'Onofrio M, Bernardoni L, Frulloni L, Iannelli M, Malleo G, Paiella S, Larghi A, Gabbrielli A. EUS-guided Radiofrequency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J Gastrointestin Liver Dis. 2018;27:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Scopelliti F, Pea A, Conigliaro R, Butturini G, Frigerio I, Regi P, Giardino A, Bertani H, Paini M, Pederzoli P, Girelli R. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 64. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 65. | Paiella S, Malleo G, Cataldo I, Gasparini C, De Pastena M, De Marchi G, Marchegiani G, Rusev B, Scarpa A, Girelli R, Giardino A, Frigerio I, D'Onofrio M, Secchettin E, Bassi C, Salvia R. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbecks Arch Surg. 2018;403:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Bas-Cutrina F, Bargalló D, Gornals JB. Small pancreatic insulinoma: Successful endoscopic ultrasound-guided radiofrequency ablation in a single session using a 22-G fine needle. Dig Endosc. 2017;29:636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Eichler K, Zangos S, Gruber-Rouh T, Vogl TJ, Mack MG. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol. 2012;46:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Francica G, Iodice G, Delle Cave M, Sarrantonio R, Lapiccirella G, Molese V, Smeraldo D, Scarano F, De Marino F. Factors predicting complete necrosis rate after ultrasound-guided percutaneous laser thermoablation of small hepatocellular carcinoma tumors in cirrhotic patients: a multivariate analysis. Acta Radiol. 2007;48:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Ishikawa T, Zeniya M, Hokari A, Kawabe T, Suzuki K, Fujise K, Toda G. An experimental study on Nd-YAG laser induced thermotherapy. Its possible application of the laser irradiation for therapy of hepatocellular carcinoma. Hepatol Res. 2002;23:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Vogl TJ, Eichler K, Straub R, Engelmann K, Zangos S, Woitaschek D, Böttger M, Mack MG. Laser-induced thermotherapy of malignant liver tumors: general principals, equipment(s), procedure(s)--side effects, complications and results. Eur J Ultrasound. 2001;13:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Mou Y, Zhao Q, Zhong L, Chen F, Jiang T. Preliminary results of ultrasound-guided laser ablation for unresectable metastases to retroperitoneal and hepatic portal lymph nodes. World J Surg Oncol. 2016;14:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Elliott JT, Samkoe KS, Gunn JR, Stewart EE, Gardner TB, Tichauer KM, Lee TY, Hoopes PJ, Pereira SP, Hasan T, Pogue BW. Perfusion CT estimates photosensitizer uptake and biodistribution in a rabbit orthotopic pancreatic cancer model: a pilot study. Acad Radiol. 2015;22:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Zhang L, Tan S, Huang S, Zhong C, Lü M, Peng Y, Tang X. The safety and efficacy of endoscopic ultrasound-guided ablation therapy for solid pancreatic tumors: a systematic review. Scand J Gastroenterol. 2020;55:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Matsumoto K, Kato H, Kawano S, Fujiwara H, Nishida K, Harada R, Fujii M, Yoshida R, Umeda Y, Hinotsu S, Yagi T, Okada H. Efficacy and safety of scheduled early endoscopic ultrasonography-guided ethanol reinjection for patients with pancreatic neuroendocrine tumors: Prospective pilot study. Dig Endosc. 2020;32:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Paik WH, Seo DW, Dhir V, Wang HP. Safety and Efficacy of EUS-Guided Ethanol Ablation for Treating Small Solid Pancreatic Neoplasm. Medicine (Baltimore). 2016;95:e2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 79. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 80. | Koulouris AI, Alexandre L, Hart AR, Clark A. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology. 2021;21:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 82. | Lu F, Dong J, Tang Y, Huang H, Liu H, Song L, Zhang K. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Support Care Cancer. 2018;26:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Bahra M, Jacob D. Surgical palliation of advanced pancreatic cancer. Recent Results Cancer Res. 2008;177:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Ge PS, Young JY, Dong W, Thompson CC. EUS-guided gastroenterostomy vs enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 85. | Xu G, Shen Y, Lv Y, Zhou X, Li W, Wang Y, Hassan S, Wang L, Zou X. Safety and efficacy of endoscopic ultrasound-guided gastroenterostomy using double balloon occlusion methods: a clinical retrospective study in 36 patients with malignant gastric outlet obstruction. Endosc Int Open. 2020;8:E1690-E1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Chen YI, Itoi T, Baron TH, Nieto J, Haito-Chavez Y, Grimm IS, Ismail A, Ngamruengphong S, Bukhari M, Hajiyeva G, Alawad AS, Kumbhari V, Khashab MA. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 87. | Perez-Miranda M, Tyberg A, Poletto D, Toscano E, Gaidhane M, Desai AP, Kumta NA, Fayad L, Nieto J, Barthet M, Shah R, Brauer BC, Sharaiha RZ, Kahaleh M. EUS-guided Gastrojejunostomy Versus Laparoscopic Gastrojejunostomy: An International Collaborative Study. J Clin Gastroenterol. 2017;51:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 88. | Fan W, Tan S, Wang J, Wang C, Xu H, Zhang L, Liu L, Fan Z, Tang X. Clinical outcomes of endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Kerdsirichairat T, Irani S, Yang J, Brewer Gutierrez OI, Moran R, Sanaei O, Dbouk M, Kumbhari V, Singh VK, Kalloo AN, Khashab MA. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7:E144-E150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 90. | Nadeau BA, Fecher LA, Owens SR, Razumilava N. Liver Toxicity with Cancer Checkpoint Inhibitor Therapy. Semin Liver Dis. 2018;38:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Rajha E, Chaftari P, Kamal M, Maamari J, Chaftari C, Yeung SJ. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol Rep (Oxf). 2020;8:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 92. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2592] [Article Influence: 370.3] [Reference Citation Analysis (0)] |
| 93. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264-iv266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 94. | Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Corrigendum: Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol. 2017;8:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Hsu C, Marshall JL, He AR. Workup and Management of Immune-Mediated Hepatobiliary Pancreatic Toxicities That Develop During Immune Checkpoint Inhibitor Treatment. Oncologist. 2020;25:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | McCarty TR, Bazarbashi AN, Njei B, Ryou M, Aslanian HR, Muniraj T. Endoscopic Ultrasound-Guided, Percutaneous, and Transjugular Liver Biopsy: A Comparative Systematic Review and Meta-Analysis. Clin Endosc. 2020;53:583-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Khurana S, Butt W, Khara HS, Johal AS, West SF, Chen ZE, Berger AL, Diehl DL. Bi-lobar liver biopsy via EUS enhances the assessment of disease severity in patients with non-alcoholic steatohepatitis. Hepatol Int. 2019;13:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Bang JY, Ward TJ, Guirguis S, Krall K, Contreras F, Jhala N, Navaneethan U, Hawes RH, Varadarajulu S. Radiology-guided percutaneous approach is superior to EUS for performing liver biopsies. Gut. 2021;70:2224-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |